Seasonal Length–Weight Relationships of European Sea Bass (Dicentrarchus labrax) in Two Aquaculture Production Systems
Abstract
1. Introduction
2. Materials and Methods
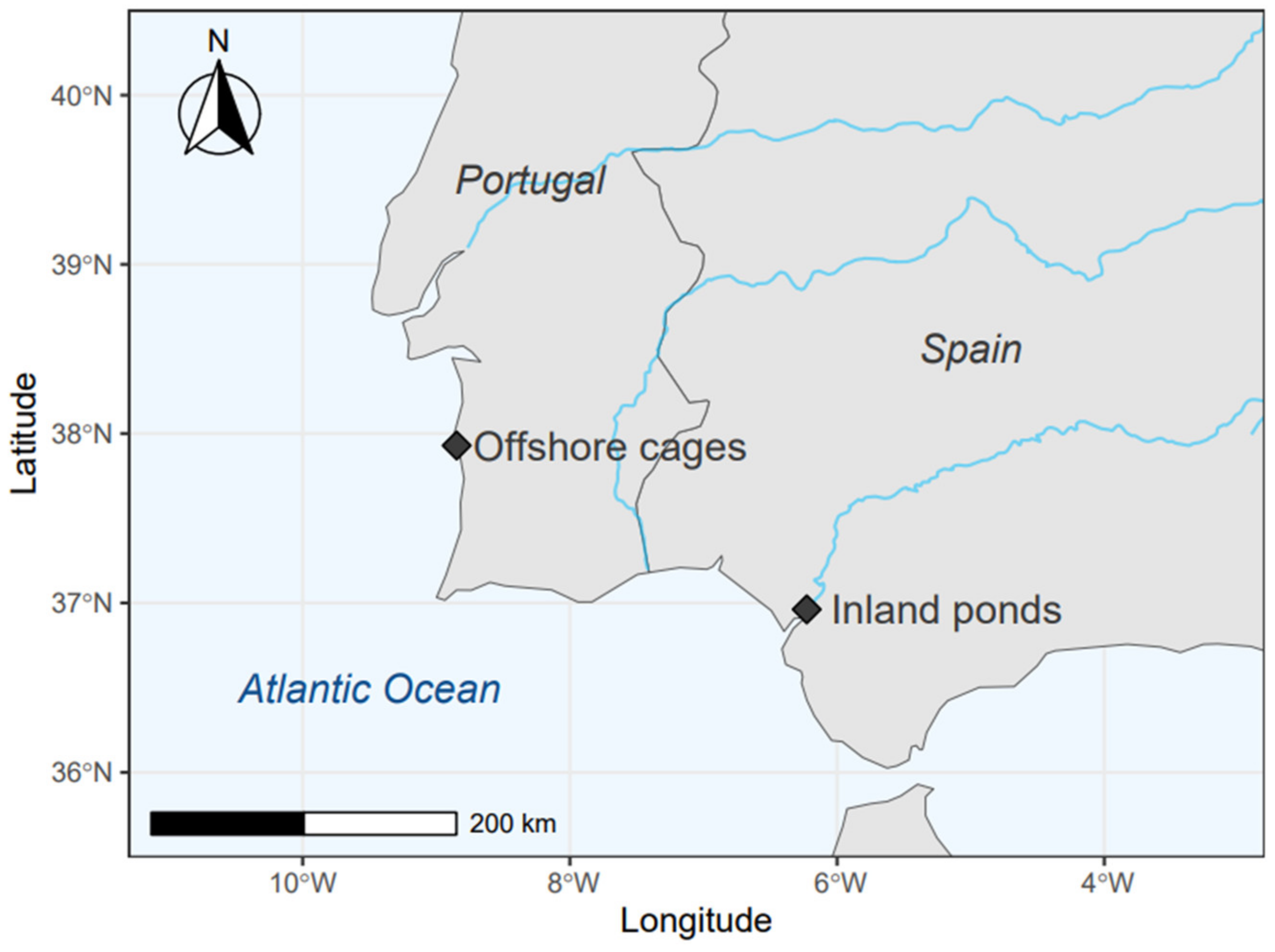
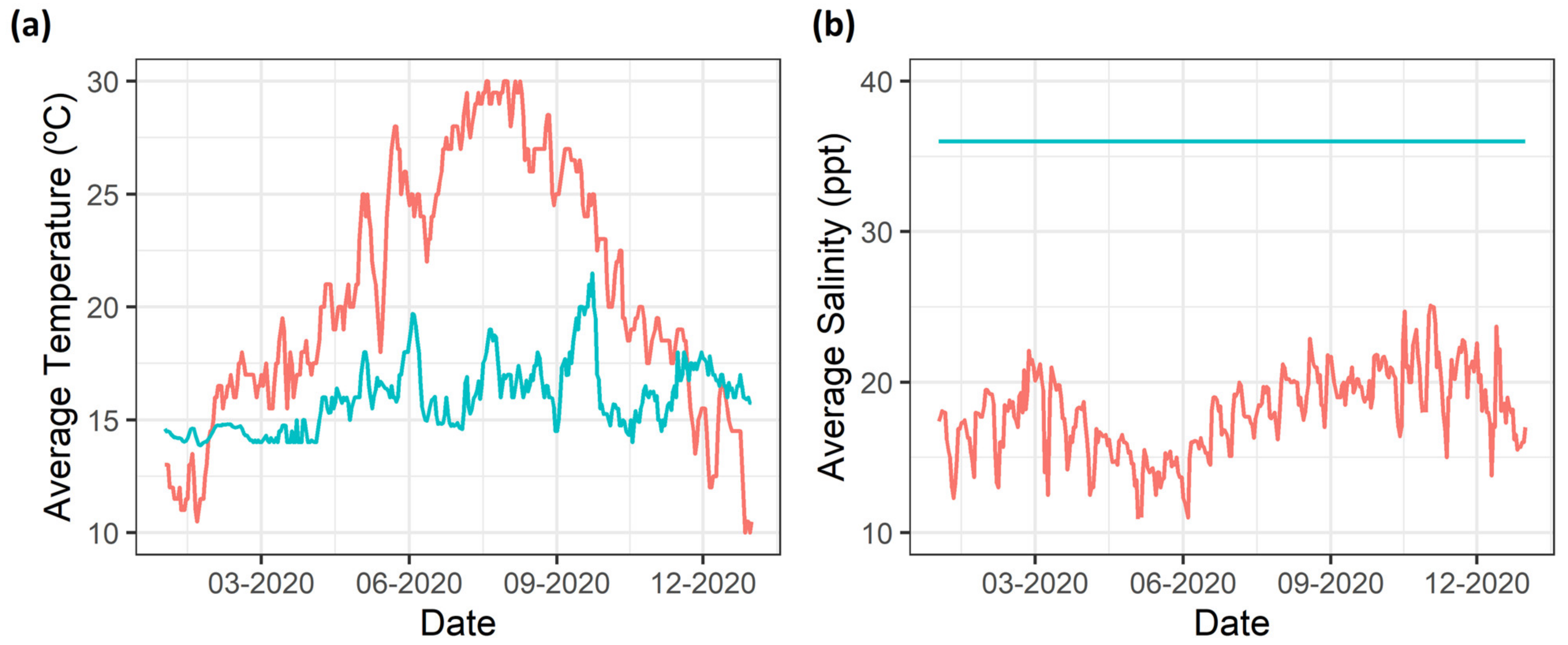
2.1. Samplings
2.2. Data Analysis
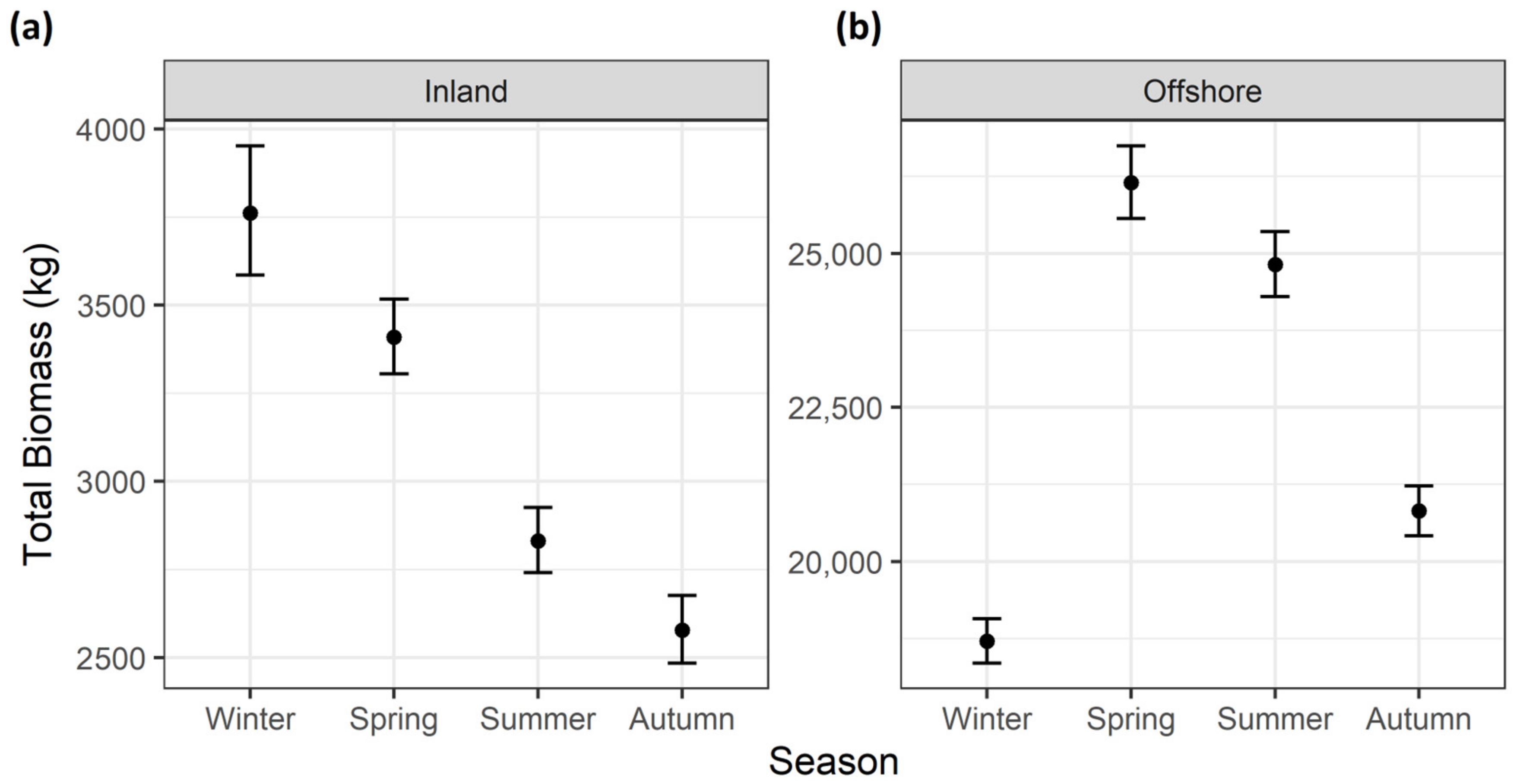
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Brugére, C.; Ridler, N. Global Aquaculture Outlook in the next Decades: An Analysis of National Aquaculture Production Forecasts to 2030. FAO Fish. Circ. 2004, 1001, 47. [Google Scholar]
- Zhou, C.; Xu, D.; Lin, K.; Sun, C.; Yang, X. Intelligent Feeding Control Methods in Aquaculture with an Emphasis on Fish: A Review. Rev. Aquac. 2018, 10, 975–993. [Google Scholar] [CrossRef]
- An, D.; Hao, J.; Wei, Y.; Wang, Y.; Yu, X. Application of Computer Vision in Fish Intelligent Feeding System—A Review. Aquac. Res. 2021, 52, 423–437. [Google Scholar] [CrossRef]
- Goldburg, R.; Naylor, R. Future Seascapes, Fishing, and Fish Farming. Front. Ecol. Environ. 2005, 3, 21–28. [Google Scholar] [CrossRef]
- Orduna, C.; Encina, L.; Rodríguez-Ruiz, A.; Rodríguez-Sánchez, V. Testing of New Sampling Methods and Estimation of Size Structure of Sea Bass (Dicentrarchus labrax) in Aquaculture Farms Using Horizontal Hydroacoustics. Aquaculture 2021, 545, 737242. [Google Scholar] [CrossRef]
- Soliveres, E.; Puig, V.; Perez-arjona, I. Acoustical Biomass Estimation Results in Mediterranean Aquaculture Sea Cages. In Proceedings of the UA2014—2nd International Conference and Exhibition on Underwater Acoustics, Rhodes, Greece, 22–27 June 2014; pp. 1423–1428. [Google Scholar]
- Pérez, J.M.; Sarasa, M.; Moço, G.; Granados, J.E.; Crampe, J.P.; Serrano, E.; Maurino, L.; Meneguz, P.G.; Afonso, A.; Alpizar-Jara, R. The Effect of Data Analysis Strategies in Density Estimation of Mountain Ungulates Using Distance Sampling. Ital. J. Zool. 2015, 82, 262–270. [Google Scholar] [CrossRef]
- Hofmeester, T.R.; Rowcliffe, J.M.; Jansen, P.A. A Simple Method for Estimating the Effective Detection Distance of Camera Traps. Remote Sens. Ecol. Conserv. 2016, 3, 81–89. [Google Scholar] [CrossRef]
- Føre, M.; Alver, M.; Alfredsen, J.A.; Marafioti, G.; Senneset, G.; Birkevold, J.; Willumsen, F.V.; Lange, G.; Espmark, Å.; Terjesen, B.F. Modelling Growth Performance and Feeding Behaviour of Atlantic Salmon (Salmo salar L.) in Commercial-Size Aquaculture Net Pens: Model Details and Validation through Full-Scale Experiments. Aquaculture 2016, 464, 268–278. [Google Scholar] [CrossRef]
- Orduna, C.; Encina, L.; Rodríguez-Ruiz, A.; Rodríguez-Sánchez, V. Hydroacoustics for Density and Biomass Estimations in Aquaculture Ponds. Aquaculture 2021, 545, 737240. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, V.; Rodríguez-Ruiz, A.; Pérez-Arjona, I.; Encina-Encina, L. Horizontal Target Strength-Size Conversion Equations for Sea Bass and Gilt-Head Bream. Aquaculture 2018, 490, 178–184. [Google Scholar] [CrossRef]
- Harvey, E.; Cappo, M.; Shortis, M.; Robson, S.; Buchanan, J.; Speare, P. The Accuracy and Precision of Underwater Measurements of Length and Maximum Body Depth of Southern Bluefin Tuna (Thunnus maccoyii) with a Stereo-Video Camera System. Fish. Res. 2003, 63, 315–326. [Google Scholar] [CrossRef]
- Espinosa, V.; Ramis, J.; Alba, J. Evaluación de La Sonda Ultrasónica EY-500 de Simrad Para El Control de Explotaciones de Dorada Sparus Auratus Linnaeus, 1758. Bol. Inst. Esp. Oceanogr. 2002, 18, 15–19. [Google Scholar]
- Conti, S.G.; Roux, P.; Fauvel, C.; Maurer, B.D.; Demer, D.A. Acoustical Monitoring of Fish Density, Behavior, and Growth Rate in a Tank. Aquaculture 2006, 251, 314–323. [Google Scholar] [CrossRef]
- The Humane Society of the United States. The Welfare of Animals in the Aquaculture Industry. Hsus Rep. 2008, 5, 24. [Google Scholar]
- Godlewska, M.; Frouzova, J.; Kubecka, J.; Wiśniewolski, W.; Szlakowski, J. Comparison of Hydroacoustic Estimates with Fish Census in Shallow Malta Reservoir—Which TS/L Regression to Use in Horizontal Beam Applications? Fish. Res. 2012, 123, 90–97. [Google Scholar] [CrossRef]
- Dennerline, D.E.; Jennings, C.A.; Degan, D.J. Relationships between Hydroacoustic Derived Density and Gill Net Catch: Implications for Fish Assessments. Fish. Res. 2012, 123, 78–89. [Google Scholar] [CrossRef]
- Greenstreet, S.P.R.; Holland, G.J.; Guirey, E.J.; Armstrong, E.; Fraser, H.M.; Gibb, I.M. Combining Hydroacoustic Seabed Survey and Grab Sampling Techniques to Assess “Local” Sandeel Population Abundance. ICES J. Mar. Sci. 2010, 67, 971–984. [Google Scholar] [CrossRef]
- Koliada, I.; Balk, H.; Tušer, M.; Ptáček, L.; Kubečka, J. Limitations of Target Detection in Horizontal Acoustic Surveys of Extremely Shallow Water Bodies. Fish. Res. 2019, 218, 94–104. [Google Scholar] [CrossRef]
- Simmonds, J.; MacLennan, D. Fisheries Acoustics: Theory and Practice; Blackwell Science Ltd.: Hoboken, NJ, USA, 2005; ISBN 978-0-632-05994-2. [Google Scholar]
- Rodríguez-Sánchez, V.; Encina-Encina, L.; Rodríguez-Ruiz, A.; Sánchez-Carmona, R. Do Close Range Measurements Affect the Target Strength (TS) of Fish in Horizontal Beaming Hydroacoustics? Fish. Res. 2016, 173, 4–10. [Google Scholar] [CrossRef]
- Lilja, J.; Marjomäki, T.J.; Riikonen, R.; Jurvelius, J. Side-Aspect Target Strength of Atlantic Salmon (Salmo salar), Brown Trout (Salmo trutta), Whitefish (Coregonus lavaretus), and Pike (Esox lucius). Aquat. Living Resour. 2000, 13, 355–360. [Google Scholar] [CrossRef]
- Frouzova, J.; Kubecka, J.; Balk, H.; Frouz, J. Target Strength of Some European Fish Species and Its Dependence on Fish Body Parameters. Fish. Res. 2005, 75, 86–96. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, V.; Encina-Encina, L.; Rodríguez-Ruiz, A.; Monteoliva, A.; Sánchez-Carmona, R. Horizontal Target Strength of Cyprinus Carpio Using 200 kHz and 430 kHz Split-Beam Systems. Fish. Res. 2016, 174, 136–142. [Google Scholar] [CrossRef]
- Harvey, E.; Fletcher, D.; Shortis, M. Estimation of Reef Fish Length by Divers and by Stereo-Video. Fish. Res. 2002, 57, 255–265. [Google Scholar] [CrossRef]
- Costa, C.; Loy, A.; Cataudella, S.; Davis, D.; Scardi, M. Extracting Fish Size Using Dual Underwater Cameras. Aquac. Eng. 2006, 35, 218–227. [Google Scholar] [CrossRef]
- De La Gandara, F.; Espinosa, V. Estimación Del Número y La Biomasa de Individuos Por Métodos Acústicos En El Cultivo Del Atún Rojo (Thunnus thynnus). Collect. Vol. Sci. Pap. ICCAT 2012, 68, 276–283. [Google Scholar]
- Islamadina, R.; Pramita, N.; Arnia, F.; Munadi, K. Estimating Fish Weight Based on Visual Captured. In Proceedings of the 2018 International Conference on Information and Communications Technology (ICOIACT), Yogyakarta, Indonesia, 6–7 March 2018; pp. 366–372. [Google Scholar] [CrossRef]
- Muñoz-Benavent, P.; Andreu-García, G.; Valiente-González, J.M.; Atienza-Vanacloig, V.; Puig-Pons, V.; Espinosa, V. Enhanced Fish Bending Model for Automatic Tuna Sizing Using Computer Vision. Comput. Electron. Agric. 2018, 150, 52–61. [Google Scholar] [CrossRef]
- Martinez-De Dios, J.R.; Serna, C.; Ollero, A. Computer Vision and Robotics Techniques in Fish Farms. Robotica 2003, 21, 233–243. [Google Scholar] [CrossRef]
- Person-Le Ruyet, J.; Mahé, K.; Le Bayon, N.; Le Delliou, H. Effects of Temperature on Growth and Metabolism in a Mediterranean Population of European Sea Bass, Dicentrarchus labrax. Aquaculture 2004, 237, 269–280. [Google Scholar] [CrossRef]
- Llorente, I.; Fernández-Polanco, J.; Baraibar-Diez, E.; Odriozola, M.D.; Bjørndal, T.; Asche, F.; Guillen, J.; Avdelas, L.; Nielsen, R.; Cozzolino, M.; et al. Assessment of the Economic Performance of the Seabream and Seabass Aquaculture Industry in the European Union. Mar. Policy 2020, 117, 103876. [Google Scholar] [CrossRef]
- Elliot, R. Faking Nature. Inquiry 1982, 25, 81–93. [Google Scholar] [CrossRef]
- Hidalgo, F.; Alliot, E.; Thebault, H. Influence of Water Temperature on Food Intake, Food Efficiency and Gross Composition of Juvenile Sea Bass, Dicentrarchus labrax. Aquaculture 1987, 64, 199–207. [Google Scholar] [CrossRef]
- Deane, E.E.; Woo, N.Y.S. Modulation of Fish Growth Hormone Levels by Salinity, Temperature, Pollutants and Aquaculture Related Stress: A Review. Rev. Fish Biol. Fish. 2009, 19, 97–120. [Google Scholar] [CrossRef]
- Besson, M.; Vandeputte, M.; van Arendonk, J.A.M.; Aubin, J.; de Boer, I.J.M.; Quillet, E.; Komen, H. Influence of Water Temperature on the Economic Value of Growth Rate in Fish Farming: The Case of Sea Bass (Dicentrarchus labrax) Cage Farming in the Mediterranean. Aquaculture 2016, 462, 47–55. [Google Scholar] [CrossRef]
- Claireaux, G.; Lagardère, J.P. Influence of Temperature, Oxygen and Salinity on the Metabolism of the European Sea Bass. J. Sea Res. 1999, 42, 157–168. [Google Scholar] [CrossRef]
- Reynolds, W.W.; Casterlin, M.E. Behavioral Thermoregulation and the “Final Preferendum” Paradigm. Integr. Comp. Biol. 1979, 19, 211–224. [Google Scholar] [CrossRef]
- Zanuy, S.; Carrillo, M. Annual Cycles of Growth, Feeding Rate, Gross Conversion Efficiency and Hematocrit Levels of Sea Bass (Dicentrarchus labrax L.) Adapted to Two Different Osmotic Media. Aquaculture 1985, 44, 11–25. [Google Scholar] [CrossRef]
- Dalla Via, J.; Villani, P.; Gasteiger, E.; Niederstätter, H. Oxygen Consumption in Sea Bass Fingerling Dicentrarchus labrax Exposed to Acute Salinity and Temperature Changes: Metabolic Basis for Maximum Stocking Density Estimations. Aquaculture 1998, 169, 303–313. [Google Scholar] [CrossRef]
- Von Oertzen, J.A. Influence of Steady-State and Fluctuating Salinities on the Oxygen Consumption and Activity of Some Brackish Water Shrimps and Fishes. J. Exp. Mar. Biol. Ecol. 1984, 80, 29–46. [Google Scholar] [CrossRef]
- Rao, G.M.M. Oxygen Consumption of Rainbow Trout (Salmo gairdneri) in Relation to Activity and Salinity. Can. J. Zool. 1968, 46, 781–786. [Google Scholar] [CrossRef]
- Farmer, G.J.; Beamish, F.W.H. Oxygen Consumption of Tilapia Nilotica in Relation to Swimming Speed and Salinity. J. Fish. Res. Board Canada 1969, 26, 2807–2821. [Google Scholar] [CrossRef]
- Boyd, C.E.; Torrans, E.L.; Tucker, C.S. Dissolved Oxygen and Aeration in Ictalurid Catfish Aquaculture. J. World Aquac. Soc. 2018, 49, 7–70. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, W. Design of Intelligent Monitoring System for Aquaculture Water Dissolved Oxygen. In Proceedings of the 2018 IEEE 3rd Advanced Information Technology, Electronic and Automation Control Conference (IAEAC), Chongqing, China, 12–14 October 2018; pp. 414–418. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Wu, J.; Zhu, L.; Yue, J. A Hybrid Model for Dissolved Oxygen Prediction in Aquaculture Based on Multi-Scale Features. Inf. Process. Agric. 2018, 5, 11–20. [Google Scholar] [CrossRef]
- Dendrinos, P.; Thorpe, J.P. Effects of Reduced Salinity on Growth and Body Composition in the European Bass Dicentrarchus labrax (L.). Aquaculture 1985, 49, 333–358. [Google Scholar] [CrossRef]
- Eroldoǧan, O.T.; Kumlu, M.; Aktaş, M. Optimum Feeding Rates for European Sea Bass Dicentrarchus labrax L. Reared in Seawater and Freshwater. Aquaculture 2004, 231, 501–515. [Google Scholar] [CrossRef]
- Gomes, M.; Correia, A.; Pinto, L.; Sá, C.; Brotas, V.; Mateus, M. Coastal Water Quality in an Atlantic Sea Bass Farm Site (Sines, Portugal): A First Assessment. Front. Mar. Sci. 2020, 7, 175. [Google Scholar] [CrossRef]
- Mosqueira, M.; Pombo, A.; Borges, C.; Brito, A.C.; Zacarias, N.; Esteves, R.; Palma, C. Potential for Coastal and Offshore Aquaculture in Portugal: Insights from Physico-Chemical and Oceanographic Conditions. Appl. Sci. 2022, 12, 2742. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Austria R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Ogle, D.H.; Doll, J.C.; Wheeler, A.P.; Dinno, A. FSA: Simple Fisheries Stock Assessment Methods. 2023. Available online: https://cran.r-project.org/package=FSA (accessed on 30 March 2023).
- Jobling, M. The Thermal Growth Coefficient (TGC) Model of Fish Growth: A Cautionary Note. Aquac. Res. 2003, 34, 581–584. [Google Scholar] [CrossRef]
- Jauralde, I.; Martínez-Llorens, S.; Tomás, A.; Ballestrazzi, R.; Jover, M. A Proposal for Modelling the Thermal-Unit Growth Coefficient and Feed Conversion Ratio as Functions of Feeding Rate for Gilthead Sea Bream (Sparus aurata, L.) in Summer Conditions. Aquac. Res. 2013, 44, 242–253. [Google Scholar] [CrossRef]
- Bramato, S.; Losada, M.A.; Contreras, E.; Polo, M. An Integrated Database Manager to Forecast Estuarine Dynamics and Water Quality in the Guadalquivir River (Spain). River Flow 2010, 2, 1415–1420. [Google Scholar]
- Contreras, E.; Polo, M.J. Measurement Frequency and Sampling Spatial Domains Required to Characterize Turbidity and Salinity Events in the Guadalquivir Estuary (Spain). Nat. Hazards Earth Syst. Sci. 2012, 12, 2581–2589. [Google Scholar] [CrossRef]
- Precht, H.; Christophersen, J.; Hensel, H. Temperatur Und Leben; Springer: Berlin/Heidelberg, Germany, 1955; p. 514. [Google Scholar]
- Georgakopoulou, E.; Sfakianakis, D.G.; Kouttouki, S.; Divanach, P.; Kentouri, M.; Koumoundouros, G. The Influence of Temperature during Early Life on Phenotypic Expression at Later Ontogenetic Stages in Sea Bass. J. Fish Biol. 2007, 70, 278–291. [Google Scholar] [CrossRef]
- Boswell, K.M.; Wilson, M.P.; Wilson, C.A. Hydroacoustics as a Tool for Assessing Fish Biomass and Distribution Associated with Discrete Habitats in a Louisiana Estuary. Estuaries Coasts 2007, 30, 607–617. [Google Scholar] [CrossRef]
- Álvarez-Ellacuría, A.; Palmer, M.; Catalán, I.A.; Lisani, J.L. Image-Based, Unsupervised Estimation of Fish Size from Commercial Landings Using Deep Learning. ICES J. Mar. Sci. 2020, 77, 1330–1339. [Google Scholar] [CrossRef]
- Hsieh, C.L.; Chang, H.Y.; Chen, F.H.; Liou, J.H.; Chang, S.K.; Lin, T. Te A Simple and Effective Digital Imaging Approach for Tuna Fish Length Measurement Compatible with Fishing Operations. Comput. Electron. Agric. 2011, 75, 44–51. [Google Scholar] [CrossRef]
- Shi, C.; Wang, Q.; He, X.; Zhang, X.; Li, D. An Automatic Method of Fish Length Estimation Using Underwater Stereo System Based on LabVIEW. Comput. Electron. Agric. 2020, 173, 105419. [Google Scholar] [CrossRef]
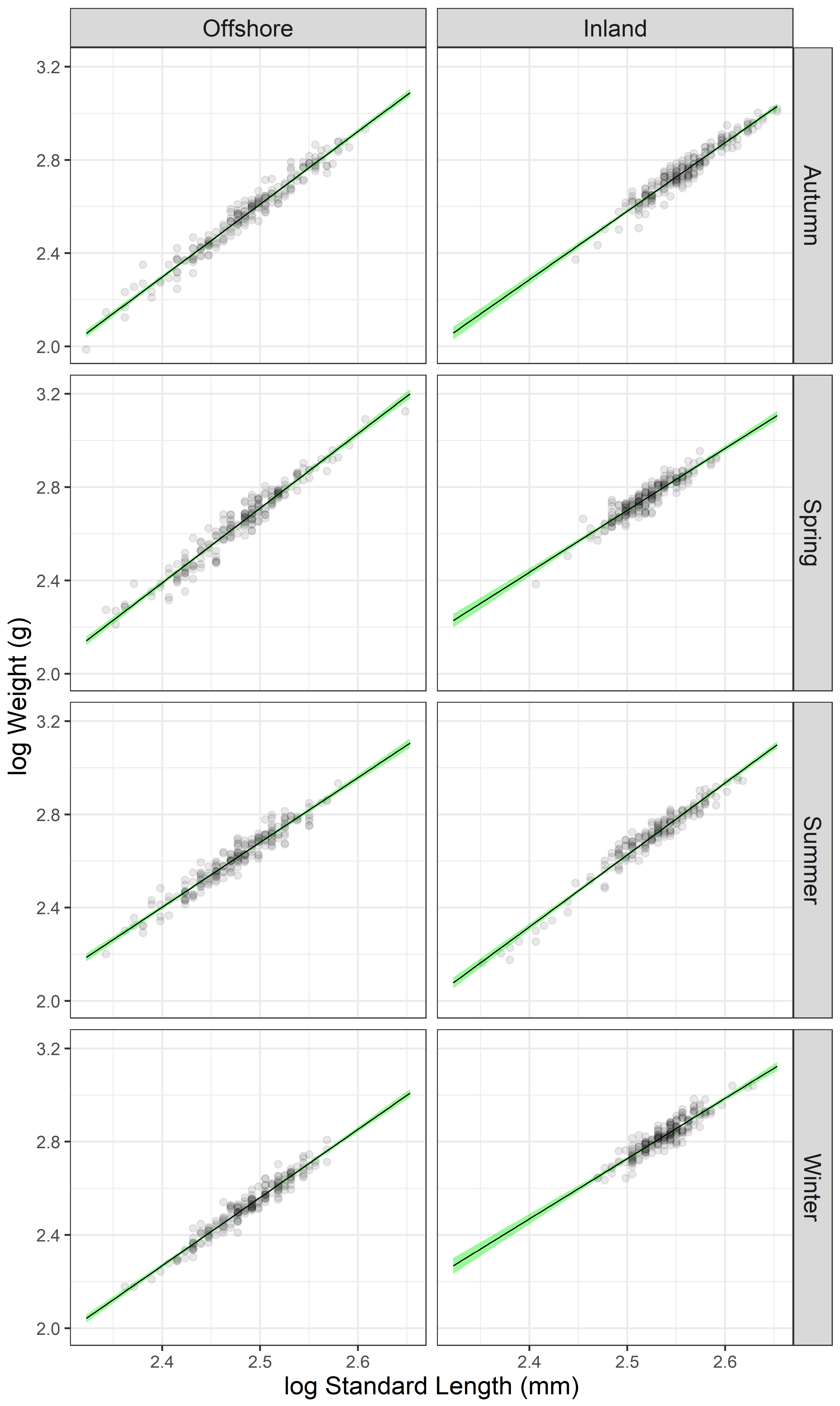
| Facility | Season | n | SL (mm) | W (g) | log(a) | 95% C.I. log(a) | b | 95% C.I. b | r2 | Growth |
|---|---|---|---|---|---|---|---|---|---|---|
| Offshore | Winter | 196 | 308 ± 28 | 345 ± 92 | −4.740 | −4.990; −4.490 | 2.920 | 2.820–3.021 | 0.944 * | Isometric |
| Offshore | Spring | 197 | 303 ± 35 | 470 ± 179 | −5.292 | −5.564; −5.019 | 3.201 | 3.091–3.311 | 0.944 * | Allometric (+) |
| Offshore | Summer | 200 | 300 ± 30 | 426 ± 120 | −4.262 | −4.528; −3.996 | 2.777 | 2.700–2.884 | 0.929 * | Allometric (−) |
| Offshore | Autumn | 197 | 309 ± 37 | 397 ± 148 | −5.193 | −5.424; −4.961 | 3.121 | 3.028–3.214 | 0.957 * | Allometric (+) |
| Inland | Winter | 198 | 346 ± 22 | 678 ± 120 | −3.736 | −4.132; −3.340 | 2.585 | 2.429–2.742 | 0.844 * | Allometric (−) |
| Inland | Spring | 200 | 331 ± 22 | 575 ± 107 | −3.940 | −4.305; −3.576 | 2.656 | 2.512–2.801 | 0.868 * | Allometric (−) |
| Inland | Summer | 166 | 336 ± 34 | 526 ± 149 | −5.087 | −5.366; −4.809 | 3.085 | 2.975–3.196 | 0.949 * | Isometric |
| Inland | Autumn | 200 | 364 ± 32 | 587 ± 157 | −4.763 | −5.057; −4.470 | 2.937 | 2.823–3.052 | 0.928 * | Isometric |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orduna, C.; de Meo, I.; Rodríguez-Ruiz, A.; Cid-Quintero, J.R.; Encina, L. Seasonal Length–Weight Relationships of European Sea Bass (Dicentrarchus labrax) in Two Aquaculture Production Systems. Fishes 2023, 8, 227. https://doi.org/10.3390/fishes8050227
Orduna C, de Meo I, Rodríguez-Ruiz A, Cid-Quintero JR, Encina L. Seasonal Length–Weight Relationships of European Sea Bass (Dicentrarchus labrax) in Two Aquaculture Production Systems. Fishes. 2023; 8(5):227. https://doi.org/10.3390/fishes8050227
Chicago/Turabian StyleOrduna, Carlos, Ilaria de Meo, Amadora Rodríguez-Ruiz, Juan Ramón Cid-Quintero, and Lourdes Encina. 2023. "Seasonal Length–Weight Relationships of European Sea Bass (Dicentrarchus labrax) in Two Aquaculture Production Systems" Fishes 8, no. 5: 227. https://doi.org/10.3390/fishes8050227
APA StyleOrduna, C., de Meo, I., Rodríguez-Ruiz, A., Cid-Quintero, J. R., & Encina, L. (2023). Seasonal Length–Weight Relationships of European Sea Bass (Dicentrarchus labrax) in Two Aquaculture Production Systems. Fishes, 8(5), 227. https://doi.org/10.3390/fishes8050227







