Assessing the Effects of Physical Barriers and Hypoxia on Red Drum Movement Patterns to Develop More Effective Management Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Model Species
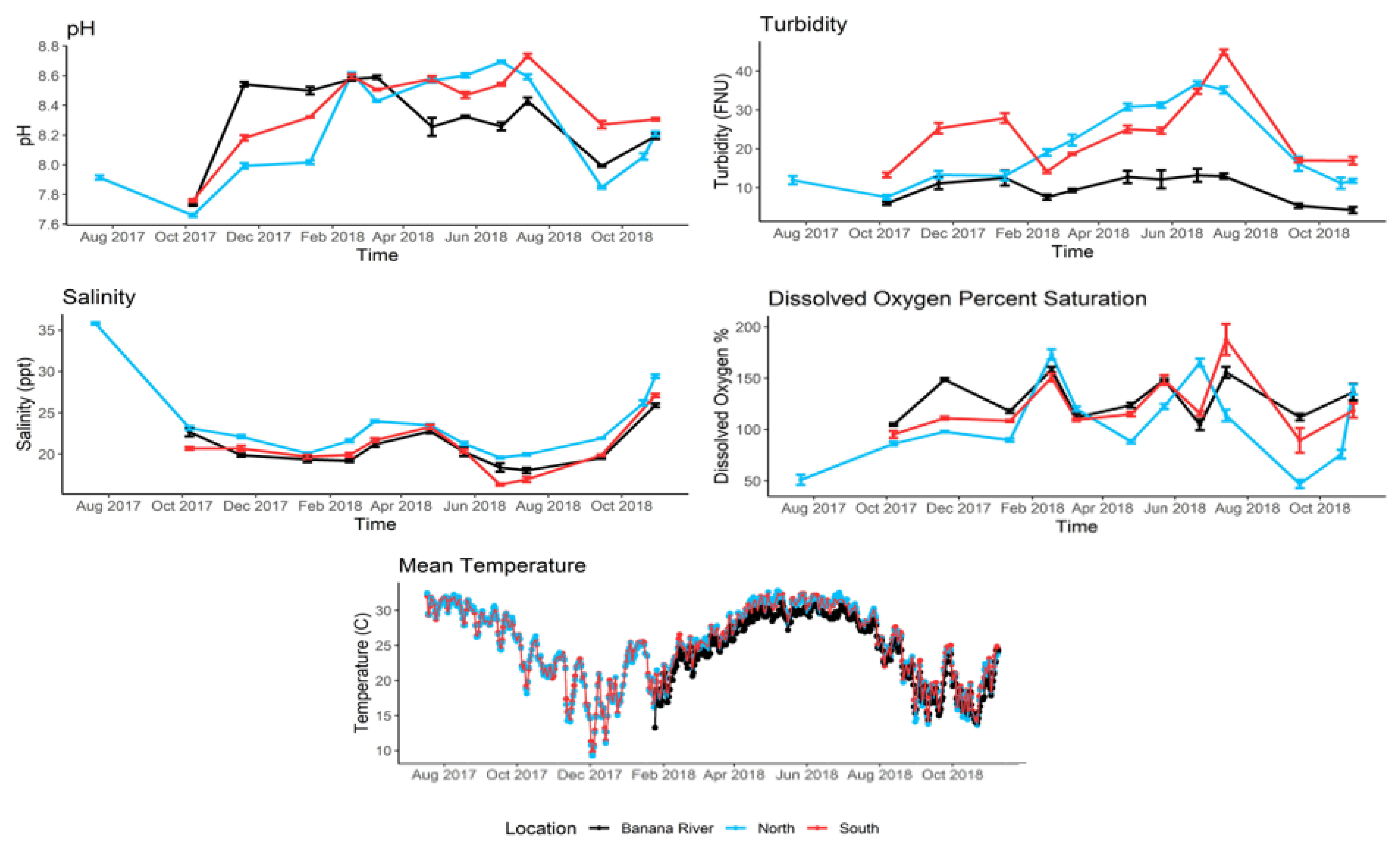
2.3. Water Quality Characterization
2.4. Fish Tagging and Receiver Deployment
2.5. Movement Analysis
2.6. Fish Response to Hypoxia
3. Results
3.1. Water Quality Characterization
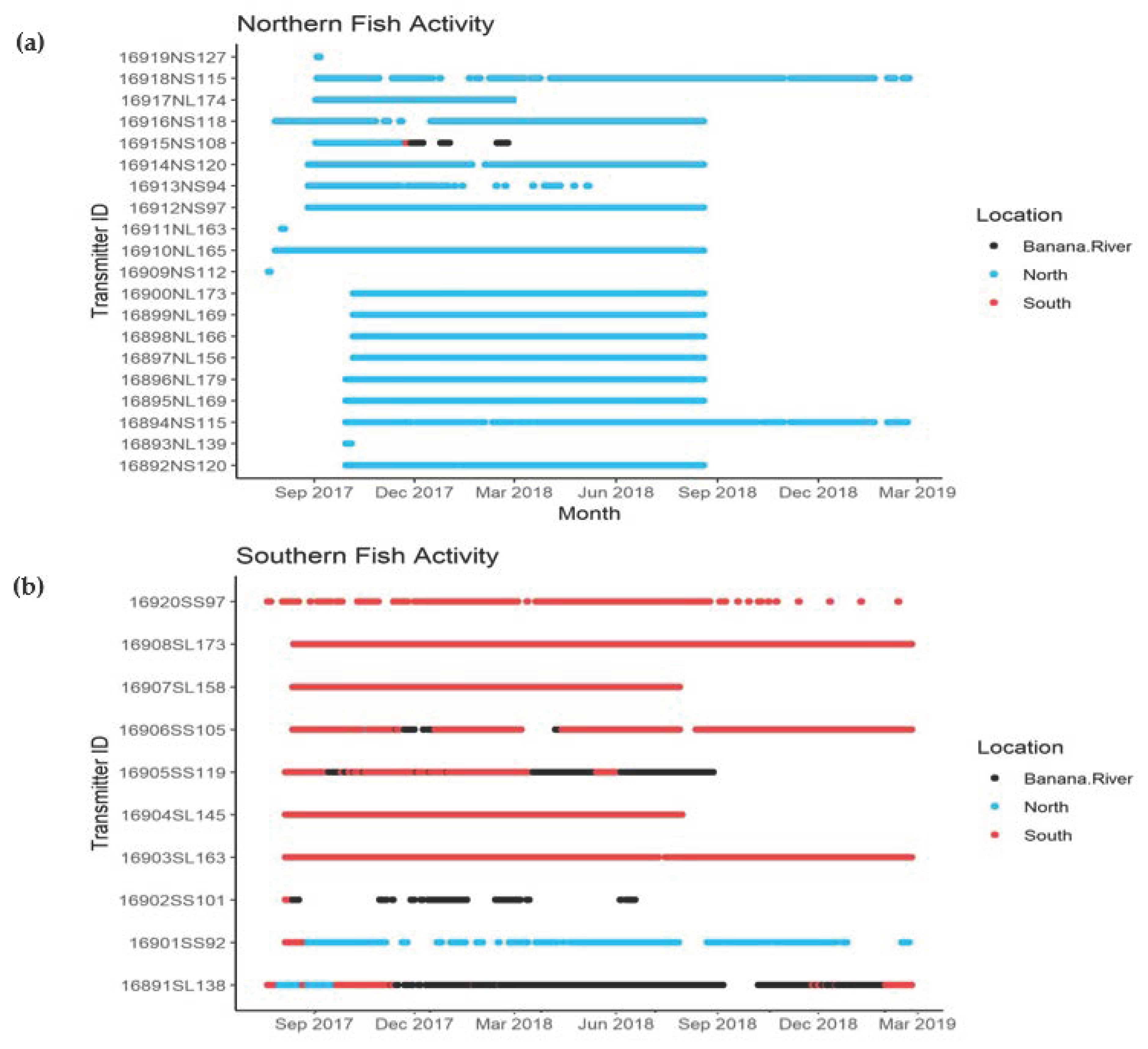
3.2. Movement Analysis
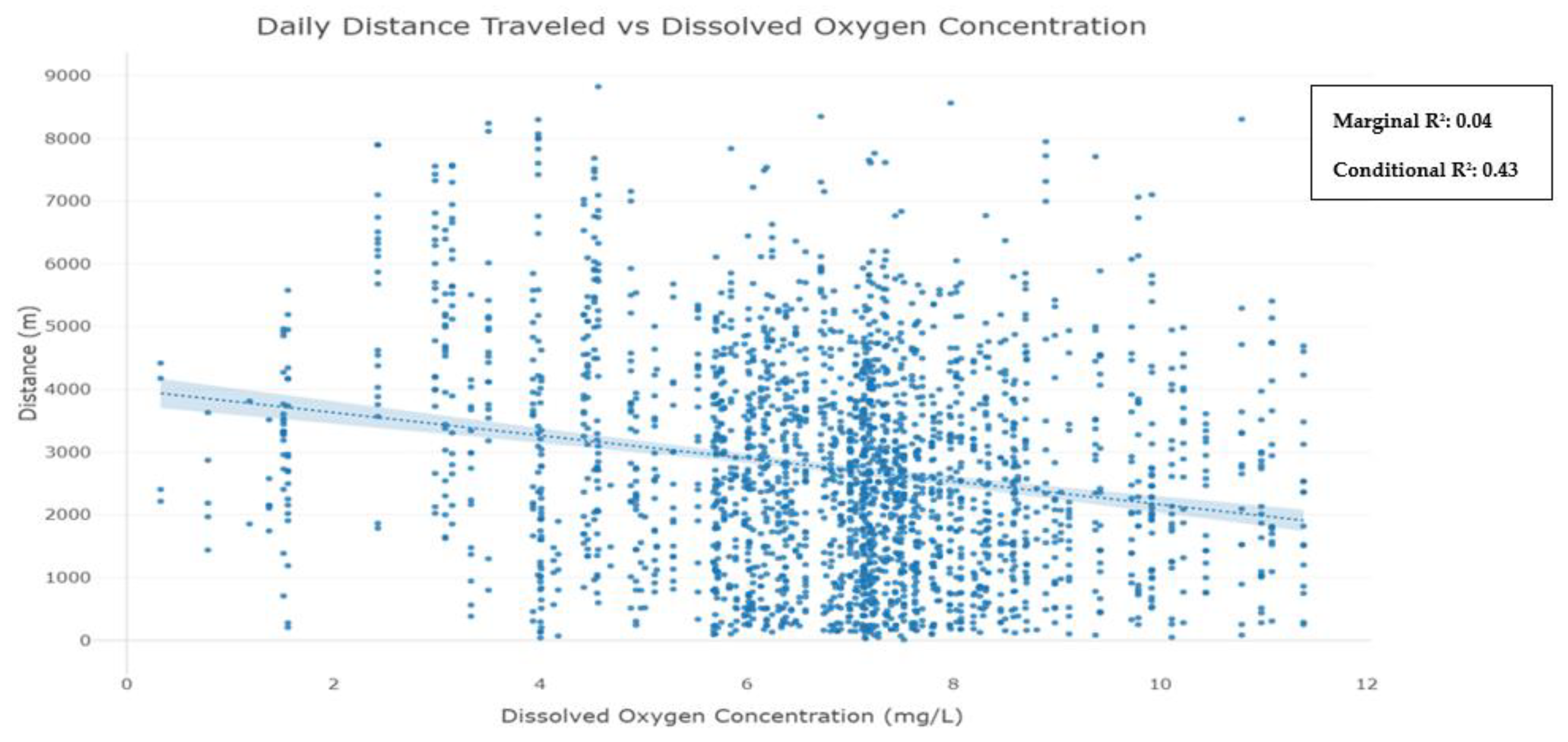
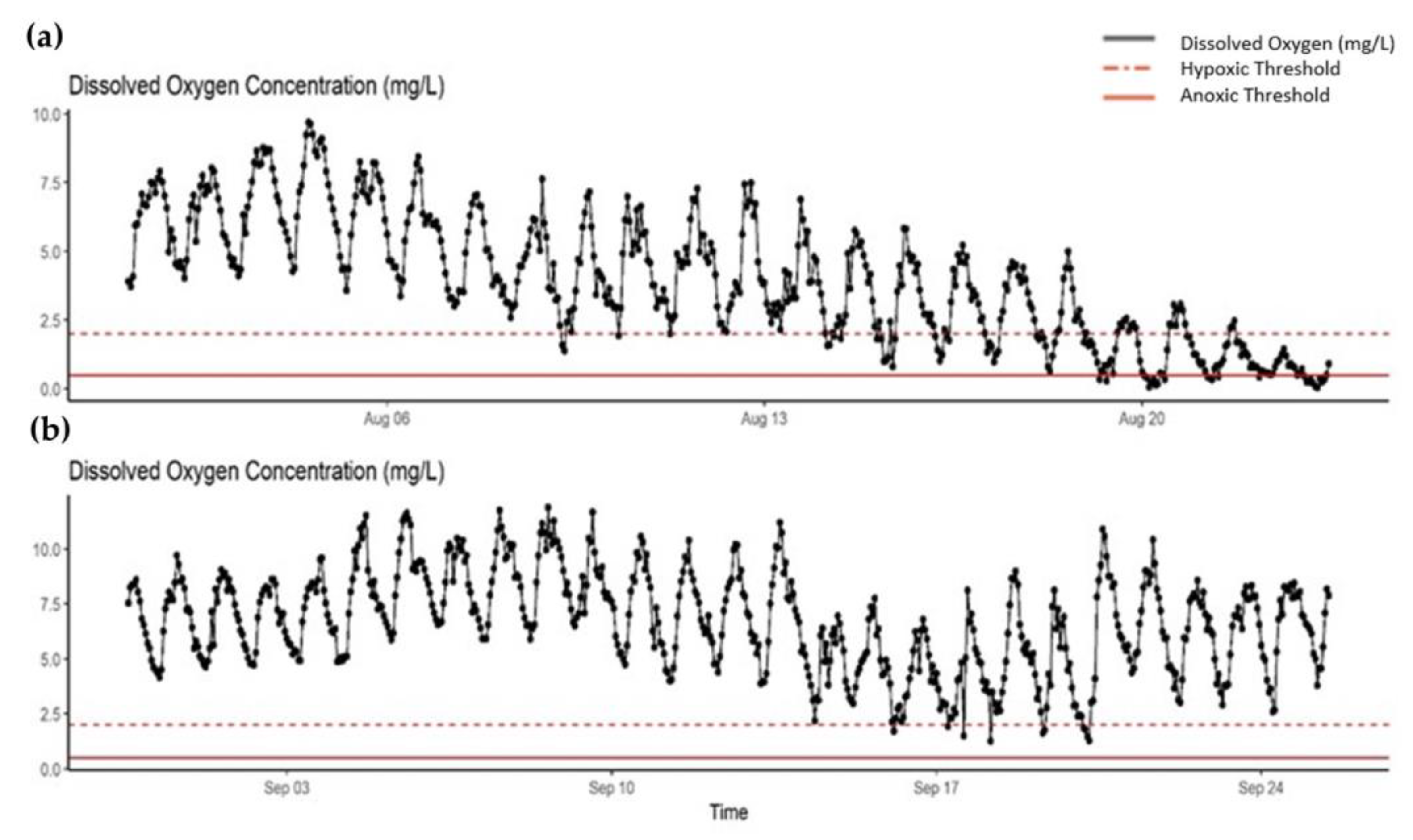
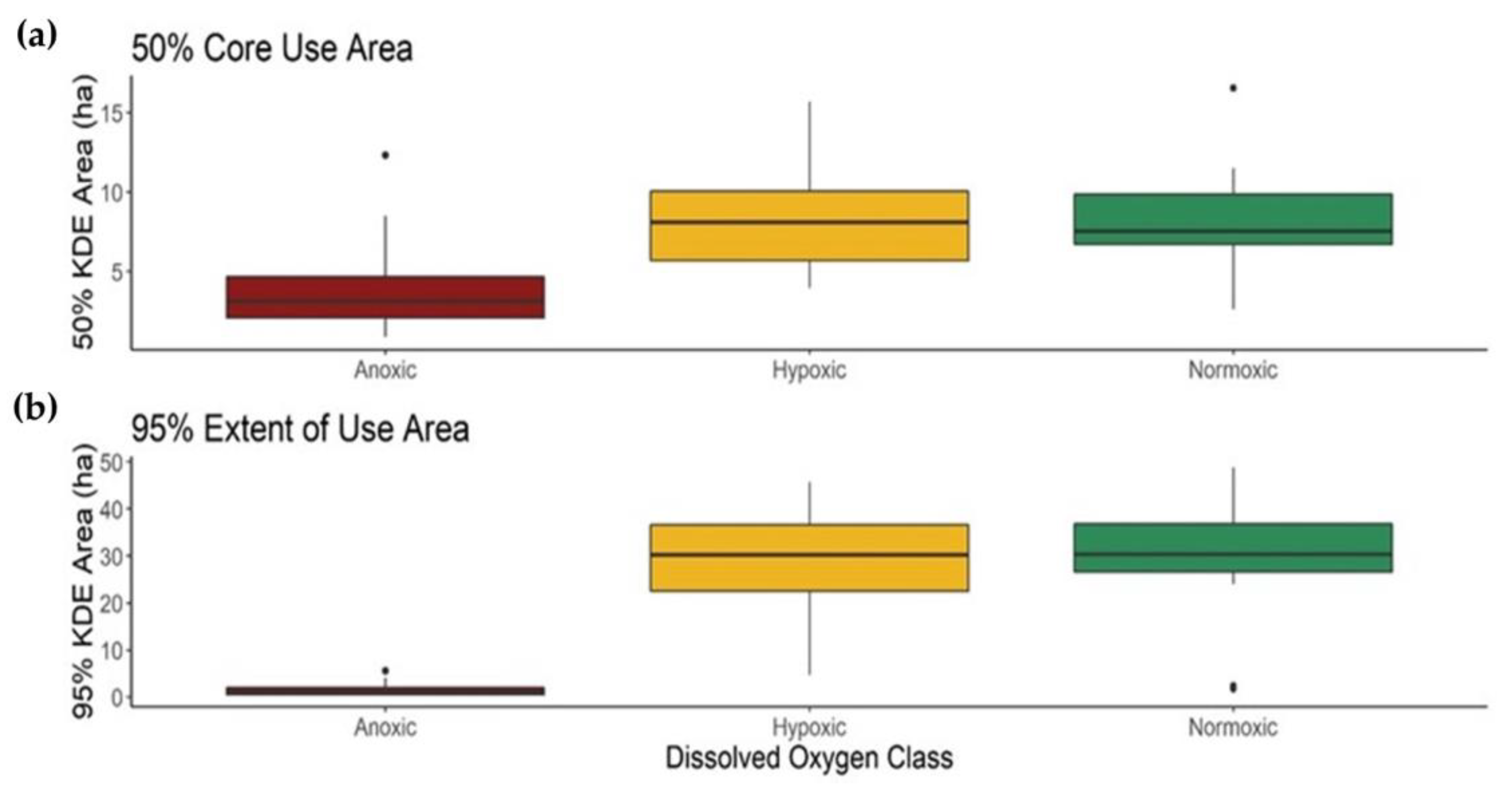
3.3. Fish Response to Hypoxia
4. Discussion
4.1. Fish Movement and Habitat Use
4.2. Red Drum Movement in Response to Hypoxia
4.3. Management Strategies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Home Range Areas
| Fish ID | Date | h Value | Points | 50% KDE Area (ha) | 95% KDE Area (ha) |
|---|---|---|---|---|---|
| 16891SL138 | 9/2017 | 30.67 | 295 | 1.36 | 5.31 |
| 16891SL138 | 10/2017 | 42.78 | 1288 | 3.73 | 19.59 |
| 16891SL138 | 11/2017 | 44.44 | 1166 | 4.92 | 18.89 |
| 16891SL138 | 12/2017 | 32.92 | 1343 | 2.15 | 8.23 |
| 16891SL138 | 1/2018 | 76.02 | 340 | 5.68 | 7.30 |
| 16891SL138 | 2/2018 | 33.82 | 907 | 1.34 | 6.44 |
| 16891SL138 | 3/2018 | 18.58 | 1071 | 0.17 | 1.63 |
| 16891SL138 | 4/2018 | 35.39 | 1034 | 0.57 | 3.02 |
| 16891SL138 | 5/2018 | 22.02 | 775 | 0.46 | 2.57 |
| 16891SL138 | 6/2018 | 106.95 | 550 | 5.91 | 31.57 |
| 16891SL138 | 7/2018 | 47.61 | 813 | 1.90 | 11.15 |
| 16891SL138 | 8/2018 | 24.91 | 1026 | 1.14 | 4.79 |
| 16891SL138 | 9/2018 | 61.00 | 620 | 3.40 | 18.04 |
| 16891SL138 | Total | 58.60 | 11,384 | 6.34 | 35.32 |
| 16892NS120 | 9/2017 | 67.94 | 27 | 5.10 | 15.00 |
| 16892NS120 | 10/2017 | 48.15 | 981 | 3.93 | 28.25 |
| 16892NS120 | 11/2017 | 61.71 | 768 | 8.37 | 35.90 |
| 16892NS120 | 12/2017 | 59.90 | 765 | 7.49 | 29.97 |
| 16892NS120 | 1/2018 | 67.45 | 451 | 6.68 | 27.07 |
| 16892NS120 | 2/2018 | 50.91 | 582 | 1.95 | 15.38 |
| 16892NS120 | 3/2018 | 4.06 | 562 | 0.01 | 0.04 |
| 16892NS120 | 4/2018 | 34.39 | 556 | 0.84 | 13.77 |
| 16892NS120 | 5/2018 | 41.27 | 907 | 2.35 | 16.86 |
| 16892NS120 | 6/2018 | 47.23 | 1153 | 7.38 | 26.62 |
| 16892NS120 | 7/2018 | 50.88 | 1134 | 8.36 | 30.50 |
| 16892NS120 | 8/2018 | 59.78 | 742 | 10.86 | 36.48 |
| 16892NS120 | Total | 37.78 | 8628 | 4.93 | 25.18 |
| 16894NS115 | 9/2017 | 102.43 | 21 | 10.72 | 24.84 |
| 16894NS115 | 10/2017 | 79.48 | 380 | 6.73 | 14.75 |
| 16894NS115 | 12/2017 | 72.12 | 215 | 12.04 | 39.62 |
| 16894NS115 | 1/2018 | 55.67 | 187 | 5.58 | 28.62 |
| 16894NS115 | 2/2018 | 51.74 | 82 | 2.69 | 18.52 |
| 16894NS115 | 3/2018 | 54.49 | 170 | 4.32 | 22.51 |
| 16894NS115 | 4/2018 | 46.67 | 286 | 4.89 | 22.71 |
| 16894NS115 | 5/2018 | 77.59 | 356 | 11.36 | 40.36 |
| 16894NS115 | 6/2018 | 65.60 | 403 | 9.35 | 36.46 |
| 16894NS115 | 7/2018 | 62.81 | 521 | 9.69 | 34.60 |
| 16894NS115 | 8/2018 | 78.94 | 247 | 10.20 | 34.26 |
| 16894NS115 | 9/2018 | 74.97 | 217 | 9.25 | 34.06 |
| 16894NS115 | 10/2018 | 100.15 | 90 | 14.14 | 44.24 |
| 16894NS115 | Total | 52.97 | 3244 | 10.48 | 34.11 |
| 16895NL169 | 9/2017 | 78.69 | 23 | 5.82 | 17.18 |
| 16895NL169 | 10/2017 | 52.68 | 1248 | 5.81 | 30.41 |
| 16895NL169 | 11/2017 | 56.50 | 1114 | 9.53 | 33.92 |
| 16895NL169 | 12/2017 | 55.65 | 1232 | 10.79 | 33.29 |
| 16895NL169 | 1/2018 | 63.51 | 828 | 9.94 | 32.96 |
| 16895NL169 | 2/2018 | 61.29 | 981 | 10.20 | 34.31 |
| 16895NL169 | 3/2018 | 61.61 | 843 | 9.05 | 31.92 |
| 16895NL169 | 4/2018 | 60.19 | 821 | 10.67 | 34.44 |
| 16895NL169 | 5/2018 | 56.98 | 930 | 8.98 | 33.69 |
| 16895NL169 | 6/2018 | 44.02 | 1157 | 4.56 | 22.65 |
| 16895NL169 | 7/2018 | 45.90 | 1220 | 6.64 | 27.08 |
| 16895NL169 | 8/2018 | 58.27 | 748 | 10.26 | 34.86 |
| 16895NL169 | Total | 39.58 | 11,145 | 8.82 | 28.37 |
| 16896NL179 | 9/2017 | 116.55 | 12 | 12.92 | 14.29 |
| 16896NL179 | 10/2017 | 52.44 | 1155 | 6.03 | 32.48 |
| 16896NL179 | 11/2017 | 56.06 | 1073 | 10.07 | 33.57 |
| 16896NL179 | 12/2017 | 55.67 | 1155 | 10.72 | 34.36 |
| 16896NL179 | 1/2018 | 65.91 | 774 | 11.27 | 36.98 |
| 16896NL179 | 2/2018 | 61.48 | 940 | 12.27 | 36.38 |
| 16896NL179 | 3/2018 | 62.26 | 1048 | 11.16 | 35.55 |
| 16896NL179 | 4/2018 | 69.65 | 844 | 11.43 | 38.85 |
| 16896NL179 | 5/2018 | 60.53 | 983 | 9.58 | 33.08 |
| 16896NL179 | 6/2018 | 55.84 | 1194 | 8.56 | 33.33 |
| 16896NL179 | 7/2018 | 52.01 | 1214 | 8.16 | 32.35 |
| 16896NL179 | 8/2018 | 55.46 | 725 | 9.63 | 33.77 |
| 16896NL179 | Total | 40.35 | 11,117 | 9.67 | 29.02 |
| 16897NL156 | 10/2017 | 62.02 | 800 | 7.94 | 34.78 |
| 16897NL156 | 11/2017 | 57.24 | 647 | 6.99 | 30.01 |
| 16897NL156 | 12/2017 | 51.82 | 516 | 6.36 | 24.78 |
| 16897NL156 | 1/2018 | 70.57 | 422 | 9.00 | 34.89 |
| 16897NL156 | 2/2018 | 80.79 | 420 | 10.12 | 37.65 |
| 16897NL156 | 3/2018 | 68.87 | 432 | 9.45 | 34.70 |
| 16897NL156 | 4/2018 | 66.49 | 455 | 8.26 | 36.37 |
| 16897NL156 | 5/2018 | 44.97 | 823 | 3.46 | 21.41 |
| 16897NL156 | 6/2018 | 35.14 | 1042 | 2.91 | 14.17 |
| 16897NL156 | 7/2018 | 41.41 | 1002 | 3.49 | 20.17 |
| 16897NL156 | 8/2018 | 60.85 | 726 | 10.25 | 36.19 |
| 16897NL156 | Total | 41.32 | 7285 | 5.68 | 27.66 |
| 16898NL166 | 10/2017 | 57.67 | 838 | 6.97 | 30.02 |
| 16898NL166 | 11/2017 | 57.09 | 664 | 5.67 | 25.37 |
| 16898NL166 | 12/2017 | 54.02 | 749 | 4.79 | 25.36 |
| 16898NL166 | 1/2018 | 62.42 | 781 | 10.23 | 37.66 |
| 16898NL166 | 2/2018 | 52.12 | 741 | 6.30 | 27.94 |
| 16898NL166 | 3/2018 | 53.91 | 696 | 6.17 | 29.36 |
| 16898NL166 | 4/2018 | 55.32 | 649 | 5.82 | 30.31 |
| 16898NL166 | 5/2018 | 42.10 | 1040 | 4.48 | 20.87 |
| 16898NL166 | 6/2018 | 42.07 | 1131 | 6.28 | 23.40 |
| 16898NL166 | 7/2018 | 41.09 | 1253 | 4.84 | 20.91 |
| 16898NL166 | 8/2018 | 53.71 | 788 | 7.34 | 28.50 |
| 16898NL166 | Total | 37.11 | 9330 | 5.63 | 25.11 |
| 16899NL169 | 10/2017 | 58.59 | 1089 | 7.00 | 29.21 |
| 16899NL169 | 11/2017 | 51.32 | 1285 | 5.76 | 25.50 |
| 16899NL169 | 12/2017 | 51.21 | 1427 | 6.96 | 27.83 |
| 16899NL169 | 1/2018 | 60.88 | 1222 | 11.76 | 37.06 |
| 16899NL169 | 2/2018 | 61.78 | 1194 | 9.81 | 35.43 |
| 16899NL169 | 3/2018 | 54.16 | 1259 | 8.20 | 31.82 |
| 16899NL169 | 4/2018 | 54.23 | 1069 | 8.41 | 30.16 |
| 16899NL169 | 5/2018 | 56.51 | 1251 | 8.70 | 29.72 |
| 16899NL169 | 6/2018 | 49.25 | 1293 | 7.12 | 28.11 |
| 16899NL169 | 7/2018 | 46.93 | 1353 | 7.17 | 26.04 |
| 16899NL169 | 8/2018 | 52.06 | 783 | 9.13 | 30.57 |
| 16899NL169 | Total | 37.78 | 13,225 | 8.14 | 26.12 |
| 16900NL173 | 10/2017 | 47.23 | 1143 | 3.31 | 19.21 |
| 16900NL173 | 11/2017 | 60.51 | 1213 | 8.43 | 34.71 |
| 16900NL173 | 12/2017 | 52.41 | 1370 | 5.87 | 29.14 |
| 16900NL173 | 1/2018 | 65.54 | 944 | 8.13 | 33.04 |
| 16900NL173 | 2/2018 | 57.61 | 1120 | 6.71 | 29.20 |
| 16900NL173 | 3/2018 | 59.27 | 1115 | 8.15 | 32.43 |
| 16900NL173 | 4/2018 | 61.04 | 914 | 9.02 | 31.48 |
| 16900NL173 | 5/2018 | 50.75 | 1020 | 5.93 | 24.82 |
| 16900NL173 | 6/2018 | 37.43 | 1248 | 3.11 | 13.85 |
| 16900NL173 | 7/2018 | 38.30 | 1253 | 3.83 | 19.34 |
| 16900NL173 | 8/2018 | 58.57 | 780 | 9.45 | 34.39 |
| 16900NL173 | Total | 39.64 | 12,120 | 6.86 | 27.52 |
| 16901SS92 | 10/2017 | 36.58 | 862 | 2.31 | 9.89 |
| 16901SS92 | 11/2017 | 53.69 | 136 | 4.04 | 17.60 |
| 16901SS92 | 12/2017 | 36.58 | 54 | 2.08 | 8.06 |
| 16901SS92 | 2/2018 | 36.21 | 106 | 1.13 | 9.92 |
| 16901SS92 | 4/2018 | 44.07 | 75 | 1.49 | 8.43 |
| 16901SS92 | 5/2018 | 76.96 | 101 | 7.25 | 17.93 |
| 16901SS92 | 6/2018 | 35.36 | 381 | 2.13 | 11.39 |
| 16901SS92 | 7/2018 | 42.39 | 848 | 3.59 | 19.25 |
| 16901SS92 | 8/2018 | 47.92 | 540 | 4.61 | 26.42 |
| 16901SS92 | Total | 58.16 | 3715 | 8.70 | 34.58 |
| 16902SS101 | 10/2017 | 84.07 | 894 | 4.36 | 40.44 |
| 16902SS101 | Total | 84.07 | 894 | 4.36 | 40.44 |
| 16903SL163 | 10/2017 | 33.15 | 964 | 1.81 | 7.56 |
| 16903SL163 | 11/2017 | 31.91 | 1175 | 1.86 | 3.79 |
| 16903SL163 | 12/2017 | 21.75 | 1291 | 0.39 | 2.89 |
| 16903SL163 | 1/2018 | 15.20 | 1082 | 0.10 | 1.17 |
| 16903SL163 | 2/2018 | 4.52 | 902 | 0.01 | 0.07 |
| 16903SL163 | 3/2018 | 16.98 | 1272 | 0.16 | 1.62 |
| 16903SL163 | 4/2018 | 5.80 | 906 | 0.01 | 0.07 |
| 16903SL163 | 5/2018 | 7.21 | 997 | 0.02 | 0.14 |
| 16903SL163 | 6/2018 | 11.13 | 824 | 0.06 | 0.55 |
| 16903SL163 | 7/2018 | 6.08 | 586 | 0.02 | 0.07 |
| 16903SL163 | 8/2018 | 25.49 | 216 | 0.30 | 2.65 |
| 16903SL163 | 9/2018 | 10.52 | 841 | 0.05 | 0.48 |
| 16903SL163 | 10/2018 | 12.23 | 1020 | 0.07 | 0.82 |
| 16903SL163 | Total | 15.81 | 12,076 | 0.17 | 2.28 |
| 16904SL145 | 10/2017 | 38.43 | 878 | 2.35 | 3.69 |
| 16904SL145 | 11/2017 | 32.89 | 769 | 0.81 | 3.38 |
| 16904SL145 | 12/2017 | 33.92 | 862 | 0.90 | 3.38 |
| 16904SL145 | 2/2018 | 39.52 | 819 | 1.70 | 3.44 |
| 16904SL145 | 3/2018 | 38.28 | 920 | 1.53 | 3.60 |
| 16904SL145 | 4/2018 | 34.33 | 696 | 1.14 | 3.25 |
| 16904SL145 | 5/2018 | 27.12 | 1148 | 0.73 | 4.47 |
| 16904SL145 | 6/2018 | 21.83 | 1209 | 0.45 | 3.07 |
| 16904SL145 | 7/2018 | 26.64 | 1382 | 0.92 | 4.56 |
| 16904SL145 | 8/2018 | 39.96 | 810 | 1.79 | 3.38 |
| 16904SL145 | Total | 24.55 | 10,288 | 0.90 | 2.70 |
| 16905SS119 | 10/2017 | 32.35 | 937 | 2.02 | 7.66 |
| 16905SS119 | 11/2017 | 58.95 | 1253 | 6.09 | 13.41 |
| 16905SS119 | 12/2017 | 43.03 | 1017 | 3.20 | 12.31 |
| 16905SS119 | 1/2018 | 38.73 | 894 | 2.10 | 10.19 |
| 16905SS119 | 2/2018 | 28.84 | 1032 | 1.09 | 6.03 |
| 16905SS119 | 3/2018 | 22.86 | 1204 | 0.83 | 3.88 |
| 16905SS119 | 4/2018 | 35.33 | 916 | 1.30 | 8.02 |
| 16905SS119 | 5/2018 | 19.72 | 612 | 0.38 | 1.94 |
| 16905SS119 | 6/2018 | 61.36 | 1216 | 5.19 | 11.95 |
| 16905SS119 | 7/2018 | 48.59 | 1236 | 2.27 | 16.73 |
| 16905SS119 | 8/2018 | 27.77 | 1257 | 1.07 | 2.28 |
| 16905SS119 | 9/2018 | 44.29 | 587 | 2.15 | 10.50 |
| 16905SS119 | Total | 38.11 | 12,161 | 3.52 | 16.70 |
| 16906SS105 | 10/2017 | 29.45 | 743 | 1.33 | 3.11 |
| 16906SS105 | 11/2017 | 30.02 | 1079 | 1.46 | 3.83 |
| 16906SS105 | 12/2017 | 27.45 | 1267 | 1.40 | 6.05 |
| 16906SS105 | 1/2018 | 96.14 | 426 | 11.05 | 49.08 |
| 16906SS105 | 2/2018 | 45.29 | 1027 | 2.74 | 16.24 |
| 16906SS105 | 3/2018 | 27.58 | 1195 | 1.22 | 5.03 |
| 16906SS105 | 4/2018 | 41.26 | 535 | 2.67 | 3.63 |
| 16906SS105 | 5/2018 | 64.26 | 672 | 5.04 | 23.31 |
| 16906SS105 | 6/2018 | 27.95 | 1202 | 1.30 | 5.24 |
| 16906SS105 | 7/2018 | 37.87 | 1271 | 2.59 | 3.52 |
| 16906SS105 | 8/2018 | 44.99 | 691 | 2.73 | 3.56 |
| 16906SS105 | 9/2018 | 20.39 | 896 | 0.49 | 2.22 |
| 16906SS105 | 10/2018 | 10.06 | 759 | 0.05 | 0.54 |
| 16906SS105 | Total | 29.13 | 11,763 | 2.06 | 8.94 |
| 16907SL158 | 10/2017 | 35.92 | 733 | 1.94 | 3.71 |
| 16907SL158 | 11/2017 | 37.14 | 797 | 1.90 | 3.78 |
| 16907SL158 | 12/2017 | 38.46 | 1201 | 2.50 | 3.43 |
| 16907SL158 | 1/2018 | 42.23 | 1029 | 2.59 | 3.67 |
| 16907SL158 | 2/2018 | 40.18 | 1052 | 2.71 | 3.47 |
| 16907SL158 | 3/2018 | 36.95 | 1205 | 2.47 | 3.50 |
| 16907SL158 | 4/2018 | 39.50 | 1060 | 2.62 | 3.52 |
| 16907SL158 | 5/2018 | 31.08 | 1289 | 1.70 | 3.45 |
| 16907SL158 | 6/2018 | 33.03 | 1280 | 2.13 | 3.75 |
| 16907SL158 | 7/2018 | 37.53 | 1372 | 2.40 | 3.58 |
| 16907SL158 | 8/2018 | 43.32 | 791 | 2.70 | 3.34 |
| 16907SL158 | Total | 26.42 | 11,809 | 1.75 | 3.24 |
| 16908SL173 | 10/2017 | 41.33 | 563 | 2.95 | 9.86 |
| 16908SL173 | 11/2017 | 34.70 | 939 | 1.69 | 3.44 |
| 16908SL173 | 12/2017 | 35.37 | 1166 | 2.10 | 3.83 |
| 16908SL173 | 1/2018 | 42.77 | 871 | 2.78 | 3.46 |
| 16908SL173 | 2/2018 | 42.72 | 880 | 2.76 | 3.54 |
| 16908SL173 | 3/2018 | 38.00 | 1053 | 2.36 | 3.79 |
| 16908SL173 | 4/2018 | 40.23 | 915 | 1.81 | 3.58 |
| 16908SL173 | 5/2018 | 36.83 | 1187 | 2.43 | 3.75 |
| 16908SL173 | 6/2018 | 32.84 | 1278 | 1.89 | 3.61 |
| 16908SL173 | 7/2018 | 39.08 | 1359 | 2.58 | 3.49 |
| 16908SL173 | 8/2018 | 36.32 | 1227 | 1.83 | 3.46 |
| 16908SL173 | Total | 25.11 | 13,769 | 1.42 | 5.65 |
| 16910NL165 | 8/2017 | 75.21 | 817 | 12.28 | 23.67 |
| 16910NL165 | 9/2017 | 60.36 | 1107 | 8.54 | 35.09 |
| 16910NL165 | 10/2017 | 69.03 | 1010 | 10.44 | 40.07 |
| 16910NL165 | 11/2017 | 49.17 | 810 | 5.21 | 24.28 |
| 16910NL165 | 12/2017 | 54.63 | 828 | 6.72 | 29.93 |
| 16910NL165 | 1/2018 | 70.00 | 598 | 10.55 | 36.01 |
| 16910NL165 | 2/2018 | 69.93 | 864 | 11.17 | 38.45 |
| 16910NL165 | 3/2018 | 75.23 | 922 | 14.35 | 26.72 |
| 16910NL165 | 4/2018 | 55.11 | 831 | 5.81 | 30.53 |
| 16910NL165 | 5/2018 | 53.46 | 1012 | 7.29 | 31.51 |
| 16910NL165 | 6/2018 | 65.30 | 981 | 11.45 | 36.38 |
| 16910NL165 | 7/2018 | 48.59 | 1249 | 6.55 | 26.15 |
| 16910NL165 | 8/2018 | 56.04 | 768 | 9.13 | 31.97 |
| 16910NL165 | Total | 41.58 | 11,842 | 6.97 | 28.53 |
| 16912NS97 | 8/2017 | 71.94 | 144 | 3.12 | 23.96 |
| 16912NS97 | 9/2017 | 66.14 | 986 | 7.92 | 31.78 |
| 16912NS97 | 10/2017 | 73.02 | 953 | 8.02 | 9.22 |
| 16912NS97 | 11/2017 | 23.37 | 814 | 0.55 | 4.49 |
| 16912NS97 | 12/2017 | 24.66 | 676 | 0.53 | 5.23 |
| 16912NS97 | 1/2018 | 46.17 | 573 | 1.35 | 16.76 |
| 16912NS97 | 2/2018 | 18.31 | 632 | 0.15 | 0.74 |
| 16912NS97 | 3/2018 | 34.65 | 504 | 0.62 | 7.79 |
| 16912NS97 | 4/2018 | 42.63 | 491 | 1.38 | 14.86 |
| 16912NS97 | 5/2018 | 46.09 | 685 | 2.85 | 17.34 |
| 16912NS97 | 6/2018 | 36.95 | 668 | 1.90 | 12.52 |
| 16912NS97 | 7/2018 | 33.53 | 1068 | 1.90 | 15.23 |
| 16912NS97 | 8/2018 | 54.38 | 698 | 5.77 | 29.77 |
| 16912NS97 | Total | 36.79 | 8895 | 2.14 | 21.34 |
| 16913NS94 | 8/2017 | 26.17 | 219 | 0.96 | 3.13 |
| 16913NS94 | 9/2017 | 79.74 | 425 | 9.75 | 39.29 |
| 16913NS94 | 10/2017 | 73.30 | 710 | 6.29 | 30.39 |
| 16913NS94 | 11/2017 | 85.02 | 187 | 3.09 | 7.22 |
| 16913NS94 | 12/2017 | 89.04 | 202 | 9.93 | 39.17 |
| 16913NS94 | Total | 64.41 | 1833 | 6.88 | 34.40 |
| 16914NS120 | 8/2017 | 35.42 | 215 | 1.59 | 6.52 |
| 16914NS120 | 9/2017 | 42.75 | 1063 | 3.09 | 14.61 |
| 16914NS120 | 10/2017 | 21.90 | 1297 | 0.49 | 3.40 |
| 16914NS120 | 11/2017 | 19.45 | 1297 | 0.44 | 2.52 |
| 16914NS120 | 12/2017 | 35.39 | 978 | 1.42 | 12.29 |
| 16914NS120 | 1/2018 | 64.00 | 256 | 6.25 | 30.61 |
| 16914NS120 | 2/2018 | 40.65 | 456 | 2.95 | 15.82 |
| 16914NS120 | 3/2018 | 43.66 | 1052 | 2.51 | 18.23 |
| 16914NS120 | 4/2018 | 41.01 | 1026 | 1.50 | 12.29 |
| 16914NS120 | 5/2018 | 61.18 | 462 | 4.10 | 18.90 |
| 16914NS120 | 6/2018 | 72.03 | 321 | 7.03 | 27.53 |
| 16914NS120 | 7/2018 | 61.87 | 360 | 4.89 | 22.83 |
| 16914NS120 | 8/2018 | 73.19 | 485 | 9.88 | 37.51 |
| 16914NS120 | Total | 30.36 | 9268 | 1.45 | 12.19 |
| 16915NS108 | 9/2017 | 44.87 | 1051 | 3.78 | 13.58 |
| 16915NS108 | 10/2017 | 52.49 | 1140 | 5.26 | 23.14 |
| 16915NS108 | 11/2017 | 156.66 | 725 | 21.35 | 132.50 |
| 16915NS108 | 12/2017 | 89.62 | 611 | 4.27 | 32.27 |
| 16915NS108 | 1/2018 | 16.37 | 33 | 0.24 | 0.72 |
| 16915NS108 | 2/2018 | 70.28 | 207 | 2.14 | 10.28 |
| 16915NS108 | Total | 220.61 | 3767 | 45.93 | 236.45 |
| 16916NS118 | 7/2017 | 50.42 | 126 | 4.46 | 14.96 |
| 16916NS118 | 8/2017 | 34.66 | 1275 | 2.31 | 13.35 |
| 16916NS118 | 9/2017 | 40.17 | 1240 | 3.33 | 14.62 |
| 16916NS118 | 10/2017 | 62.48 | 660 | 7.76 | 29.34 |
| 16916NS118 | 11/2017 | 56.63 | 64 | 4.32 | 18.20 |
| 16916NS118 | 12/2017 | 56.87 | 252 | 7.19 | 29.38 |
| 16916NS118 | 1/2018 | 56.38 | 436 | 8.84 | 28.21 |
| 16916NS118 | 2/2018 | 38.15 | 672 | 3.67 | 16.43 |
| 16916NS118 | 3/2018 | 46.58 | 416 | 4.28 | 23.06 |
| 16916NS118 | 4/2018 | 43.79 | 580 | 4.03 | 19.62 |
| 16916NS118 | 5/2018 | 44.03 | 540 | 3.23 | 17.42 |
| 16916NS118 | 6/2018 | 43.77 | 613 | 3.21 | 17.42 |
| 16916NS118 | 7/2018 | 37.59 | 939 | 3.25 | 14.89 |
| 16916NS118 | 8/2018 | 59.27 | 790 | 9.20 | 33.85 |
| 16916NS118 | Total | 32.10 | 8603 | 3.62 | 19.82 |
| 16917NL174 | 9/2017 | 44.73 | 613 | 3.05 | 19.15 |
| 16917NL174 | 10/2017 | 56.51 | 522 | 3.48 | 15.76 |
| 16917NL174 | 11/2017 | 45.92 | 403 | 2.11 | 12.10 |
| 16917NL174 | 12/2017 | 20.44 | 508 | 0.20 | 1.65 |
| 16917NL174 | 1/2018 | 48.46 | 376 | 1.45 | 11.52 |
| 16917NL174 | 2/2018 | 44.94 | 741 | 3.03 | 6.50 |
| 16917NL174 | 3/2018 | 29.34 | 534 | 0.44 | 4.37 |
| 16917NL174 | Total | 35.36 | 9878 | 1.60 | 9.24 |
| 16918NS115 | 9/2017 | 46.62 | 749 | 4.19 | 23.04 |
| 16918NS115 | 10/2017 | 50.21 | 500 | 3.47 | 18.79 |
| 16918NS115 | 11/2017 | 49.60 | 105 | 2.68 | 12.02 |
| 16918NS115 | 12/2017 | 39.66 | 52 | 1.34 | 8.61 |
| 16918NS115 | 1/2018 | 61.30 | 37 | 3.14 | 18.42 |
| 16918NS115 | 2/2018 | 32.84 | 247 | 1.06 | 9.26 |
| 16918NS115 | 3/2018 | 66.85 | 66 | 4.02 | 21.11 |
| 16918NS115 | 4/2018 | 58.11 | 156 | 5.32 | 26.21 |
| 16918NS115 | 5/2018 | 78.54 | 319 | 10.61 | 40.90 |
| 16918NS115 | 6/2018 | 64.81 | 387 | 9.12 | 36.24 |
| 16918NS115 | 7/2018 | 61.78 | 528 | 9.44 | 33.68 |
| 16918NS115 | 8/2018 | 81.74 | 237 | 10.74 | 36.28 |
| 16918NS115 | 9/2018 | 76.69 | 190 | 9.66 | 33.02 |
| 16918NS115 | 10/2018 | 94.66 | 107 | 12.33 | 42.17 |
| 16918NS115 | Total | 45.97 | 3680 | 7.18 | 29.14 |
| 16920SS97 | 9/2017 | 31.01 | 77 | 0.45 | 3.06 |
| 16920SS97 | 10/2017 | 55.82 | 106 | 1.46 | 1.55 |
| 16920SS97 | 1/2018 | 8.41 | 399 | 0.03 | 0.27 |
| 16920SS97 | 2/2018 | 24.71 | 652 | 0.31 | 3.46 |
| 16920SS97 | 5/2018 | 23.84 | 522 | 0.44 | 2.86 |
| 16920SS97 | 6/2018 | 23.16 | 781 | 0.30 | 2.93 |
| 16920SS97 | 7/2018 | 21.75 | 585 | 0.21 | 2.34 |
| 16920SS97 | 8/2018 | 7.71 | 311 | 0.03 | 0.16 |
| 16920SS97 | Total | 22.23 | 4068 | 0.27 | 3.11 |
Appendix B. Observations of August 2018 Fish Kill Event

Appendix C. Mean Abiotic Variables
Appendix D. Statistical Models
| KDE 50% Home Range | |||
|---|---|---|---|
| One Environmental Variable | |||
| Model | Model Variables | AIC Score | Weight |
| 50M1 | DO Concentration + (1|FishID) | 649.8 | 0.550 |
| 50M2 | Temperature + (1|FishID) | 652.2 | 0.167 |
| 50M3 | Salinity + (1|FishID) | 653.1 | 0.107 |
| 50M4 | Wind Speed + (1|FishID) | 655.1 | 0.040 |
| 50M5 | pH + (1|FishID) | 655.2 | 0.037 |
| 50M6 | Depth + (1|FishID) | 655.2 | 0.037 |
| 50M7 | Head Width + (1|FishID) | 655.5 | 0.032 |
| 50M8 | (1|FishID) | 655.5 | 0.031 |
| Two Environmental Variables | |||
| 50M9 | DO Concentration × Salinity + (1|FishID) | 647.2 | 0.205 |
| 50M10 | DO Concentration × Wind Speed + (1|FishID) | 647.7 | 0.161 |
| 50M11 | DO Concentration × pH + (1|FishID) | 648.1 | 0.131 |
| 50M12 | DO Concentration + pH + (1|FishID) | 648.1 | 0.130 |
| 50M13 | DO Concentration × Depth + (1|FishID) | 648.2 | 0.130 |
| 50M14 | DO Concentration + Salinity + (1|FishID) | 649.4 | 0.070 |
| 50M15 | DO Concentration + Head Width + (1|FishID) | 649.7 | 0.059 |
| 50M16 | DO Concentration + Wind Speed + (1|FishID) | 649.8 | 0.058 |
| 50M17 | DO Concentration + Depth + (1|FishID) | 649.8 | 0.057 |
| Three Environmental Variables | |||
| 50M18 | DO Concentration × Salinity × Wind Speed + (1|FishID) | 647.0 | 0.15 |
| 50M19 | DO Concentration × Salinity × pH + (1|FishID) | 647.0 | 0.15 |
| 50M20 | DO Concentration × Salinity × Depth + (1|FishID) | 647.0 | 0.15 |
| 50M21 | DO Concentration × Salinity + Head Width + (1|FishID) | 647.2 | 0.14 |
| 50M22 | DO Concentration × Salinity + Depth + (1|FishID) | 647.2 | 0.14 |
| 50M23 | DO Concentration × Salinity + Wind Speed + (1|FishID) | 647.2 | 0.14 |
| 50M24 | DO Concentration × Salinity + pH + (1|FishID) | 647.2 | 0.14 |
| All Environmental Variables | |||
| 50M25 | DO Concentration + Salinity + pH + Depth + Standard Length + (1|FishID) | 647.8 | 1.0 |
| 50M26 | DO Concentration + Salinity + pH + Depth + Standard Length | 731.7 | <0.001 |
| KDE 95% Home Range | |||
| One Environmental Variable | |||
| Model | Model Variables | AIC Score | Weight |
| 95M1 | Temperature + (1|FishID) | 1024.1 | 0.398 |
| 95M2 | Salinity + (1|FishID) | 1025.1 | 0.236 |
| 95M3 | DO Concentration + (1|FishID) | 1025.8 | 0.165 |
| 95M4 | pH + (1|FishID) | 1027.8 | 0.062 |
| 95M5 | Head Width + (1|FishID) | 1028.8 | 0.038 |
| 95M6 | Wind Speed + (1|FishID) | 1028.9 | 0.035 |
| 95M7 | Depth + (1|FishID) | 1029.0 | 0.035 |
| 95M8 | (1|FishID) | 1029.2 | 0.031 |
| Two Environmental Variables | |||
| 95M9 | Temperature × pH + (1|FishID) | 1023.7 | 0.14 |
| 95M10 | Temperature + pH + (1|FishID) | 1023.8 | 0.13 |
| 95M11 | Temperature × Depth + (1|FishID) | 1023.9 | 0.13 |
| 95M12 | Temperature × Salinity + (1|FishID) | 1023.9 | 0.12 |
| 95M13 | Temperature + Salinity + (1|FishID) | 1023.9 | 0.12 |
| 95M14 | Temperature × Wind Speed + (1|FishID) | 1024.0 | 0.12 |
| 95M15 | Temperature + Wind Speed + (1|FishID) | 1024.0 | 0.12 |
| 95M16 | Temperature + Depth + (1|FishID) | 1024.1 | 0.11 |
| Three Environmental Variables | |||
| 95M17 | Temperature × pH × Depth + (1|FishID) | 1021.7 | 0.220 |
| 95M18 | Temperature × pH × Wind Speed + (1|FishID) | 1021.7 | 0.220 |
| 95M19 | Temperature × pH × Salinity + (1|FishID) | 1021.7 | 0.220 |
| 95M20 | Temperature × pH + Head Width + (1|FishID) | 1023.2 | 0.102 |
| 95M21 | Temperature × pH + Wind Speed + (1|FishID) | 1023.7 | 0.081 |
| 95M22 | Temperature × pH + Depth + (1|FishID) | 1023.7 | 0.080 |
| 95M23 | Temperature × pH + Salinity + (1|FishID) | 1023.7 | 0.078 |
| 95M24 | |||
| All Environmental Variables | |||
| 95M25 | DO Concentration + Salinity + pH + Depth + Standard Length + (1|FishID) | 1023.3 | 1.0 |
| 95M26 | DO Concentration + Salinity + pH + Depth + Standard Length | 1123.7 | <0.001 |
| Daily Distance Traveled | |||
| One Environmental Variable | |||
| Model | Model Variables | AIC Score | Weight |
| ATM1 | DO Class + (1|FishID) | 19,828.7 | 1.0 |
| ATM2 | Temperature + (1|FishID) | 19,847.8 | <0.001 |
| ATM3 | Salinity + (1|FishID) | 19,912.1 | <0.001 |
| ATM4 | DO Concentration + (1|FishID) | 19,932.3 | <0.001 |
| ATM5 | Wind Speed + (1|FishID) | 20,020.4 | <0.001 |
| ATM6 | Moon Phase + (1|FishID) | 20,063.4 | <0.001 |
| ATM7 | Depth + (1|FishID) | 20,063.9 | <0.001 |
| ATM8 | Head Width + (1|FishID) | 20,066.3 | <0.001 |
| ATM9 | (1|FishID) | 20,083.0 | <0.001 |
| ATM10 | pH + (1|FishID) | 20,083.4 | <0.001 |
| Two Environmental Variables | |||
| ATM9 | DO Class × Temperature + (1|FishID) | 19,616.3 | 1.0 |
| ATM10 | DO Class + Temperature + (1|FishID) | 19,707.3 | <0.001 |
| ATM11 | DO Class × Wind Speed + (1|FishID) | 19,710.6 | <0.001 |
| ATM12 | DO Class × pH + (1|FishID) | 19,729.7 | <0.001 |
| ATM13 | DO Class × Salinity + (1|FishID) | 19,730.1 | <0.001 |
| ATM14 | DO Class + Salinity + (1|FishID) | 19,748.3 | <0.001 |
| ATM15 | DO Class + Wind Speed + (1|FishID) | 19,771.3 | <0.001 |
| ATM16 | DO Class × Depth + (1|FishID) | 19,779.8 | <0.001 |
| ATM17 | DO Class + pH + (1|FishID) | 19,808.5 | <0.001 |
| ATM18 | DO Class + Head Width + (1|FishID) | 19,810.9 | <0.001 |
| ATM19 | DO Class + Moon Phase + (1|FishID) | 19,818.8 | <0.001 |
| ATM20 | DO Class + Depth + (1|FishID) | 19,823.2 | <0.001 |
| Three Environmental Variables | |||
| ATM21 | DO Class × Temperature × pH + (1|FishID) | 19,475.0 | 0.95 |
| ATM22 | DO Class × Temperature × Wind Speed + (1|FishID) | 19,480.9 | 0.05 |
| ATM23 | DO Class × Temperature × Depth + (1|FishID) | 19,505.3 | <0.001 |
| ATM24 | DO Class × Temperature × Salinity + (1|FishID) | 19,511.6 | <0.001 |
| ATM25 | DO Class × Temperature + Wind Speed + (1|FishID) | 19,546.5 | <0.001 |
| ATM26 | DO Class × Temperature + pH + (1|FishID) | 19,547.0 | <0.001 |
| ATM27 | DO Class × Temperature + Moon Phase + (1|FishID) | 19,596.8 | <0.001 |
| ATM28 | DO Class × Temperature + Head Width + (1|FishID) | 19,598.0 | <0.001 |
| ATM29 | DO Class × Temperature + Depth + (1|FishID) | 19,610.9 | <0.001 |
| ATM30 | DO Class × Temperature + Salinity + (1|FishID) | 19,616.3 | <0.001 |
| All Environmental Variables | |||
| ATM31 | Temperature + DO Class + Salinity + pH + Standard Length + Wind Speed + Moon Phase + (1|FishID) | 19,511.1 | 1.0 |
| ATM32 | Temperature + DO Class + Salinity + pH + Standard Length + Wind Speed + Moon Phase | 20,106.6 | <0.001 |
Appendix E. Daily Distance Traveled as a Function of Dissolved Oxygen Concentration
References
- Saunders, D.A.; Hobbs, R.J.; Margules, C.R. Biological Consequences of Ecosystem Fragmentation: A Review. Conserv. Biol. 1991, 5, 18–32. [Google Scholar] [CrossRef]
- Tracey, J.A. Individual-based modeling as a tool for conserving connectivity. In Connectivity Conservation; Crooks, K.R., Sanjayan, M., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 343–368. [Google Scholar]
- Cook, G.S.; Parnell, P.E.; Levin, L.A. Population connectivity shifts at high frequency within an open-coast marine protected area network. PLoS ONE 2014, 9, e103654. [Google Scholar] [CrossRef]
- Gustafson, E.J.; Gardner, R.H. The Effect of Landscape Heterogeneity on the Probability of Patch Colonization. Ecology 1996, 77, 94–107. [Google Scholar] [CrossRef]
- Harris, J.H.; Kingsford, R.T.; Peirson, W.; Baumgartner, L.J. Mitigating the effects of barriers to freshwater fish migrations: The Australian experience. Mar. Freshw. Res. 2017, 68, 614–628. [Google Scholar] [CrossRef]
- Mahoney, R.D.; Beal, J.L.; Lewis, D.M.; Cook, G.S. Quantifying the Response of an Estuarine Nekton Community to Coastal Wetland Habitat Restoration. Sustainability 2021, 13, 13299. [Google Scholar] [CrossRef]
- Crooks, K.R.; Sanjayan, M. (Eds.) Connectivity conservation: Maintaining connections for nature. In Connectivity Conservation; Cambridge University Press: Cambridge, UK, 2006; pp. 1–20. [Google Scholar]
- Breitburg, D. Effects of hypoxia, and the balance between hypoxia and enrichment, on coastal fishes and fisheries. Estuaries 2002, 25, 767–781. [Google Scholar] [CrossRef]
- Lewis, D.M.; Thompson, K.A.; MacDonald, T.C.; Cook, G.S. Understanding shifts in estuarine fish communities following disturbances using an ensemble modeling framework. Ecol. Indic. 2021, 126, 107623. [Google Scholar] [CrossRef]
- Pan, Y.K.; Ern, R.; Esbaugh, A.J. Hypoxia tolerance decreases with body size in red drum Sciaenops ocellatus. J. Fish Biol. 2016, 89, 1488–1493. [Google Scholar] [CrossRef]
- Beatty, S.J.; Tweedley, J.R.; Cottingham, A.; Ryan, T.; Williams, J.; Lynch, K.; Morgan, D.L. Entrapment of an estuarine fish associated with a coastal surge barrier can increase the risk of mass mortalities. Ecol. Eng. 2018, 122, 229–240. [Google Scholar] [CrossRef]
- La, V.T.; Cooke, S.J. Advancing the Science and Practice of Fish Kill Investigations. Rev. Fish. Sci. 2011, 19, 21–33. [Google Scholar] [CrossRef]
- Breitburg, D.L.; Hondorp, D.W.; Davias, L.A.; Diaz, R.J. Hypoxia, Nitrogen, and Fisheries: Integrating Effects Across Local and Global Landscapes. Annu. Rev. Mar. Sci. 2009, 1, 329–349. [Google Scholar] [CrossRef] [PubMed]
- Breitburg, D.; Levin, L.A.; Oschlies, A.; Grégoire, M.; Chavez, F.P.; Conley, D.J.; Garçon, V.; Gilbert, D.; Gutiérrez, D.; Isensee, K.; et al. Declining Oxygen in the Global Ocean and Coastal Waters. Science 2018, 359, eaam7240. [Google Scholar] [CrossRef]
- Borsuk, M.E. Predictive Assessment of Fish Health and Fish Kills in the Neuse River Estuary Using Elicited Expert Judgment. Hum. Ecol. Risk Assess. Int. J. 2004, 10, 415–434. [Google Scholar] [CrossRef]
- Thronson, A.; Quigg, A. Fifty-five years of fish kills in coastal Texas. Estuaries Coasts 2008, 31, 802–813. [Google Scholar] [CrossRef]
- Gray, J.S.; Wu, R.S.-S.; Or, Y.Y. Effects of hypoxia and organic enrichment on the coastal marine environment. Mar. Ecol. Prog. Ser. 2002, 238, 249–279. [Google Scholar] [CrossRef]
- Farrell, A.P.; Richards, J.G. Defining hypoxia: An integrative synthesis of the responses of fish to hypoxia. In Fish physiology; Academic Press: Cambridge, MA, USA, 2009; Volume 27, pp. 487–503. [Google Scholar]
- Smale, M.A.; Rabeni, C.F. Hypoxia and Hyperthermia Tolerances of Headwater Stream Fishes. Trans. Am. Fish. Soc. 1995, 124, 698–710. [Google Scholar] [CrossRef]
- Keister, J.E.; Houde, E.D.; Breitburg, D.L. Effects of bottom-layer hypoxia on abundances and depth distributions of organisms in Patuxent River, Chesapeake Bay. Mar. Ecol. Prog. Ser. 2000, 205, 43–59. [Google Scholar] [CrossRef]
- Mandic, M.; Todgham, A.E.; Richards, J.G. Mechanisms and evolution of hypoxia tolerance in fish. Proc. R. Soc. Biol. Sci. 2009, 276, 735–744. [Google Scholar] [CrossRef]
- Díaz, R.J.; Rosenberg, R. Introduction to Environmental and Economic Consequences of Hypoxia. Water Resour. Dev. 2011, 27, 71–82. [Google Scholar] [CrossRef]
- Rey, J.R.; Kain, T.; Stahl, R. Wetland Impoundments of East-Central Florida. Fla. Sci. 1991, 54, 33–40. [Google Scholar]
- Brockmeyer, R., Jr.; Rey, J.R.; Virnstein, R.W.; Gilmore, R.G.; Earnest, L. Rehabilitation of impounded estuarine wetlands by hydrologic reconnection to the Indian River Lagoon, Florida (USA). Wetl. Ecol. Manag. 1997, 4, 93–109. [Google Scholar] [CrossRef]
- Montague, C.L.; Zale, A.V.; Percival, H.F. Ecological effects of coastal marsh impoundments: A review. Environ. Manag. 1987, 11, 743–756. [Google Scholar] [CrossRef]
- Taylor, D.S.; Banner, A.; Carroll, J.D. Fish and Wood Stork (Mycteria americana) Population Monitoring in Two Large Mosquito Impoundments in the Northern Indian River Lagoon, Florida: The Dynamics of Estuarine Reconnection. Fla. Sci. 2004, 67, 177–193. [Google Scholar]
- Dai, H.; Zheng, T.; Liu, D. Effects of Reservoir Impounding on Key Ecological Factors in the Three Gorges Region. Procedia 2010, 2, 15–24. [Google Scholar] [CrossRef]
- Loch, J.M.H.; Walters, L.J.; Donnelly, M.L.; Cook, G.S. Restored Coastal Habitat Can “Reel In” Juvenile Sportfish: Population and Community Responses to Habitat Restoration in the Indian River Lagoon, Florida, USA. Sustainability 2021, 13, 12832. [Google Scholar] [CrossRef]
- Troast, B.; Paperno, R.; Cook, G.S. Multidecadal shifts in fish community diversity across a dynamic biogeographic transition zone. Divers. Distrib. 2019, 26, 93–107. [Google Scholar] [CrossRef]
- Poulakis, G.R.; Shenker, J.M.; Taylor, D.S. Habitat use by fishes after tidal reconnection of an impounded estuarine wetland in the Indian River Lagoon, Florida (USA). Wetl. Ecol. Manag. 2002, 10, 51–69. [Google Scholar] [CrossRef]
- O’Shea, T.J.; Beck, C.A.; Bonde, R.K.; Kochman, H.I.; Odell, D.K. An Analysis of Manatee Mortality Patterns in Florida, 1976–1981. J. Wildl. Manag. 1985, 49, 1–11. [Google Scholar] [CrossRef]
- CERP Interagency Manatee Task Force. Guidelines for Mantee Conservation During Comprehensive Everglades Restoration Plan Implementation; CERP Interagency Manatee Task Force: Vero Beach, FL, USA, 2006. [Google Scholar]
- Satchell, E. Manatees Rescued After Mass Stranding in Florida Storm Drain ABC News; ABC News: Satellite Beach, FL, USA, 2015; Available online: http://wric.com/2015/02/24/manatees-rescued-after-mass-stranding-in-florid (accessed on 10 September 2018).
- District SJRWM. Fast Facts about the Indian River Lagoon. 2018. Available online: https://www.sjrwmd.com/waterways/indian-river-lagoon/facts/ (accessed on 10 September 2018).
- Gilmore, R.G.J. Fishes of the Indian River Lagoon and Adjacent Waters, Florida. Bull. Fla. State Mus. Biol. Sci. 1977, 22, 101–148. [Google Scholar]
- Troast, B.V.; Walters, L.J.; Cook, G.S. A multi-tiered assessment of fish community responses to habitat restoration in a coastal lagoon. Mar. Ecol. Prog. Ser. 2022, 698, 1–14. [Google Scholar] [CrossRef]
- Johnson, D.R.; Funicelli, N.A.; Bohnsack, J.A. Effectiveness of an existing estuarine no-take fish sanctuary within the Kennedy Space Center, Florida. N. Am. J. Fish. Manag. 1999, 19, 436–453. [Google Scholar] [CrossRef]
- Air Force Space & Missile Museum. Integrate-Transfer-Launch (ITL) Complex. Available online: http://afspacemuseum.org/ccafs/ITL/ (accessed on 10 August 2015).
- Reyier, E.A.; Shenker, J.M.; Christian, D. Role of an estuarine fisheries reserve in the production and export of ichthyoplankton. Mar. Ecol. Prog. Ser. 2008, 359, 249–260. [Google Scholar]
- Reyier, E.A.; Scheidt, D.M.; Stolen, E.D.; Lowers, R.H.; Holloway-adkins, K.G.; Ahr, B.J. Residency and Dispersal of Three Sport Fish Species From a Coastal Marine Reserve: Insights From a Regional-Scale Acoustic Telemetry Network. In Global Ecology and Conservation; Elsevier Ltd.: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Myers, O.M.; Reyier, E.; Ahr, B.; Cook, G.S. Striped Mullet Migration Patterns in the Indian River Lagoon: A Network Analysis Approach to Spatial Fisheries Management. Mar. Coast. Fish. 2020, 12, 423–440. [Google Scholar] [CrossRef]
- Garreau, C.M. Development of Methods for Non-Lethal Health Assessment of the Red Drum (Sciaenops ocellatus) Inside NASA’s Kennedy Space Center No-Take Fisheries Reserve; University of Florida: Gainesville, FL, USA, 2012. [Google Scholar]
- Baker, S.M. Assessing the Effects of Habitat and Manatee Exclusion Devices on Red Drum (Sciaenops ocellatus) Movement Patterns in Estuarine Impoundments. Master’s Thesis, University of Central Florida, Orlando, FL, USA, 2019. [Google Scholar]
- Overstreet, R.M.; Heard, R.W. Food of the Red Drum, Sciaenops ocellata, from Mississippi Sound. Gulf Res. Rep. 1978, 6, 131–135. [Google Scholar] [CrossRef]
- Ross, J.L.; Stevens, T.M.; Vaughan, D.S. Age, Growth, Mortality, and Reproductive Biology of Red Drums in North Carolina Waters. Trans. Am. Fish. Soc. 1995, 124, 37–54. [Google Scholar] [CrossRef]
- Johnson, D.R.; Funicelli, N.A. Spawning of the Red Drum in Mosquito Lagoon, East-Central Florida. Estuaries 1991, 14, 74–79. [Google Scholar] [CrossRef]
- Reyier, E.A.; Lowers, R.H.; Scheidt, D.M.; Adams, D.H. Movement patterns of adult red drum, Sciaenops ocellatus, in shallow Florida lagoons as inferred through autonomous acoustic telemetry. Environ. Biol. Fishes 2010, 90, 343–360. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Murphy, M.D.; Taylor, R.G. Reproduction, Growth, and Mortality of Red Drum Sciaenops ocellatus in Florida Waters. Fish. Bull. 1990, 88, 531–542. [Google Scholar]
- Hobday, A.J.; Pincock, D. Estimating Detection Probabilities for Linear Acoustic Monitoring Arrays. Am. Fish. Soc. Symp. 2011, 76, 1–22. [Google Scholar]
- Huveneers, C.; Simpfendorfer, C.A.; Kim, S.; Semmens, J.M.; Hobday, A.J.; Pederson, H.; Stieglitz, T.; Vallee, R.; Webber, D.; Heupel, M.R.; et al. The influence of environmental parameters on the performance and detection range of acoustic receivers. Methods Ecol. Evol. 2016, 7, 825–835. [Google Scholar] [CrossRef]
- Young, J.M.; Bowers, M.E.; Reyier, E.A.; Morley, D.; Ault, E.R.; Pye, J.D.; Gallagher, R.M.; Ellis, R.D. The FACT Network: Philosophy, Evolution, and Management of a Collaborative Coastal Tracking Network. Mar. Coast. Fish. 2020, 12, 258–271. [Google Scholar]
- Campbell, H.A.; Watts, M.E.; Dwyer, R.G.; Franklin, C.E. V-Track: Software for analysing and visualising animal movement from acoustic telemetry detections. Mar. Freshw. Res. 2012, 63, 815–820. [Google Scholar]
- Holbrook, C.; Hayden, T.; Pye, J.; Nunes, A. Glatos: A Package for the Great Lakes Acoustic Telemetry Observation System. Available online: https://rdrr.io/github/jsta/glatos/ (accessed on 14 March 2023).
- Signer, J.; Balkenhol, N. Reproducible home ranges (rhr): A new, user-friendly R package for analyses of wildlife telemetry data. Wildl. Soc. Bull. 2015, 39, 358–363. [Google Scholar]
- Calenge, C. The package “adehabitat” for the R software: A tool for the analysis of space and habitat use by animals. Ecol. Model. 2006, 197, 516–519. [Google Scholar] [CrossRef]
- White, E.; Mihoff, M.; Jones, B.; Bajona, L.; Halfyard, E. White-Mihoff False Filtering Tool. 2014. Available online: https://studylib.net/doc/6977022/white-mihoff-false-filtering-tool (accessed on 14 March 2023).
- Simpfendorfer, C.A.; Heupel, M.R.; Hueter, R.E. Estimation of short-term centers of activity from an array of omnidirectional hydrophones and its use in studying animal movements. Can. J. Fish. Aquat. Sci. 2002, 59, 23–32. [Google Scholar] [CrossRef]
- Zuur, A.F.; Leno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2011. [Google Scholar]
- Bunt, C.M.; Castro-Santos, T.; Haro, A. Performance of fish passage structures at upstream barriers to migration. River Res. Appl. 2012, 28, 457–478. [Google Scholar] [CrossRef]
- Kimball, M.E.; Boswell, K.M.; Rozas, L.P. Estuarine fish behavior around slotted water control structures in a managed salt marsh. Wetl. Ecol. Manag. 2017, 25, 299–312. [Google Scholar]
- Rooker, J.; Holt, S. Utilization of subtropical seagrass meadows by newly settled red drum Sciaenops ocellatus: Patterns of distribution and growth. Mar. Ecol. Prog. Ser. 1997, 158, 139–149. [Google Scholar] [CrossRef]
- Moulton, D.L.; Dance, M.A.; Williams, J.A.; Sluis, M.Z.; Stunz, G.W.; Rooker, J.R. Habitat Partitioning and Seasonal Movement of Red Drum and Spotted Seatrout. Estuaries Coasts 2017, 40, 905–916. [Google Scholar]
- Lewis, D.M.; Troast, B.V.; Glomb, J.C.; Cook, G.S. An ecological characterization of fish communities in the Mosquito Lagoon, Florida. Southeast. Nat. 2020, 19, 491–510. [Google Scholar]
- Yarbro, L.; Carlson, P., Jr. Seagrass Integrated Mapping and Monitoring: Program Mapping and Monitoring Report No. 2; Florida Fish and Wildlife Conservation Commission: St. Petersburg, FL, USA, 2016. [Google Scholar]
- Adams, D.H.; Tremain, D.M.; Paperno, R.; Sonne, C. Florida lagoon at risk of ecosystem collapse. Science 2019, 365, 991–992. [Google Scholar] [PubMed]
- Eby, L.A.; Crowder, L.B.; Mcclellan, C.M.; Peterson, C.H.; Powers, M.J.; Eby, L.A.; Crowder, L.B.; Mcclellan, C.M.; Peterson, C.H.; Powers, M.J. Habitat degradation from intermittent hypoxia: Impacts on demersal fishes. Mar. Ecol. Prog. Ser. 2015, 291, 249–261. [Google Scholar]
- Eggleston, D.B.; Bell, G.W. Species-specific avoidance responses by blue crabs and fish to chronic and episodic hypoxia. Mar. Biol. 2005, 146, 761–770. [Google Scholar]
- Herbert, N.A.; Steffensen, J.F. The response of Atlantic cod, Gadus morhua, to progressive hypoxia: Fish swimming speed and physiological stress. Mar. Biol. 2005, 147, 1403–1412. [Google Scholar]
- Tyler, R.M.; Targett, T.E. Juvenile weakfish Cynoscion regalis distribution in relation to diel-cycling dissolved oxygen in an estuarine tributary. Mar. Ecol. Prog. Ser. 2007, 333, 257–269. [Google Scholar] [CrossRef]
- Switzer, T.S.; Chesney, E.J.; Baltz, D.M. Habitat selection by flat fishes in the northern Gulf of Mexico: Implications for susceptibility to hypoxia. J. Exp. Mar. Biol. Ecol. 2009, 381, 51–64. [Google Scholar]
- Craig, J. Aggregation on the edge: Effects of hypoxia avoidance on the spatial distribution of brown shrimp and demersal fishes in the Northern Gulf of Mexico. Mar. Ecol. Prog. Ser. 2012, 445, 75–95. [Google Scholar]
- Neill, H. Environmental Requirements of Red Drum; The National Council for Agricultural Education: Alexandria, VA, USA, 1990. [Google Scholar]
- Perez-Dominguez, R.; Holt, S.; Holt, G. Environmental variability in seagrass meadows: Effects of nursery environment cycles on growth and survival in larval red drum Sciaenops ocellatus. Mar. Ecol. Prog. Ser. 2006, 321, 41–53. [Google Scholar] [CrossRef]
- Michaud, J.P. A Citizen’s Guide to Understanding and Monitoring Lakes and Streams; Washington State Department of Ecology: Olympia, WA, USA, 1991. [Google Scholar]
- Lewis, D.M.; Durham, K.E.; Walters, L.J.; Cook, G.S. A Resident Fish Guild as a Higher Trophic Level Indicator of Oyster Reef Restoration Success. Sustainability 2021, 13, 13004. [Google Scholar] [CrossRef]
- Searles, A.R.; Gipson, E.E.; Walters, L.J.; Cook, G.S. Oyster reef restoration facilitates the recovery of macroinvertebrate abundance, diversity, and composition in estuarine communities. Sci. Rep. 2022, 12, 8163. [Google Scholar] [CrossRef]



| SL (cm) | FL (cm) | TL (cm) | HW (cm) | Mass (kg) | Max SL (cm) | Min SL (cm) | Max HW (cm) | Min HW (cm) | |
|---|---|---|---|---|---|---|---|---|---|
| NI Large (n = 10) | 91.5 (±6.2) | 101.9 (±6.0) | 106.6 (±8.5) | 16.5 (±1.1) | 14.1 (±2.8) | 99.0 | 79.5 | 17.9 | 13.9 |
| NI Small (n = 10) | 66.3 (±4.1) | 75.5 (±4.1) | 79.9 (±4.3) | 11.3 (±1.0) | 5.2 (±1.1) | 71.0 | 58.0 | 12.7 | 9.4 |
| SI Large (n = 5) | 90.1 (±8.9) | 100.1 (±9.9) | 104.9 (±10.9) | 15.5 (±1.4) | 12.6 (±2.8) | 99.0 | 80.0 | 17.3 | 13.8 |
| SI Small (n = 5) | 61.9 (±5.8) | 69.9 (±5.9) | 74.0 (±5.4) | 10.3 (±1.0) | 4.5 (±0.9) | 70.0 | 56.0 | 11.9 | 9.2 |
| All Large (n = 15) | 91.0 (±6.9) | 101.3 (±7.3) | 106.0 (±8.9) | 16.2 (±1.3) | 13.6 (±2.8) | 99.0 | 79.5 | 17.9 | 13.8 |
| All Small (n = 15) | 64.8 (±4.9) | 73.6 (±5.3) | 77.9 (±5.3) | 10.9 (±1.1) | 4.9 (±1.1) | 71.0 | 56.0 | 12.7 | 9.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baker, S.M.; Reyier, E.A.; Ahr, B.J.; Cook, G.S. Assessing the Effects of Physical Barriers and Hypoxia on Red Drum Movement Patterns to Develop More Effective Management Strategies. Fishes 2023, 8, 171. https://doi.org/10.3390/fishes8040171
Baker SM, Reyier EA, Ahr BJ, Cook GS. Assessing the Effects of Physical Barriers and Hypoxia on Red Drum Movement Patterns to Develop More Effective Management Strategies. Fishes. 2023; 8(4):171. https://doi.org/10.3390/fishes8040171
Chicago/Turabian StyleBaker, Steven M., Eric A. Reyier, Bonnie J. Ahr, and Geoffrey S. Cook. 2023. "Assessing the Effects of Physical Barriers and Hypoxia on Red Drum Movement Patterns to Develop More Effective Management Strategies" Fishes 8, no. 4: 171. https://doi.org/10.3390/fishes8040171
APA StyleBaker, S. M., Reyier, E. A., Ahr, B. J., & Cook, G. S. (2023). Assessing the Effects of Physical Barriers and Hypoxia on Red Drum Movement Patterns to Develop More Effective Management Strategies. Fishes, 8(4), 171. https://doi.org/10.3390/fishes8040171






