Morphometric Variations of Nile Tilapia (Oreochromis niloticus) (Linnaeus, 1758) Local Strains Collected from Different Fish Farms in South Western Highland Agro-Ecological Zone (SWHAEZ), Uganda: Screening Strains for Aquaculture
Abstract
1. Introduction
2. Materials and Methods
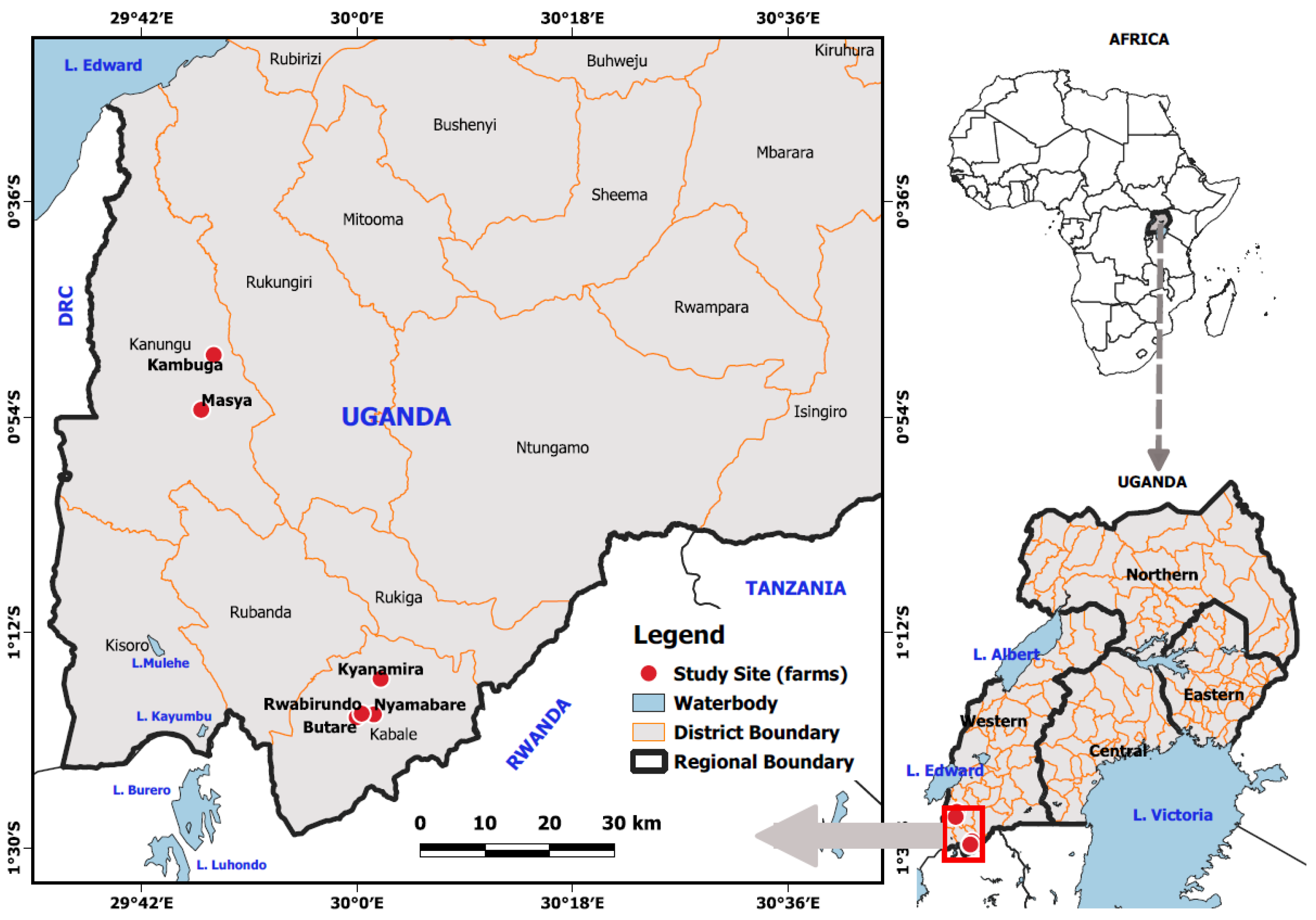
2.1. The Study Area
2.2. Research Design
2.3. Data Analysis
3. Results
3.1. Morphometric Traits of the O. niloticus Populations
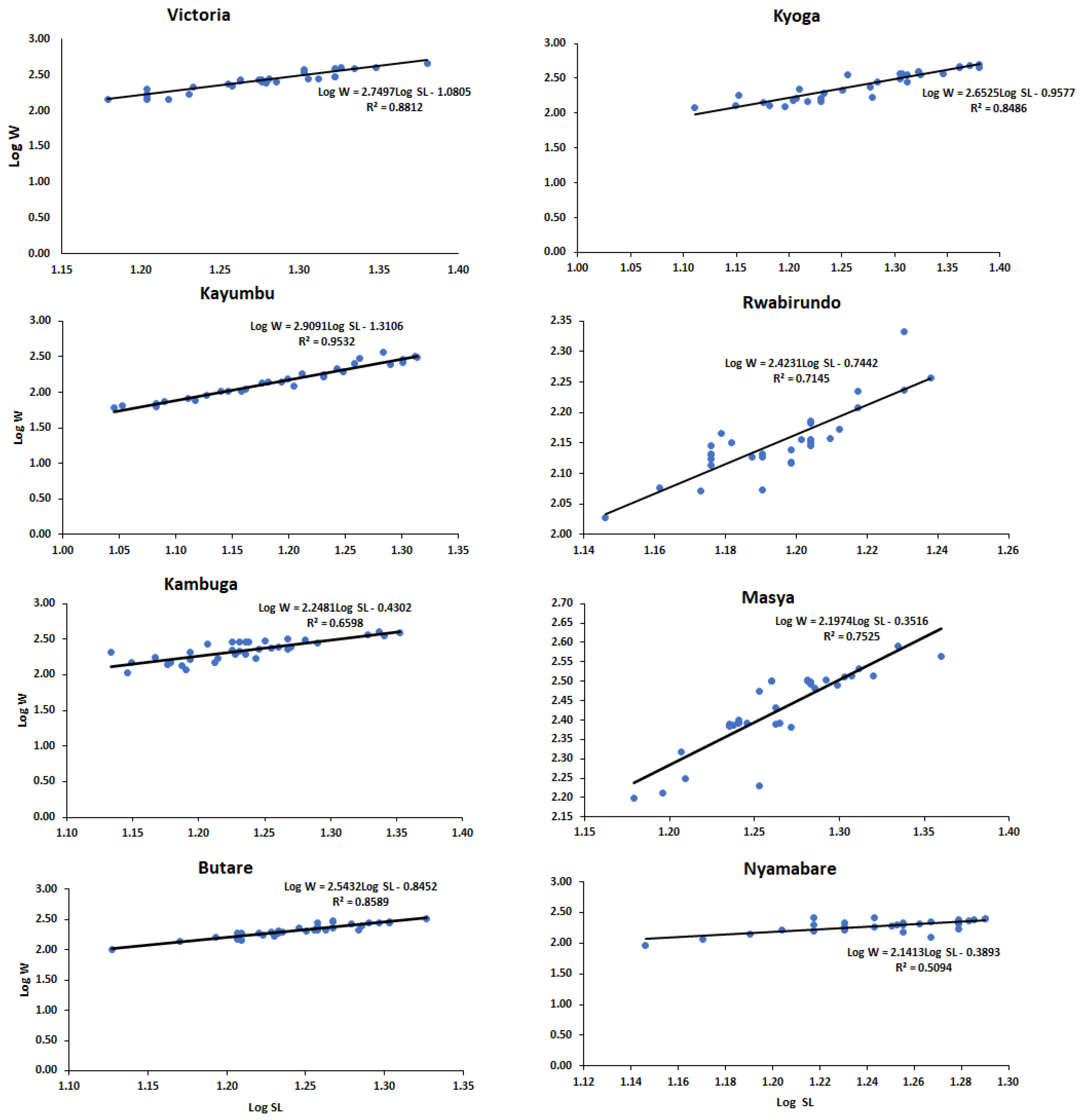
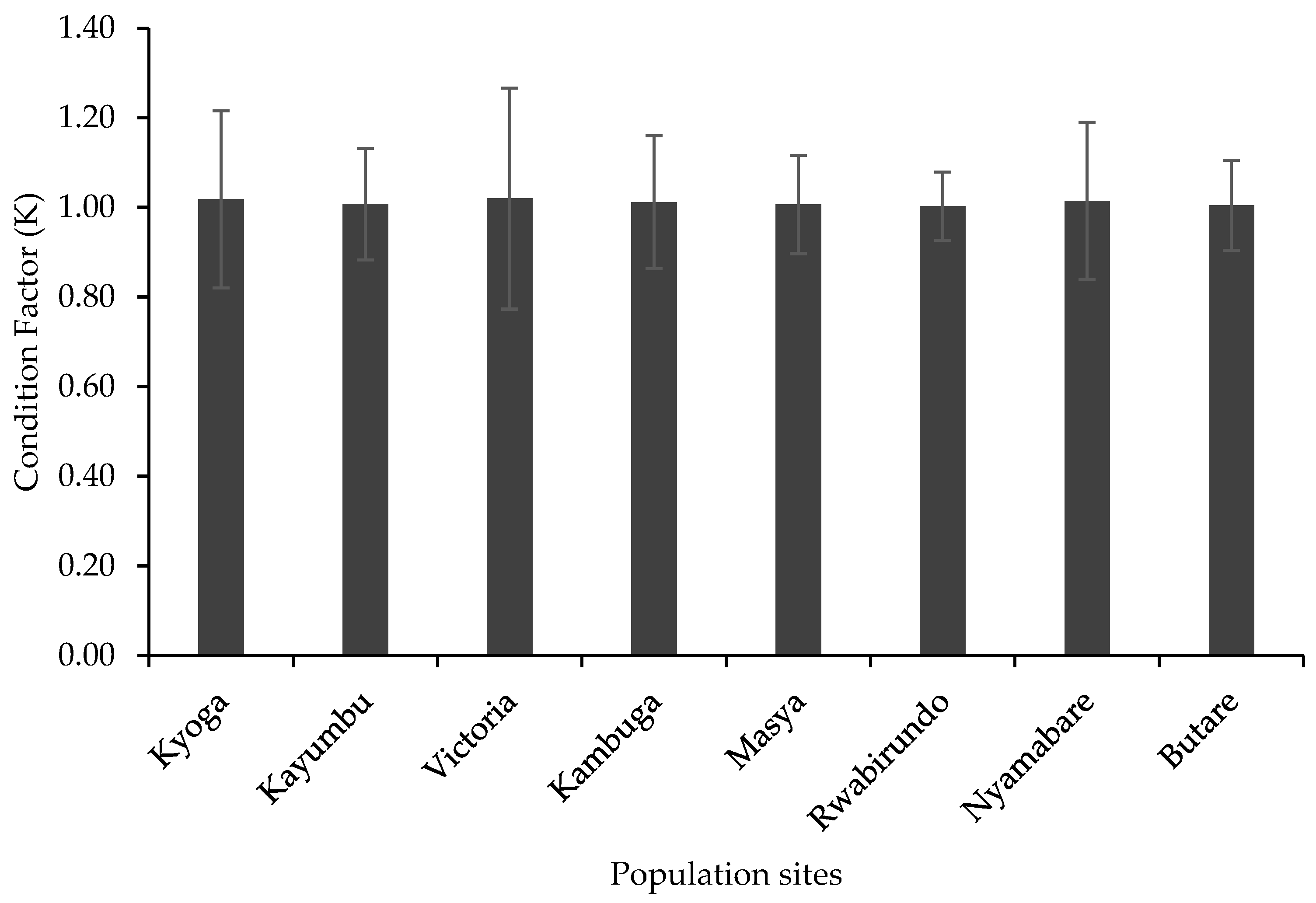
3.2. Length–Weight Relationship and Condition Factor
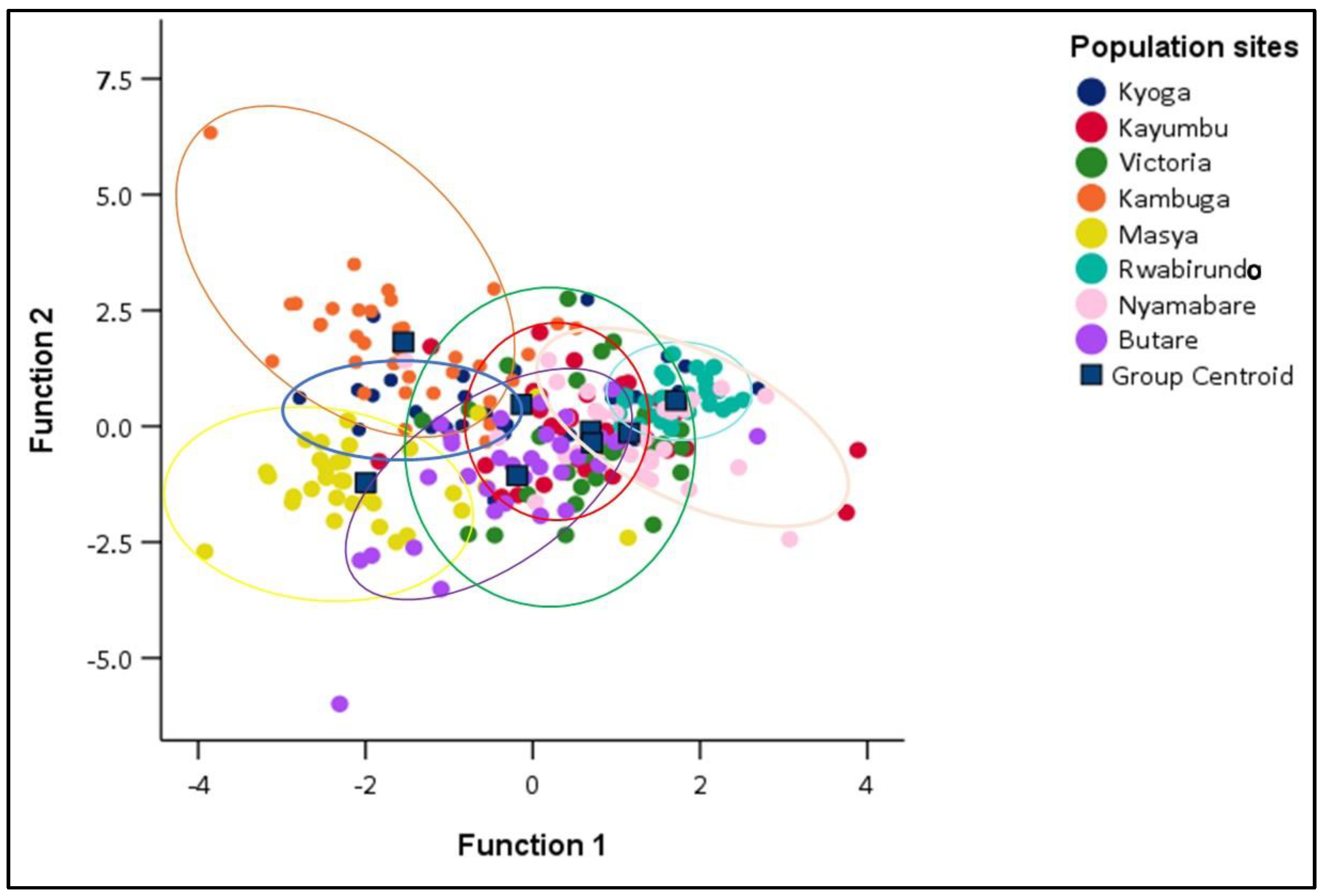
3.3. Similitude in the Morphometric Traits among the Oreochromis niloticus Populations
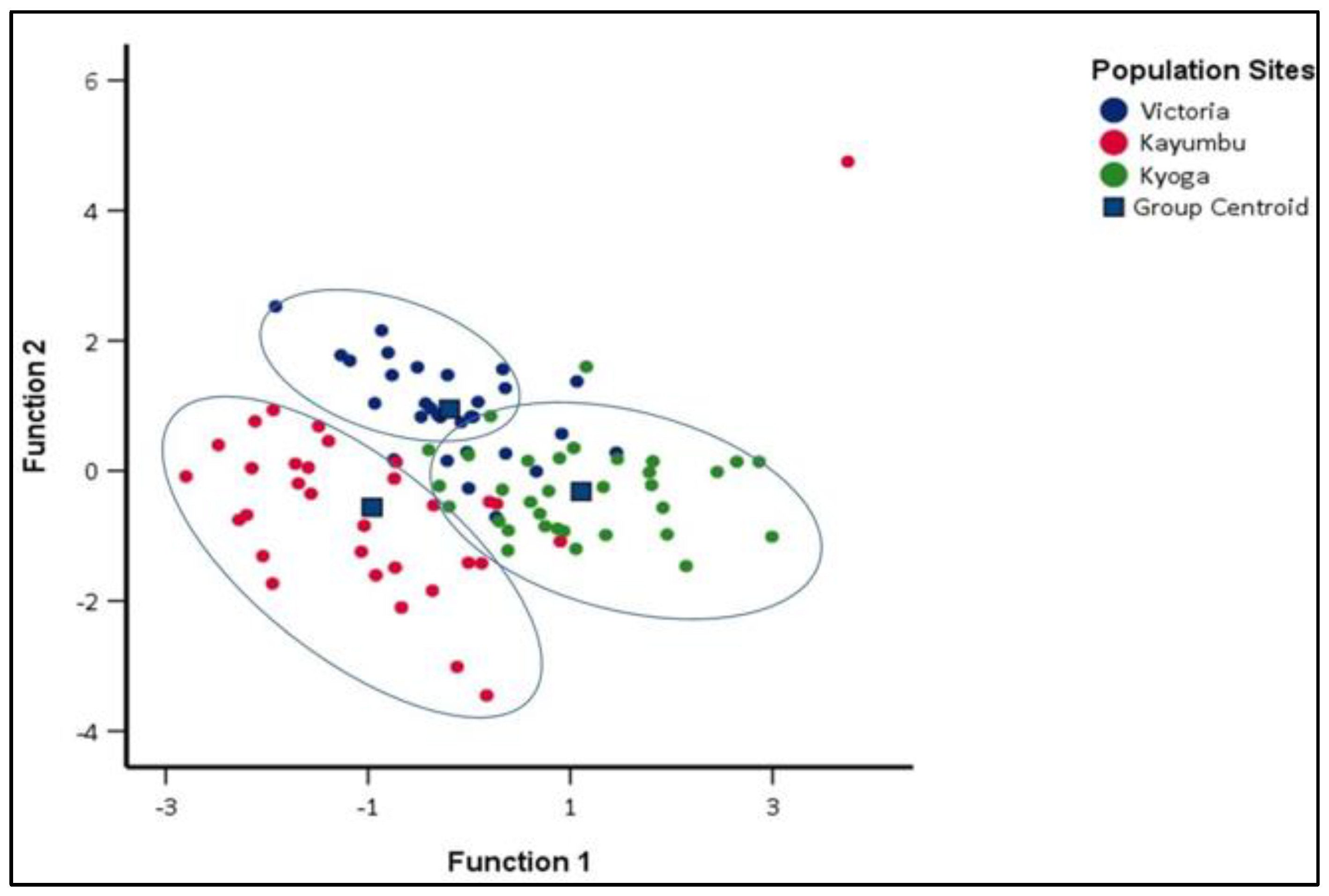
3.3.1. Discriminant Function Analysis (DFA) of different Oreochromis niloticus populations
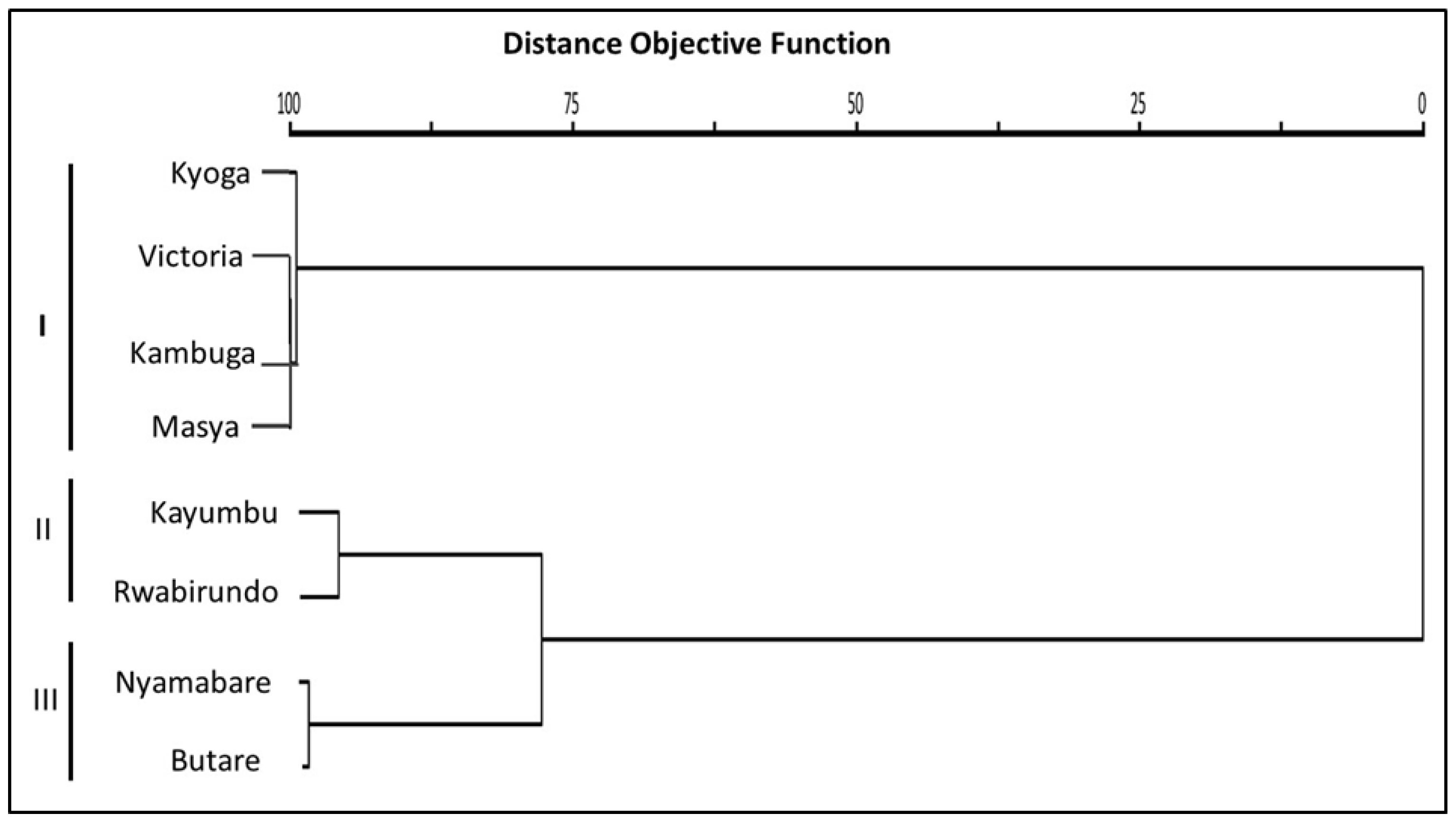
3.3.2. Hierarchical Cluster Analysis
4. Discussion
4.1. Morphometric Traits of the O. niloticus Populations
4.2. Length–Weight Relationship and Condition Factor
4.3. Similitude in the Morphometric Traits among the Oreochromis niloticus Populations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Subasinghe, R.; Soto, D.; Jia, J. Global aquaculture and its role in sustainable development. Rev. Aquac. 2009, 1, 2–9. [Google Scholar] [CrossRef]
- IMARC Group. Tilapia Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2021–2026. 2021. Available online: https://www.imarcgroup.com/tilapia-market (accessed on 23 June 2022).
- Miao, W.; Wang, W.E. Trends of aquaculture production and trade: Carp, tilapia, and shrimp. Asian Fish. Sci. 2020, 33, 1–10. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, R.; Ding, M.; Tao, L.; Liu, L.; Tao, N.; Wang, X.; Zhong, J. Effect of extraction methods on the structural characteristics, functional properties, and emulsion stabilization ability of Tilapia skin gelatins. Food Chem. 2020, 328, 127114. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. The State of the World Fisheries and Aquaculture; FAO: Rome, Italy, 2020; pp. 1–224. [Google Scholar]
- Hassanien, H.A.; Kamel, E.A.; Salem, M.A.; Dorgham, A.S. Multivariate analysis of morphometric parameters in wild and cultured Nile tilapia Oreochromis niloticus. J. Arab. Aquac. Soc. 2011, 6, 424–440. [Google Scholar]
- Opiyo, M.A.; Marijani, E.; Muendo, P.; Odede, R.; Leschen, W.; Charo-Karisa, H. A review of aquaculture production and health management practices of farmed fish in Kenya. Int. J. Vet. Sci. Med. 2018, 6, 141–148. [Google Scholar] [CrossRef]
- Tibihika, P.D.; Curto, M.; Alemayehu, E.; Waidbacher, H.; Masembe, C.; Akoll, P.; Meimberg, H. Molecular genetic diversity and differentiation of Nile tilapia (Oreochromis niloticus, L. 1758) in East African natural and stocked populations. BMC Evol. Biol. 2020, 20, 16. [Google Scholar] [CrossRef]
- Zengeya, T.A.; Booth, A.J.; Bastos, A.D.; Chimimba, C.T. Trophic interrelationships between the exotic Nile tilapia, Oreochromis niloticus and indigenous tilapiine cichlids in a subtropical African river system (Limpopo River, South Africa). Environ. Biol. Fishes 2011, 92, 479–489. [Google Scholar] [CrossRef]
- Nyirenda, S.M. Abudance, Growth and Reproductive Biology of Oreochromis niloticus (Linneaus, 1758) Compared with Tilapiines Indigenous to the Middle Zambezi. Ph.D. Thesis, The University of Zambia, Lusaka, Zambia, 2017. [Google Scholar]
- Trewavas, E. Tilapiine Fishes of the Genera Sarotherodon, Oreochromis, and Danakilia; British Museum (Natural History): London, UK, 1983. [Google Scholar]
- Makeche, M.C.; Muleya, W.; Nhiwatiwa, T. Characterization of Oreochromis niloticus strains of Lake Kariba culture fisheries using morphological and meristic methods. Am. Sci. Res. J. Eng. Technol. Sci. 2020, 74, 31–40. [Google Scholar]
- MAAIF. Essentials of Aquaculture Production, Management and Development in Uganda; Ministry of Agriculture, Animal Industry and Fisheries (MAAIF): Entebbe, Uganda, 2018.
- Mwanja, M.; Ondhoro, C.; Sserwada, M.; Achieng, P.; Ddungu, R.; Mwanja, W. Morphological variation of Nile tilapia populations from major water bodies of Uganda. Uganda J. Agric. Sci. 2016, 17, 21–32. [Google Scholar] [CrossRef]
- Kwikiriza, G.; Barekye, A.; Aheisibwe, A.R.; Byakora, E.; Tibihika, P.D. Comparative growth performance and proximate nutrient composition of three local strains of Nile tilapia (Oreochromis niloticus L.) collected from different locations in Uganda. Fish. Aquac. J. 2017, 8, 1–5. [Google Scholar]
- Samaddar, A. Recent trends on Tilapia cultivation and its major socioeconomic impact among some developing nations: A Review. Asian J. Fish. Aquat. Res. 2022, 8, 1–10. [Google Scholar] [CrossRef]
- Anane-Taabeah, G.; Frimpong, E.A.; Hallerman, E. Aquaculture-mediated invasion of the genetically improved farmed Tilapia (Gift) into the lower Volta Basin of Ghana. Diversity 2019, 11, 188. [Google Scholar] [CrossRef]
- Beardmore, J.A.; Mair, G.C.; Lewis, R.I. Monosex male production in finfish as exemplified by tilapia: Applications, problems, and prospects. Reprod. Biotechnol. Finfish Aquac. 2001, 1, 283–301. [Google Scholar]
- Brummett, R.E.; Ponzoni, R.W. Concepts, alternatives, and environmental considerations in the development and use of improved strains of tilapia in African aquaculture. Rev. Fish. Sci. 2009, 17, 70–77. [Google Scholar] [CrossRef]
- Deines, A.M.; Bbole, I.; Katongo, C.; Feder, J.L.; Lodge, D.M. Hybridisation between native Oreochromis species and introduced Nile tilapia O. niloticus in the Kafue River, Zambia. Afr. J. Aquat. Sci. 2014, 39, 23–34. [Google Scholar] [CrossRef]
- Bradbeer, S.J.; Harrington, J.; Watson, H.; Warraich, A.; Shechonge, A.; Smith, A.; Tamatamah, R.; Ngatunga, B.P.; Turner, G.F.; Genner, M.J. Limited hybridization between introduced and Critically Endangered indigenous tilapia fishes in northern Tanzania. Hydrobiologia 2019, 832, 257–268. [Google Scholar] [CrossRef]
- Fuerst, P.A.; Mwanja, W.W.; Kaufman, L. The genetic history of the introduced Nile tilapia of Lake Victoria (Uganda–E. Africa): The population structure of Oreochromis niloticus (Pisces: Cichlidae) revealed by DNA microsatellite markers. In Tilapia Aquaculture in the 21st Century Proceedings from the Fifth International Symposium on Tilapia in Aquaculture; Panorama da Aquicultura Magazine: Rio de Janeiro, Brazil, 2000; pp. 30–40. [Google Scholar]
- Natugonza, V.; Nyamweya, C.; Sturludóttir, E.; Musinguzi, L.; Ogutu-Ohwayo, R.; Bassa, S.; Mplaponi, E.; Tomasson, T.; Stefansson, G. Spatiotemporal variation in fishing patterns and fishing pressure in Lake Victoria (East Africa) in relation to balanced harvest. Fish. Res. 2022, 252, 106355. [Google Scholar] [CrossRef]
- Diedericks, G.; Maetens, H.; Van Steenberge, M.; Snoeks, J. Testing for hybridization between Nile tilapia (Oreochromis niloticus) and blue spotted tilapia (Oreochromis leucostictus) in the Lake Edward system. J. Gt. Lakes Res. 2021, 47, 1446–1452. [Google Scholar] [CrossRef]
- Bamwerinde, W.; Bashaasha, B.; Ssembajjwe, W.; Place, F. Determinants of land use in the densely populated Kigezi highlands of southwestern Uganda; No. 1004-2016-78549; Ag Econ. In Proceedings of the International Association of Agricultural Economists (IAAE) Conference, Gold Coast, QLD, Australia, 12–18 August 2006. [Google Scholar]
- Elliott, J.M.; Hurley, M.A. The functional relationship between body size and growth rate in fish. Funct. Ecol. 1995, 1, 625–627. [Google Scholar] [CrossRef]
- Langerhans, R.B.; DeWitt, T.J. Shared and unique features of evolutionary diversification. Am. Nat. 2004, 164, 335–349. [Google Scholar] [CrossRef]
- Rogers, S.M.; Bernatchez, L. The genetic architecture of ecological speciation and the association with signatures of selection in natural lake whitefish (Coregonus sp. Salmonidae) species pairs. Mol. Biol. Evol. 2007, 24, 1423–1438. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.C. Manual of Fisheries Survey Methods II: With Periodic Updates; Michigan Department of Natural Resources, Fisheries Division: Lansing, MI, USA, 2000.
- LeCren, E.D. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol. 1951, 1, 201–219. [Google Scholar]
- Jawad, L.A.; Ibáñez, A.L.; Kiki, M.; Gnohossou, P. Determination of body shape and meristic characters variations in wild and cultured populations of cichlid fish, Oreochromis niloticus, from the Republic of Benin, West of Africa. Fish. Aquat. Sci. 2020, 3, 23–28. [Google Scholar] [CrossRef]
- Asmamaw, B.; Tessema, M. Morphometric Variations of Nile tilapia (Oreochromis niloticus) (Linnaeus, 1758) (Perciformes, Cichlidae) collected from three rift valley Lakes in Ethiopia. JAFH 2021, 10, 341–355. [Google Scholar] [CrossRef]
- Vehanen, T.; Huusko, A. Brown trout Salmo trutta express different morphometrics due to divergence in the rearing environment. J. Fish Biol. 2011, 79, 1167–1181. [Google Scholar] [CrossRef]
- Tumwesigye, Z.; Tumwesigye, W.; Opio, F.; Kemigabo, C.; Mujuni, B. The effect of water quality on aquaculture productivity in Ibanda District, Uganda. Aquac. Res. 2022, 2, 23–36. [Google Scholar] [CrossRef]
- Froese, R. Cube law, condition factor and weight–length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Datta, S.N.; Kaur, V.I.; Dhawan, A.; Jassal, G. Estimation of length-weight relationship and condition factor of spotted snakehead Channa punctata (Bloch) under different feeding regimes. SpringerPlus 2013, 2, 436. [Google Scholar] [CrossRef]
- Otieno, O.N.; Kitaka, N.; Njiru, J.M. Length-weight relationship, condition factor, length at first maturity and sex ratio of Nile tilapia, Oreochromis niloticus in Lake Naivasha, Kenya. Int. J. Fish. Aquat. 2014, 2, 67–72. [Google Scholar]
- Popoola, O.M.; Ebiwonjumi, A. Assessment of morphological variations in wild populations of Nile tilapia from South West Nigeria. J. Agric. Sci. Technol. 2021, 13, 1313–8820. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. Early environment influences later performance in fishes. J. Fish Biol. 2014, 85, 151–188. [Google Scholar] [CrossRef] [PubMed]
- Ogutu-Ohwayo, R. The decline of the native fishes of lakes Victoria and Kyoga (East Africa) and the impact of introduced species, especially the Nile perch, Lates niloticus, and the Nile tilapia, Oreochromis niloticus. Environ. Biol. Fishes 1990, 27, 81–96. [Google Scholar] [CrossRef]


| Populations | SL | BW | TL | DL | CL | HL | WID | PEC | PEL |
|---|---|---|---|---|---|---|---|---|---|
| Kyoga | 18.68 ± 3.19 | 283.20 ± 91.41 | 80.74 ± 13.62 | 84.92 ± 12.64 | 27.53 ± 2.05 | 32.49 ± 2.53 | 42.02 ± 3.38 | 39.59 ± 2.61 | 28.50 ± 3.10 |
| Kayumbu | 15.88 ± 2.81 | 167.05 ± 87.86 | 87.98 ± 52.91 | 67.28 ± 8.43 | 27.89 ± 5.39 | 33.09 ± 3.04 | 40.10 ± 4.12 | 36.48 ± 3.81 | 27.64 ± 2.57 |
| Victoria | 18.98 ± 2.16 | 281.69 ± 89.73 | 78.24 ± 6.45 | 67.49 ± 7.01 | 27.14 ± 3.06 | 33.86 ± 3.14 | 41.35 ± 2.54 | 36.29 ± 3.03 | 28.28 ± 3.58 |
| Kambuga | 17.27 ± 2.21 | 233.39 ± 78.01 | 82.06 ± 1.42 | 56.33 ± 3.55 | 24.31 ± 2.25 | 32.66 ± 2.19 | 36.57 ± 6.95 | 39.18 ± 2.91 | 27.948 ± 2.25 |
| Masya | 18.47 ± 1.70 | 274.12 ± 58.45 | 80.49 ± 1.36 | 59.01 ± 2.40 | 26.09 ± 1.53 | 30.42 ± 1.36 | 39.48 ± 2.71 | 33.38 ± 2.54 | 33.72 ± 3.24 |
| Rwabirundo | 15.69 ± 0.76 | 143.18 ± 21.10 | 79.07 ± 4.23 | 75.88 ± 4.02 | 29.24 ± 1.28 | 33.97 ± 1.31 | 40.96 ± 1.70 | 42.38 ± 1.73 | 30.35 ± 1.72 |
| Nyamabare | 17.68 ± 1.41 | 195.33 ± 43.21 | 78.83 ± 1.86 | 76.12 ± 4.10 | 27.92 ± 1.63 | 32.46 ± 1.70 | 40.35 ± 1.74 | 39.99 ± 2.82 | 29.16 ± 2.26 |
| Butare | 17.64 ± 1.64 | 215.80 ± 53.44 | 83.74 ± 14.34 | 66.81 ± 15.51 | 26.53 ± 4.51 | 31.22 ± 2.90 | 40.44 ± 4.34 | 34.57 ± 7.23 | 28.88 ± 4.78 |
| Average | 17.56 ± 2.29 | 227.95 ± 86.43 | 87.53 ± 25.09 | 68.92 ± 10.56 | 27.02 ± 3.34 | 32.49 ± 2.63 | 40.11 ± 4.14 | 37.65 ± 674 | 29.30 ± 3.58 |
| P7.250 | 0.000 | 0.000 | 0.000 | 0.040 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| F | 11.30 | 17.84 | 20.74 | 2.13 | 7.54 | 8.34 | 6.13 | 21.19 | 13.31 |
| Population | N | Standard Length (cm) | Body Weight (g) | a | b |
|---|---|---|---|---|---|
| Kyoga | 32 | 18.68 ± 3.19 | 283.20 ± 130.41 | −0.96 | 2.65 |
| Kayumbu | 32 | 15.88 ± 2.81 | 167.05 ± 87.86 | −1.31 | 2.91 |
| Victoria | 30 | 18.98 ± 2.16 | 281.69 ± 89.73 | −1.08 | 2.75 |
| Kambuga | 35 | 17.27 ± 2.21 | 233.39 ± 78.01 | −0.43 | 2.25 |
| Masya | 33 | 18.47 ± 1.70 | 274.12 ± 58.45 | −0.35 | 2.20 |
| Rwabirundo | 30 | 15.69 ± 0.76 | 143.18 ± 21.10 | −0.74 | 2.42 |
| Nyamabare | 30 | 17.68 ± 1.41 | 195.33 ± 43.21 | −0.39 | 2.14 |
| Butare | 34 | 17.64 ± 1.64 | 215.80 ± 53.44 | −0.85 | 2.54 |
| Function | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| SL | 1.35 | −1.11 | −0.15 | 0.62 | −0.44 | −1.29 | 0.25 |
| BW | −1.72 | 1.17 | 0.49 | 0.08 | 0.04 | 1.09 | 0.09 |
| TL | 0.18 | −0.03 | 0.79 | 0.08 | 0.44 | −0.03 | −0.15 |
| DL | 0.06 | 0.03 | 0.01 | 0.29 | −0.30 | 0.20 | 0.13 |
| CL | 0.50 | −0.08 | −0.07 | 0.13 | −0.02 | 0.38 | 0.69 |
| HL | 0.46 | 0.35 | 0.19 | −0.02 | 0.16 | 0.11 | 0.26 |
| WID | 0.34 | −0.62 | 0.10 | 0.00 | −0.27 | 0.43 | −0.68 |
| PEC | 0.29 | 0.78 | −0.19 | 0.47 | 0.16 | −0.19 | −0.23 |
| PEL | −0.33 | −0.29 | −0.44 | 0.46 | 0.67 | 0.06 | −0.07 |
| Eigenvalue | 1.51 a | 0.89 a | 0.80 a | 0.25 a | 0.18 a | 0.08 a | 0.00 a |
| % of Variance | 40.50 | 24.20 | 21.60 | 6.80 | 4.70 | 2.10 | 0.10 |
| Cumulative % | 40.50 | 64.70 | 86.30 | 93.10 | 97.90 | 99.90 | 100.00 |
| Canonical Correlation | 0.775 | 0.69 | 0.69 | 0.45 | 0.39 | 0.27 | 0.06 |
| Predicted Group Membership | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Kyoga | Kayumbu | Victoria | Kambuga | Masya | Rwabirundo | Nyamabare | Butare | Total | ||
| Count | Kyoga | 14 | 2 | 1 | 2 | 1 | 6 | 4 | 2 | 32 |
| Kayumbu | 4 | 13 | 1 | 1 | 0 | 3 | 7 | 3 | 32 | |
| Victoria | 0 | 1 | 29 | 1 | 0 | 0 | 0 | 1 | 32 | |
| Kambuga | 5 | 0 | 0 | 27 | 1 | 0 | 1 | 1 | 35 | |
| Masya | 0 | 1 | 0 | 0 | 28 | 0 | 1 | 3 | 33 | |
| Rwabirundo | 0 | 0 | 0 | 0 | 0 | 27 | 3 | 0 | 30 | |
| Nyamabare | 1 | 2 | 0 | 1 | 0 | 8 | 15 | 3 | 30 | |
| Butare | 3 | 4 | 0 | 0 | 6 | 1 | 4 | 16 | 34 | |
| % | Kyoga | 43.8 | 6.3 | 3.1 | 6.3 | 3.1 | 18.8 | 12.5 | 6.3 | 100.0 |
| Kayumbu | 12.5 | 40.6 | 3.1 | 3.1 | 0.0 | 9.4 | 21.9 | 9.4 | 100.0 | |
| Victoria | 0.0 | 3.1 | 90.6 | 3.1 | 0.0 | 0.0 | 0.0 | 3.1 | 100.0 | |
| Kambuga | 14.3 | 0.0 | 0.0 | 77.1 | 2.9 | 0.0 | 2.9 | 2.9 | 100.0 | |
| Masya | 0.0 | 3.0 | 0.0 | 0.0 | 84.8 | 0.0 | 3.0 | 9.1 | 100.0 | |
| Rwabirundo | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 90.0 | 10.0 | 0.0 | 100.0 | |
| Nyamabare | 3.3 | 6.7 | 0.0 | 3.3 | 0.0 | 26.7 | 50.0 | 10.0 | 100.0 | |
| Butare | 8.8 | 11.8 | 0.0 | 0.0 | 17.6 | 2.9 | 11.8 | 47.1 | 100.0 | |
| Predicted Membership | |||||
|---|---|---|---|---|---|
| Population Sites | Butare | Rwabirundo | Nyamabare | Total | |
| Count | Butare | 30 | 0 | 4 | 34 |
| Rwabirundo | 0 | 29 | 1 | 30 | |
| Nyamabare | 5 | 6 | 19 | 30 | |
| % | Butare | 88.2 | 0.0 | 11.8 | 100.0 |
| Rwabirundo | 0.0 | 96.7 | 3.3 | 100.0 | |
| Nyamabare | 16.7 | 20.0 | 63.3 | 100.0 | |
| Predicted Group Membership | |||||
|---|---|---|---|---|---|
| Site | Victoria | Kayumbu | Kyoga | Total | |
| Count | Victoria | 24 | 2 | 4 | 30 |
| Kayumbu | 3 | 26 | 3 | 32 | |
| Kyoga | 4 | 2 | 27 | 33 | |
| % | Victoria | 80.0 | 6.7 | 13.3 | 100.0 |
| Kayumbu | 9.4 | 81.3 | 9.4 | 100.0 | |
| Kyoga | 12.1 | 6.1 | 81.8 | 100.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwikiriza, G.; Yegon, M.J.; Byamugisha, N.; Beingana, A.; Atukwatse, F.; Barekye, A.; Nattabi, J.K.; Meimberg, H. Morphometric Variations of Nile Tilapia (Oreochromis niloticus) (Linnaeus, 1758) Local Strains Collected from Different Fish Farms in South Western Highland Agro-Ecological Zone (SWHAEZ), Uganda: Screening Strains for Aquaculture. Fishes 2023, 8, 217. https://doi.org/10.3390/fishes8040217
Kwikiriza G, Yegon MJ, Byamugisha N, Beingana A, Atukwatse F, Barekye A, Nattabi JK, Meimberg H. Morphometric Variations of Nile Tilapia (Oreochromis niloticus) (Linnaeus, 1758) Local Strains Collected from Different Fish Farms in South Western Highland Agro-Ecological Zone (SWHAEZ), Uganda: Screening Strains for Aquaculture. Fishes. 2023; 8(4):217. https://doi.org/10.3390/fishes8040217
Chicago/Turabian StyleKwikiriza, Gerald, Mourine J. Yegon, Nelson Byamugisha, Apulnal Beingana, Faith Atukwatse, Alex Barekye, Juliet K. Nattabi, and Harald Meimberg. 2023. "Morphometric Variations of Nile Tilapia (Oreochromis niloticus) (Linnaeus, 1758) Local Strains Collected from Different Fish Farms in South Western Highland Agro-Ecological Zone (SWHAEZ), Uganda: Screening Strains for Aquaculture" Fishes 8, no. 4: 217. https://doi.org/10.3390/fishes8040217
APA StyleKwikiriza, G., Yegon, M. J., Byamugisha, N., Beingana, A., Atukwatse, F., Barekye, A., Nattabi, J. K., & Meimberg, H. (2023). Morphometric Variations of Nile Tilapia (Oreochromis niloticus) (Linnaeus, 1758) Local Strains Collected from Different Fish Farms in South Western Highland Agro-Ecological Zone (SWHAEZ), Uganda: Screening Strains for Aquaculture. Fishes, 8(4), 217. https://doi.org/10.3390/fishes8040217






