Assessment of Growth-Related Parameters, Immune-Biochemical Profile, and Expression of Selected Genes of Red Tilapia Fed with Roselle Calyces (Hibiscus sabdariffa) Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Validation
2.2. Roselle Calyx Collection and Chemical Extraction
2.3. (GC-MS) Analysis of AME Extract
2.4. Experiment Design
2.5. Growth Parameters
2.6. Morphometric Assessment of Intestine
2.7. Blood Sampling
2.8. Haemato-Biochemical Analyses
2.9. Immune and Oxidative Stress Activities
2.10. Total Carotene Measurement
2.11. QRT-PCR
2.12. Statistical Analysis
3. Results
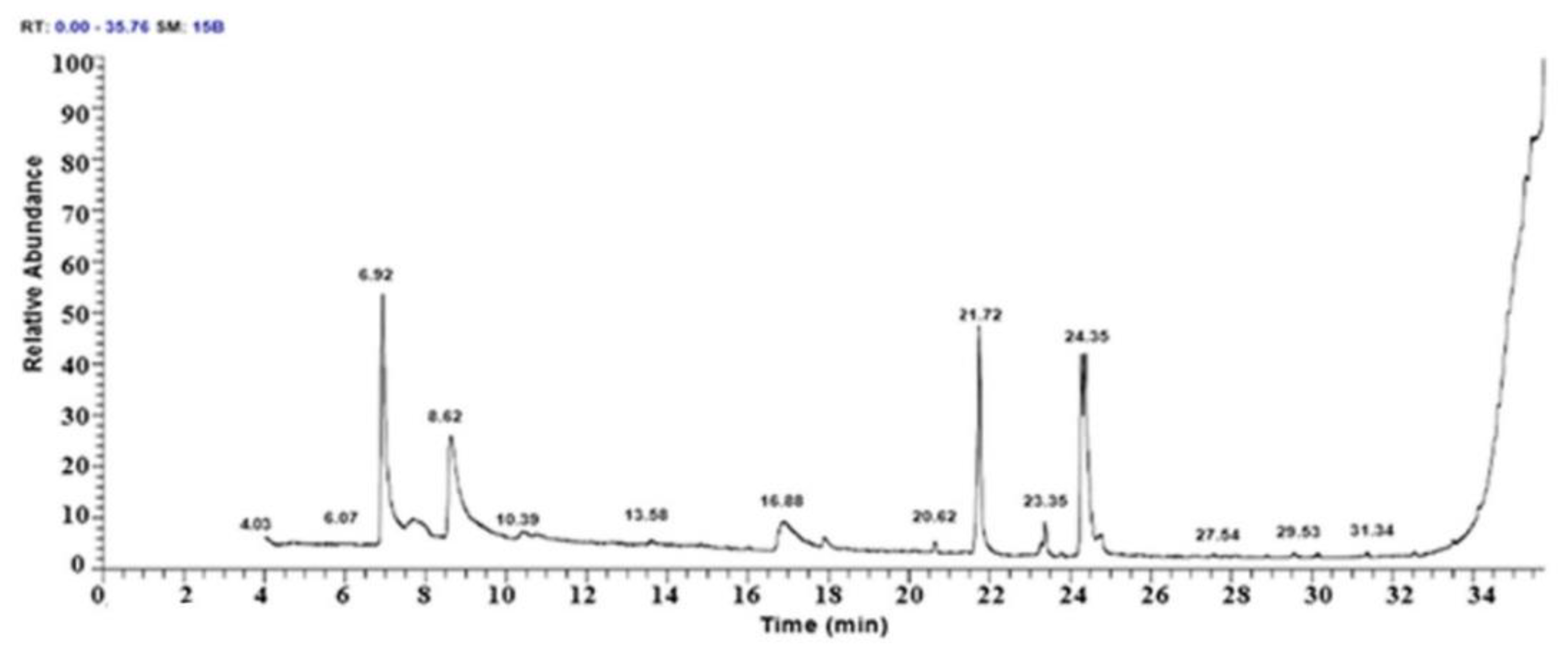
3.1. The GCMs Analysis of Roselle Ethyl Extract
3.2. Growth Parameters
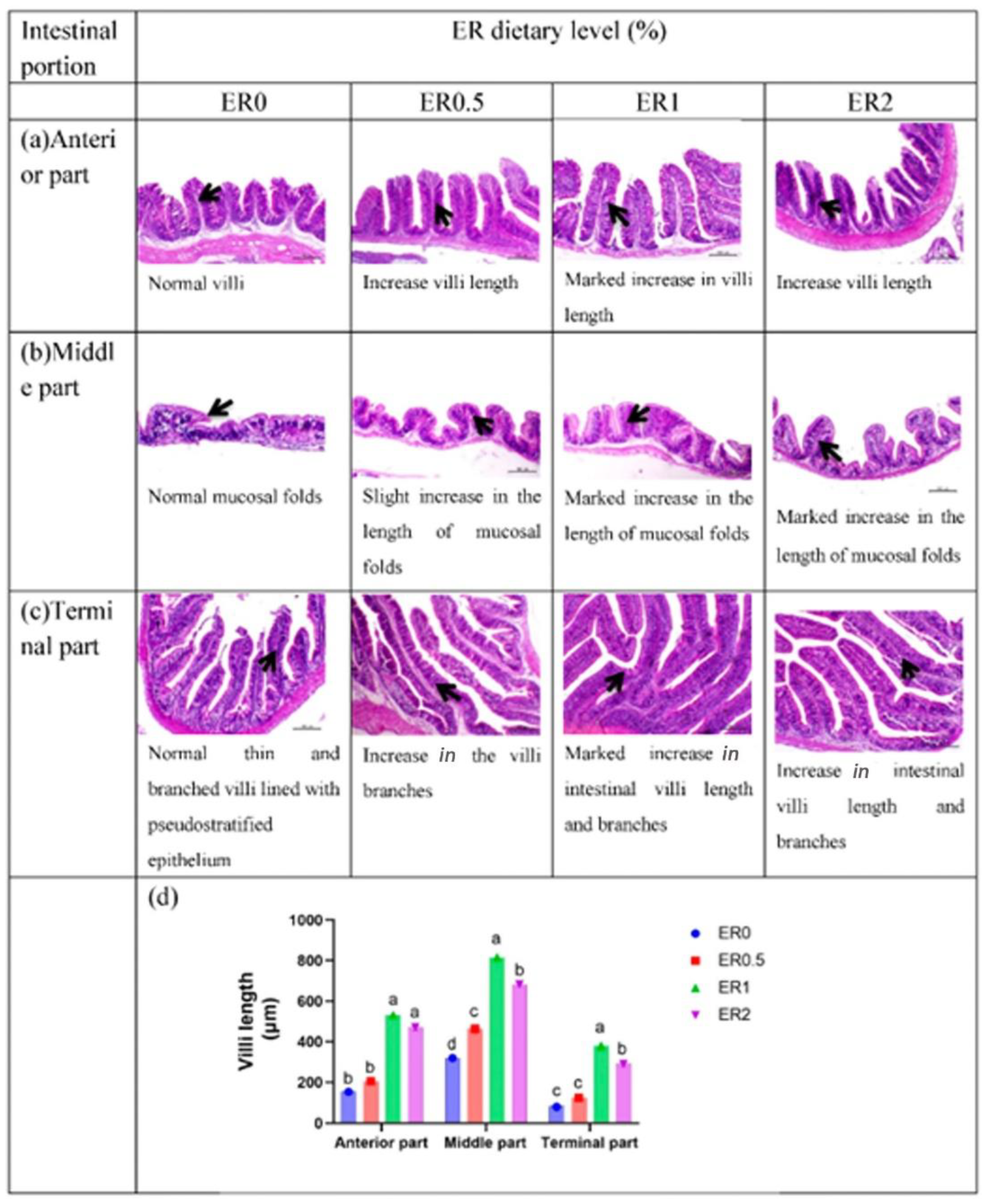
3.3. Whole Intestinal Morphometry
3.4. Blood and Biochemical Parameters
3.5. Immune Assay
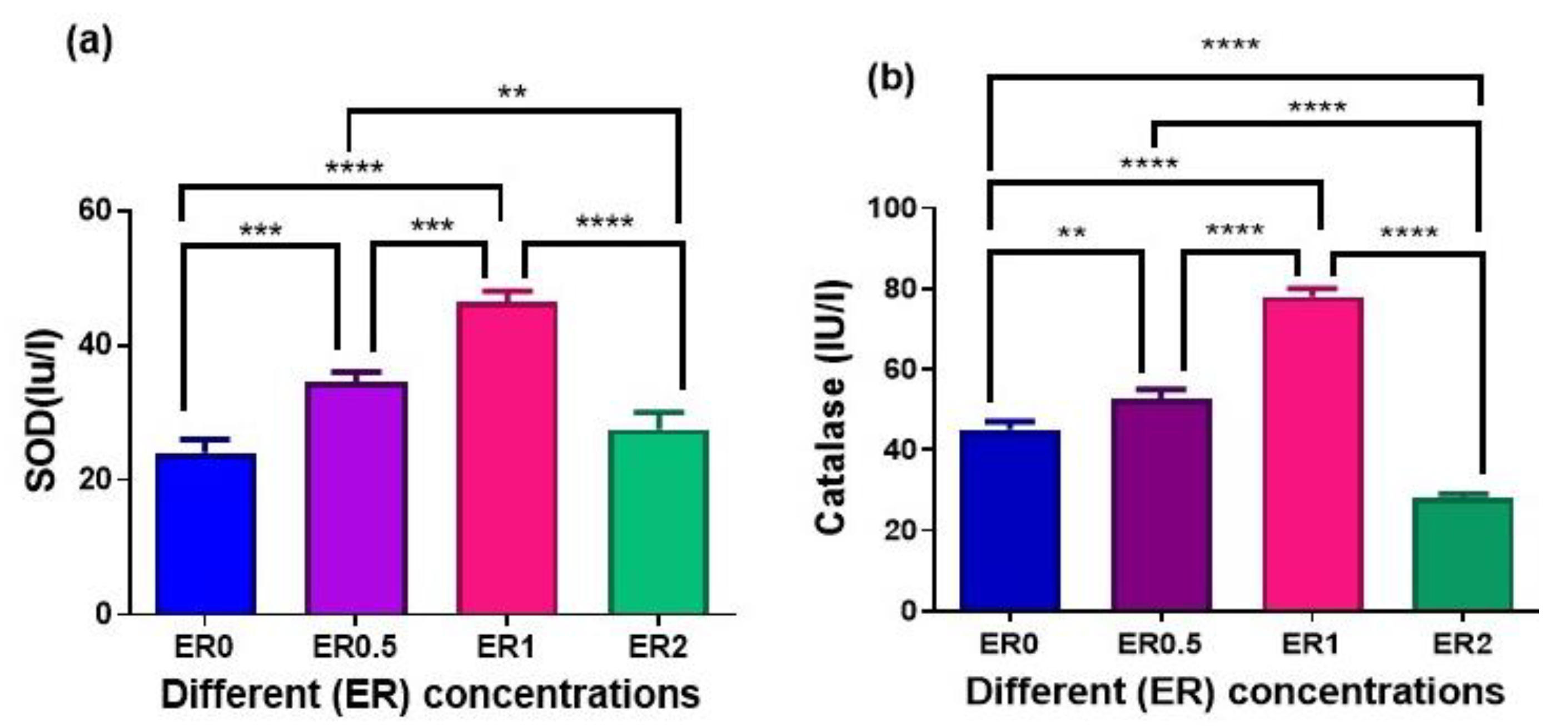
3.6. Activity of Antioxidant Enzymes
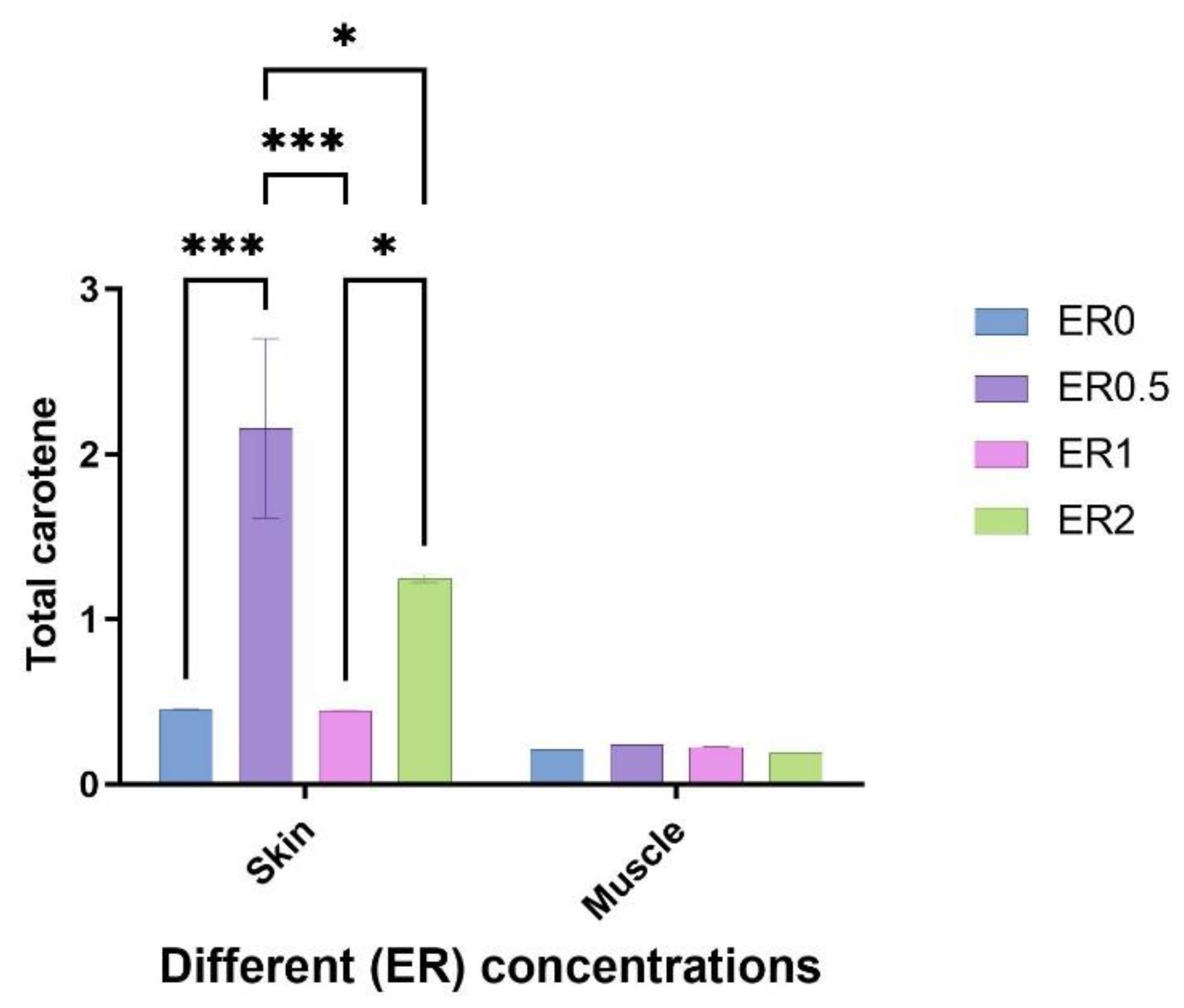
3.7. Total Carotene Measurement
3.8. Time-Series Expression of Studied Genes in Response to Roselle Extract
4. Discussion
5. Conclusions and Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020: Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Antonucci, F.; Costa, C. Precision aquaculture: A short review on engineering innovations. Aquac. Int. 2020, 28, 41–57. [Google Scholar] [CrossRef]
- Pirozzi, I.; Booth, M.A.; Pankhurst, P.M. The effect of stocking density and repeated handling on the growth of juvenile mulloway, Argyrosomus japonicus (Temminck & Schlegel 1843). Aquac. Int. 2009, 17, 199–205. [Google Scholar]
- Tosun, D.D. Crocodile farming and its present state in global aquaculture. J. Fish. Sci. 2013, 7, 43. [Google Scholar] [CrossRef]
- Pankhurst, N.; Ludke, S.; King, H.; Peter, R. The relationship between acute stress, food intake, endocrine status and life history stage in juvenile farmed Atlantic salmon, Salmo salar. Aquaculture 2008, 275, 311–318. [Google Scholar] [CrossRef]
- Santos, G.; Schrama, J.; Mamauag, R.; Rombout, J.; Verreth, J. Chronic stress impairs performance, energy metabolism and welfare indicators in European seabass (Dicentrarchus labrax): The combined effects of fish crowding and water quality deterioration. Aquaculture 2010, 299, 73–80. [Google Scholar] [CrossRef]
- Jittinandana, S.; Kenney, P.; Slider, S.; Mazik, P.; Bebak-Williams, J.; Hankins, J. Effect of fish attributes and handling stress on quality of smoked arctic char fillets. J. Food Sci. 2003, 68, 57–63. [Google Scholar] [CrossRef]
- Abo-Raya, M.H.; Alshehri, K.M.; Abdelhameed, R.F.; Elbialy, Z.I.; Elhady, S.S.; Mohamed, R.A. Assessment of growth-related parameters and immune-biochemical profile of nile tilapia (oreochromis niloticus) fed dietary ulva fasciata extract. Aquac. Res. 2021, 52, 3233–3246. [Google Scholar] [CrossRef]
- Austin, B.; Austin, D. Bacterial Fish Pathogens: Diseases of Farmed and Wild Fish; Springer-Praxis: Godalming, UK, 2007. [Google Scholar]
- Moustafa, E.M.; Farrag, F.A.; Dawood, M.A.; Shahin, K.; Hamza, A.; Decamp, O.; Mohamed, R.; Elsabagh, M.; Eltholth, M.; Omar, A.A. Efficacy of Bacillus probiotic mixture on the immunological responses and histopathological changes of Nile tilapia (Oreochromis niloticus, L) challenged with Streptococcus iniae. Aquac. Res. 2021, 52, 2205–2219. [Google Scholar] [CrossRef]
- Akinrotimi, A.; Gabriel, U.; Anyanwu, P.; Anyanwu, A. Influence of sex, acclimation methods and period on Haematology of Sarotherodon melanotheron (Cichilidae). Res. J. Biol. Sci. 2007, 2, 248–352. [Google Scholar]
- Batista, S.; Ramos, M.; Cunha, S.; Barros, R.; Cristóvão, B.; Rema, P.; Pires, M.; Valente, L.; Ozório, R. Immune responses and gut morphology of Senegalese sole (Solea senegalensis, K aup 1858) fed monospecies and multispecies probiotics. Aquac. Nutr. 2015, 21, 625–634. [Google Scholar] [CrossRef]
- Wu, Y.-R.; Gong, Q.-F.; Fang, H.; Liang, W.-W.; Chen, M.; He, R.-J. Effect of Sophora flavescens on non-specific immune response of tilapia (GIFT Oreochromis niloticus) and disease resistance against Streptococcus agalactiae. Fish Shellfish Immunol. 2013, 34, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, Q.E.; Zhou, X.; Wang, F.-H.; Muurinen, J.; Virta, M.P.; Brandt, K.K.; Zhu, Y.-G. Antibiotic resistome in the livestock and aquaculture industries: Status and solutions. Crit. Rev. Environ. Sci. Technol. 2020, 51, 2159–2196. [Google Scholar] [CrossRef]
- Lulijwa, R.; Rupia, E.J.; Alfaro, A.C. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: A review of the top 15 major producers. Rev. Aquac. 2020, 12, 640–663. [Google Scholar] [CrossRef]
- Maqsood, S.; Singh, P.; Samoon, M.H.; Munir, K. Emerging role of immunostimulants in combating the disease outbreak in aquaculture. Int. Aquat. Res. 2011, 3, 147–163. [Google Scholar]
- Portillo-Torres, L.A.; Bernardino-Nicanor, A.; Mercado-Monroy, J.; Gómez-Aldapa, C.A.; González-Cruz, L.; Rangel-Vargas, E.; Castro-Rosas, J. Antimicrobial Effects of Aqueous Extract from Calyces of Hibiscus sabdariffa in CD-1 Mice Infected with Multidrug-Resistant Enterohemorrhagic Escherichia coli and Salmonella Typhimurium. J. Med. Food 2021, 25, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Jomeh, R.; Chitsaz, H.; Akrami, R. Effect of anthocyanin extract from Roselle, Hibiscus sabdariffa, calyx on haematological, biochemical and immunological parameters of rainbow trout, Oncorhynchus mykiss. Aquac. Res. 2021, 52, 3736–3744. [Google Scholar] [CrossRef]
- Yousefi, M.; Vatnikov, Y.A.; Kulikov, E.V.; Ahmadifar, E.; Mirghaed, A.T.; Hoseinifar, S.H.; Van Doan, H. Effects of dietary Hibiscus sabdariffa supplementation on biochemical responses and inflammatory-related genes expression of rainbow trout, Oncorhynchus mykiss, to ammonia toxicity. Aquaculture 2021, 533, 736095. [Google Scholar] [CrossRef]
- El Mesallamy, A.M.; Ahmad, M.H.; Souleman, A.M.; El Morsy, A.T.; Abd El-Naby, A.S. Effects of Roselle calyx (Hibiscus sabdariffa L.)-supplemented diets on growth and disease (Aeromonas hydrophila) resistance in Nile tilapia (Oreochromis niloticus L.). Egypt. Pharm. J. 2016, 15, 78. [Google Scholar]
- Yin, G.; Cao, L.; Xu, P.; Jeney, G.; Nakao, M. Hepatoprotective and antioxidant effects of Hibiscus sabdariffa extract against carbon tetrachloride-induced hepatocyte damage in Cyprinus carpio. Vitr. Cell. Dev. Biol. Anim. 2011, 47, 10–15. [Google Scholar] [CrossRef]
- Siddiqui, A.Q.; Al-Harbi, A.H. Evaluation of three species of tilapia, red tilapia and a hybrid tilapia as culture species in Saudi Arabia. Aquaculture 1995, 138, 145–157. [Google Scholar] [CrossRef]
- Haque, M.R.; Islam, M.A.; Wahab, M.A.; Hoq, M.E.; Rahman, M.M.; Azim, M.E. Evaluation of production performance and profitability of hybrid red tilapia and genetically improved farmed tilapia (GIFT) strains in the carbon/nitrogen controlled periphyton-based (C/N-CP) on-farm prawn culture system in Bangladesh. Aquac. Rep. 2016, 4, 101–111. [Google Scholar] [CrossRef]
- Aly, H.; Abdel-Rahim, M.; Lotfy, A.; Abdelaty, B. Impact of different colors of artificial light on pigmentation and growth performance of hybrid Red Tilapia (Oreochromis mosambicus × O. hornorum) reared in saline well water. J. Mar. Sci. Res. Dev. 2017, 7, 229. [Google Scholar]
- Tayel, A.A.; Bahnasy, A.G.; Mazrou, K.E.; Alasmari, A.; El Rabey, H.A.; Elboghashy, S.A.; Diab, A.M. Biopreservation and quality enhancement of fish surimi using colorant plant extracts. J. Food Qual. 2021, 2021, 6624565. [Google Scholar] [CrossRef]
- Abd El-Kareem, M.S.; Rabbih, M.A.E.F.; Selim, E.T.M.; Elsherbiny, E.A.E.-m.; El-Khateeb, A.Y. Application of GC/EIMS in combination with semi-empirical calculations for identification and investigation of some volatile components in basil essential oil. Int. J. Anal. Mass Spectrom. Chromatogr. 2016, 4, 14–25. [Google Scholar] [CrossRef]
- Bancroft, J.; Layton, C. The hematoxylins and eosin. In Bancroft’s Theory and Practice of Histological Techniques; Elsevier: Amsterdam, The Netherlands, 2013; pp. 173–186. [Google Scholar]
- Al-Deriny, S.H.; Dawood, M.A.; Abou Zaid, A.A.; Wael, F.; Paray, B.A.; Van Doan, H.; Mohamed, R.A. The synergistic effects of Spirulina platensis and Bacillus amyloliquefaciens on the growth performance, intestinal histomorphology, and immune response of Nile tilapia (Oreochromis niloticus). Aquac. Rep. 2020, 17, 100390. [Google Scholar] [CrossRef]
- Feldman, B.; Zinkl, J.; Jain, N. Schalm’s Veterinary Hematology; Lippincott Williams & Wilkins: Baltimore, PA, USA, 2000. [Google Scholar]
- Thrall, M.; Baker, D.; Lassen, E. Veterinary Haematology and Clinical Chemistry; Lippincott Williams & Wilkins: Baltimore, PA, USA, 2004. [Google Scholar]
- Doumas, B.T.; Bayse, D.D.; Carter, R.J.; Peters, T., Jr.; Schaffer, R. A candidate reference method for determination of total protein in serum. I. Development and validation. Clin. Chem. 1981, 27, 1642–1650. [Google Scholar] [CrossRef]
- Doumas, B.T.; Watson, W.A.; Biggs, H.G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta 1971, 31, 87–96. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Heinegård, D.; Tiderström, G. Determination of serum creatinine by a direct colorimetric method. Clin. Chim. Acta 1973, 43, 305–310. [Google Scholar] [CrossRef]
- Coulombe, J.; Favreau, L. A new simple semimicro method for colorimetric determination of urea. Clin. Chem. 1963, 9, 102–108. [Google Scholar] [CrossRef]
- Fynn-Aikins, K.; Hung, S.S.; Liu, W.; Li, H. Growth, lipogenesis and liver composition of juvenile white sturgeon fed different levels of D-glucose. Aquaculture 1992, 105, 61–72. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Samir, F.; Abd El-Naby, A.S.; Monier, M.N. Antioxidative and immunostimulatory effect of dietary cinnamon nanoparticles on the performance of Nile tilapia, Oreochromis niloticus (L.) and its susceptibility to hypoxia stress and Aeromonas hydrophila infection. Fish Shellfish Immunol. 2018, 74, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Hao, K.; Ullah, H.; Jarwar, A.R.; Nong, X.; Tu, X.; Zhang, Z. Functional identification of an FMRFamide-related peptide gene on diapause induction of the migratory locust, Locusta migratoria L. Genomics 2020, 112, 1821–1828. [Google Scholar] [CrossRef]
- Kawahara, E.; Ueda, T.; Nomura, S. In vitro phagocytic activity of white-spotted char blood cells after injection with Aeromonas salmonicida extracellular products. Fish Pathol. 1991, 26, 213–214. [Google Scholar] [CrossRef]
- Demers, N.E.; Bayne, C.J. The immediate effects of stress on hormones and plasma lysozyme in rainbow trout. Dev. Comp. Immunol. 1997, 21, 363–373. [Google Scholar] [CrossRef]
- Torrissen, O.J.; Naevdal, G. Pigmentation of salmonids—Genetical variation in carotenoid deposition in rainbow trout. Aquaculture 1984, 38, 59–66. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Qiang, J.; He, J.; Yang, H.; Wang, H.; Kpundeh, M.; Xu, P.; Zhu, Z. Temperature modulates hepatic carbohydrate metabolic enzyme activity and gene expression in juvenile GIFT tilapia (Oreochromis niloticus) fed a carbohydrate-enriched diet. J. Therm. Biol. 2014, 40, 25–31. [Google Scholar] [CrossRef]
- Tian, J.; Wu, F.; Yang, C.-G.; Jiang, M.; Liu, W.; Wen, H. Dietary lipid levels impact lipoprotein lipase, hormone-sensitive lipase, and fatty acid synthetase gene expression in three tissues of adult GIFT strain of Nile tilapia, Oreochromis niloticus. Fish Physiol. Biochem. 2015, 41, 1–18. [Google Scholar] [CrossRef]
- Elkatatny, N.A.; Elbialy, Z.I.; El-Nahas, A.F.; Mahmoud, S. Characterization of Myostatin Gene in Nile Tilapia (Oreochromis niloticus), the Possible Association of BsmI-exon 2 Polymorphism with Its Growth. Am. J. Life Sci. 2016, 4, 82–86. [Google Scholar] [CrossRef]
- Costa, L.S.; Rosa, P.V.; Fortes-Silva, R.; Sánchez-Vázquez, F.J.; López-Olmeda, J.F. Daily rhythms of the expression of genes from the somatotropic axis: The influence on tilapia (Oreochromis niloticus) of feeding and growth hormone administration at different times. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 181, 27–34. [Google Scholar] [CrossRef]
- Aanyu, M.; Betancor, M.B.; Monroig, O. Effects of dietary limonene and thymol on the growth and nutritional physiology of Nile tilapia (Oreochromis niloticus). Aquaculture 2018, 488, 217–226. [Google Scholar] [CrossRef]
- Han, C.Y.; Zheng, Q.M.; Sun, Z.T. Gene expression and activities of antioxidant enzymes in liver of hybrid Tilapia, Oreochromis niloticus × Oreochromis aureus, under acute pH stress. J. World Aquac. Soc. 2016, 47, 260–267. [Google Scholar] [CrossRef]
- Qiang, J.; He, J.; Yang, H.; Xu, P.; Habte-Tsion, H.M.; Ma, X.; Zhu, Z. The changes in cortisol and expression of immune genes of GIFT tilapia Oreochromis niloticus (L.) at different rearing densities under Streptococcus iniae infection. Aquac. Int. 2016, 24, 1365–1378. [Google Scholar] [CrossRef]
- Salah, A.S.; El Nahas, A.F.; Mahmoud, S. Modulatory effect of different doses of β-1, 3/1, 6-glucan on the expression of antioxidant, inflammatory, stress and immune-related genes of Oreochromis niloticus challenged with Streptococcus iniae. Fish Shellfish Immunol. 2017, 70, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Assefa, A.; Abunna, F. Maintenance of fish health in aquaculture: Review of epidemiological approaches for prevention and control of infectious disease of fish. Vet. Med. Int. 2018, 2018, 5432497. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Pourmohammadi Fallah, H.; Yousefi, M.; Dawood, M.A.; Hoseinifar, S.H.; Adineh, H.; Yilmaz, S.; Paolucci, M.; Doan, H.V. The gene regulatory roles of herbal extracts on the growth, immune system, and reproduction of fish. Animals 2021, 11, 2167. [Google Scholar] [CrossRef]
- Amer, S.A.; Al-Khalaifah, H.S.; Gouda, A.; Osman, A.; Goda, N.I.; Mohammed, H.A.; Darwish, M.I.; Hassan, A.M.; Mohamed, S.K.A. Potential Effects of Anthocyanin-Rich Roselle (Hibiscus sabdariffa L.) Extract on the Growth, Intestinal Histomorphology, Blood Biochemical Parameters, and the Immune Status of Broiler Chickens. Antioxidants 2022, 11, 544. [Google Scholar] [CrossRef]
- Adewole, A. Organosomatic indices and histopathological response of Clarias gariepinus fed Roselle (Hibiscus sabdariffa) meal diets. Niger. J. Anim. Prod. 2018, 45, 199–206. [Google Scholar] [CrossRef]
- Van Hai, N.; Fotedar, R. Comparison of the effects of the prebiotics (Bio-Mos® and β-1, 3-D-glucan) and the customised probiotics (Pseudomonas synxantha and P. aeruginosa) on the culture of juvenile western king prawns (Penaeus latisulcatus Kishinouye, 1896). Aquaculture 2009, 289, 310–316. [Google Scholar] [CrossRef]
- Imran, S.M.; Ali, A.H.; Najim, S.M. Effect of Dietary Prebiotic Safmannan and Bio-antibiotic Fluconazole on some Growth and Haemato-immunological Parameters of Common Carp Cyprinus carpio Linnaeus. Basrah J. Agric. Sci. 2019, 32, 176–192. [Google Scholar] [CrossRef]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The Interactions between polyphenols and microorganisms, especially gut microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef]
- Naiel, M.A.; Alagawany, M.; Patra, A.K.; El-Kholy, A.I.; Amer, M.S.; Abd El-Hack, M.E. Beneficial impacts and health benefits of macroalgae phenolic molecules on fish production. Aquaculture 2021, 534, 736186. [Google Scholar] [CrossRef]
- Adewole, A.M. Effects of roselle as dietary additive on growth performance and production economy of Clarias gariepinus. J. Emerg. Trends Eng. Appl. Sci. 2014, 5, 1–8. [Google Scholar]
- Hoseini, S.M.; Hoseinifar, S.H.; Van Doan, H. Growth performance and hematological and antioxidant characteristics of rainbow trout, Oncorhynchus mykiss, fed diets supplemented with Roselle, Hibiscus sabdariffa. Aquaculture 2021, 530, 735827. [Google Scholar] [CrossRef]
- Akbary, P.; Aminikhoei, Z. Effect of water-soluble polysaccharide extract from the green alga Ulva rigida on growth performance, antioxidant enzyme activity, and immune stimulation of grey mullet Mugil cephalus. J. Appl. Phycol. 2018, 30, 1345–1353. [Google Scholar] [CrossRef]
- Elsabagh, M.; Mohamed, R.; Moustafa, E.M.; Hamza, A.; Farrag, F.; Decamp, O.; Dawood, M.A.; Eltholth, M. Assessing the impact of Bacillus strains mixture probiotic on water quality, growth performance, blood profile and intestinal morphology of Nile tilapia, Oreochromis niloticus. Aquac. Nutr. 2018, 24, 1613–1622. [Google Scholar] [CrossRef]
- Lauriano, E.; Pergolizzi, S.; Capillo, G.; Kuciel, M.; Alesci, A.; Faggio, C. Immunohistochemical characterization of Toll-like receptor 2 in gut epithelial cells and macrophages of goldfish Carassius auratus fed with a high-cholesterol diet. Fish Shellfish Immunol. 2016, 59, 250–255. [Google Scholar] [CrossRef]
- Banan Khojasteh, S.M. The morphology of the post-gastric alimentary canal in teleost fishes: A brief review. Int. J. Aquat. Sci. 2012, 3, 71–88. [Google Scholar]
- Dan, T.M.; Shakib, A. Jus hibiscus bukan sekadar minuman biasa [Malaysia]. Dewan Ekon. 1995, 2, 12–13. [Google Scholar]
- Kamboh, A.; Arain, M.A.; Mughal, M.J.; Zaman, A.; Arain, Z.; Soomro, A. Flavonoids: Health promoting phytochemicals for animal production-a review. J. Anim. Health Prod 2015, 3, 6–13. [Google Scholar] [CrossRef]
- Adel, M.; Yeganeh, S.; Dadar, M.; Sakai, M.; Dawood, M.A. Effects of dietary Spirulina platensis on growth performance, humoral and mucosal immune responses and disease resistance in juvenile great sturgeon (Huso huso Linnaeus, 1754). Fish Shellfish Immunol. 2016, 56, 436–444. [Google Scholar] [CrossRef]
- Carbone, D.; Faggio, C. Importance of prebiotics in aquaculture as immunostimulants. Effects on immune system of Sparus aurata and Dicentrarchus labrax. Fish Shellfish Immunol. 2016, 54, 172–178. [Google Scholar] [CrossRef]
- Ogueji, E.; Iheanacho, S.; Dada, A.; Yaji, A.; Ifejimalu, A.; Ibrahim, B.; Mbah, E.; Okafor, E.; Nnatuanya, I. Effect of Roselle (Hibiscus sabdariffa) and ginger (Zingiber officinale) as feed additives, on growth and haematology of Clarias gariepinus Juvenile. Afr. J. Biotechnol. 2017, 16, 2242–2247. [Google Scholar] [CrossRef]
- Lie, Ø.; Syed, M.; Solbu, H. Improved agar plate assays of bovine lysozyme and haemolytic complement activity. Acta Vet. Scand. 1986, 27, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Asaniyan, E.; Akinduro, V. Haematology and serum biochemistry of broiler chickens offered extracts of dried roselle plant (Hibiscus sabdariffa) calyx in drinking water. Ife J. Sci. 2020, 22, 149–157. [Google Scholar] [CrossRef]
- Yahaya, T.; Okpuzor, J.; Ajayi, T. The prophylactic efficacy of roselle [H. sabdariffa], moringa [Moringa oleifera], ginger [Z. officinale] and ugwu [T. occidentalis] on the hematology and serum protein of albino rats [Rattus norvegicus] exposed to cement dust. Res. J. Med. Plant 2012, 6, 189–196. [Google Scholar] [CrossRef]
- Sewaka, M.; Trullas, C.; Chotiko, A.; Rodkhum, C.; Chansue, N.; Boonanuntanasarn, S.; Pirarat, N. Efficacy of synbiotic Jerusalem artichoke and Lactobacillus rhamnosus GG-supplemented diets on growth performance, serum biochemical parameters, intestinal morphology, immune parameters and protection against Aeromonas veronii in juvenile red tilapia (Oreochromis spp.). Fish Shellfish Immunol. 2019, 86, 260–268. [Google Scholar]
- Ugwu, D.; Jiwuba, P.; Ubogu, V.; Akazue, R. Phytochemical properties of Hibiscus sabdariffa Calyx and the effects of its aqueous extract supplementation on haematological and serum biochemical indices of broiler birds. Niger. J. Anim. Sci. 2020, 22, 165–172. [Google Scholar]
- Iheanacho, S.; Ogueji, E.; Yaji, A.; Dada, O.; Mbah, C.; Ifejimalu, A.; Ibrahim, B.-U. Effects of herbal plants (Zingiber officinale and Hibiscus sabdariffa) as dietary additives on serum biochemistry and some metabolites in Clarias gariepinus (Burchell, 1822). J. Coast. Life Med. 2017, 5, 516–520. [Google Scholar] [CrossRef]
- Halliwell, B.; Clement, M.V.; Long, L.H. Hydrogen peroxide in the human body. FEBS Lett. 2000, 486, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-H.; Chen, J.-H.; Wang, C.-J. Chemopreventive properties and molecular mechanisms of the bioactive compounds in Hibiscus sabdariffa Linne. Curr. Med. Chem. 2011, 18, 1245–1254. [Google Scholar] [CrossRef]
- Li, X.; Cui, K.; Fang, W.; Chen, Q.; Xu, D.; Mai, K.; Zhang, Y.; Ai, Q. High level of dietary olive oil decreased growth, increased liver lipid deposition and induced inflammation by activating the p38 MAPK and JNK pathways in large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2019, 94, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Witztum, J.L. Atherosclerosis: The road ahead. Cell 2001, 104, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Ajeniyi, S.; Solomon, R. Urea and creatinine of Clarias gariepinus in three different commercial ponds. Nat. Sci. 2014, 12, 124–138. [Google Scholar]
- Dacie, J.V.; Lewis, S.M. Practical Haematology; Churchill Livingstone: London, UK, 1995; p. 609. [Google Scholar]
- Bilen, S.; Filogh, A.M.; Ali, A.B.; Kenanoğlu, O.N.; Zoral, M.A. Effect of common mallow (Malva sylvestris) dietary supplementation on growth performance, digestive enzyme activities, haemotological and immune responses of common carp (Cyprinus carpio). Aquac. Int. 2020, 28, 73–84. [Google Scholar] [CrossRef]
- Xu, A.; Shang-Guan, J.; Li, Z.; Gao, Z.; Huang, Y.C.; Chen, Q. Effects of dietary Chinese herbal medicines mixture on feeding attraction activity, growth performance, nonspecific immunity and digestive enzyme activity of Japanese seabass (Lateolabrax japonicus). Aquac. Rep. 2020, 17, 100304. [Google Scholar] [CrossRef]
- Ghiasi, F.; Mirzargar, S.; Badakhshan, H.; Shamsi, S. Lysozyme in Serum, Leukocyte Count and Phagocytic Index in Cyprinus carpio under the Wintering Conditions. J. Fish. Aquat. Sci. 2010, 5, 113–119. [Google Scholar]
- Khanjani, M.H.; Ghaedi, G.; Sharifinia, M. Effects of diets containing β-glucan on survival, growth performance, haematological, immunity and biochemical parameters of rainbow trout (Oncorhynchus mykiss) fingerlings. Aquac. Res. 2022, 53, 1842–1850. [Google Scholar] [CrossRef]
- Coulter, A.; Lombardini, J.; Sufrin, J.R.; Talalay, P. Structural and Conformational Analogues of L-Methionine as Inhibitors of the Enzymatic Synthesis of S-Adenosyl-L-methionine: III. Carbocyclic and Heterocyclic Amino Acids. Mol. Pharmacol. 1974, 10, 319–334. [Google Scholar]
- Mahfudh, N.; Hadi, A.; Solechan, R. Immunomodulatory activity of yogurt fortified with roselle (Hibiscus sabdariffa L.) extract. Int. Food Res. J. 2021, 28, 255–261. [Google Scholar] [CrossRef]
- Hirunpanich, V.; Utaipat, A.; Morales, N.P.; Bunyapraphatsara, N.; Sato, H.; Herunsale, A.; Suthisisang, C. Hypocholesterolemic and antioxidant effects of aqueous extracts from the dried calyx of Hibiscus sabdariffa L. in hypercholesterolemic rats. J. Ethnopharmacol. 2006, 103, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Aphirakchatsakun, W.; Angkanaporn, K.; Kijparkorn, S. The Effect of Roselle (Hibicus sabdariffa Linn.) Calyx as antioxidant and acidifier on growth performance in postweaning pigs. Asian-Australas. J. Anim. Sci. 2008, 21, 574–581. [Google Scholar] [CrossRef]
- Yin, G.; Cao, L.; Xu, P.; Jeney, G.; Nakao, M.; Lu, C. Hepatoprotective and antioxidant effects of Glycyrrhiza glabra extract against carbon tetrachloride (CCl4)-induced hepatocyte damage in common carp (Cyprinus carpio). Fish Physiol. Biochem. 2011, 37, 209–216. [Google Scholar] [CrossRef]
- Gomaa, M.; Hifney, A.F.; Fawzy, M.A.; Abdel-Gawad, K.M. Use of seaweed and filamentous fungus derived polysaccharides in the development of alginate-chitosan edible films containing fucoidan: Study of moisture sorption, polyphenol release and antioxidant properties. Food Hydrocoll. 2018, 82, 239–247. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Mirghaed, A.T.; Iri, Y.; Ghelichpour, M. Effects of dietary cineole administration on growth performance, hematological and biochemical parameters of rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 495, 766–772. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Chien, Y.-H.; Pan, C.-H. Effects of dietary supplementation of carotenoids on survival, growth, pigmentation, and antioxidant capacity of characins, Hyphessobrycon callistus. Aquaculture 2006, 261, 641–648. [Google Scholar] [CrossRef]
- Bendich, A.; Olson, J.A. Biological actions of carotenoids 1. FASEB J. 1989, 3, 1927–1932. [Google Scholar] [CrossRef]
- Christiansen, R.; Glette, J.; Lie, Ø.; Torrissen, O.; Waagbø, R. Antioxidant status and immunity in Atlantic salmon, Salmo salar L., fed semi-purified diets with and without astaxanthin supplementation. J. Fish Dis. 1995, 18, 317–328. [Google Scholar] [CrossRef]
- Al-Sayed, H.M.; Hegab, S.A.; Youssef, M.A.; Khalafalla, M.Y.; Almaroai, Y.A.; Ding, Z.; Eissa, M.A. Evaluation of quality and growth of roselle (Hibiscus sabdariffa L.) as affected by bio-fertilizers. J. Plant Nutr. 2020, 43, 1025–1035. [Google Scholar] [CrossRef]
- Wong, P.K.; Yusof, S.; Ghazali, H.; Man, Y.C. Physico-chemical characteristics of roselle (Hibiscus sabdariffa L.). Nutr. Food Sci. 2002, 32, 68–73. [Google Scholar] [CrossRef]
- Hu, X.; Jandacek, R.J.; White, W.S. Intestinal absorption of β-carotene ingested with a meal rich in sunflower oil or beef tallow: Postprandial appearance in triacylglycerol-rich lipoproteins in women. Am. J. Clin. Nutr. 2000, 71, 1170–1180. [Google Scholar] [CrossRef]
- Zeb, A. Effects of β-carotene on the thermal oxidation of fatty acids. Afr. J. Biotechnol. 2011, 10, 15346–15352. [Google Scholar] [CrossRef]
- van het Hof, K.H.; West, C.E.; Weststrate, J.A.; Hautvast, J.G. Dietary factors that affect the bioavailability of carotenoids. J. Nutr. 2000, 130, 503–506. [Google Scholar] [CrossRef]
- Mitrofanova, O.; Dementeva, N.; Krutikova, A.; Yurchenko, O.; Vakhrameev, A.; Terletskiy, V. Association of polymorphic variants in MSTN, PRL, and DRD2 genes with intensity of young animal growth in Pushkin breed chickens. Cytol. Genet. 2017, 51, 179–184. [Google Scholar] [CrossRef]
- Elbialy, Z.I.; Salah, A.S.; Elsheshtawy, A.; Rizk, M.; Abualreesh, M.H.; Abdel-Daim, M.M.; Salem, S.M.R.; Askary, A.E.; Assar, D.H. Exploring the Multimodal Role of Yucca schidigera Extract in Protection against Chronic Ammonia Exposure Targeting: Growth, Metabolic, Stress and Inflammatory Responses in Nile Tilapia (Oreochromis niloticus L.). Animals 2021, 11, 2072. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-D.; Kim, S.; Zhu, H.-Y.; Jin, L.; Guo, Q.; Li, X.-C.; Zhang, Y.-C.; Xing, X.-X.; Xuan, M.-F.; Zhang, G.-L. Generation of cloned adult muscular pigs with myostatin gene mutation by genetic engineering. RSC Adv. 2017, 7, 12541–12549. [Google Scholar] [CrossRef]
- Lv, Q.; Yuan, L.; Deng, J.; Chen, M.; Wang, Y.; Zeng, J.; Li, Z.; Lai, L. Efficient generation of myostatin gene mutated rabbit by CRISPR/Cas9. Sci. Rep. 2016, 6, 25029. [Google Scholar] [CrossRef]
- Abdel-Latif, S.; El-Yamany, A.; Edaly, E.A. Evaluation of using different levels and sources of medicinal herbs in growing Japanese quail diets. Egypt. J. Nutr. Feed. 2004, 7, 69–81. [Google Scholar]
- Murray, R.; Granner, D.; Mayes, P.; Rodwell, V. The Text Book of Harper’s Biochemistry; Appleton and Large: Norwalk, CT, USA; Las Altos, CA, USA, 1991. [Google Scholar]
- Chamorro, S.; Viveros, A.; Centeno, C.; Romero, C.; Arija, I.; Brenes, A. Effects of dietary grape seed extract on growth performance, amino acid digestibility and plasma lipids and mineral content in broiler chicks. Animal 2013, 7, 555–561. [Google Scholar] [CrossRef]
- Brenes, A.; Montoro, A.V.; Cambrodón, I.G.; Centeno, C.; Calixto, F.S.; Arija, I. Effect grape seed extract on growth performance, protein and polyphenol digestibilities, and antioxidant activity in chickens. Span. J. Agric. Res. 2010, 8, 326–333. [Google Scholar] [CrossRef]
- Lopez-Huertas, E. Health effects of oleic acid and long chain omega-3 fatty acids (EPA and DHA) enriched milks. A review of intervention studies. Pharmacol. Res. 2010, 61, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Vraskou, Y.; Roher, N.; Díaz, M.; Antonescu, C.N.; MacKenzie, S.A.; Planas, J.V. Direct involvement of tumor necrosis factor-α in the regulation of glucose uptake in rainbow trout muscle cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R716–R723. [Google Scholar] [CrossRef]
- Elbahnaswy, S.; Elshopakey, G.E. Differential gene expression and immune response of Nile tilapia (Oreochromis niloticus) challenged intraperitoneally with Photobacterium damselae and Aeromonas hydrophila demonstrating immunosuppression. Aquaculture 2020, 526, 735364. [Google Scholar] [CrossRef]
- Tavaria, M.; Gabriele, T.; Kola, I.; Anderson, R.L. A hitchhiker’s guide to the human Hsp70 family. Cell Stress Chaperones 1996, 1, 23. [Google Scholar] [CrossRef] [PubMed]
- Morano, K.A. New tricks for an old dog: The evolving world of Hsp70. Ann. N. Y. Acad. Sci. 2007, 1113, 1–14. [Google Scholar] [CrossRef]
- Ečimović, S.; Velki, M.; Vuković, R.; Čamagajevac, I.Š.; Petek, A.; Bošnjaković, R.; Grgić, M.; Engelmann, P.; Bodó, K.; Filipović-Marijić, V. Acute toxicity of selenate and selenite and their impacts on oxidative status, efflux pump activity, cellular and genetic parameters in earthworm Eisenia andrei. Chemosphere 2018, 212, 307–318. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Mohammady, E.Y.; Soaudy, M.R.; Sabae, S.A.; Mahmoud, A.M.; El-Haroun, E.R. Comparative study on the effect of dietary β-carotene and phycocyanin extracted from Spirulina platensis on immune-oxidative stress biomarkers, genes expression and intestinal enzymes, serum biochemical in Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2021, 108, 63–72. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Yousefi, S.; Capillo, G.; Paknejad, H.; Khalili, M.; Tabarraei, A.; Van Doan, H.; Spanò, N.; Faggio, C. Mucosal immune parameters, immune and antioxidant defence related genes expression and growth performance of zebrafish (Danio rerio) fed on Gracilaria gracilis powder. Fish Shellfish Immunol. 2018, 83, 232–237. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Ren, X.; Liu, W.; Liu, Y. Effects of fluconazole on the clinical outcome and immune response in fungal co-infected tuberculosis patients. Microb. Pathog. 2018, 117, 148–152. [Google Scholar] [CrossRef]
- Damon, M.; Louveau, I.; Lefaucheur, L.; Lebret, B.; Vincent, A.; Leroy, P.; Sanchez, M.P.; Herpin, P.; Gondret, F. Number of intramuscular adipocytes and fatty acid binding protein-4 content are significant indicators of intramuscular fat level in crossbred Large White× Duroc pigs. J. Anim. Sci. 2006, 84, 1083–1092. [Google Scholar] [CrossRef]
- Mobbs, C.V.; Makimura, H. Block the FAS, lose the fat. Nat. Med. 2002, 8, 335–336. [Google Scholar] [CrossRef]
- Albalat, A.; Saera-Vila, A.; Capilla, E.; Gutiérrez, J.; Pérez-Sánchez, J.; Navarro, I. Insulin regulation of lipoprotein lipase (LPL) activity and expression in gilthead sea bream (Sparus aurata). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 148, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.G.; Zasłona, Z.; Galván-Peña, S.; Koppe, E.L.; Sévin, D.C.; Angiari, S.; Triantafilou, M.; Triantafilou, K.; Modis, L.K.; O’Neill, L.A. An unexpected link between fatty acid synthase and cholesterol synthesis in proinflammatory macrophage activation. J. Biol. Chem. 2018, 293, 5509–5521. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Novosyadlyy, R.; Wu, Y.; Yakar, S.; LeRoith, D. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Horm. IGF Res. 2010, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.A.; Elbialy, Z.I.; Abd El Latif, A.S.; Shukry, M.; Assar, D.H.; El Nokrashy, A.M.; Elsheshtawy, A.; Dawood, M.A.; Paray, B.A.; Van Doan, H. Dietary clenbuterol modifies the expression of genes involved in the regulation of lipid metabolism and growth in the liver, skeletal muscle, and adipose tissue of Nile tilapia (Oreochromis niloticus). Aquac. Rep. 2020, 17, 100319. [Google Scholar] [CrossRef]
- Kim-Kang, H.; Bova, A.; Crouch, L.S.; Wislocki, P.G.; Robinson, R.A.; Wu, J. Tissue distribution, metabolism, and residue depletion study in Atlantic salmon following oral administration of [3H] emamectin benzoate. J. Agric. Food Chem. 2004, 52, 2108–2118. [Google Scholar] [CrossRef]
- Peng, M.; Xu, W.; Mai, K.; Zhou, H.; Zhang, Y.; Liufu, Z.; Zhang, K.; Ai, Q. Growth performance, lipid deposition and hepatic lipid metabolism related gene expression in juvenile turbot (Scophthalmus maximus L.) fed diets with various fish oil substitution levels by soybean oil. Aquaculture 2014, 433, 442–449. [Google Scholar] [CrossRef]
- Alvarez, M.; Diez, A.; Lopez-Bote, C.; Gallego, M.; Bautista, J. Short-term modulation of lipogenesis by macronutrients in rainbow trout (Oncorhynchus mykiss) hepatocytes. Br. J. Nutr. 2000, 84, 619–628. [Google Scholar] [CrossRef]
- Peng, S.; Shi, Z.; Gao, Q.; Zhang, C.; Wang, J. Dietary n-3 LC-PUFA s affect lipoprotein lipase (LPL) and fatty acid synthase (FAS) activities and mRNA expression during vitellogenesis and ovarian fatty acid composition of female silver pomfret (Pampus argenteus) broodstock. Aquac. Nutr. 2017, 23, 692–701. [Google Scholar] [CrossRef]
- Kim, H.-K.; Choi, S.; Choi, H. Suppression of hepatic fatty acid synthase by feeding α-linolenic acid rich perilla oil lowers plasma triacylglycerol level in rats. J. Nutr. Biochem. 2004, 15, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Richard, N.; Mourente, G.; Kaushik, S.; Corraze, G. Replacement of a large portion of fish oil by vegetable oils does not affect lipogenesis, lipid transport and tissue lipid uptake in European seabass (Dicentrarchus labrax L.). Aquaculture 2006, 261, 1077–1087. [Google Scholar] [CrossRef]
- Richard, N.; Kaushik, S.; Larroquet, L.; Panserat, S.; Corraze, G. Replacing dietary fish oil by vegetable oils has little effect on lipogenesis, lipid transport and tissue lipid uptake in rainbow trout (Oncorhynchus mykiss). Br. J. Nutr. 2006, 96, 299–309. [Google Scholar] [CrossRef]
- Hudgins, L.C.; Baday, A.; Hellerstein, M.K.; Parker, T.S.; Levine, D.M.; Seidman, C.E.; Neese, R.A.; Tremaroli, J.D.; Hirsch, J. The effect of dietary carbohydrate on genes for fatty acid synthase and inflammatory cytokines in adipose tissues from lean and obese subjects. J. Nutr. Biochem. 2008, 19, 237–245. [Google Scholar] [CrossRef]
- Wang, A.; Han, G.; Qi, Z.; Lv, F.; Yu, Y.; Huang, J.; Wang, T.; Xu, P. Cloning of lipoprotein lipase (LPL) and the effects of dietary lipid levels on LPL expression in GIFT tilapia (Oreochromis niloticus). Aquac. Int. 2013, 21, 1219–1232. [Google Scholar] [CrossRef]
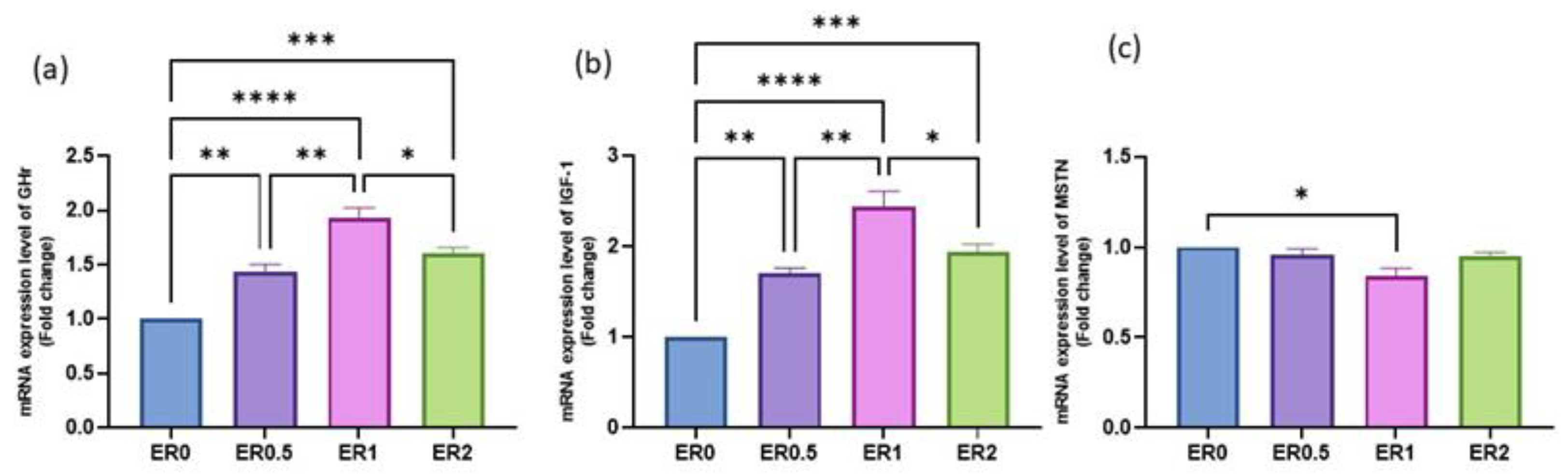
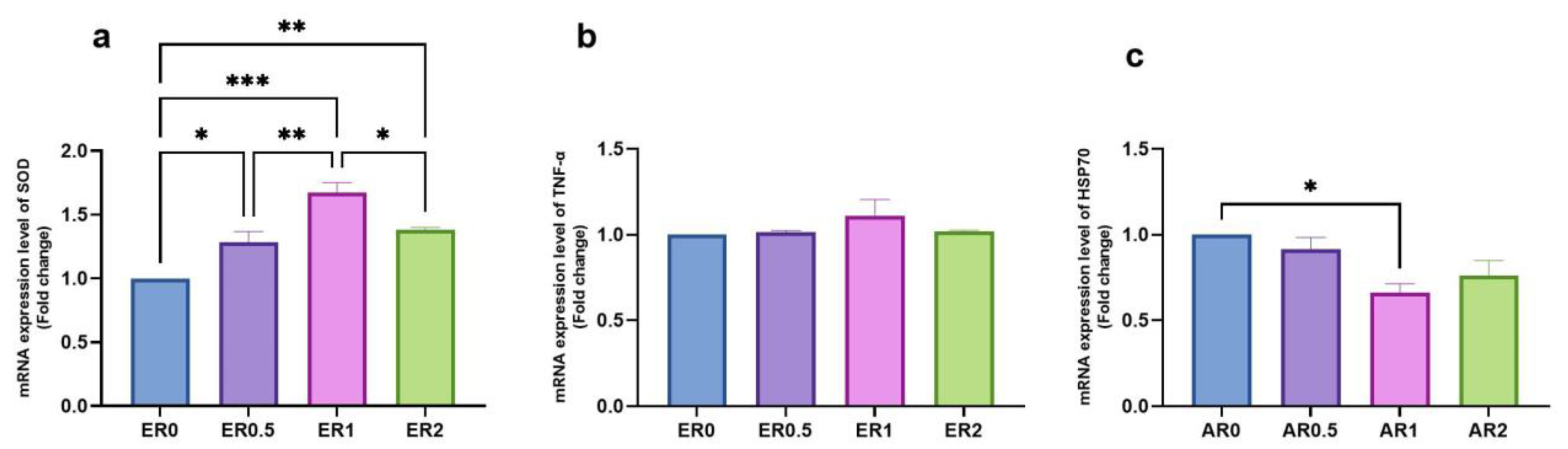
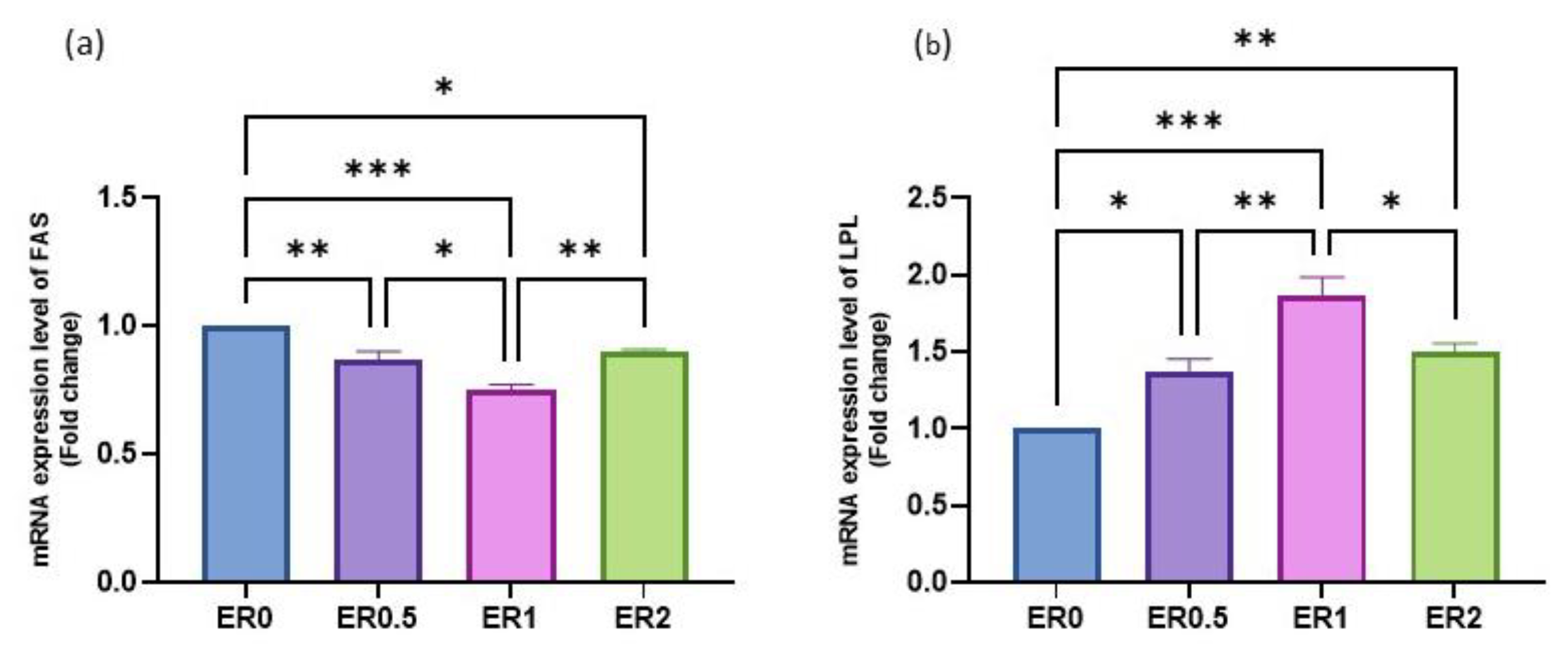
| ER Dietary Level (%) | ||||
|---|---|---|---|---|
| ER0 | ER0.5 | ER1 | ER2 | |
| Feed Ingredients (%) | ||||
| Fish meal (72% CP) | 2.5 | 2.5 | 2.5 | 2.5 |
| Soybean meal | 41 | 41 | 41 | 41 |
| Yellow corn | 19 | 19 | 19 | 19 |
| Wheat middling | 12 | 12 | 12 | 12 |
| Rice bran | 10 | 10 | 10 | 10 |
| Gluten 60 | 5 | 5 | 5 | 5 |
| Linseed meal | 3.6 | 3.6 | 3.6 | 3.6 |
| Meat meal | 4 | 4 | 4 | 4 |
| Choline chloride | 0.1 | 0.1 | 0.1 | 0.1 |
| Soya oil | 1 | 1 | 1 | 1 |
| Calcium carbonate | 0.6 | 0.6 | 0.6 | 0.6 |
| Sod. Bicarbonate | 0.1 | 0.1 | 0.1 | 0.1 |
| Lysine | 0.5 | 0.5 | 0.5 | 0.5 |
| Methionine | 0.3 | 0.3 | 0.3 | 0.3 |
| Vitam. and min. mix.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Anti-mycosis | 0.1 | 0.1 | 0.1 | 0.1 |
| Vit. C | 0.1 | 0.1 | 0.1 | 0.1 |
| Dietary ER (g/kg) | 0 | 0.5 | 1 | 2 |
| Proximate composition | ||||
| Dry matter (%) | 88.84 | 88.65 | 88.93 | 89.02 |
| Crude protein (%) | 30.87 | 30.94 | 30.64 | 30.20 |
| Ether extract (%) | 6.38 | 6.81 | 7.58 | 7.34 |
| Crude fiber (%) | 5.18 | 6.01 | 5.90 | 5.67 |
| Ash (%) | 6.16 | 9.28 | 8.96 | 9.18 |
| NFE (%) | 51.41 | 46.96 | 46.92 | 47.61 |
| Growth energy2 (kcal/kg) | 4516.56 | 4373.36 | 4427.49 | 4409.07 |
| No. | Gene | Primer Sequence (5’-3’) | Accession Number | Reference |
|---|---|---|---|---|
| 1 | β-actin | F: CCACACAGTGCCCATCTACGA R: CCACGCTCTGTCAGGATCTTCA | XM_003455949.2 | [43] |
| 2 | FAS | F: TGAAACTGAAGCCTTGTGTGCC R: TCCCTGTGAGCGGAGGTGATTA | GU433188 | [44] |
| 3 | LPL | F: TGCTAATGTGATTGTGGTGGAC R: GCTGATTTTGTGGTTGGTAAGG | FJ623077 | [44] |
| 4 | MSTN | F: GCATCTGTCTCAGATCGTGCT R: TGCCATCATTACAATTGTCTCCG | XM_003458832 | [45] |
| 5 | IGF-1 | F: TCCTGTAGCCACACCCTCTC R: ACAGCTTTGGAAGCAGCACT | XM_00344805 | [46] |
| 6 | GHR-1 | F: CAGACTTCTACGCTCAGGTC R: CTGGATTCTGAGTTGCTGTC | AY973232.1 | [47] |
| 7 | SOD | F: CCACGCTCTGTCAGGATCTTCA R: CATGCCTTCGGAGACAACAC | AY491056.1 | [48] |
| 8 | TNF-α | F: ACCTTCTCGTGGATCACCAT R: GGAAGCAGCTCCACTCTGATGA | JF957373.1 | [49] |
| 9 | HSP-70 | F: CATCGCCTACGGTCTGGACAA R: TGCCGTCTTCAATGGTCAGGAT | FJ207463.1 | [49] |
| n. | Post-Derivatization Component Naming | Molecular Formula | Rt (min) | Molecular Weight (m/z) | Peak Area (%) | Prior to Derivatization Compound Name |
|---|---|---|---|---|---|---|
| 1 | Cyclopentane carboxylic acid, 2-oxo-, ethyl ester | C8H12O3 | 6.91 | 156 | 21.82 | Cyclopentane carboxylic acids |
| 2 | Hexadecanoic acid, ethyl ester | C18H36O2 | 21.72 | 284 | 18.35 | Ethyl palmitate |
| 3 | 9,12-Octadecadienoic acid (Z,Z)- | C18H32O2 | 24.35 | 280 | 16.51 | Linoleic acid |
| 4 | 9,12-Octadecadienoic acid, ethyl ester | C20H36O2 | 24.26 | 308 | 14.10 | Linolelaidic acid |
| 5 | Cyclohexane carboxylic acid, 2-ethylcyclohexyl ester | C15H26O2 | 8.60 | 238 | 11.83 | Cyclohexane carboxylic acid |
| 6 | 2-(2,4-difluorophenyl)-1,3-bis(1,2,4-triazol-1-yl)propan-2-ol | C13H12F2N6O | 16.84 | 306 | 4.93 | Fluconazole |
| 7 | Butanedioic acid, 3-hydroxy-2,2-dimethyl-, diethyl ester | C10H18O5 | 16.84 | 218 | 4.93 | Succinic acid |
| 8 | 9,12-Octadecadienoyl chloride, (Z,Z)- | C18H31ClO | 23.35 | 298 | 3.06 | Linoleic acid chloride |
| 9 | 4,6-O-Ethylidene-D-glucopyranose | C8H14O6 | 17.87 | 206.19 | 0.97 | Glycopyranoside |
| 10 | 8-Benzoyloxy quinoline | C16H11NO2 | 17.87 | 249 | 0.97 | Benzoxyquinone |
| Parameters | ER Dietary Level (%) | p-Value | |||
|---|---|---|---|---|---|
| ER0 | ER0.5 | ER1 | ER2 | ||
| Initial weight (g) | 4.850 ± 0.017 | 4.817 ± 0.049 | 4.783 ± 0.083 | 4.883 ± 0.017 | 0.556 |
| Final weight (g) | 33.67 ± 0.333 a | 33.67 ± 0.333 a | 34.67 ± 0.166 a | 31.20 ± 0.057 b | <0.0001 |
| Weight gain (g) | 28.82 ± 0.317 a | 28.85 ± 0.356 a | 29.88 ± 0.249 a | 26.32 ± 0.055 b | <0.0001 |
| Weight gain rate (%) | 594.1 ± 4.631 a | 599.2 ± 11.96 a | 625.3 ± 16.35 a | 538.9 ± 2.289 b | 0.002 |
| SGR (%/day) | 3.229 ± 0.011 a | 3.241 ± 0.028 a | 3.302 ± 0.037 a | 3.091 ± 0.005 b | 0.001 |
| FCR | 1.430 ± 0.022 a | 1.286 ± 0.014 b | 1.156 ± 0.001 c | 1.330 ± 0.002 b | <0.0001 |
| HSI (%) | 2.579 ± 0.253 a,b | 2.406 ± 0.024 a,b | 1.817 ± 0.008 b | 2.798 ± 0.244 a | 0.022 |
| VSI (%) | 2.487 ± 0.2150 | 2.704 ± 0.02704 | 2.654 ± 0.02515 | 2.788 ± 0.1882 | 0.540 |
| SSI (%) | 0.584 ± 0.017 | 0.484 ± 0.052 | 0.458 ± 0.077 | 0.371 ± 0.038 | 0.100 |
| Survival (%) | 95.56 ± 2.222 | 100.0 ± 0.000 | 100.0 ± 0.000 | 100.0 ± 0.000 | 0.051 |
| Intestinal Segment | Variable | ER Dietary Level (%) | p Values | |||
|---|---|---|---|---|---|---|
| ER0 | ER0.5 | ER1 | ER2 | |||
| Anterior part | Villi length (μm) | 153.2 ± 14.110 b | 205.2 ± 17.140 b | 531.3 ± 16.550 a | 471.4 ± 18.940 a | <0.0001 |
| Villi width (μm) | 92.28 ± 5.640 | 79.61 ± 5.978 | 76.64 ± 5.947 | 80.19 ± 10.110 | 0.469 | |
| Goblet cell (no/mm2) | 20.67 ± 1.453 b | 27.67 ± 2.404 a,b | 33.00 ± 1.732 a | 28.33 ± 0.881 a,b | 0.006 | |
| Middle part | Villi length (μm) | 319.20 ± 11.10 d | 464.0 ± 29.860 c | 815.9 ± 32.250 a | 683.0 ± 14.260 b | <0.0001 |
| Villi width (μm) | 64.22 ± 4.772 | 69.96 ± 7.694 | 83.16 ± 6.252 | 77.69 ± 6.020 | 0.229 | |
| Goblet cell (no/mm2) | 30.00 ± 1.732 c | 36.00 ± 1.155 b,c | 51.33 ± 4.631 a | 48.33 ± 2.028 a,b | 0.001 | |
| Terminal part | Villi length (μm) | 79.63 ± 0.910 c | 123.2 ± 4.300 c | 380.3 ± 24.180 a | 290.3 ± 16.920 b | <0.0001 |
| Villi width (μm) | 120.6 ± 10.520 | 104.7 ± 6.425 | 132.6 ± 25.440 | 123.5 ± 20.880 | 0.731 | |
| Goblet cell (no/mm2) | 8.0 ± 0.577 b | 12.33 ± 1.856 b | 20.33 ± 1.453 a | 23.33 ± 1.453 a | 0.0002 | |
| ER Dietary Level (%) | p-Value | ||||
|---|---|---|---|---|---|
| ER0 | ER0.5 | ER1 | ER2 | ||
| PCV (%) | 24.05 ± 0.548 | 25.45 ± 2.916 | 23.90 ± 1.790 | 22.20 ± 0.923 | 0.661 |
| RBCs (×10/mm3) | 1.985 ± 0.049 | 1.975 ± 0.089 | 1.975 ± 0.089 | 1.850 ± 0.028 | 0.500 |
| Hb (g/100 mL) | 8.450 ± 0.028 | 9.550 ± 0.894 | 8.750 ± 0.375 | 8.650 ± 0.028 | 0.445 |
| TP (g/dL) | 6.350 ± 0.721 a | 7.450 ± 0.548 a | 2.900 ± 0.115 b | 2.650 ± 0.490 b | 0.0003 |
| Albumin (g/dL) | 3.650 ± 0.664 a | 3.200 ± 0.808 a,b | 1.500 ± 0.173 a,b | 0.850 ± 0.028 b | 0.016 |
| ALT (U/L) | 87.50 ± 7.217 a | 13.50 ± 0.866 b | 29.50 ± 1.443 b | 75.00 ± 14.430 a | 0.0005 |
| AST (U/L) | 283.0 ± 52.540 a,b | 73.00 ± 9.815 c | 186.0 ± 8.083 b,c | 389.0 ± 69.860 a | 0.005 |
| Creatinine (mg/dL) | 0.320 ± 0.103 b | 0.110 ± 0.017 b | 0.400 ± 0.057 b | 2.150 ± 0.606 a | 0.005 |
| Urea (mg/dL) | 21.50 ± 0.866 a | 9.200 ± 0.173 b | 15.40 ± 3.522 a,b | 22.85 ± 2.800 a | 0.010 |
| Cholesterol (mg/dL) | 279.5 ± 86.310 a | 53.50 ± 4.907 b | 110.0 ± 5.774 a,b | 255.0 ± 2.887 a | 0.015 |
| Triglycerides (mg/dL) | 127.0 ± 9.815 | 217.0 ± 1.732 | 259.0 ± 99.880 | 347.5 ± 153.9 | 0.443 |
| Glucose (mg/dL) | 83.50 ± 26.850 | 88.00 ± 36.950 | 44.00 ± 6.928 | 33.50 ± 2.598 | 0.307 |
| Amylase (U/L) | 41.60 ± 4.850 b | 82.05 ± 2.281 a | 72.00 ± 4.041 a | 24.45 ± 4.705 b | <0.0001 |
| Lipase (U/L) | 91.00 ± 2.887 a | 118.0 ± 12.120 a | 89.00 ± 0.577 a | 38.00 ± 4.041 b | 0.0002 |
| ER Dietary Level (%) | p-Value | ||||
|---|---|---|---|---|---|
| ER0 | ER0.5 | ER1 | ER2 | ||
| Phagocytic activity (μg Ml−1) | 5.500 ± 0.692 b | 12.05 ± 0.490 a | 10.95 ± 0.317 a | 10.65 ± 0.721 a | 0.0002 |
| Phagocytic index | 1.040 ± 0.034 | 1.315 ± 0.164 | 1.200 ± 0.000 | 1.010 ± 0.063 | 0.131 |
| Lysozyme activity (μg mL−1) | 0.985 ± 0.002 b | 1.015 ± 0.002 a | 0.880 ± 0.005 c | 0.550 ± 0.005 d | <0.0001 |
| WBCs (×103/mm3) | 43.10 ± 1.042 | 46.71 ± 6.521 | 39.40 ± 2.930 | 35.95 ± 1.299 | 0.264 |
| Neutrophils (%) | 1.800 ± 0.057 a | 0.850 ± 0.086 b | 1.250 ± 0.202 b | 1.000 ± 0.000 b | 0.001 |
| Lymphocytes (%) | 91.65 ± 0.433 b | 93.55 ± 0.144 a | 92.95 ± 0.375 a,b | 92.70 ± 0.173 a,b | 0.014 |
| Monocytes (%) | 6.450 ± 0.317 | 5.600 ± 0.057 | 5.800 ± 0.173 | 6.300 ± 0.173 | 0.053 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diab, A.M.; Eldeghaidy, E.E.; Abo-Raya, M.H.; Shukry, M.; Abdeen, A.; Ibrahim, S.F.; Fericean, L.; Abdo, M.; Khalafalla, M.M. Assessment of Growth-Related Parameters, Immune-Biochemical Profile, and Expression of Selected Genes of Red Tilapia Fed with Roselle Calyces (Hibiscus sabdariffa) Extract. Fishes 2023, 8, 172. https://doi.org/10.3390/fishes8040172
Diab AM, Eldeghaidy EE, Abo-Raya MH, Shukry M, Abdeen A, Ibrahim SF, Fericean L, Abdo M, Khalafalla MM. Assessment of Growth-Related Parameters, Immune-Biochemical Profile, and Expression of Selected Genes of Red Tilapia Fed with Roselle Calyces (Hibiscus sabdariffa) Extract. Fishes. 2023; 8(4):172. https://doi.org/10.3390/fishes8040172
Chicago/Turabian StyleDiab, Amany M., Eslam E. Eldeghaidy, Mohamed H. Abo-Raya, Mustafa Shukry, Ahmed Abdeen, Samah F. Ibrahim, Liana Fericean, Mohamed Abdo, and Malik M. Khalafalla. 2023. "Assessment of Growth-Related Parameters, Immune-Biochemical Profile, and Expression of Selected Genes of Red Tilapia Fed with Roselle Calyces (Hibiscus sabdariffa) Extract" Fishes 8, no. 4: 172. https://doi.org/10.3390/fishes8040172
APA StyleDiab, A. M., Eldeghaidy, E. E., Abo-Raya, M. H., Shukry, M., Abdeen, A., Ibrahim, S. F., Fericean, L., Abdo, M., & Khalafalla, M. M. (2023). Assessment of Growth-Related Parameters, Immune-Biochemical Profile, and Expression of Selected Genes of Red Tilapia Fed with Roselle Calyces (Hibiscus sabdariffa) Extract. Fishes, 8(4), 172. https://doi.org/10.3390/fishes8040172








