Quantitative Analysis of Ovarian Dynamics of European Sardine Sardina pilchardus (Walbaum, 1792) during Its Spawning Period
Abstract
:1. Introduction
2. Materials and Methods
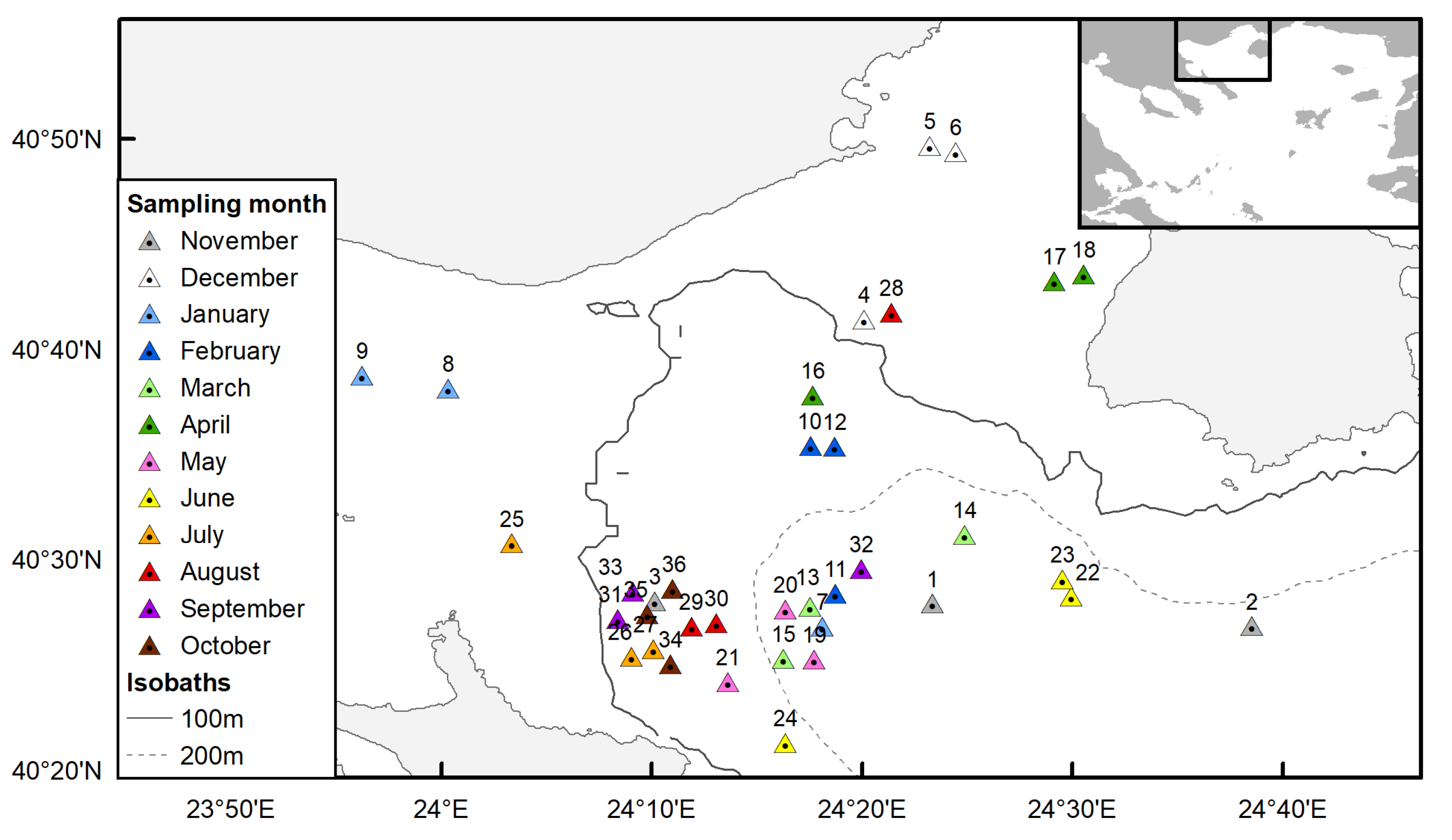
2.1. Ovarian Sampling and Processing
2.2. Oocyte Packing Density Theory
2.2.1. Volume Fraction
2.2.2. Ovarian Density
2.2.3. Volume-Based Oocyte Diameter
2.2.4. Correction for Oocyte Shrinkage—Whole Mount Analysis
2.2.5. Stagei Oocytes in the Whole Ovary
2.3. Trends along Ovulation Cycle and Reproductive Period
2.3.1. Oocyte Size Trends
2.3.2. Oocyte Number Trends
3. Results
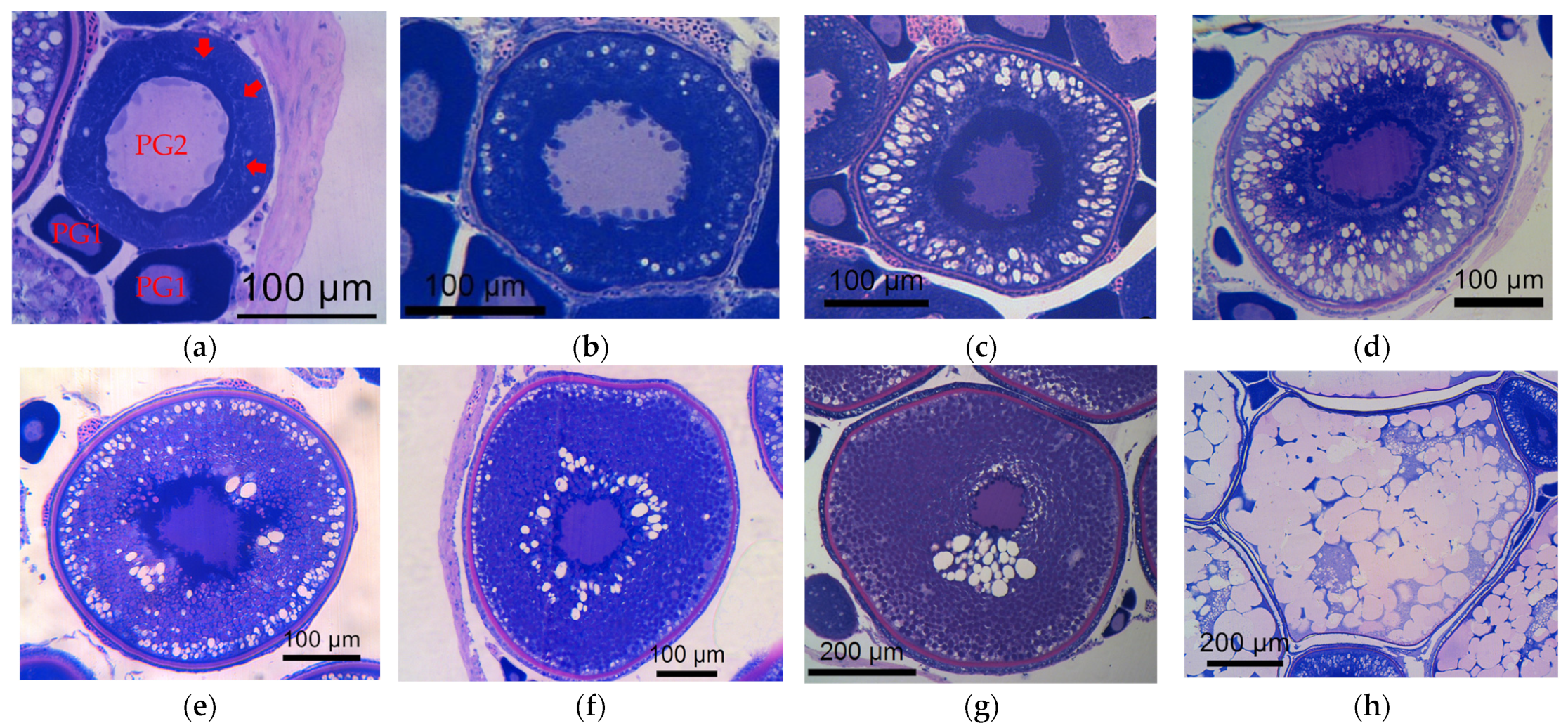
3.1. Histological Analysis
3.2. Oocyte Packing Density Theory Parameters
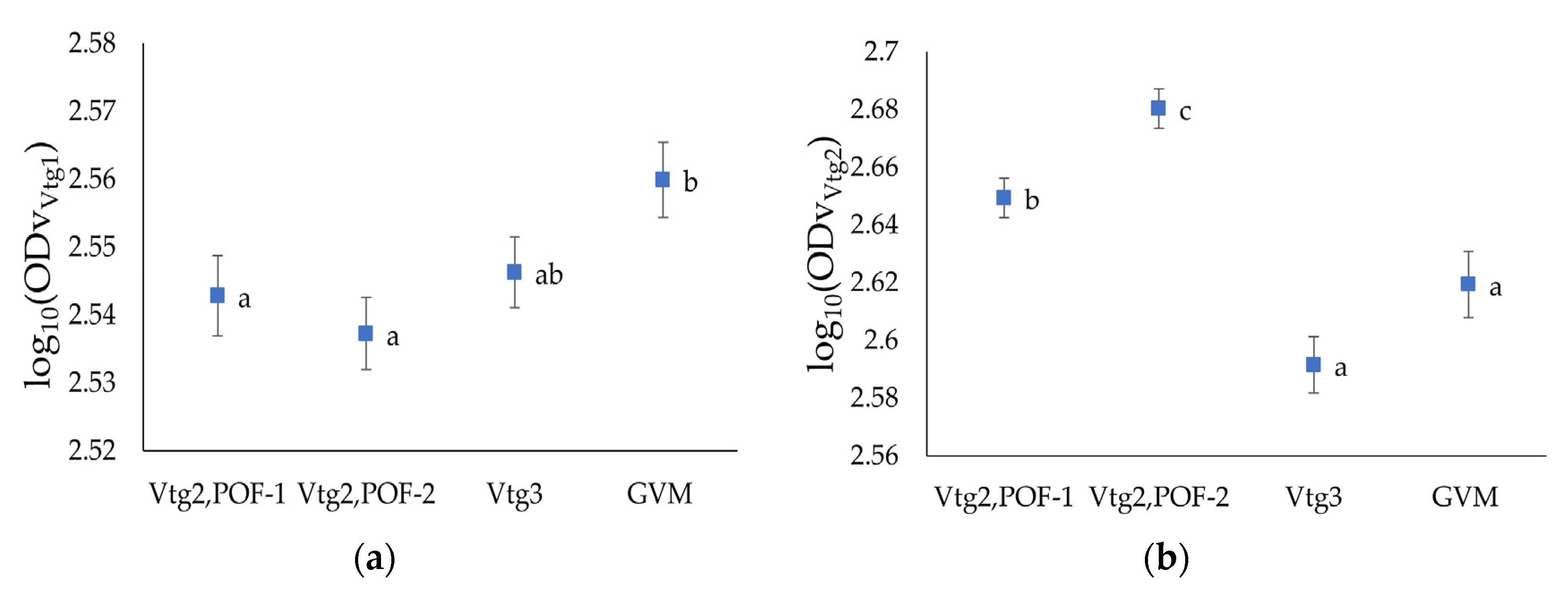
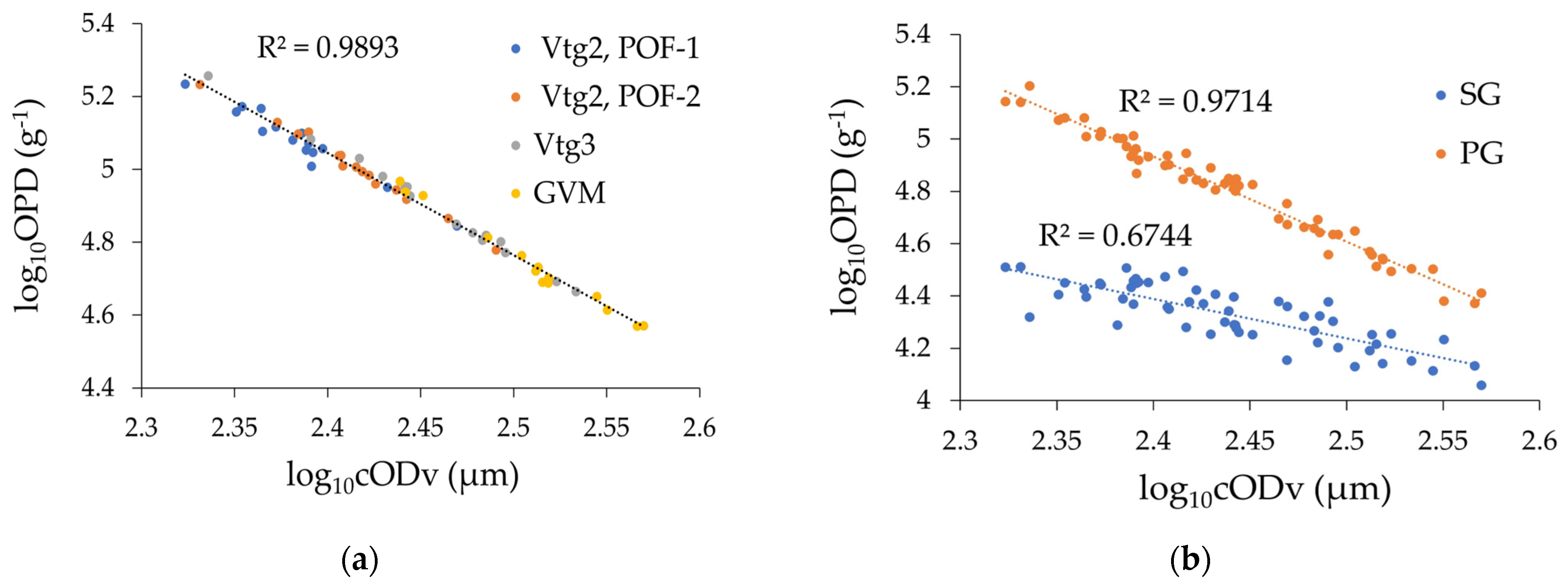
3.2.1. Ovarian Density
3.2.2. Volume Fraction
3.2.3. Histology Shrinkage Factor
3.3. Oocyte Sizes
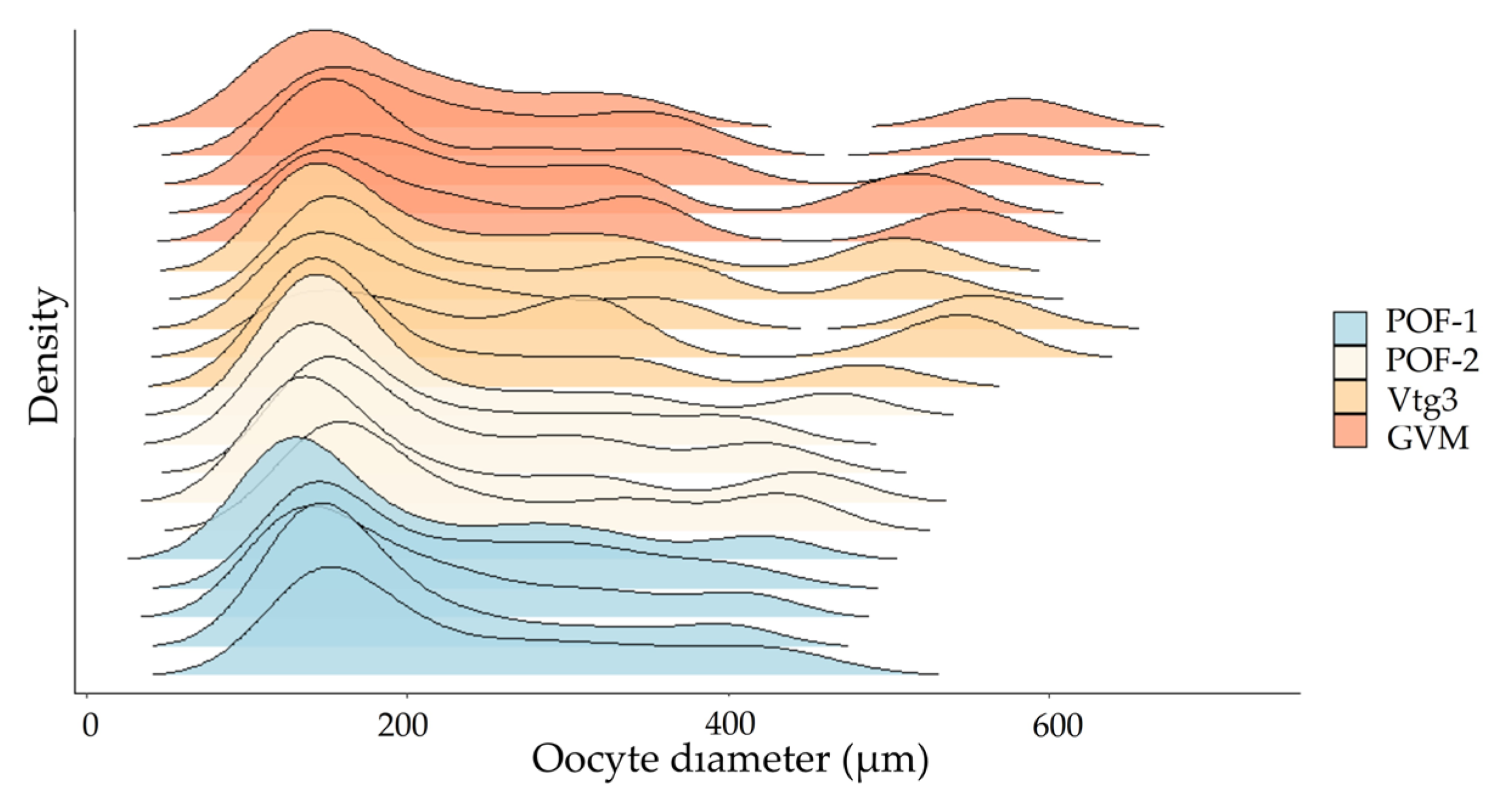
3.3.1. Oocyte Size Frequency Distributions
3.3.2. Oocyte Size Trends
3.4. Oocyte Numbers
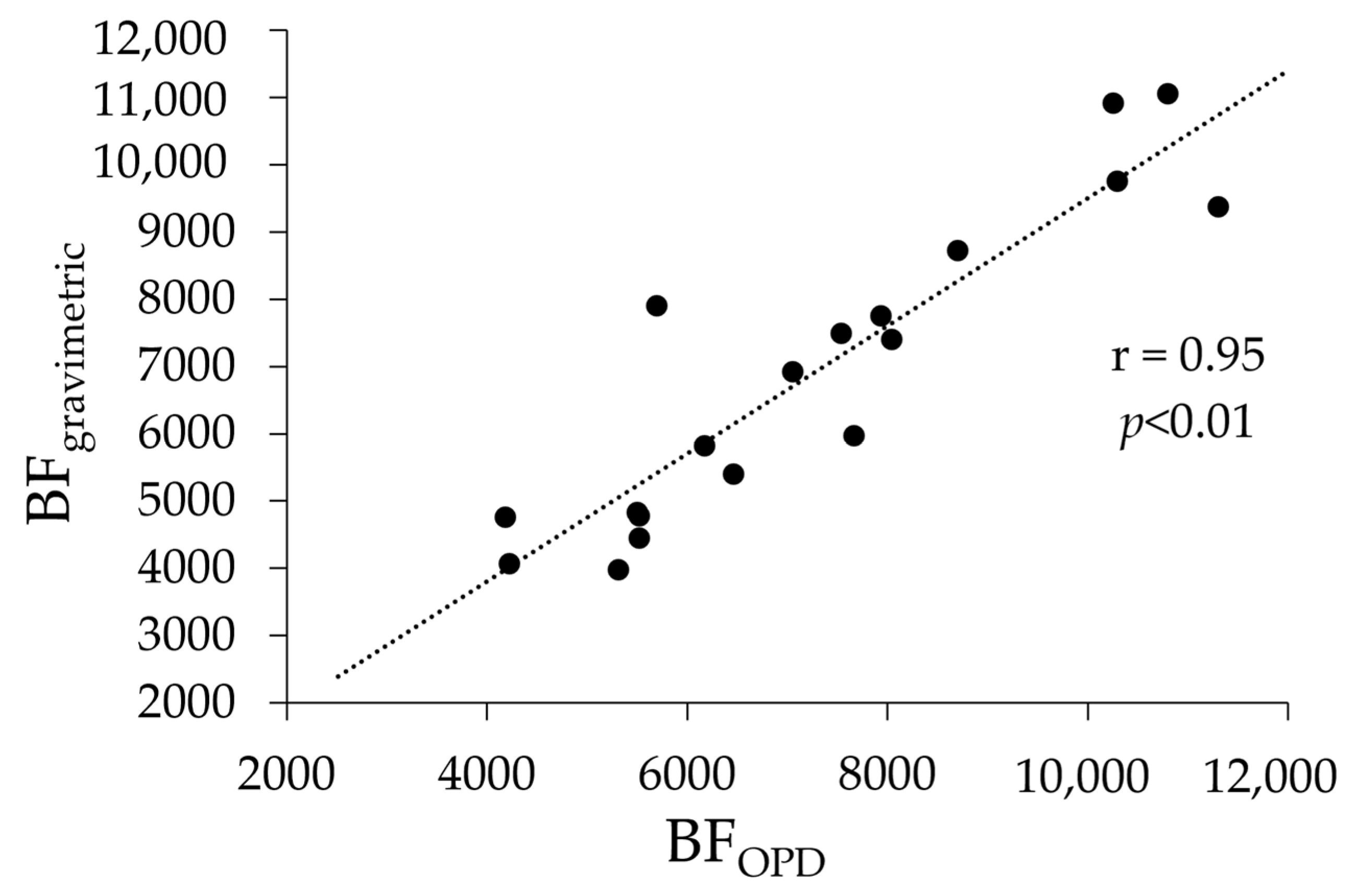
3.4.1. Oocyte Packing Density and Batch Fecundity
3.4.2. Oocyte Numbers Trends
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tyler, C.R.; Sumpter, J.P. Oocyte growth and development in teleosts. Rev. Fish. Biol. Fish. 1996, 6, 287–318. [Google Scholar] [CrossRef]
- Wallace, R.A.; Selman, K. Cellular and Dynamic Aspect of Oocyte Growth in Teleosts. Am. Zool. 1981, 21, 325–343. [Google Scholar] [CrossRef]
- Guraya, S.S. Recent Advances in the Morphology, Cytochemistry, and Function of Balbiani’s Vitelline Body in Animal Oocytes. Int. Rev. Cytol. 1979, 59, 249–321. [Google Scholar]
- Brown-Peterson, N.J.; Wyanski, D.M.; Saborido-Rey, F.; Macewicz, B.J.; Lowerre-Barbieri, S.K. A Standardized Terminology for Describing Reproductive Development in Fishes. Mar. Coast. Fish. 2011, 3, 52–70. [Google Scholar] [CrossRef]
- Hunter, R.J.; Macewicz, B.J.; Chyan-huel Lo, N.; Kimbrell, C.A. Fecundity, spawning, and maturity of female Dover sole Microstomus pacificus, with an evaluation of assumptions and precision. Fish. Bull. 1992, 90, 101–128. [Google Scholar]
- Murua, H.; Saborido-Rey, F. Female Reproductive Strategies of Marine Fish Species of the North Atlantic. J. Northwest Atl. Fish. Sci. 2003, 33, 23–31. [Google Scholar] [CrossRef]
- Ganias, K.; Lowerre-Barbieri, S. Oocyte recruitment and fecundity type in fishes: Refining terms to reflect underlying processes and drivers. Fish Fish. 2018, 19, 562–572. [Google Scholar] [CrossRef]
- Korta, M.; Murua, H.; Kurita, Y.; Kjesbu, O.S. How are the oocytes recruited in an indeterminate fish? Applications of stereological techniques along with advanced packing density theory on European hake (Merluccius merluccius L.). Fish. Res. 2010, 104, 56–63. [Google Scholar] [CrossRef]
- Kurita, Y.; Kjesbu, O.S. Fecundity estimation by oocyte packing density formulae in determinate and indeterminate spawners: Theoretical considerations and applications. J. Sea Res. 2009, 61, 188–196. [Google Scholar] [CrossRef]
- Schismenou, E.; Somarakis, S.; Thorsen, A.; Kjesbu, O.S. Dynamics of de novo vitellogenesis in fish with indeterminate fecundity: An application of oocyte packing density theory to European anchovy, Engraulis encrasicolus. Mar. Biol. 2012, 159, 757–768. [Google Scholar] [CrossRef]
- Serrat, A.; Saborido-Rey, F.; Garcia-Fernandez, C.; Muñoz, M.; Lloret, J.; Thorsen, A.; Kjesbu, O.S. New insights in oocyte dynamics shed light on the complexities associated with fish reproductive strategies. Sci. Rep. 2019, 9, 18411. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Schmidt, T.C.; Thorsen, A.; Slotte, A.; Nøttestad, L.; Kjesbu, O.S. First thorough assessment of de novo oocyte recruitment in a teleost serial spawner, the Northeast Atlantic mackerel (Scomber scombrus) case. Sci. Rep. 2021, 11, 21795. [Google Scholar] [CrossRef]
- Thorsen, A.; Kjesbu, O.S. A rapid method for estimation of oocyte size and potential fecundity in Atlantic cod using a computer-aided particle analysis system. J. Sea Res. 2001, 46, 295–308. [Google Scholar] [CrossRef]
- Mouchlianitis, F.A.; Belo, A.F.; Vieira, A.R.; Quintella, B.R.; Almeida, P.R.; Ganias, K. Primary and secondary oocyte growth dynamics in anadromous semelparous Allis shad Alosa alosa. J. Fish. Biol. 2019, 95, 1447–1456. [Google Scholar] [CrossRef]
- Mouchlianitis, F.A.; Minos, G.; Ganias, K. Timing of oocyte recruitment within the ovulatory cycle of Macedonian shad, Alosa macedonica, a batch spawning fish with indeterminate fecundity. Theriogenology 2020, 146, 31–38. [Google Scholar] [CrossRef]
- Somarakis, S.; Giannoulaki, M.; Markakis, K.; Tsiaras, K.; Schismenou, E.; Peristeraki, P. Ovarian dynamics, batch fecundity and spawning phenology of the Lessepsian migrant Etrumeus golanii DiBattista, Randall & Bowen, 2012 (Clupeidae: Dussumieriinae). Mediterr. Mar. Sci. 2021, 22, 466–479. [Google Scholar] [CrossRef]
- Ganias, K.; Somarakis, S.; Koutsikopoulos, C.; Machias, A. Factors affecting the spawning period of sardine in two highly oligotrophic Seas. Mar. Biol. 2007, 151, 1559–1569. [Google Scholar] [CrossRef]
- Somarakis, S.; Tsoukali, S.; Giannoulaki, M.; Schismenou, E.; Nikolioudakis, N. Spawning stock, egg production and larval survival in relation to small pelagic fish recruitment. Mar. Ecol. Prog. Ser. 2019, 617, 113–139. [Google Scholar] [CrossRef]
- Ganias, K.; Somarakis, S.; Machias, A.; Theodorou, A.J. Evaluation of spawning frequency in a Mediterranean sardine population (Sardina pilchardus sardina). Mar. Biol. 2003, 142, 1169–1179. [Google Scholar] [CrossRef]
- Ganias, K.; Somarakis, S.; Machias, A.; Theodorou, A. Pattern of oocyte development and batch fecundity in the Mediterranean sardine. Fish. Res. 2004, 67, 13–23. [Google Scholar] [CrossRef]
- Bennett, H.S.; Wyrick, A.D.; Lee, S.W.; McNeil, H. Science and art in preparing tissues embebbed in plastic for light microscopy, with special reference to glycol methacrylate, glass knives and simple stains. Stain. Technol. 1976, 51, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Pèrez, N.; Figueiredo, I.; Lo, N.C.H. Batch fecundity of Sardina pilchardus (Walb.) off the Atlantic Iberian coast. Bol. Ins. Esp. Oceanogr. 1992, 8, 155–162. [Google Scholar]
- Charitonidou, K.; Panteris, E.; Ganias, K. Balbiani body formation and cytoplasmic zonation during early oocyte development in two Clupeiform fishes. J. Fish. Biol. 2022, 100, 1223–1232. [Google Scholar] [CrossRef]
- Hunter, R.J.; Macewicz, B.J. Measurement of Spawning Frequency in Multiple Spawning Fishes. NOAA Tech. Rep. 1985, 36, 79–94. [Google Scholar]
- Saber, S.; Macías, D.; Ortiz de Urbina, J.; Kjesbu, O.S. Stereological comparison of oocyte recruitment and batch fecundity estimates from paraffin and resin sections using spawning albacore (Thunnus alalunga) ovaries as a case study. J. Sea Res. 2015, 95, 226–238. [Google Scholar] [CrossRef]
- Howard, V.; Reed, M.G. Unbiased Stereology: Three-Dimensional Measurement in Microscopy, 1st ed.; BIOS Scientific Publishers: Oxford, UK, 1998. [Google Scholar]
- ImageJ. Available online: https://imagej.nih.gov/ij/ (accessed on 27 July 2021).
- ObjectJ. Available online: https://sils.fnwi.uva.nl/bcb/objectj/ (accessed on 27 July 2021).
- Emerson, L.S.; Greer, M.; Witthames, P.R. A stereological method for estimating fish fecundity. J. Fish. Biol. 1990, 36, 721–730. [Google Scholar] [CrossRef]
- Hunter, R.J.; Lo, N.C.H.; Leong, R.J.H. Batch Fecundity in Multiple Spawning Fishes. NOAA Tech. Rep. 1985, 36, 67–77. [Google Scholar]
- Gkanasos, A.; Somarakis, S.; Tsiaras, K.; Kleftogiannis, D.; Giannoulaki, M.; Schismenou, E.; Sofianos, S.; Triantafyllou, G. Development, application and evaluation of a 1-D full life cycle anchovy and sardine model for the North Aegean Sea (Eastern Mediterranean). PLoS ONE 2019, 14, e0219671. [Google Scholar] [CrossRef]
- Geladakis, G.; Nikolioudakis, N.; Koumoundouros, G.; Somarakis, S. Morphometric discrimination of pelagic fish stocks challenged by variation in body condition. ICES J. Mar. Sci. 2018, 75, 711–718. [Google Scholar] [CrossRef]
- Somarakis, S.; Ganias, K.; Siapatis, A.; Koutsikopoulos, C.; Machias, A.; Papaconstantinou, C. Spawning habitat and daily egg production of sardine (Sardina pilchardus) in the eastern Mediterranean. Fish Oceanogr. 2006, 15, 281–292. [Google Scholar] [CrossRef]
- Tsikliras, A.C.; Antonopoulou, E.; Stergiou, K.I. Spawning period of Mediterranean marine fishes. Rev. Fish Biol. Fish. 2010, 20, 499–538. [Google Scholar] [CrossRef]
- Somarakis, S.; Ganias, K.; Tserpes, G.; Koutsikopoulos, C. Ovarian allometry and the use of the gonosomatic index: A case study in the Mediterranean sardine, Sardina pilchardus. Mar. Biol. 2004, 146, 181–189. [Google Scholar] [CrossRef]
- Daoulas, C.; Economou, A.N. Seasonal variation of egg size in the sardine, Sardina pilchardus Walb., of the Saronikos Gulf: Causes and a probable explanation. J. Fish. Biol. 1986, 28, 449–457. [Google Scholar] [CrossRef]
- Mandić, M.; Regner, S. Variation in fish egg size in several pelagic fish species. Stud. Mar. 2014, 17, 31–46. [Google Scholar]
- Charitonidou, K.; Panteris, E.; Ganias, K. Oogonial proliferation and early oocyte dynamics during the reproductive cycle of two Clupeiform fish species. J. Fish. Biol. 2022, 102, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Charitonidou, K.; Kjesbu, O.S.; Nunes, C.; Angélico, M.M.; Dominguez-Petit, R.; Garabana, D.; Ganias, K. Linking the dynamic organization of the ovary with spawning dynamics in pelagic fishes. Mar. Biol. 2022, 169, 47. [Google Scholar] [CrossRef]
- Ganias, K.; Lowerre-Barbieri, S.K.; Cooper, W. Understanding the determinate-indeterminate fecundity dichotomy in fish populations using a temperature dependent oocyte growth model. J. Sea Res. 2015, 96, 1–10. [Google Scholar] [CrossRef]
- Dos Santos Schmidt, T.C.; Slotte, A.; Kennedy, J.; Sundby, S.; Johannessen, A.; Óskarsson, G.J.; Kurita, Y.; Kjesbu, O.S. Oogenesis and reproductive investment of Atlantic herring are functions of not only present but long-ago environmental influences as well. Proc. Natl. Acad. Sci. USA 2017, 114, 2634–2639. [Google Scholar] [CrossRef]
- Ganias, K. Linking sardine spawning dynamics to environmental variability. Estuar. Coast. Shelf Sci. 2009, 84, 402–408. [Google Scholar] [CrossRef]
- Thomé, R.G.; Domingos, F.F.T.; Santos, H.B.; Martinelli, P.M.; Sato, Y.; Rizzo, E.; Bazzoli, N. Apoptosis, cell proliferation and vitellogenesis during the folliculogenesis and follicular growth in teleost fish. Tissue Cell. 2012, 44, 54–62. [Google Scholar] [CrossRef]
- Bernal, M.; Somarakis, S.; Witthames, P.R.; van Damme, C.J.G.; Uriarte, A.; Lo, N.C.H.; Dickey-Collas, M. Egg production methods in marine fisheries: An introduction. Fish. Res. 2012, 117, 1–5. [Google Scholar] [CrossRef]
- Dickey-Collas, M.; Somarakis, S.; Witthames, P.R.; van Damme, C.J.G.; Uriarte, A.; Lo, N.C.H.; Bernal, M. Where do egg production methods for estimating fish biomass go from here? Fish. Res. 2012, 117, 6–11. [Google Scholar] [CrossRef]
- Saraux, C.; Van Beveren, E.; Brosset, P.; Queiros, Q.; Bourdeix, J.H.; Dutto, G.; Gasset, E.; Jac, C.; Bonhommeau, S.; Fromentin, J.M. Small pelagic fish dynamics: A review of mechanisms in the Gulf of Lions. Deep Sea Res. Part. II Top. Stud. Oceanogr. 2019, 159, 52–61. [Google Scholar] [CrossRef]
- Brosset, P.; Fromentin, J.-M.; Van Beveren, E.; Lloret, J.; Marques, V.; Basilone, G.; Bonanno, A.; Carpi, P.; Donato, F.; Čikeš Keč, V.; et al. Spatio-temporal patterns and environmental controls of small pelagic fish body condition from contrasted Mediterranean areas. Prog. Oceanogr. 2017, 151, 149–162. [Google Scholar] [CrossRef]
- Albo-Puigserver, M.; Pennino, M.G.; Bellido, J.M.; Colmenero, A.I.; Giráldez, A.; Hidalgo, M.; Gabriel Ramírez, J.; Steenbeek, J.; Torres, P.; Cousido-Rocha, M.; et al. Changes in life history traits of small pelagic fish in the western Mediterranean sea. Front. Mar. Sci. 2021, 8, 570354. [Google Scholar] [CrossRef]

| Ovarian Phase | Gonad Stage | Oocyte Stage | Month | OPD | WM | |
|---|---|---|---|---|---|---|
 | Spawning capable | Secondary with POF-1,(Vtg2, POF-1) Spawned the previous night | Vtg2 | November | 3 | 2 |
| December | 3 | 1 | ||||
| January | 3 | 1 | ||||
| February | 3 | 1 | ||||
| March | 3 | 1 | ||||
 | Spawning capable | Secondary with POF-2,(Vtg2, POF-2) Spawned two days before sampling | Vtg2 | November | 3 | 1 |
| December | 3 | 1 | ||||
| January | 3 | 1 | ||||
| February | 3 | 1 | ||||
| March | 3 | 1 | ||||
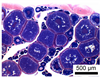 | Spawning capable | Tertiary, (Vtg3) | Vtg3 | November | 3 | 1 |
| December | 3 | 1 | ||||
| January | 3 | 1 | ||||
| February | 3 | 1 | ||||
| March | 3 | 1 | ||||
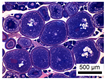 | Spawning capable (Active spawning subphase) | Germinal vesicle migration, (GVM) | GVM | November | 3 | 1 |
| December | 2 | 1 | ||||
| January | 3 | 1 | ||||
| February | 3 | 1 | ||||
| March | 3 | 1 | ||||
| Total | 59 | 21 |
| Year | Month | Ovarian Phase | ||||
|---|---|---|---|---|---|---|
| IR | D | SC (+AS) | R | Ν | ||
| 2018 | November | - | 40.3% | 43.0% (+16.7%) | - | 72 |
| 2018 | December | - | 57.5% | 39.7% (+2.7%) | - | 73 |
| 2019 | January | - | 31.9% | 58.3% (+9.7%) | - | 72 |
| 2019 | February | - | 24.6% | 43.1% (+32.3%) | - | 65 |
| 2019 | March | - | 26.9% | 64.2% (+8.9%) | - | 67 |
| 2019 | April | 6.8% | 16.9% | 27.1% | 49.2% | 59 |
| 2019 | May | 84.4% | 0.44% | - | 11.1% | 45 |
| 2019 | June | 100% | - | - | - | 62 |
| 2019 | July | 100% | - | - | - | 64 |
| 2019 | August | 100% | - | - | - | 64 |
| 2019 | September | 69.4% | 30.6% | - | - | 62 |
| 2019 | October | 10.8% | 80% | 7.7% | 1.5% | 65 |
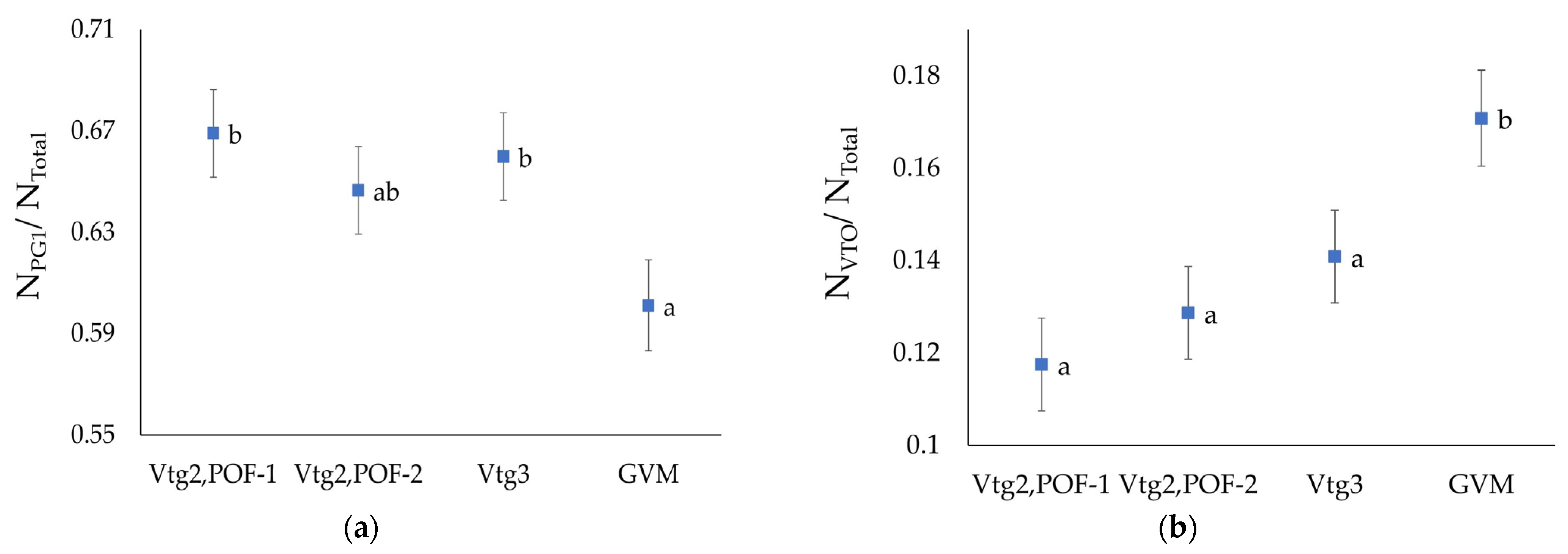
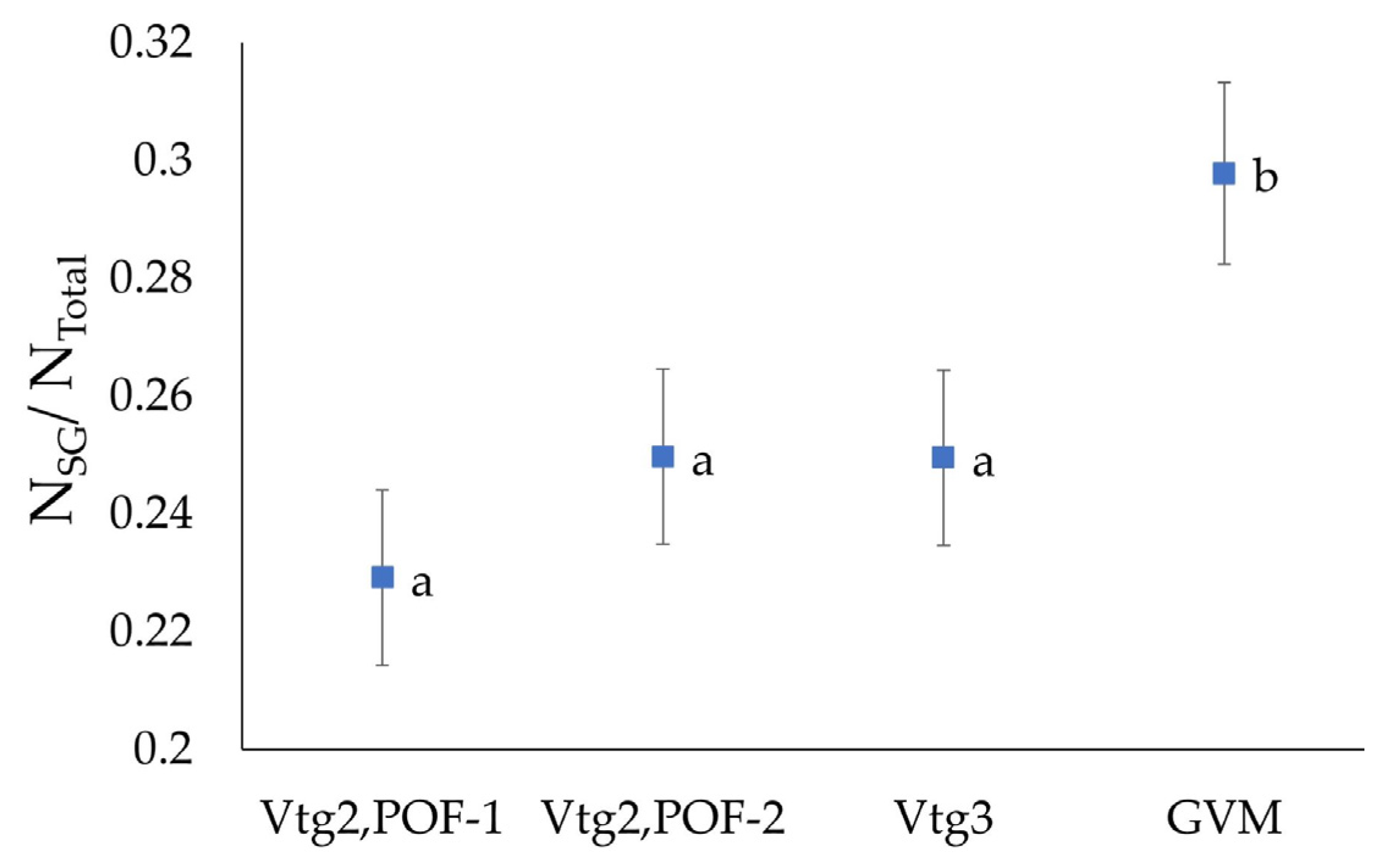
| Gonad Stage | PG1/Total (±SE) | PG2/Total (±SE) | (VTO)/Total (±SE) | (SG)/Total (±SE) |
|---|---|---|---|---|
| Vtg2, POF-1 | 0.669 (±0.017) | 0.102 (±0.009) | 0.117 (±0.01) | 0.229 (±0.015) |
| Vtg2, POF-2 | 0.646 (±0.017) | 0.104 (±0.009) | 0.129 (±0.01) | 0.25 (±0.015) |
| Vtg3 | 0.66 (±0.017) | 0.091 (±0.009) | 0.141 (±0.01) | 0.25 (±0.015) |
| GVM | 0.601 (±0.018) | 0.101 (±0.01) | 0.171 (±0.01) | 0.298 (±0.015) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Servou, E.; Schismenou, E.; Somarakis, S. Quantitative Analysis of Ovarian Dynamics of European Sardine Sardina pilchardus (Walbaum, 1792) during Its Spawning Period. Fishes 2023, 8, 226. https://doi.org/10.3390/fishes8050226
Servou E, Schismenou E, Somarakis S. Quantitative Analysis of Ovarian Dynamics of European Sardine Sardina pilchardus (Walbaum, 1792) during Its Spawning Period. Fishes. 2023; 8(5):226. https://doi.org/10.3390/fishes8050226
Chicago/Turabian StyleServou, Eleni, Eudoxia Schismenou, and Stylianos Somarakis. 2023. "Quantitative Analysis of Ovarian Dynamics of European Sardine Sardina pilchardus (Walbaum, 1792) during Its Spawning Period" Fishes 8, no. 5: 226. https://doi.org/10.3390/fishes8050226
APA StyleServou, E., Schismenou, E., & Somarakis, S. (2023). Quantitative Analysis of Ovarian Dynamics of European Sardine Sardina pilchardus (Walbaum, 1792) during Its Spawning Period. Fishes, 8(5), 226. https://doi.org/10.3390/fishes8050226







