Effects of Aging Uncertainty on the Estimation of Growth Functions of Major Tuna Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Operating Models
2.2. Observation Models
2.3. Estimation Models
2.4. Evaluation Models
3. Results
3.1. Estimation Uncertainty of the Growth Curve
3.2. Parameter Distributions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clear, N.; Francis, M.; Tsuji, S.; Krusic-Golub, K.; Itoh, T.; Tzeng, W.N.; Sutton, C.; Findlay, J.; Hirai, A.; Shiao, J.C.; et al. A manual for age determination of southern bluefin tuna Thunnus maccoyii, Otolith sampling, preparation and interpretation. In Proceedings of the Direct Age Estimation Workshop CCSBT, Victoria, Australia, 11–14 June 2002. [Google Scholar]
- Shimose, T.; Farley, J.H. Age, Growth and Reproductive Biology of Bluefin Tunas. In Biology and Ecology of Bluefin Tuna; CRC Press: London, UK, 2015. [Google Scholar]
- Dortel, E.; Massiot-Granier, F.; Rivot, E.; Million, J.; Hallier, J.P.; Morize, E.; Munaron, J.-M.; Bousquet, N.; Chassot, E. Accounting for age uncertainty in growth modeling, the case study of yellowfin tuna (Thunnus albacares) of the Indian Ocean. PLoS ONE 2013, 8, e60886. [Google Scholar] [CrossRef]
- Murua, H.; Rodriguez-Marin, E.; Neilson, J.D.; Farley, J.H.; Juan-Jordá, M.J. Fast versus slow growing tuna species: Age, growth, and implications for population dynamics and fisheries management. Rev. Fish Biol. Fish. 2017, 27, 733–773. [Google Scholar] [CrossRef]
- Katsanevakis, S. Modelling fish growth: Model selection, multi-model inference and model selection uncertainty. Fish. Res. 2006, 81, 229–235. [Google Scholar] [CrossRef]
- Cope, J.M.; Punt, A.E. Admitting ageing error when fitting growth curves: An example using the von Bertalanffy growth function with random effects. Can. J. Fish. Aquat. Sci. 2007, 64, 205–218. [Google Scholar] [CrossRef]
- Gunn, J.S.; Clear, N.P.; Carter, T.I.; Rees, A.J.; Stanley, C.A.; Farley, J.H.; Kalish, J.M. Age and growth in southern bluefin tuna, Thunnus maccoyii (Castelnau): Direct estimation from otoliths, scales and vertebrae. Fish. Res. 2008, 92, 207–220. [Google Scholar] [CrossRef]
- Stéquert, B.; Conand, F. Preliminary studies of age and growth of bigeye tuna (Thunnus obesus) in the western Indian Ocean. In IOTC Proceedings, 7th Expert Consultation on Indian Ocean Tunas, Victoira, Seychelles, 9–14 Nomvember 1998; IOTC: Victoria, Seychelles, 1998. [Google Scholar]
- Thorogood, J. Age and growth rate determination of southern bluefin tuna, Thunnus maccoyii, using otolith banding. J. Fish Biol. 1987, 30, 7–14. [Google Scholar] [CrossRef]
- Wells, R.D.; Kohin, S.; Teo, S.L.; Snodgrass, O.E.; Uosaki, K. Age and growth of North Pacific albacore (Thunnus alalunga): Implications for stock assessment. Fish. Res. 2013, 147, 55–62. [Google Scholar] [CrossRef]
- ISSF. Status of the World Fisheries for Tuna: September 2021; ISSF Technical Report 2021-13; ISSF: Munich, Germany, 2021. [Google Scholar]
- Khan, S.; Khan, M.A. Importance of age and growth studies in fisheries management. In Reviewed Proceedings of National Seminar on NGSV; Next Generation Sciences: Rohtak, India, 2020; pp. 194–201. [Google Scholar]
- Farley, J.; Krusic-Golub, K.; Eveson, P.; Clear, N.; Roupsard, F.; Sanchez, C.; Nicol, S.; Hampton, J. Age and growth of yellowfin and bigeye tuna in the western and central Pacific Ocean from otoliths. WCPFC-SC16-2020/SA-WP-02 2020, 11, 20.
- Andrews, A.H.; Pacicco, A.; Allman, R.; Falterman, B.J.; Lang, E.T.; Golet, W. Age validation of yellowfin (Thunnus albacares) and bigeye (Thunnus obesus) tuna of the northwestern Atlantic Ocean. Can. J. Fish. Aquat. Sci. 2020, 77, 637–643. [Google Scholar] [CrossRef]
- Nikolic, N.; Morandeau, G.; Hoarau, L.; West, W.; Arrizabalaga, H.; Hoyle, S.; Nicol, S.J.; Bourjea, J.; Puech, A.; Farley, J.H.; et al. Review of albacore tuna, Thunnus alalunga, biology, fisheries and management. Rev. Fish Biol. Fish. 2017, 27, 775–810. [Google Scholar] [CrossRef]
- Spence, M.A.; Turtle, A.J. Making the most of survey data: Incorporating age uncertainty when fitting growth parameters. Ecol. Evol. 2017, 7, 7058–7068. [Google Scholar] [CrossRef]
- Chen, K.S.; Shimose, T.; Tanabe, T.; Chen, C.Y.; Hsu, C.C. Age and growth of albacore Thunnus alalunga in the North Pacific Ocean. J. Fish Biol. 2012, 80, 2328–2344. [Google Scholar] [CrossRef]
- Xia, M. Stock Assessment and Management Strategy Evaluation of Striped Marlin (Tetrapturus audax) in the Indian Ocean; Shanghai Ocean University: Shanghai, China, 2020. [Google Scholar]
- Punt, A.E.; Butterworth, D.S.; de Moor, C.L.; De Oliveira, J.A.; Haddon, M. Management strategy evaluation: Best practices. Fish Fish. 2016, 17, 303–334. [Google Scholar] [CrossRef]
- Dichmont, C.; Deng, A.; Punt, A.E.; Ellis, N.; Venables, W.N.; Kompas, T.; Ye, Y.; Zhou, S.; Bishop, J. Beyond biological performance measures in management strategy evaluation: Bringing in economics and the effects of trawling on the benthos. Fish. Res. 2008, 94, 238–250. [Google Scholar] [CrossRef]
- Farley, J.H.; Williams, A.J.; Clear, N.P.; Davies, C.R.; Nicol, S.J. Age estimation and validation for South Pacific albacore Thunnus alalunga. J. Fish Biol. 2013, 82, 1523–1544. [Google Scholar] [CrossRef]
- Jiang, J.J.; Xu, L.X.; Zhu, G.P. Preliminary study on age and growth of north Pacific albacore Thunnus alalunga. Mar. Fish. 2016, 38, 1–8. [Google Scholar]
- Sardenne, F.; Dortel, E.; Le Croizier, G.; Million, J.; Labonne, M.; Leroy, B.; Bodin, N.; Chassot, E. Determining the age of tropical tunas in the Indian Ocean from otolith microstructures. Fish. Res. 2015, 163, 44–57. [Google Scholar] [CrossRef]
- Eveson, J.P.; Million, J.; Sardenne, F.; Le Croizier, G. Estimating growth of tropical tunas in the Indian Ocean using tag-recapture data and otolith-based age estimates. Fish. Res. 2015, 163, 58–68. [Google Scholar] [CrossRef]
- Pacicco, A.E.; Allman, R.J.; Lang, E.T.; Murie, D.J.; Falterman, B.J.; Ahrens, R.; Walter, J.F., III. Age and Growth of Yellowfin Tuna in the US Gulf of Mexico and Western Atlantic. Mar. Coast. Fish. 2021, 13, 345–361. [Google Scholar] [CrossRef]
- Dortel, E.; Sardenne, F.; Bousquet, N.; Rivot, E.; Million, J.; Le Croizier, G.; Chassot, E. An integrated Bayesian modeling approach for the growth of Indian Ocean yellowfin tuna. Fish. Res. 2015, 163, 69–84. [Google Scholar] [CrossRef]
- Lehodey, P.; Leroy, B. Age and Growth of Yellowfin Tuna (Thunnus albacares) from the Western and Central Pacific Ocean as Indicated by Daily Growth Increments and Tagging Data; Secretariat of the Pacific Community: Noumea, New Caledonia, 1999. [Google Scholar]
- Hearn, W.S.; Polacheck, T. Estimating long-term growth-rate changes of southern bluefin tuna (Thunnus maccoyii) from two periods of tag-return data. Fish. Bull. Natl. Ocean. Atmos. Adm. 2003, 101, 58–74. [Google Scholar]
- Polacheck, T.; Eveson, J.P.; Laslett, G.M. Increase in growth rates of southern bluefin tuna (Thunnus maccoyii) over four decades: 1960 to 2000. Can. J. Fish. Aquat. Sci. 2004, 61, 307–322. [Google Scholar] [CrossRef]
- Gaikov, V.V.; Chur, V.N.; Zharov, V.L.; Fedoseev, Y.P. On age and growth of the Atlantic bigeye tuna. ICCAT Collect. Vol. Sci. Pap. 1980, 9, 294–302. [Google Scholar]
- Farley, J.; Krusic-Golub, K.; Eveson, P.; Clear, N.; Luque, P.; Artetxe-Arrate, I.; Fraile, I.; Zudaire, I.; Vidot, A.; Govindn, R.; et al. Estimating the age and growth of bigeye tuna (Thunnus obesus) in the Indian Ocean from counts of daily and annual increments in otoliths. IOTC-2021-WPTT23-18_Rev1 2021, 1–33.
- Labelle, M.; Hampton, J.; Bailey, K.; Murray, T.; Fournier, D.A.; Sibert, J.R. Determination of age and growth of south Pacific albacore (Thunnus alalunga) using three methodologies. Fish. Bull. 1993, 91, 649–663. [Google Scholar]
- Compean-Jimenez, G.; Bard, F.X. Age and growth of East Atlantic bluefin tuna as determined by reading of fin rays cross section. Collect. Vol. Sci. Pap ICCAT 1980, 9, 547–552. [Google Scholar]
- Neilson, J.D.; Campana, S.E. A validated description of age and growth of western Atlantic bluefin tuna (Thunnus thynnus). Can. J. Fish. Aquat. Sci. 2008, 65, 1523–1527. [Google Scholar] [CrossRef]
- Adams, J.L.; Kerstetter, D.W. Age and growth of three coastal-pelagic tunas (Actinopterygii: Perciformes: Scombridae) in the Florida Straits, USA: Blackfin tuna, Thunnus atlanticus, little tunny, Euthynnus alletteratus, and skipjack tuna, Katsuwonus pelamis. Acta Ichthyol. Piscat. 2014, 44, 201. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, L.; Wang, X.; Zhu, G.; Wan, R.; Zang, Y.; Song, P.; Zhang, T. Direct age estimation, interpretation and precision of bigeye tuna (Thunnus obesus) in the Southern Indian Ocean using vertebrae. In AIP Conference Proceedings; AIP Publishing LLC: New York, NY, USA, 2020; Volume 2208, p. 020005. [Google Scholar] [CrossRef]
- Zhu, G.; Zhou, Y.; Xu, L.; Dai, X. Growth and mortality of bigeye tuna Thunnus obesus (Scombridae) in the eastern and central tropical Pacific Ocean. Environ. Biol. Fishes 2009, 85, 127–137. [Google Scholar] [CrossRef]
- Campana, S.E. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J. Fish Biol. 2001, 59, 197–242. [Google Scholar] [CrossRef]
- Lang, E.T.; Falterman, B.J.; Kitchens, L.L.; Marshall, C.D. Age and growth of yellowfin tuna (Thunnus albacares) in the northern Gulf of Mexico. Collect. Vol. Sci. Pap. ICCAT. 2017, 73, 423–433. [Google Scholar]
- Duarte-Neto, P.; Higa, F.M.; Lessa, R.P. Age and growth estimation of bigeye tuna, Thunnus obesus (Teleostei: Scombridae) in the southwestern Atlantic. Neotrop. Ichthyol. 2012, 10, 148–158. [Google Scholar] [CrossRef]
- Ku, J.E.; Lee, S.I.; Kim, D.N. Age and Growth of Southern Bluefin Tuna, Thunnus maccoyii, Based on Otolith Microstructure. Ocean. Sci. J. 2021, 56, 413–423. [Google Scholar] [CrossRef]
- Farley, J.; Krusic-Golub, K.; Eveson, P.; Luque, P.; Clear, N.; Fraile, I.; Artetxe-Arrate, I.; Zudaire, I.; Vidot, A.; Govindn, R.; et al. Estimating the age and growth of yellowfin tuna (Thunnus albacares) in the Indian Ocean from counts of daily and annual increments in otoliths. IOTC-2021-WPTT23-05_Rev1 2021, 1–34.
- Lee, L.K.; Yeh, S.Y. Age and growth of South Atlantic albacore–a revision after the revelation of otolith daily ring counts. Collect. Vol. Sci. Pap. ICCAT 2007, 60, 443–456. [Google Scholar]
- Farber, M.I.; Lee, D.W. Ageing western Atlantic bluefin tuna, Thunnus thynnus, using tagging data, caudal vertebrae and otoliths. ICCAT Col. Vol. Sci. Pap. 1981, 15, 288–301. [Google Scholar]
- Schaefer, K.M.; Fuller Daniel, W. Estimates of Age and Growth of Bigeye Tuna (Thunnus obesus) in the Eastern Pacific Ocean Based on Otolith Increments and Tagging Data; Inter-American Tropical Tuna Commission: San Diego, CA, USA, 2006. [Google Scholar]
- Lessa, R.; Duarte-Neto, P. Age and growth of yellowfin tuna (Thunnus albacares) in the western equatorial Atlantic, using dorsal fin spines. Fish. Res. 2004, 69, 157–170. [Google Scholar] [CrossRef]
- Shimose, T.; Tanabe, T.; Chen, K.S.; Hsu, C.C. Age determination and growth of Pacific bluefin tuna, Thunnus orientalis, off Japan and Taiwan. Fish. Res. 2009, 100, 134–139. [Google Scholar] [CrossRef]
- Milatou, N.; Megalofonou, P. Age structure and growth of bluefin tuna (Thunnus thynnus, L.) in the capture-based aquaculture in the Mediterranean Sea. Aquaculture 2014, 424, 35–44. [Google Scholar] [CrossRef]
- Wilson, K.L.; Matthias, B.G.; Barbour, A.B.; Ahrens, R.N.; Tuten, T.; Allen, M.S. Combining samples from multiple gears helps to avoid fishy growth curves. N. Am. J. Fish. Manag. 2015, 35, 1121–1131. [Google Scholar] [CrossRef]
- Hazin, H.G.; da Silva, G.B. Age and growth of skipjack tuna (Katsuwonus pelamis) in the western equatorial Atlantic based on dorsal spines analysis. Bol. Inst. Pesca 2022, 48, e686. [Google Scholar] [CrossRef]
- Tanabe, T. Precise age determination of young to adult skipjack tuna (Katsuonus pelamis) with validation of otolith daily increment. In Proceedings of the 16th Meeting of Sanding Committee on Tuna and Billfish, Mooloolaba, Australia, 9–16 July 2003; pp. 1–10. [Google Scholar]
- Farley, J.H.; Clear, N.P.; Leroy, B.; Davis, T.L.; McPherson, G. Age, growth and preliminary estimates of maturity of bigeye tuna, Thunnus obesus, in the Australian region. Mar. Freshw. Res. 2006, 57, 713–724. [Google Scholar] [CrossRef]
- Turner, S.C.; Restrepo, V.R. A review of the growth rate of west Atlantic bluefin tuna, Thunnus thynnus, estimated from marked and recaptured fish. Collect. Vol. Sci. Pap. ICCAT 1994, 42, 170–172. [Google Scholar]
- Smart, J.J.; Grammer, G.L. Modernising fish and shark growth curves with Bayesian length-at-age models. PLoS ONE 2021, 16, e0246734. [Google Scholar] [CrossRef] [PubMed]
- Juan-Jordá, M.J.; Mosqueira, I.; Freire, J.; Dulvy, N.K. Population declines of tuna and relatives depend on their speed of life. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150322. [Google Scholar] [CrossRef] [PubMed]
- Aires-da-Silva, A.M.; Maunder, M.N.; Schaefer, K.M.; Fuller, D.W. Improved growth estimates from integrated analysis of direct aging and tag–recapture data: An illustration with bigeye tuna (Thunnus obesus) of the eastern Pacific Ocean with implications for management. Fish. Res. 2015, 163, 119–126. [Google Scholar] [CrossRef]
- Reeves, S.A. A simulation study of the implications of age-reading errors for stock assessment and management advice. ICES J. Mar. Sci. 2003, 60, 314–328. [Google Scholar] [CrossRef]
- Williams, A.J.; Farley, J.H.; Hoyle, S.D.; Davies, C.R.; Nicol, S.J. Spatial and sex-specific variation in growth of albacore tuna (Thunnus alalunga) across the South Pacific Ocean. PLoS ONE 2012, 7, e39318. [Google Scholar] [CrossRef]
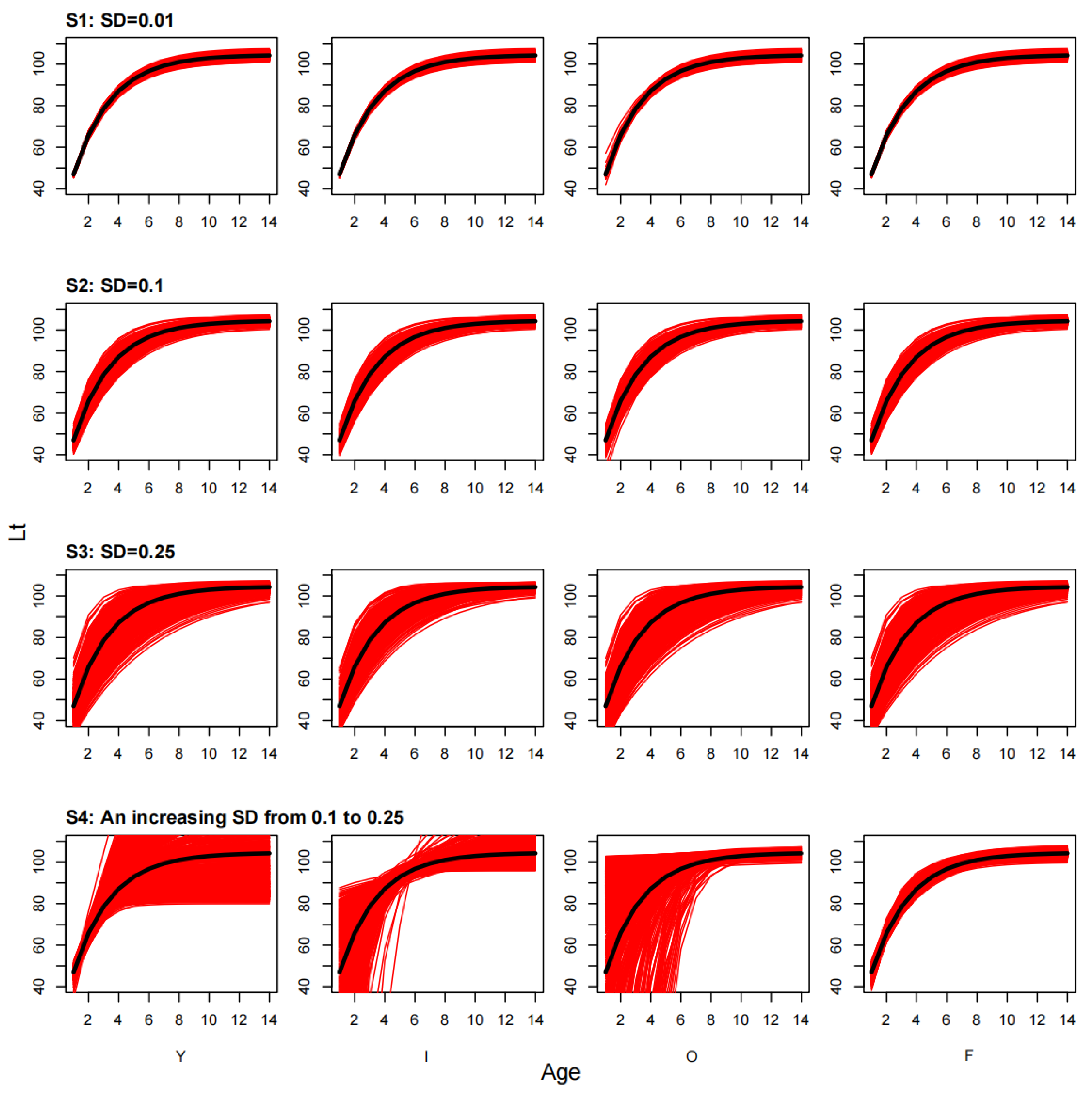


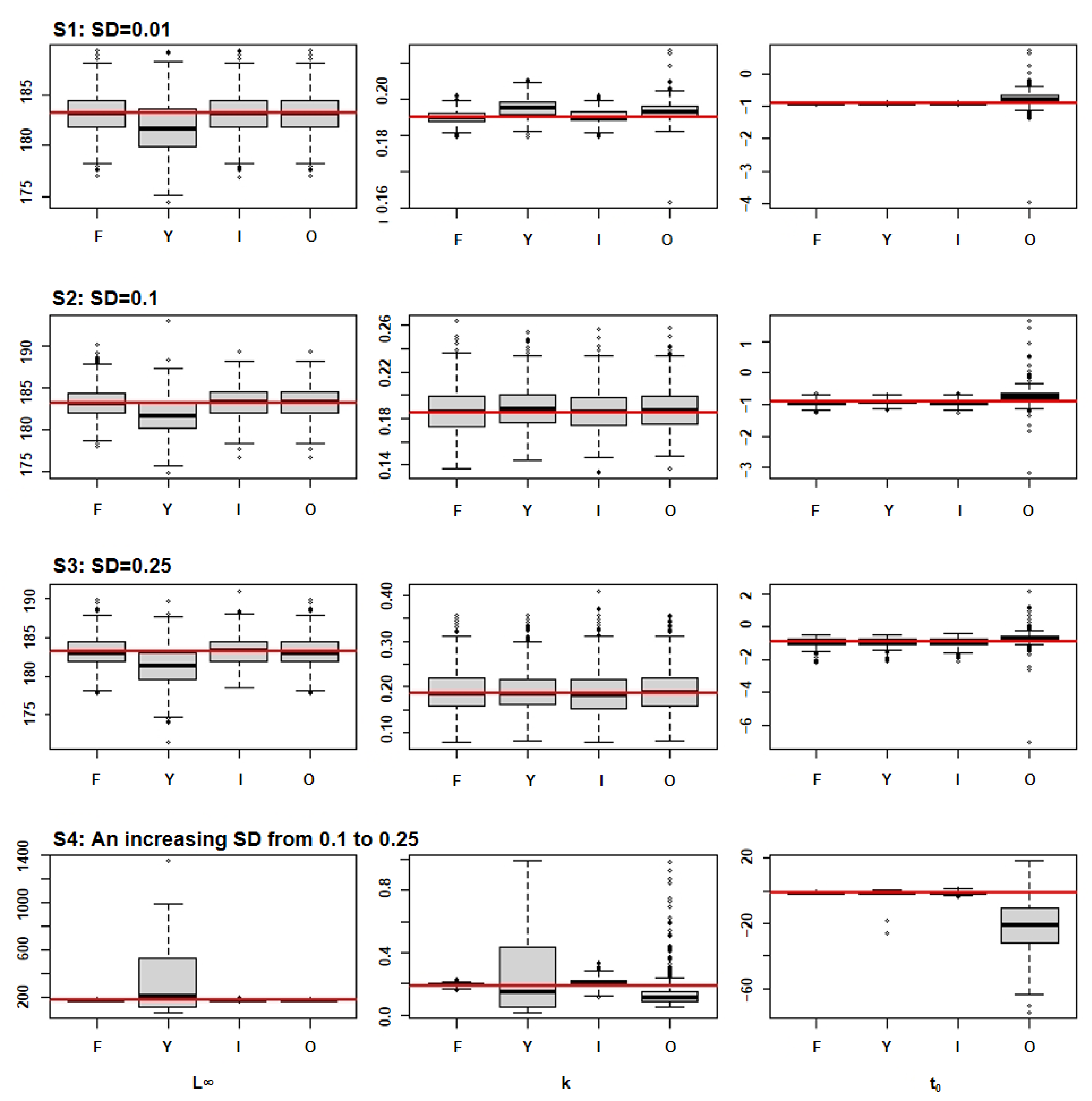
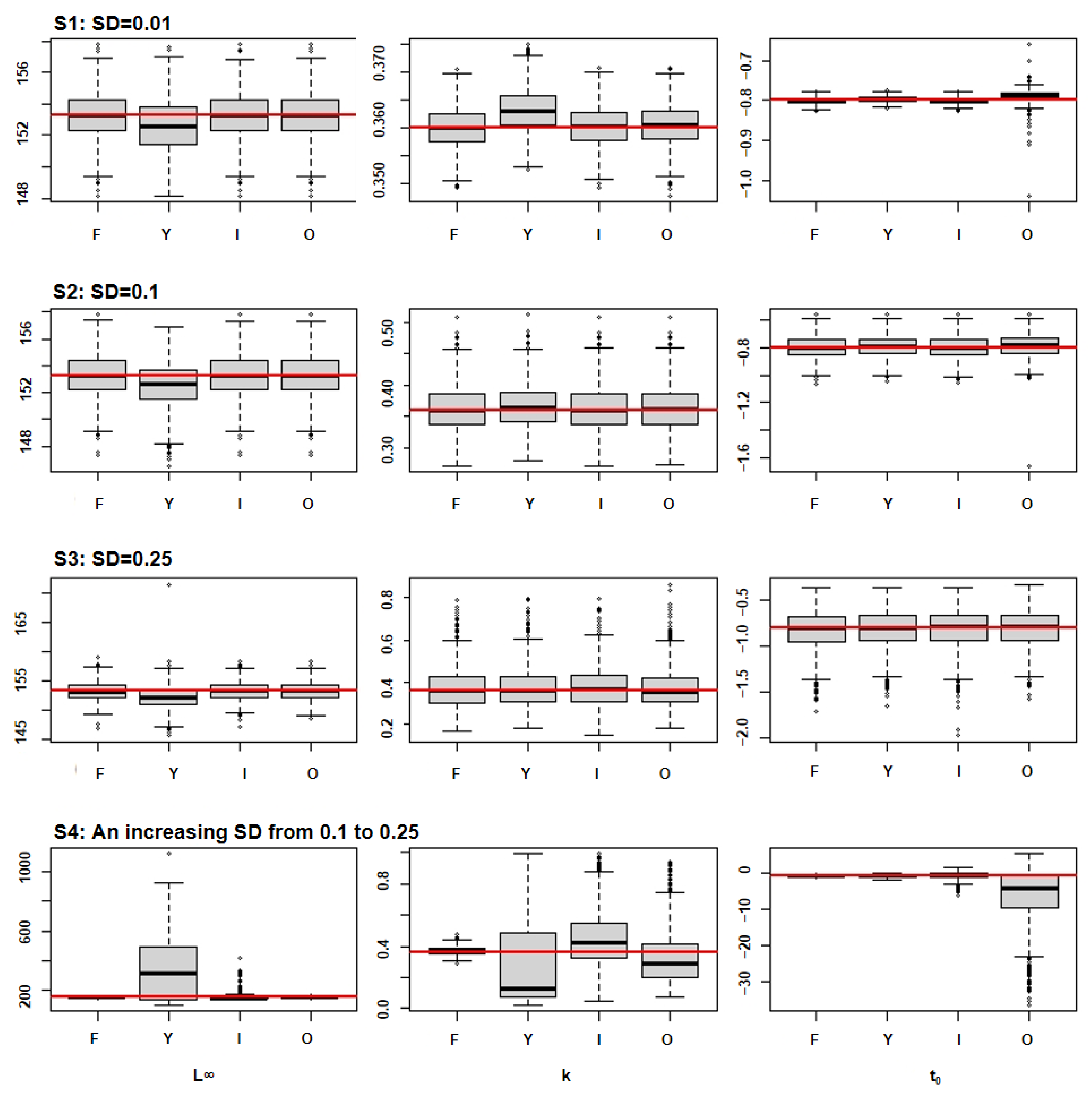

| Species 1 | L∞ (cm) | K (Year 1) | t0 (Year 1) | Inflection Points (Year) | Longevity (Year) |
|---|---|---|---|---|---|
| Alb | 104.52 | 0.4 | −0.49 | 3 and 7~8 | 14 |
| BET | 151.1 | 0.386 | −0.410 | 2~3 and 8 | 16 |
| YFT | 153.3 | 0.36 | −0.8 | 2~3 and 7~8 | 18 |
| SBT | 183.18 | 0.185 | −0.923 | 3 and 20 | 41 |
| SKJ | 122.5 | 0.12 | −1.69 | 1 | 6~7 |
| Specie | Young Group | Intermediate Group | Old Group | Full-Age Group |
|---|---|---|---|---|
| ALB | 1~3 | 4~7 | 8~14 | 1~14 |
| BET | <2.5 | 3~7 | 8~16 | 0.5~16 |
| YFT | <1.5 | 2~7 | 8~18 | 0.5~18 |
| SBT | 1~3 | 4~20 | 21~41 | 1~41 |
| SKJ | <1 | – | 2~7 | 0.25~7 |
| Scenario | Error | Age Groups |
|---|---|---|
| S1 | Young group | |
| Intermediate group | ||
| Old group | ||
| Full-age group | ||
| S2 | Young group | |
| Intermediate group | ||
| Old group | ||
| Full-age group | ||
| S3 | Young group | |
| Intermediate group | ||
| Old group | ||
| Full-age group | ||
| S4 | Young group | |
| Intermediate group | ||
| Old group | ||
| Full-age group |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, D.; Lin, Q.; Zhu, J.; Zhang, F. Effects of Aging Uncertainty on the Estimation of Growth Functions of Major Tuna Species. Fishes 2023, 8, 131. https://doi.org/10.3390/fishes8030131
Lu D, Lin Q, Zhu J, Zhang F. Effects of Aging Uncertainty on the Estimation of Growth Functions of Major Tuna Species. Fishes. 2023; 8(3):131. https://doi.org/10.3390/fishes8030131
Chicago/Turabian StyleLu, Dongqi, Qinqin Lin, Jiangfeng Zhu, and Fan Zhang. 2023. "Effects of Aging Uncertainty on the Estimation of Growth Functions of Major Tuna Species" Fishes 8, no. 3: 131. https://doi.org/10.3390/fishes8030131
APA StyleLu, D., Lin, Q., Zhu, J., & Zhang, F. (2023). Effects of Aging Uncertainty on the Estimation of Growth Functions of Major Tuna Species. Fishes, 8(3), 131. https://doi.org/10.3390/fishes8030131






