Stress Response to Entrainment Flow Speed near Pump Inlet Fish Screens in Two Model Teleost Species, Anguilla anguilla and Oncorhynchus mykiss
Abstract
1. Introduction
2. Materials and Methods
2.1. Desktop Analysis of Fish Swimming Fatigue Curves
2.2. Experimental Setup
2.3. Sample Collection
2.4. Cortisol Quantification
2.5. Data Analyses
3. Results
3.1. Fish Morphometry
3.2. Physical Damage
3.3. Cortisol Quantification
3.4. Blood Serum Cortisol Levels
3.5. Skin Mucus Cortisol Levels
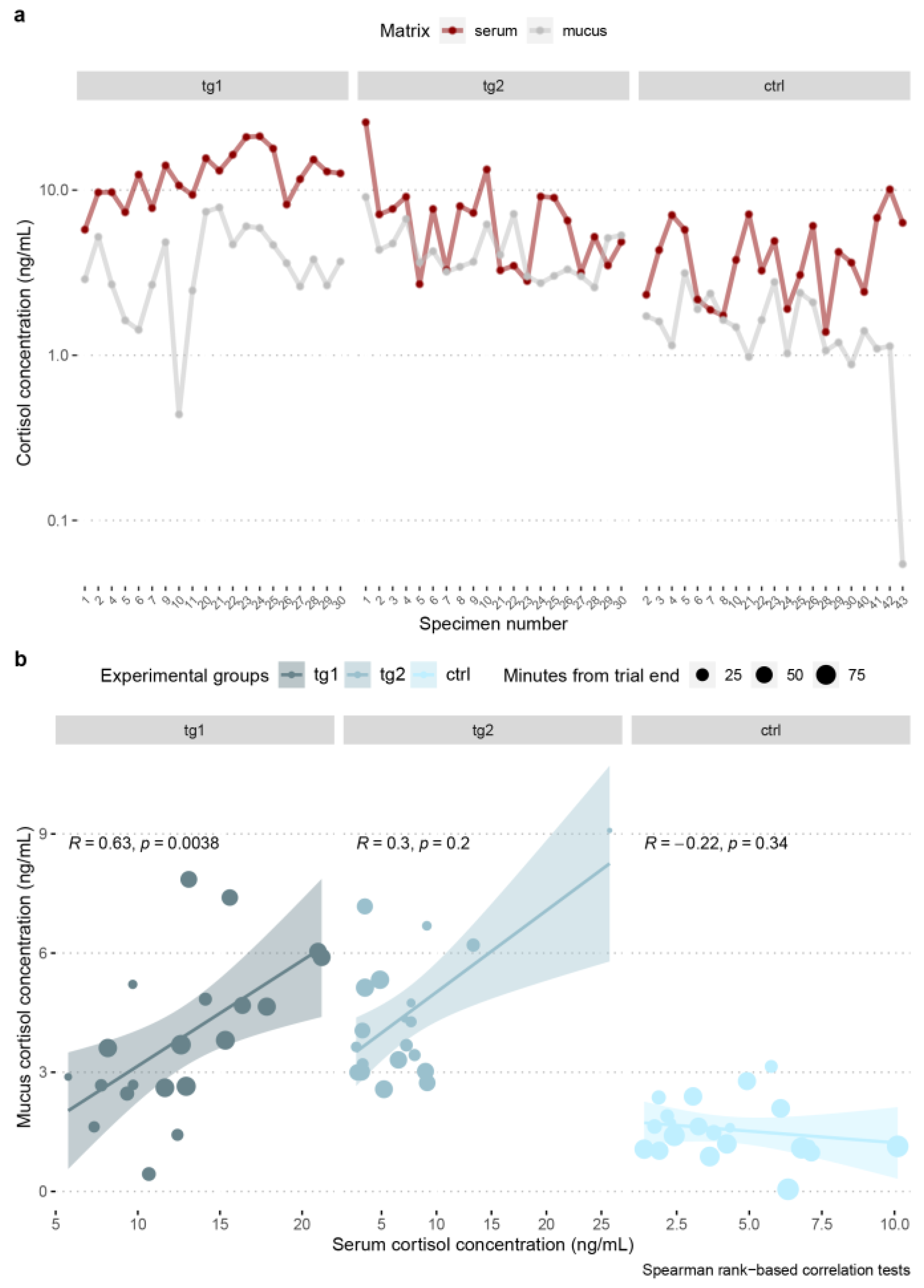
3.6. Blood Serum–Skin Mucus Cortisol Correlation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruiz, R.A.; De Vilder, L.H.; Prasasti, E.B.; Aouad, M.; De Luca, A.; Geisseler, B.; Terheiden, K.; Scanu, S.; Miccoli, A.; Roeber, V.; et al. Low-Head Pumped Hydro Storage: A Review on Civil Structure Designs, Legal and Environmental Aspects to Make Its Realization Feasible in Seawater. Renew. Sustain. Energy Rev. 2022, 160, 112281. [Google Scholar] [CrossRef]
- European Union Commission Delivering the European Green Deal. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal/delivering-european-green-deal_en (accessed on 28 January 2023).
- European Union Commission. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 Establishing a Framework for Community Action in the Field of Marine Environmental Policy (Marine Strategy Framework Directive. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008L0056 (accessed on 28 January 2023).
- Knott, J.; Mueller, M.; Pander, J.; Geist, J. Fish Passage and Injury Risk at a Surface Bypass of a Small-Scale Hydropower Plant. Sustainability 2019, 11, 6037. [Google Scholar] [CrossRef]
- Mueller, M.; Sternecker, K.; Milz, S.; Geist, J. Assessing Turbine Passage Effects on Internal Fish Injury and Delayed Mortality Using X-ray Imaging. PeerJ 2020, 8, ee9977. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Pander, J.; Geist, J. Evaluation of External Fish Injury Caused by Hydropower Plants Based on a Novel Field-Based Protocol. Fish. Manag. Ecol. 2017, 24, 240–255. [Google Scholar] [CrossRef]
- Piper, A.T.; Rosewarne, P.J.; Wright, R.M.; Kemp, P.S. The Impact of an Archimedes Screw Hydropower Turbine on Fish Migration in a Lowland River. Ecol. Eng. 2018, 118, 31–42. [Google Scholar] [CrossRef]
- Richmond, M.C.; Serkowski, J.A.; Ebner, L.L.; Sick, M.; Brown, R.S.; Carlson, T.J. Quantifying Barotrauma Risk to Juvenile Fish during Hydro-Turbine Passage. Fish. Res. 2014, 154, 152–164. [Google Scholar] [CrossRef]
- Saylor, R.; Sterling, D.; Bevelhimer, M.; Pracheil, B. Within and Among Fish Species Differences in Simulated Turbine Blade Strike Mortality: Limits on the Use of Surrogacy for Untested Species. Water 2020, 12, 701. [Google Scholar] [CrossRef]
- Pflugrath, B.D.; Mueller, R.P.; Engbrecht, K.; Colotelo, A.H. American Eel Resilience to Simulated Fluid Shear Associated with Passage through Hydroelectric Turbines. Knowl. Manag. Aquat. Ecosyst. 2021, 422, 20. [Google Scholar] [CrossRef]
- Saylor, R.; Fortner, A.; Bevelhimer, M. Quantifying Mortality and Injury Susceptibility for Two Morphologically Disparate Fishes Exposed to Simulated Turbine Blade Strike. Hydrobiologia 2019, 842, 55–75. [Google Scholar] [CrossRef]
- Romero-Gomez, P.; Richmond, M.C. Simulating Blade-Strike on Fish Passing through Marine Hydrokinetic Turbines. Renew. Energy 2014, 71, 401–413. [Google Scholar] [CrossRef]
- Calles, O.; Olsson, I.C.; Comoglio, C.; Kemp, P.S.; Blunden, L.; Schmitz, M.; Greenberg, L.A. Size-Dependent Mortality of Migratory Silver Eels at a Hydropower Plant, and Implications for Escapement to the Sea. Freshw. Biol. 2010, 55, 2167–2180. [Google Scholar] [CrossRef]
- Li, X.; Deng, Z.D.; Brown, R.S.; Fu, T.; Martinez, J.J.; McMichael, G.A.; Skalski, J.R.; Townsend, R.L.; Trumbo, B.A.; Ahmann, M.L.; et al. Migration Depth and Residence Time of Juvenile Salmonids in the Forebays of Hydropower Dams Prior to Passage through Turbines or Juvenile Bypass Systems: Implications for Turbine-Passage Survival. Conserv. Physiol. 2015, 3, cou064. [Google Scholar] [CrossRef] [PubMed]
- Jansen, H.M.; Winter, H.V.; Bruijs, M.C.M.; Polman, H.J.G. Just Go with the Flow? Route Selection and Mortality during Downstream Migration of Silver Eels in Relation to River Discharge. ICES J. Mar. Sci. 2007, 64, 1437–1443. [Google Scholar] [CrossRef]
- Barton, B.A. Stress in Fishes: A Diversity of Responses with Particular Reference to Changes in Circulating Corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Bevacqua, D.; Melià, P.; Gatto, M.; De Leo, G.A. A Global Viability Assessment of the European Eel. Glob. Chang. Biol. 2015, 21, 3323–3335. [Google Scholar] [CrossRef]
- Piper, A.T.; Wright, R.M.; Walker, A.M.; Kemp, P.S. Escapement, Route Choice, Barrier Passage and Entrainment of Seaward Migrating European Eel, Anguilla anguilla, within a Highly Regulated Lowland River. Ecol. Eng. 2013, 57, 88–96. [Google Scholar] [CrossRef]
- Flodmark, L.E.W.; Urke, H.A.; Halleraker, J.H.; Arnekleiv, J.V.; Vøllestad, L.A.; Poléo, A.B.S. Cortisol and Glucose Responses in Juvenile Brown Trout Subjected to a Fluctuating Flow Regime in an Artificial Stream. J. Fish Biol. 2002, 60, 238–248. [Google Scholar] [CrossRef]
- Di Rocco, R.; Gervais, R. SPOT: Swim Performance Online Tools. Available online: http://www.fishprotectiontools.ca/ (accessed on 28 January 2023).
- Katopodis, C.; Gervais, R. Fish Swimming Performance Database and Analyses; DFO Can. Sci. Advis. Sec. Res. Doc. 2016/002., 550. Available online: http://www.dfo-mpo.gc.ca/csas-sccs/Publications/ResDocs-DocRech/2016/2016_002-eng.html (accessed on 28 January 2023).
- Scruton, D.A.; Pennell, C.J.; Robertson, M.J.; Clarke, K.D.; Eddy, W.; McKinley, R.S. Telemetry Studies of the Passage Route and Entrainment of Downstream Migrating Wild Atlantic Salmon (Salmo salar) Smolts at Two Hydroelectric Installations on the Exploits River, Newfoundland, Canada. Aquat. Telem. Adv. Appl. Proc. Fifth Conf. Fish Telem. 2005, 91–101. [Google Scholar]
- Calles, O.; Elghagen, J.; Nyqvist, D.; Harbicht, A.; Nilsson, P.A. Efficient and Timely Downstream Passage Solutions for European Silver Eels at Hydropower Dams. Ecol. Eng. 2021, 170, 106350. [Google Scholar] [CrossRef]
- Sanz-Ronda, F.J.; Fuentes-Pérez, J.F.; García-Vega, A.; Bravo-Córdoba, F.J. Fishways as Downstream Routes in Small Hydropower Plants: Experiences with a Potamodromous Cyprinid. Water 2021, 13, 1041. [Google Scholar] [CrossRef]
- Peter, A.; Schoelzel, N.; Wilmsmeier, L.; Albayrak, I.; Bravo-Córdoba, F.J.; García-Vega, A.; Fuentes-Pérez, J.F.; Valbuena-Castro, J.; Carazo-Cea, O.; Escudero-Ortega, C.; et al. The Attractiveness of Fishways and Bypass Facilities. In Novel Developments for Sustainable Hydropower; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Olsen, Y.A.; Einarsdottir, I.E.; Nilssen, K.J. Metomidate Anaesthesia in Atlantic Salmon, Salmo salar, Prevents Plasma Cortisol Increase during Stress. Aquaculture 1995, 134, 155–168. [Google Scholar] [CrossRef]
- Priborsky, J.; Velisek, J. A Review of Three Commonly Used Fish Anesthetics. Rev. Fish. Sci. Aquac. 2018, 26, 417–442. [Google Scholar] [CrossRef]
- Javahery, S.; Nekoubin, H.; Moradlu, A.H. Effect of Anaesthesia with Clove Oil in Fish (Review). Fish Physiol. Biochem. 2012, 38, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, C.C.; Cardinaletti, G.; Sigelaki, I.; Polzonetti-Magni, A. Comparative Efficacy of Clove Oil and 2-Phenoxyethanol as Anesthetics in the Aquaculture of European Sea Bass (Dicentrarchus labrax) and Gilthead Sea Bream (Sparus aurata) at Different Temperatures. Aquaculture 2005, 246, 467–481. [Google Scholar] [CrossRef]
- Panel, A. Opinion of the Scientific Panel on Animal Health and Welfare (AHAW) on a Request from the Commission Related to the Aspects of the Biology and Welfare of Animals Used for Experimental and Other Scientific Purposes. EFSA J. 2005, 3, 292. [Google Scholar] [CrossRef]
- Anderson, W.G.; McKinley, R.S.; Colavecchia, M. The Use of Clove Oil as an Anesthetic for Rainbow Trout and Its Effects on Swimming Performance. North Am. J. Fish. Manag. 1997, 17, 301–307. [Google Scholar] [CrossRef]
- King, V.W.; Hooper, B.; Hillsgrove, S.; Benton, C.; Berlinsky, D.L. The Use of Clove Oil, Metomidate, Tricaine Methanesulphonate and 2-Phenoxyethanol for Inducing Anaesthesia and Their Effect on the Cortisol Stress Response in Black Sea Bass (Centropristis striata L.). Aquac. Res. 2005, 36, 1442–1449. [Google Scholar] [CrossRef]
- Iversen, M.H.; Økland, F.; Thorstad, E.B.; Finstad, B. The Efficacy of Aqui-S Vet. (Iso-Eugenol) and Metomidate as Anaesthetics in European Eel (Anguilla anguilla L.), and Their Effects on Animal Welfare and Primary and Secondary Stress Responses. Aquac. Res. 2013, 44, 1307–1316. [Google Scholar] [CrossRef]
- Renault, S.; Daverat, F.; Pierron, F.; Gonzalez, P.; Dufour, S.; Lanceleur, L.; Schäfer, J.; Baudrimont, M. The Use of Eugenol and Electro-Narcosis as Anaesthetics: Transcriptional Impacts on the European Eel (Anguilla anguilla L.). Ecotoxicol. Environ. Saf. 2011, 74, 1573–1577. [Google Scholar] [CrossRef]
- Wickham, H.; Bryan, J. Readxl: Read Excel Files, R Package Version 1.3.1. 2019. Available online: https://readxl.tidyverse.org/ (accessed on 28 January 2023).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Robinson, D.; Hayes, A.; Couch, S. Broom: Convert Statistical Objects into Tidy Tibbles, R Package Version 0.7.11. 2022. Available online: https://broom.tidymodels.org/ (accessed on 28 January 2023).
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests, R Package Version 0.7.0. 2021. Available online: https://cran.r-project.org/web/packages/rstatix/index.html (accessed on 28 January 2023).
- Kassambara, A. Ggpubr: “ggplot2” Based Publication Ready Plots, R Package Version 0.4.0. 2022. Available online: https://cran.r-project.org/web/packages/ggpubr/index.html (accessed on 28 January 2023).
- Wilke, C.O.; Wiernik, B.M. Ggtext: Improved Text Rendering Support for “Ggplot2”, R Package Version 0.1.2. 2022. Available online: https://cran.r-project.org/web/packages/ggtext/index.html (accessed on 28 January 2023).
- Grimaldo, L.F.; Sommer, T.; Van Ark, N.; Jones, G.; Holland, E.; Moyle, P.B.; Herbold, B.; Smith, P. Factors Affecting Fish Entrainment into Massive Water Diversions in a Tidal Freshwater Estuary: Can Fish Losses Be Managed? N. Am. J. Fish. Manag. 2009, 29, 1253–1270. [Google Scholar] [CrossRef]
- Boys, C.A.; Rayner, T.S.; Baumgartner, L.J.; Doyle, K.E. Native Fish Losses Due to Water Extraction in Australian Rivers: Evidence, Impacts and a Solution in Modern Fish- and Farm-friendly Screens. Ecol. Manag. Restor. 2021, 22, 134–144. [Google Scholar] [CrossRef]
- Unwin, M.J.; Webb, M.; Barker, R.J.; Link, W.A. Quantifying Production of Salmon Fry in an Unscreened Irrigation System: A Case Study on the Rangitata River, New Zealand. North Am. J. Fish. Manag. 2005, 25, 619–634. [Google Scholar] [CrossRef]
- Black, E.C. Hyperactivity as a Lethal Factor in Fish. J. Fish. Res. Board Can. 1958, 15, 573–586. [Google Scholar] [CrossRef]
- Ben Ammar, I.; Cornet, V.; Houndji, A.; Baekelandt, S.; Antipine, S.; Sonny, D.; Mandiki, S.N.M.; Kestemont, P. Impact of Downstream Passage through Hydropower Plants on the Physiological and Health Status of a Critically Endangered Species: The European Eel Anguilla anguilla. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 254, 110876. [Google Scholar] [CrossRef]
- Pracheil, B.M.; DeRolph, C.R.; Schramm, M.P.; Bevelhimer, M.S. A Fish-Eye View of Riverine Hydropower Systems: The Current Understanding of the Biological Response to Turbine Passage. Rev. Fish Biol. Fish. 2016, 26, 153–167. [Google Scholar] [CrossRef]
- Bevelhimer, M.S.; Pracheil, B.M.; Fortner, A.M.; Saylor, R.; Deck, K.L. Mortality and Injury Assessment for Three Species of Fish Exposed to Simulated Turbine Blade Strike. Can. J. Fish. Aquat. Sci. 2019, 76, 2350–2363. [Google Scholar] [CrossRef]
- Van Ginneken, V.J.T.; Balm, P.; Sommandas, V.; Onderwater, M.; Van Den Thillart, G. Acute Stress Syndrome of the Yellow European Eel (Anguilla anguilla Linnaeus) When Exposed to a Graded Swimming-Load. Neth. J. Zool. 2002, 52, 29–42. [Google Scholar] [CrossRef]
- Pickering, A.D.; Pottinger, T.G. Stress Responses and Disease Resistance in Salmonid Fish: Effects of Chronic Elevation of Plasma Cortisol. Fish Physiol. Biochem. 1989, 7, 253–258. [Google Scholar] [CrossRef]
- Barton, B.A. Salmonid Fishes Differ in Their Cortisol and Glucose Responses to Handling and Transport Stress. N. Am. J. Aquac. 2000, 62, 12–18. [Google Scholar] [CrossRef]
- Samaras, A.; Dimitroglou, A.; Sarropoulou, E.; Papaharisis, L.; Kottaras, L.; Pavlidis, M. Repeatability of Cortisol Stress Response in the European Sea Bass (Dicentrarchus labrax) and Transcription Differences between Individuals with Divergent Responses. Sci. Rep. 2016, 6, 34858. [Google Scholar] [CrossRef] [PubMed]
- Schreck, C.B.; Tort, L. The Concept of Stress in Fish; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 35, ISBN 9780128027288. [Google Scholar]
- Bertotto, D.; Poltronieri, C.; Negrato, E.; Majolini, D.; Radaelli, G.; Simontacchi, C. Alternative Matrices for Cortisol Measurement in Fish. Aquac. Res. 2010, 41, 1261–1267. [Google Scholar] [CrossRef]
- King, G.D.; Chapman, J.M.; Cooke, S.J.; Suski, C.D. Stress in the Neighborhood: Tissue Glucocorticoids Relative to Stream Quality for Five Species of Fish. Sci. Total Environ. 2016, 547, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Inoue, K.; Takei, Y. Steroidogenic Acute Regulatory Protein in Eels: CDNA Cloning and Effects of ACTH and Seawater Transfer on Its mRNA Expression. Zool. Sci. 2003, 20, 211–219. [Google Scholar] [CrossRef]
- Donaldson, E.M. The Pituitary-Interrenal Axis as an Indicator of Stress in Fish. In Stress and Fish; Pickering, A.D., Ed.; Academic Press: London, UK, 1981; pp. 11–47. [Google Scholar]
- Bernier, N.J.; Flik, G.; Klaren, P.H.M. Regulation and Contribution of Corticotropic, Melanotropic and Thyrotropic Axes to The Stress Response in Fishes. In Fish Physiology; Bernier, N.J., Van Der Kraak, G., Farrell, A., Brauner, C.J., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2009; ISBN 978-0-12-374631-3. [Google Scholar]
- Wendelaar Bonga, S.E. The Stress Response in Fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef]
- Mommsen, T.P.; Vijayan, M.M.; Moon, T.W. Cortisol in Teleosts: Dynamics, Mechanisms of Action, and Metabolic Regulation. Rev. Fish Biol. Fish. 1999, 9, 211–268. [Google Scholar] [CrossRef]
- McCormick, S.D. Endocrine Control of Osmoregulation in Teleost Fish. Am. Zool. 2001, 41, 781–794. [Google Scholar] [CrossRef]
- Bernier, N.J. The Corticotropin-Releasing Factor System as a Mediator of the Appetite-Suppressing Effects of Stress in Fish. Gen. Comp. Endocrinol. 2006, 146, 45–55. [Google Scholar] [CrossRef]
- Chang, J.P.; Wong, A.O.L. Growth Hormone Regulation in Fish. In Fish Physiology; Bernier, N.J., Van Der Kraak, G., Farrell, A., Brauner, C.J., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Harris, J.; Bird, D.J. Modulation of the Fish Immune System by Hormones. Vet. Immunol. Immunopathol. 2000, 77, 163–176. [Google Scholar] [CrossRef]
- Vizzini, A.; Vazzana, M.; Cammarata, M.; Parrinello, N. Peritoneal Cavity Phagocytes from the Teleost Sea Bass Express a Glucocorticoid Receptor (Cloned and Sequenced) Involved in Genomic Modulation of the in vitro Chemiluminescence Response to Zymosan. Gen. Comp. Endocrinol. 2007, 150, 114–123. [Google Scholar] [CrossRef]
- Sen Huang, Y.; Rousseau, K.; Sbaihi, M.; Le Belle, N.; Schmitz, M.; Dufour, S. Cortisol Selectively Stimulates Pituitary Gonadotropin β-Subunit in a Primitive Teleost, Anguilla Anguilla. Endocrinology 1999, 140, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Uren Webster, T.M.; Rodriguez-Barreto, D.; Martin, S.A.M.; Van Oosterhout, C.; Orozco-terWengel, P.; Cable, J.; Hamilton, A.; Garcia De Leaniz, C.; Consuegra, S. Contrasting Effects of Acute and Chronic Stress on the Transcriptome, Epigenome, and Immune Response of Atlantic Salmon. Epigenetics 2018, 13, 1191–1207. [Google Scholar] [CrossRef] [PubMed]
- Uren Webster, T.M.; Rodriguez-Barreto, D.; Consuegra, S.; Garcia de Leaniz, C. Cortisol-Related Signatures of Stress in the Fish Microbiome. Front. Microbiol. 2020, 11, 1621. [Google Scholar] [CrossRef] [PubMed]
- Sadoul, B.; Geffroy, B. Measuring Cortisol, the Major Stress Hormone in Fishes. J. Fish Biol. 2019, 94, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Madaro, A.; Nilsson, J.; Whatmore, P.; Roh, H.J.; Grove, S.; Stien, L.H.; Olsen, R.E. Acute Stress Response on Atlantic Salmon: A Time-Course Study of the Effects on Plasma Metabolites, Mucus Cortisol Levels, and Head Kidney Transcriptome Profile. Fish Physiol. Biochem. 2022, 49, 97–116. [Google Scholar] [CrossRef]
- Samaras, A.; Pavlidis, M. Fish Scales Produce Cortisol upon Stimulation with ACTH. Animals 2022, 12, 3510. [Google Scholar] [CrossRef] [PubMed]
- Vercauteren, M.; Ampe, B.; Devriese, L.; Moons, C.P.H.; Decostere, A.; Aerts, J.; Chiers, K. Explorative Study on Scale Cortisol Accumulation in Wild Caught Common Dab (Limanda limanda). BMC Vet. Res. 2022, 18, 324. [Google Scholar] [CrossRef]
- Ellis, T.; Yildiz, H.Y.; López-Olmeda, J.; Spedicato, M.T.; Tort, L.; Øverli, Ø.; Martins, C.I.M. Cortisol and Finfish Welfare. Fish Physiol. Biochem. 2012, 38, 163–188. [Google Scholar] [CrossRef]
- Madaro, A.; Olsen, R.E.; Kristiansen, T.S.; Ebbesson, L.O.E.; Nilsen, T.O.; Flik, G.; Gorissen, M. Stress in Atlantic Salmon: Response to Unpredictable Chronic Stress. J. Exp. Biol. 2015, 218, 2538–2550. [Google Scholar] [CrossRef]
- Laberge, F.; Yin-Liao, I.; Bernier, N.J. Temporal Profiles of Cortisol Accumulation and Clearance Support Scale Cortisol Content as an Indicator of Chronic Stress in Fish. Conserv. Physiol. 2019, 7, coz052. [Google Scholar] [CrossRef]
- Pfalzgraff, T.; Lund, I.; Skov, P.V. Prolonged Cortisol Elevation Alters Whole Body and Tissue Metabolism in Rainbow Trout (Oncorhynchus mykiss). Comp. Biochem. Physiol.-Part A Mol. Integr. Physiol. 2022, 263, 111098. [Google Scholar] [CrossRef] [PubMed]
- Madison, B.N.; Tavakoli, S.; Kramer, S.; Bernier, N.J. Chronic Cortisol and the Regulation of Food Intake and the Endocrine Growth Axis in Rainbow Trout. J. Endocrinol. 2015, 226, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, B.S.; Spear, A.R.; Philip, A.M.; Leaman, D.W.; Stepien, C.A.; Sepulveda-Villet, O.J.; Palmquist, D.E.; Vijayan, M.M. Effects of Cortisol and Lipopolysaccharide on Expression of Select Growth-, Stress- and Immune-Related Genes in Rainbow Trout Liver. Fish Shellfish Immunol. 2018, 74, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, N.W. The Endocrinology of Stress in Fish: An Environmental Perspective. Gen. Comp. Endocrinol. 2011, 170, 265–275. [Google Scholar] [CrossRef]
- Cutler, C.P.; Cramb, G. Branchial Expression of an Aquaporin 3 (AQP-3) Homologue Is Downregulated in the European Eel Anguilla anguilla Following Seawater Acclimation. J. Exp. Biol. 2002, 205, 2643–2651. [Google Scholar] [CrossRef]
- Cao, Q.; Gu, J.; Wang, D.; Liang, F.; Zhang, H.; Li, X.; Yin, S. Physiological Mechanism of Osmoregulatory Adaptation in Anguillid Eels. Fish Physiol. Biochem. 2018, 44, 423–433. [Google Scholar] [CrossRef]
- Cutler, C.P.; Phillips, C.; Hazon, N.; Cramb, G. Cortisol Regulates Eel (Anguilla anguilla) Aquaporin 3 (AQP3) mRNA Expression Levels in Gill. Gen. Comp. Endocrinol. 2007, 152, 310–313. [Google Scholar] [CrossRef]
- Carbajal, A.; Reyes-López, F.E.; Tallo-Parra, O.; Lopez-Bejar, M.; Tort, L. Comparative Assessment of Cortisol in Plasma, Skin Mucus and Scales as a Measure of the Hypothalamic-Pituitary-Interrenal Axis Activity in Fish. Aquaculture 2019, 506, 410–416. [Google Scholar] [CrossRef]
- Guardiola, F.A.; Cuesta, A.; Esteban, M.Á. Using Skin Mucus to Evaluate Stress in Gilthead Seabream (Sparus aurata L.). Fish Shellfish Immunol. 2016, 59, 323–330. [Google Scholar] [CrossRef]
- De Mercado, E.; Larrán, A.M.; Pinedo, J.; Tomás-Almenar, C. Skin Mucous: A New Approach to Assess Stress in Rainbow Trout. Aquaculture 2018, 484, 90–97. [Google Scholar] [CrossRef]
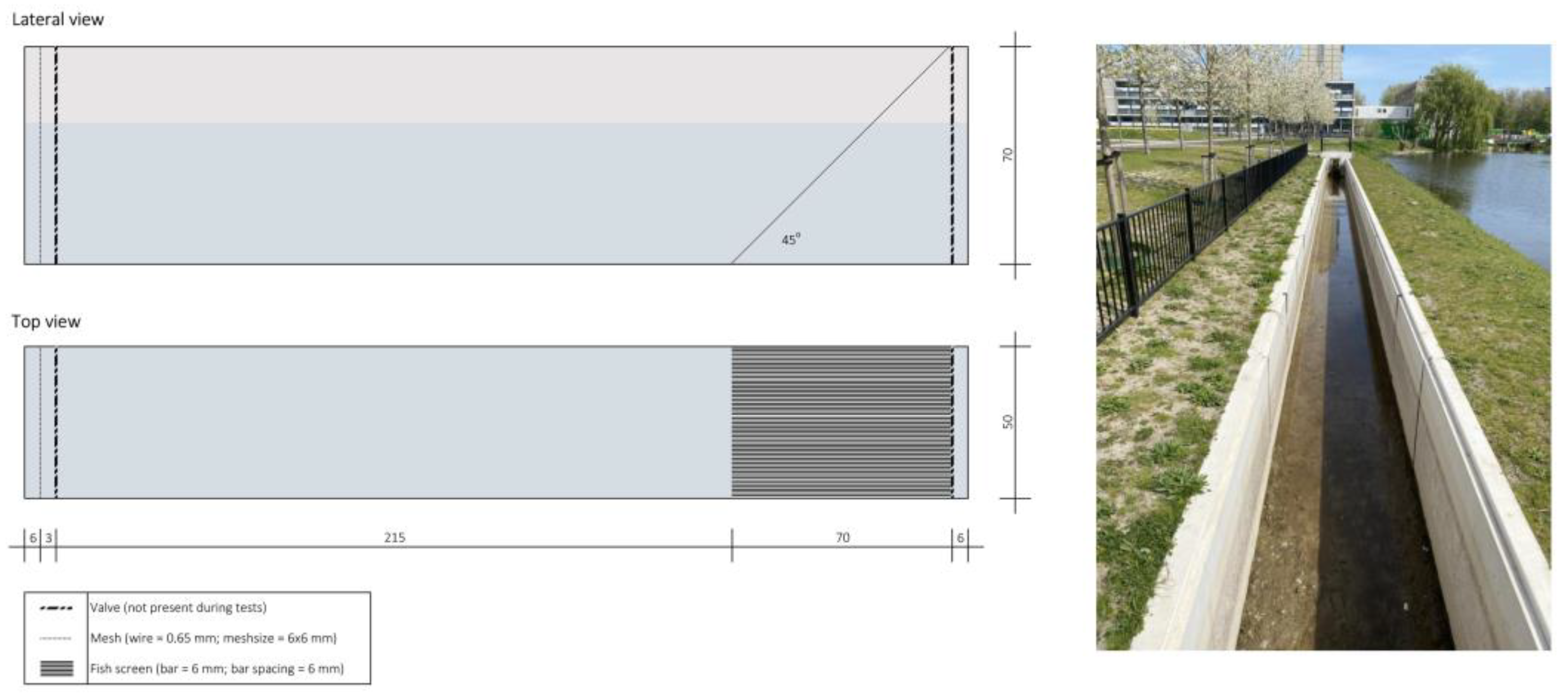
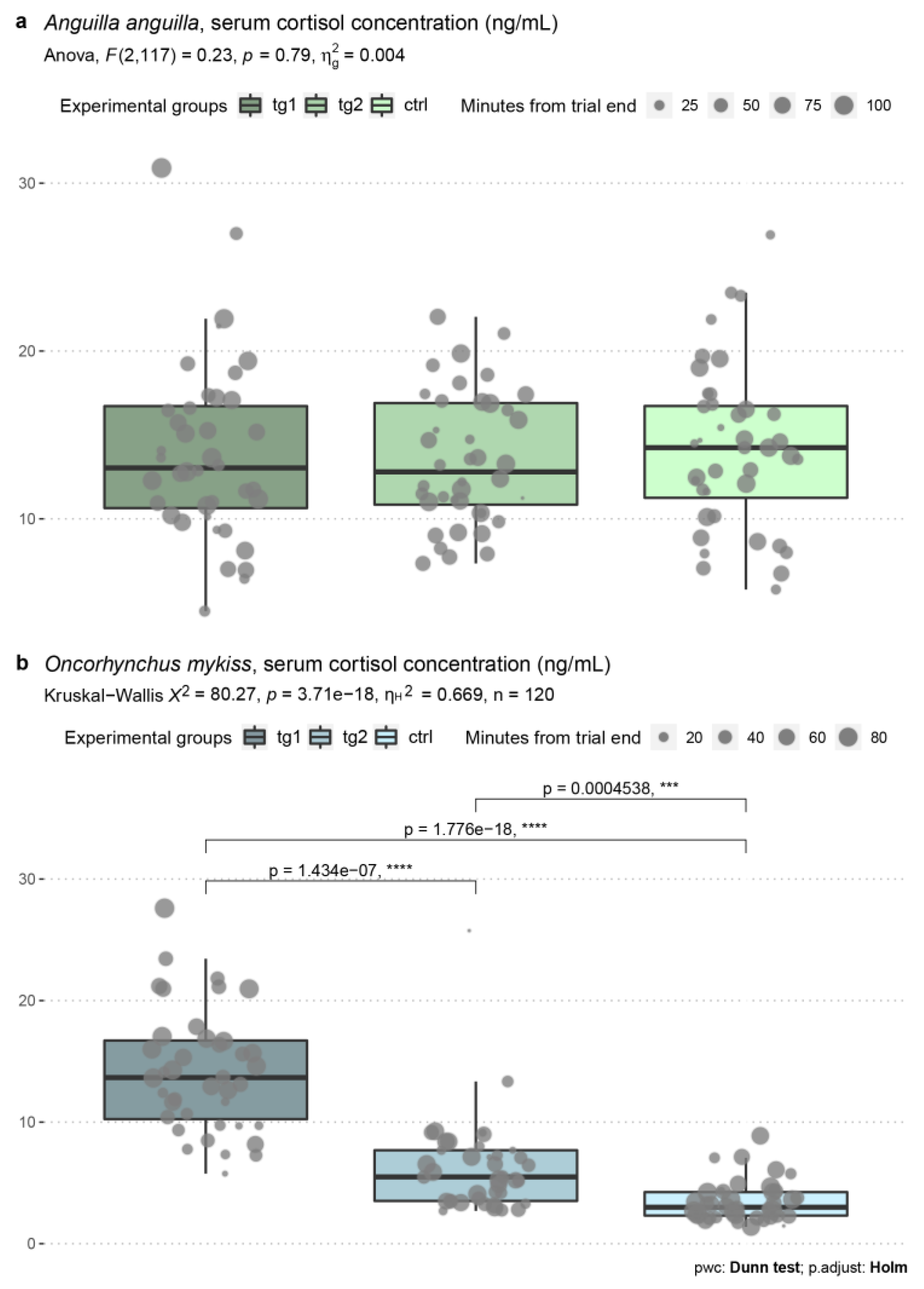
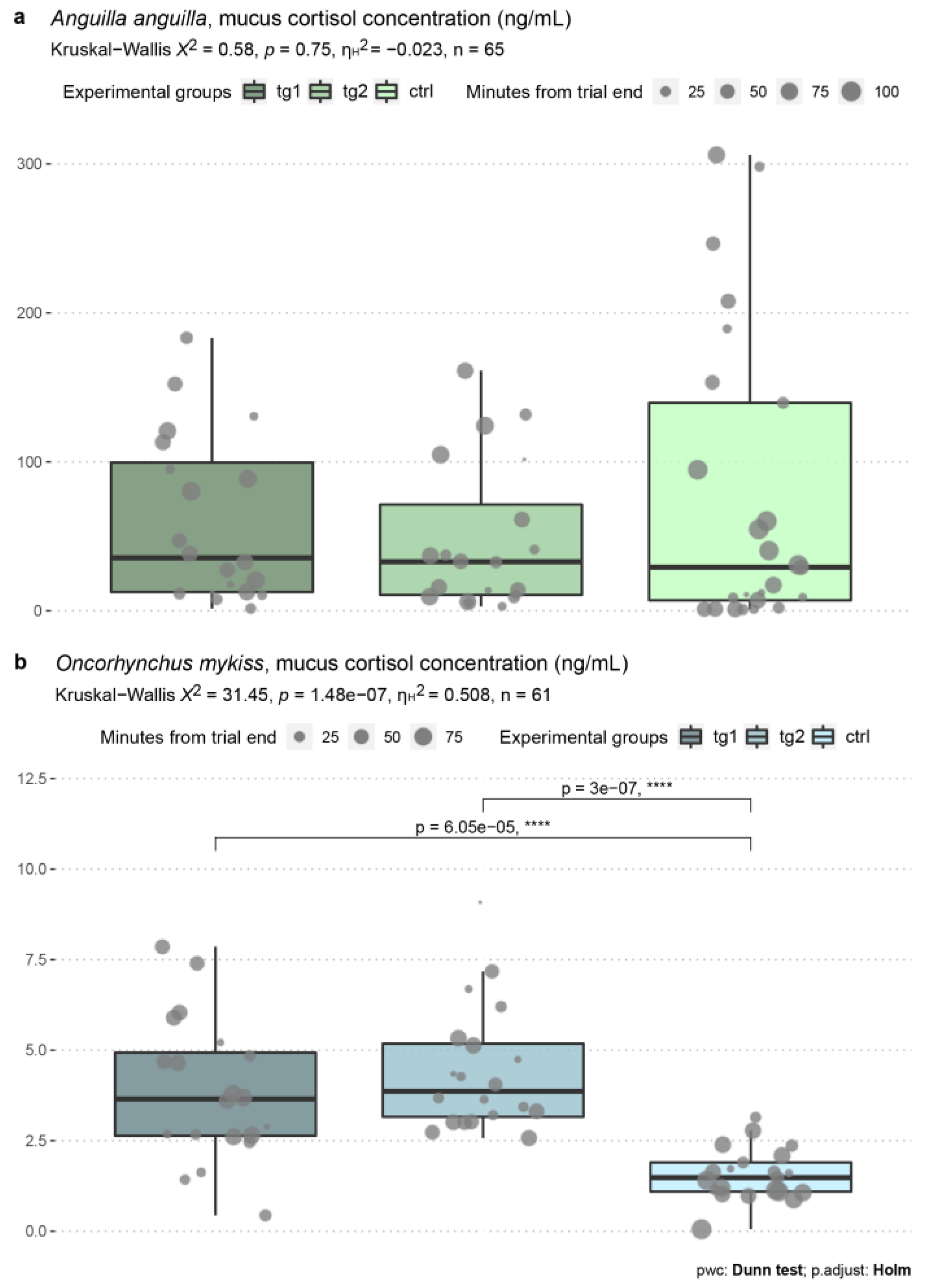

| Test n. | Date | Species | Test Group | Test Regime (m s−1) | Average Test Regime (m s−1) | Start Time | End Time | Water Temp. (°C) | Dissolved O2 (mg mL−1) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 19/10/2022 | A. anguilla | 1 | 0.25 | 0.27 | 09:00 | 09:30 | 13.5 | 10.7 |
| 2 | 19/10/2022 | A. anguilla | 2 | 0.15 | 0.16 | 11:45 | 12:15 | 13.8 | 10.6 |
| 3 | 19/10/2022 | A. anguilla | ctrl | 0 | 0 | 14:30 | 15:00 | 14.1 | 10.8 |
| 4 | 20/10/2022 | O. mykiss | 1 | 0.4 | 0.40 | 09:03 | 09:33 | 12.7 | 10.4 |
| 5 | 20/10/2022 | O. mykiss | 2 | 0.2 | 0.19 | 11:40 | 12:10 | 13.1 | 10.4 |
| 6 | 20/10/2022 | O. mykiss | ctrl | 0 | 0 | 14:02 | 14:32 | 13.3 | 10.2 |
| Date | Species | Assay | Matrix | Model | AICC | r | SE | α | θ | η | κ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 05/11/2022 | O. mykiss | tg1 | serum | DR-Hill | −49.999327 | 0.99997959521394 | 0.029352300693504 | −4.73 × 10−2 | 1.25 × 101 | −3.10 × 100 | 2.21 × 10−1 |
| 07/11/2022 | O. mykiss | tg2 | serum | DR-Hill | −52.919306 | 0.999971475135835 | 0.0310407373702673 | 0 | 1.02 × 101 | −4.55 × 100 | 2.66 × 10−1 |
| 08/11/2022 | O. mykiss | ctrl | serum | DR-Hill | −48.649550 | 0.999951357104034 | 0.0405347747446875 | 0 | 1.16 × 101 | −4.68 × 100 | 2.36 × 10−1 |
| 14/11/2022 | A. anguilla | tg1 | serum | DR-Hill | −67.501116 | 0.999997711179028 | 0.00983069005901328 | −8.19 × 10−2 | 1.84 × 101 | −2.58 × 100 | 1.29 × 10−1 |
| 15/11/2022 | A. anguilla | tg2 | serum | DR-Hill | −40.225637 | 0.99993076509465 | 0.0540671371859781 | −4.87 × 10−2 | 1.91 × 102 | −2.26 × 100 | 4.82 × 10−2 |
| 21/11/2022 | A. anguilla | ctrl | serum | DR-Hill | −56.574998 | 0.999991030724768 | 0.0194605917542529 | −1.11 × 10−1 | 1.83 × 101 | −2.38 × 100 | 1.29 × 10−1 |
| 22/11/2022 | A. anguilla | tg1–tg2–ctrl (10 samples each) | serum | DR-Hill | −57.422141 | 0.99999193195216 | 0.0184570248435207 | −6.65 × 10−2 | 1.61 × 101 | −2.96 × 100 | 2.24 × 10−1 |
| 24/11/2022 | O. mykiss | tg1–tg2–ctrl (10 samples each) | serum | DR-Hill | −43.465033 | 0.999907000646847 | 0.0560471069924146 | 0 | 2.24 × 101 | −2.73 × 100 | 1.02 × 10−1 |
| 05/12/2022 | A. anguilla | tg1–tg2–ctrl | mucus | DR-Hill | −59.640103 | 0.999993885485762 | 0.016067882856762 | −3.07 × 10−2 | 1.21 × 101 | −3.73 × 100 | 1.79 × 10−1 |
| 12/12/2022 | O. mykiss | tg1–tg2–ctrl | mucus | DR-Hill | −60.478106 | 0.999994493579905 | 0.0152479834979885 | −1.05 × 10−1 | 1.60 × 101 | −2.86 × 100 | 1.66 × 10−1 |
| Species | Group | r | Statistic | p |
|---|---|---|---|---|
| A. anguilla | tg1 | 0.16 | 1116 | 0.496 |
| A. anguilla | tg2 | −0.14 | 1522 | 0.542 |
| A. anguilla | ctrl | −0.13 | 2934 | 0.539 |
| O. mykiss | tg1 | 0.63 | 496 | 3.79 × 10−3 |
| O. mykiss | tg2 | 0.3 | 928 | 0.195 |
| O. mykiss | ctrl | −0.22 | 1879 | 0.337 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miccoli, A.; De Luca, A.; Bricker, J.; Vriese, F.T.; Moll, R.; Scapigliati, G. Stress Response to Entrainment Flow Speed near Pump Inlet Fish Screens in Two Model Teleost Species, Anguilla anguilla and Oncorhynchus mykiss. Fishes 2023, 8, 139. https://doi.org/10.3390/fishes8030139
Miccoli A, De Luca A, Bricker J, Vriese FT, Moll R, Scapigliati G. Stress Response to Entrainment Flow Speed near Pump Inlet Fish Screens in Two Model Teleost Species, Anguilla anguilla and Oncorhynchus mykiss. Fishes. 2023; 8(3):139. https://doi.org/10.3390/fishes8030139
Chicago/Turabian StyleMiccoli, Andrea, Antonio De Luca, Jeremy Bricker, Frederik Tijmen Vriese, Roelof Moll, and Giuseppe Scapigliati. 2023. "Stress Response to Entrainment Flow Speed near Pump Inlet Fish Screens in Two Model Teleost Species, Anguilla anguilla and Oncorhynchus mykiss" Fishes 8, no. 3: 139. https://doi.org/10.3390/fishes8030139
APA StyleMiccoli, A., De Luca, A., Bricker, J., Vriese, F. T., Moll, R., & Scapigliati, G. (2023). Stress Response to Entrainment Flow Speed near Pump Inlet Fish Screens in Two Model Teleost Species, Anguilla anguilla and Oncorhynchus mykiss. Fishes, 8(3), 139. https://doi.org/10.3390/fishes8030139






