The Effect of Cu2+ Exposure on the Nrf2 Signaling Pathway of Tilapia Hepatocyte, Base on Experiments In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Primary Culture of Tilapia Hepatocytes
2.3. The Effect of Copper Ions on the Hepatocyte Viability of Tilapia
2.4. MTT Assay for Cell Viability
2.5. Biochemical Indices
2.6. qPCR
2.7. Statistical Analysis
3. Results
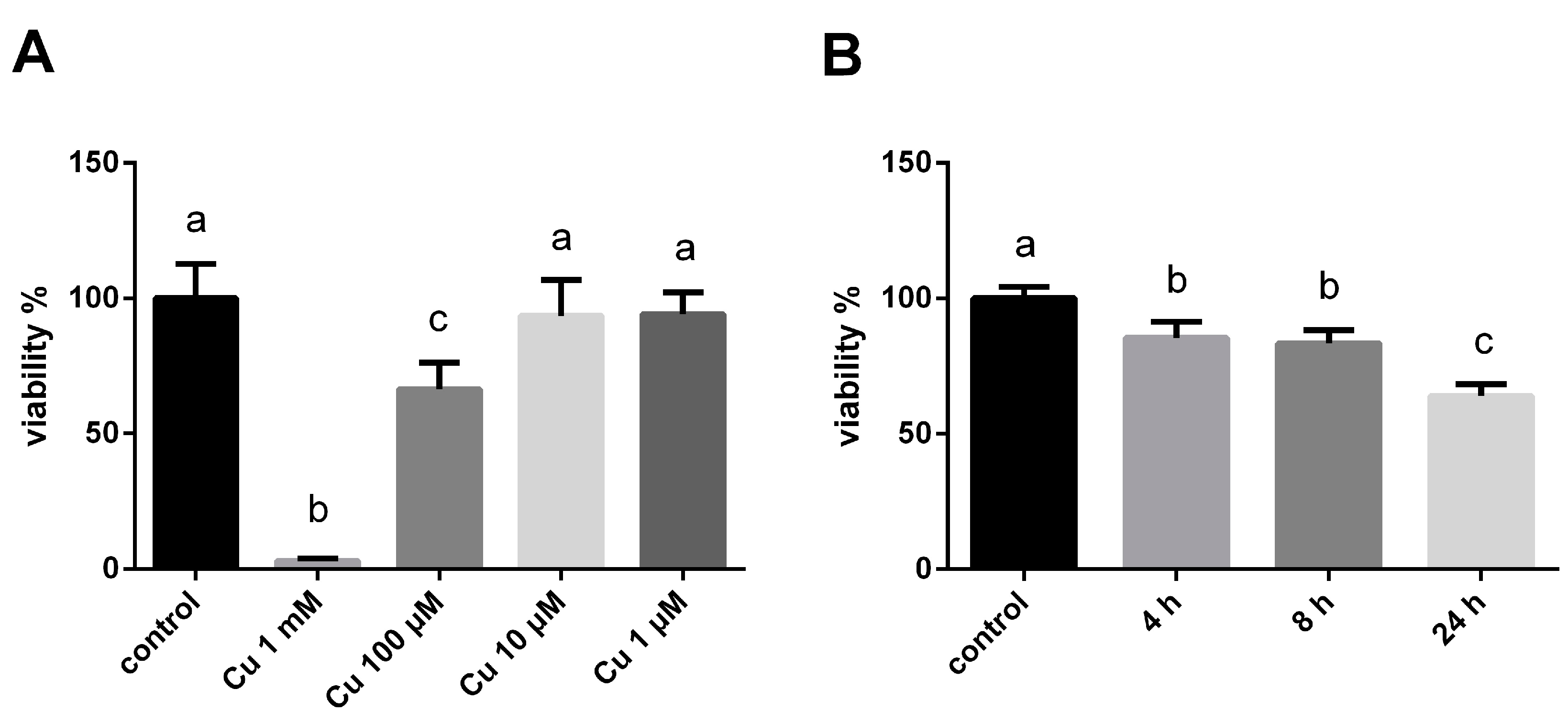
3.1. The Effect of Copper Ions on the Hepatocyte Activity of Tilapia
3.2. The Effect of Copper Ions on the Biochemical Indices of Tilapia Hepatocytes
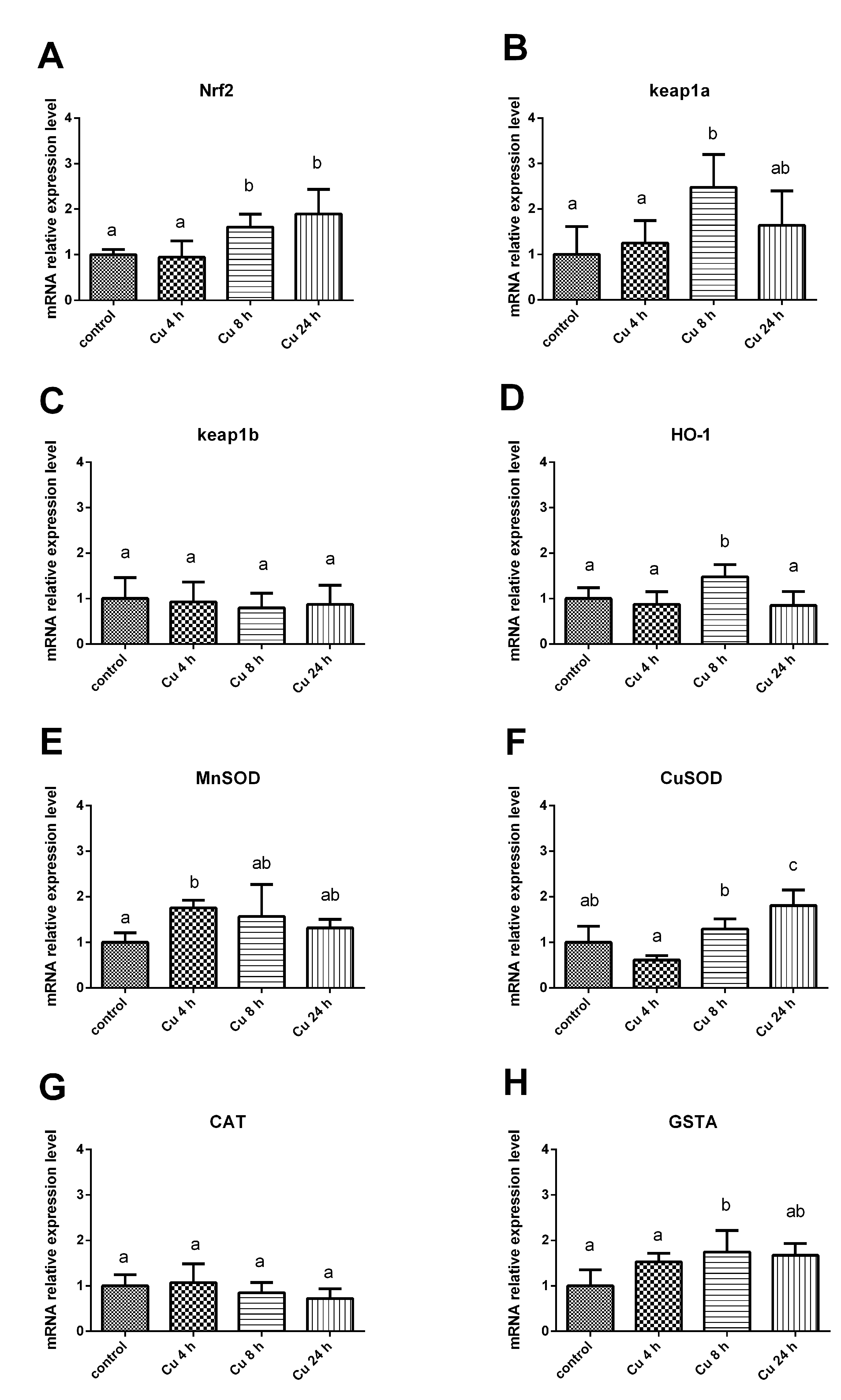
3.3. The Effect of Copper Ions on Genes Expression in Nrf2 Signaling Pathway of Tilapia Hepatocytes
4. Discussion
4.1. Copper Inhibited the Activity of Tilapia Hepatocytes and Caused Oxidative Stress
4.2. Activate Nrf2 Signaling Pathway to Resist Oxidative Stress Caused by Copper Ions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, M.Q.; Cui, H.; Fang, W.H.; Hu, H.J.; Miao, L.; Jin, S.; Xie, J.S.; Ma, R.R. The effects of CuSO4 on Cryptocaryon irritans tomonts and its potential mechanism. Aquaculture 2022, 560, 738578. [Google Scholar] [CrossRef]
- Yang, Z.G.; Lian, W.; Waiho, K.; Zhu, L.L.; Chen, A.Q.; Cheng, Y.X.; Wang, Y.J. Effects of copper exposure on lipid metabolism and SREBP pathway in the Chinese mitten crab Eriocheir sinensis. Chemosphere 2022, 308, 136556. [Google Scholar] [CrossRef] [PubMed]
- Monier, M.N.; El-Naby, A.S.A.; Samir, F.; Abdel-Tawwab, M. Positive effects of dietary nanosized sodium butyrate on growth performance, immune, antioxidant indices, and resistance of Nile tilapia to waterborne copper toxicity. Aquac. Rep. 2022, 26, 101323. [Google Scholar] [CrossRef]
- Chen, S.J.; Liu, Y.T.; Xie, S.W.; Guo, Y.C.; Yang, H.R.; Wei, Y.R.; Xu, Q.; Ye, T.; Meng, B.S.; Huang, R.B.; et al. Role of myo-inositol supplementation against toxicity of excessive dietary copper in Pacific white shrimp Litopenaeus vannamei. Ecotoxicol. Environ. Saf. 2022, 241, 113712. [Google Scholar] [CrossRef]
- Liu, X.J.; Luo, Z.; Xiong, B.X.; Liu, X.; Zhao, Y.H.; Hu, G.F.; Lv, G.J. Effect of waterborne copper exposure on growth, hepatic enzymatic activities and histology in Synechogobius hasta. Ecotoxicol. Environ. Saf. 2010, 73, 1286–1291. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Yang, M.; Yang, F.; Wang, G.; Liu, D.; Li, X.; Yang, L.; Wang, Z. Copper-induced oxidative stress, transcriptome changes, intestinal microbiota, and histopathology of common carp (Cyprinus carpio). Ecotoxicol. Environ. Saf. 2022, 246, 114136. [Google Scholar] [CrossRef]
- Osburn, W.O.; Kensler, T.W. Nrf2 signaling: An adaptive response pathway for protection against environmental toxic insults. Mutat. Res. Rev. Mutat. Res. 2008, 659, 31–39. [Google Scholar] [CrossRef]
- Kobayashi, M.; Itoh, K.; Suzuki, T.; Osanai, H.; Nishikawa, K.; Katoh, Y.; Takagi, Y.; Yamamoto, M. Identification of the interactive interface and phylogenic conservation of the Nrf2-Keap1 system. Genes Cells 2002, 7, 807–820. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yamamoto, M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid. Redox Signal. 2005, 7, 385–394. [Google Scholar] [CrossRef]
- Bao, S.A.; Nie, X.P.; Ou, R.K.; Wang, C.; Ku, P.J.; Li, K.B. Effects of diclofenac on the expression of Nrf2 and its downstream target genes in mosquito fish (Gambusia affinis). Aquat. Toxicol. 2017, 188, 43–53. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, L.; Jiang, W.D.; Liu, Y.; Jiang, J.; Kuang, S.Y.; Li, S.W.; Tang, L.; Tang, W.E.; Zhou, X.Q.; et al. Dietary Vitamin A Improved the Flesh Quality of Grass Carp (Ctenopharyngodon idella) in Relation to the Enhanced Antioxidant Capacity through Nrf2/Keap 1a Signaling Pathway. Antioxidants 2022, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yuan, Y.M.; Dai, Y.Y.; Gong, Y.C. Economic profitability of tilapia farming in China. Aquac. Int. 2017, 25, 1253–1264. [Google Scholar] [CrossRef]
- Li, L.m.; Zhang, Z.p.; Huang, Y.f. Integrative transcriptome analysis and discovery of signaling pathways involved in the protective effects of curcumin against oxidative stress in tilapia hepatocytes. Aquat. Toxicol. 2020, 224, 105516. [Google Scholar] [CrossRef] [PubMed]
- Morozesk, M.; Franqui, L.S.; Mansano, A.S.; Martinez, D.S.T.; Fernandes, M.N. Interactions of oxidized multiwalled carbon nanotube with cadmium on zebrafish cell line: The influence of two co-exposure protocols on in vitro toxicity tests. Aquat. Toxicol. 2018, 200, 136–147. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.T.; Sun, Y.L.; Guo, M.X.; Feng, J.J.; Wang, Y.L.; Zhang, Z.P. Regulatory effect of heat shock transcription factor-1 gene on heat shock proteins and its transcriptional regulation analysis in small abalone Haliotis diversicolor. BMC Mol. Cell Biol. 2020, 21, 83. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.L.; Luo, Z.; Zhuo, M.Q.; Tan, X.Y.; Sun, L.D.; Zheng, J.L.; Chen, Q.L. In vitro exposure to copper influences lipid metabolism in hepatocytes from grass carp (Ctenopharyngodon idellus). Fish Physiol. Biochem. 2014, 40, 595–605. [Google Scholar] [CrossRef]
- Wang, T.; Chen, X.; Long, X.; Liu, Z.; Yan, S. Copper Nanoparticles and Copper Sulphate Induced Cytotoxicity in Hepatocyte Primary Cultures of Epinephelus coioides. PLoS ONE 2016, 11, e0149484. [Google Scholar] [CrossRef]
- Yin, G.J.; Cao, L.P.; Xu, P.; Jeney, G.; Nakao, M.; Lu, C.P. Hepatoprotective and antioxidant effects of Glycyrrhiza glabra extract against carbon tetrachloride (CCl4)-induced hepatocyte damage in common carp (Cyprinus carpio). Fish Physiol. Biochem. 2011, 37, 209–216. [Google Scholar] [CrossRef]
- Shaw, B.J.; Al-Bairuty, G.; Handy, R.D. Effects of waterborne copper nanoparticles and copper sulphate on rainbow trout, (Oncorhynchus mykiss): Physiology and accumulation. Aquat. Toxicol. 2012, 116–117, 90–101. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, Q.B.; Lin, C.G.; He, L.; Wei, L.L. Histological alterations, oxidative stress, and inflammatory response in the liver of swamp eel (Monopterus albus) acutely exposed to copper. Fish Physiol. Biochem. 2021, 47, 1865–1878. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, J.; Zhang, J.; Chen, H.; Li, D.; Li, L.; Cao, J.; Xie, L.; Luo, Y. Effects of dietary Cu and Zn on the accumulation, oxidative stress and the expressions of immune-related genes in the livers of Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2020, 100, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Akbary, P.; Sartipi Yarahmadi, S.; Jahanbakhshi, A. Hematological, hepatic enzymes’ activity and oxidative stress responses of gray mullet (Mugil cephalus) after sub-acute exposure to copper oxide. Environ. Sci. Pollut. Res. Int. 2018, 25, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Dinkova Kostova, A.T. The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.K.; Itoh, K.; Yamamoto, M.; Kensler, T.W. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: Role of antioxidant response element-like sequences in the nrf2 promoter. Mol. Cell. Biol. 2002, 22, 2883–2892. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; He, X.; Fang, H.; Liao, S.; Liu, Y.; Tian, L.; Niu, J. Identification of heme oxygenase-1 from golden pompano (Trachinotus ovatus) and response of Nrf2/HO-1 signaling pathway to copper-induced oxidative stress. Chemosphere 2020, 253, 126654. [Google Scholar] [CrossRef]
- Liang, H.L.; Ji, K.; Ge, X.P.; Mi, H.F.; Xi, B.W.; Ren, M.C. Effects of dietary copper on growth, antioxidant capacity and immune responses of juvenile blunt snout bream (Megalobrama amblycephala) as evidenced by pathological examination. Aquac. Rep. 2020, 17, 100296. [Google Scholar] [CrossRef]
- Jiang, W.D.; Liu, Y.; Hu, K.; Jiang, J.; Li, S.H.; Feng, L.; Zhou, X.Q. Copper exposure induces oxidative injury, disturbs the antioxidant system and changes the Nrf2/ARE (CuZnSOD) signaling in the fish brain: Protective effects of myo-inositol. Aquat. Toxicol. 2014, 155, 301–313. [Google Scholar] [CrossRef]
- Wu, L.; Yu, Q.G.; Zhang, G.; Wu, F.L.; Zhang, Y.Y.; Yuan, C.; Zhang, T.; Wang, Z.Z. Single and combined exposures of waterborne Cu and Cd induced oxidative stress responses and tissue injury in female rare minnow (Gobiocypris rarus). Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2019, 222, 90–99. [Google Scholar] [CrossRef]
- Lee, O.H.; Jain, A.K.; Papusha, V.; Jaiswal, A.K. An auto-regulatory loop between stress sensors INrf2 and Nrf2 controls their cellular abundance. J. Biol. Chem. 2007, 282, 36412–36420. [Google Scholar] [CrossRef]
- Giuliani, M.E.; Regoli, F. Identification of the Nrf2-Keap1 pathway in the European eel Anguilla anguilla: Role for a transcriptional regulation of antioxidant genes in aquatic organisms. Aquat. Toxicol. 2014, 150, 117–123. [Google Scholar] [CrossRef]
- Wang, B.; Feng, L.; Jiang, W.D.; Wu, P.; Kuang, S.Y.; Jiang, J.; Tang, L.; Tang, W.N.; Zhang, Y.A.; Liu, Y.; et al. Copper-induced tight junction mRNA expression changes, apoptosis and antioxidant responses via NF-κB, TOR and Nrf2 signaling molecules in the gills of fish: Preventive role of arginine. Aquat. Toxicol. 2015, 158, 125–137. [Google Scholar] [CrossRef] [PubMed]

| Gene | Acc. NO. (GenBank) | Primers | Primer Sequence (5′–3′) |
|---|---|---|---|
| Keap1a | XM_003442916.5 | Keap1a-f | CCCTCAACATCCCCAGGAAC |
| Keap1a-r | GGATCCCACTTTTCCACCGT | ||
| Keap1b | XM_003447926.4 | Keap1b-f | CGGTGGAGCGCTATGATGTA |
| Keap1b-r | GCCATCGTAACCTCCCAACA | ||
| HO-1 | XM_013272963.3 | HO-1-f | GACAGGAACTCGGACCATCC |
| HO-1-r | AGTGGCTGCAGGAATGACAA | ||
| MnSOD | XM_003449940.5 | MnSOD-f | TCACAGCAAGCACCATGCTA |
| MnSOD-r | ATGTGGCCGCCTCCATTAAA | ||
| CuZnSOD | XM_003446807.5 | CuZnSOD-f | GGAGACAACACAAACGGGTG |
| CuZnSOD-r | TCTGCTGCAGTCACATTCCC | ||
| CAT | XM_003447521.5 | CAT-f | GGACCATTATTCGAGCCATCAG |
| CAT-r | AAACTGCAAGTGCTGCTGAAA | ||
| β-ACTIN | XM_003443127.5 | β-ACTIN-f | TGCGGGATATCATTTGCCTGA |
| β-ACTIN-r | GAATCCGGCCTTGCACATAC | ||
| Nrf2 | XM_003447296.3 | Nrf2-f | CAGCCCAGAACTGCCGTAAA |
| Nrf2-r | GCCAAAGACCTCCAGGTACA | ||
| GSTA | XM_019350593.2 | GSTA-f | GACAGACCCAGCATCAAAGC |
| GSTA-r | CTCATCAGCTGACAAGCCAATC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Wang, R.; Zhang, Z. The Effect of Cu2+ Exposure on the Nrf2 Signaling Pathway of Tilapia Hepatocyte, Base on Experiments In Vitro. Fishes 2023, 8, 280. https://doi.org/10.3390/fishes8060280
Li L, Wang R, Zhang Z. The Effect of Cu2+ Exposure on the Nrf2 Signaling Pathway of Tilapia Hepatocyte, Base on Experiments In Vitro. Fishes. 2023; 8(6):280. https://doi.org/10.3390/fishes8060280
Chicago/Turabian StyleLi, Linming, Ruoxuan Wang, and Ziping Zhang. 2023. "The Effect of Cu2+ Exposure on the Nrf2 Signaling Pathway of Tilapia Hepatocyte, Base on Experiments In Vitro" Fishes 8, no. 6: 280. https://doi.org/10.3390/fishes8060280
APA StyleLi, L., Wang, R., & Zhang, Z. (2023). The Effect of Cu2+ Exposure on the Nrf2 Signaling Pathway of Tilapia Hepatocyte, Base on Experiments In Vitro. Fishes, 8(6), 280. https://doi.org/10.3390/fishes8060280





