Cinnamaldehyde Decreases the Pathogenesis of Aeromonas hydrophila by Inhibiting Quorum Sensing and Biofilm Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Reagents
2.2. Determination of Minimum Inhibitory Concentrations (MICs)
2.3. Growth Curves Assay
2.4. Hemolysis
2.5. Immuno-Blot
2.6. Lipase Assay
2.7. Protease Activity Assay
2.8. Swarming Motility Assay
2.9. Biofilm Formation
2.10. qPCR Assay
2.11. AHLs Production Assay
2.12. Cell Viability Assays
2.13. Animal Study
2.14. Statistical Analysis
3. Results
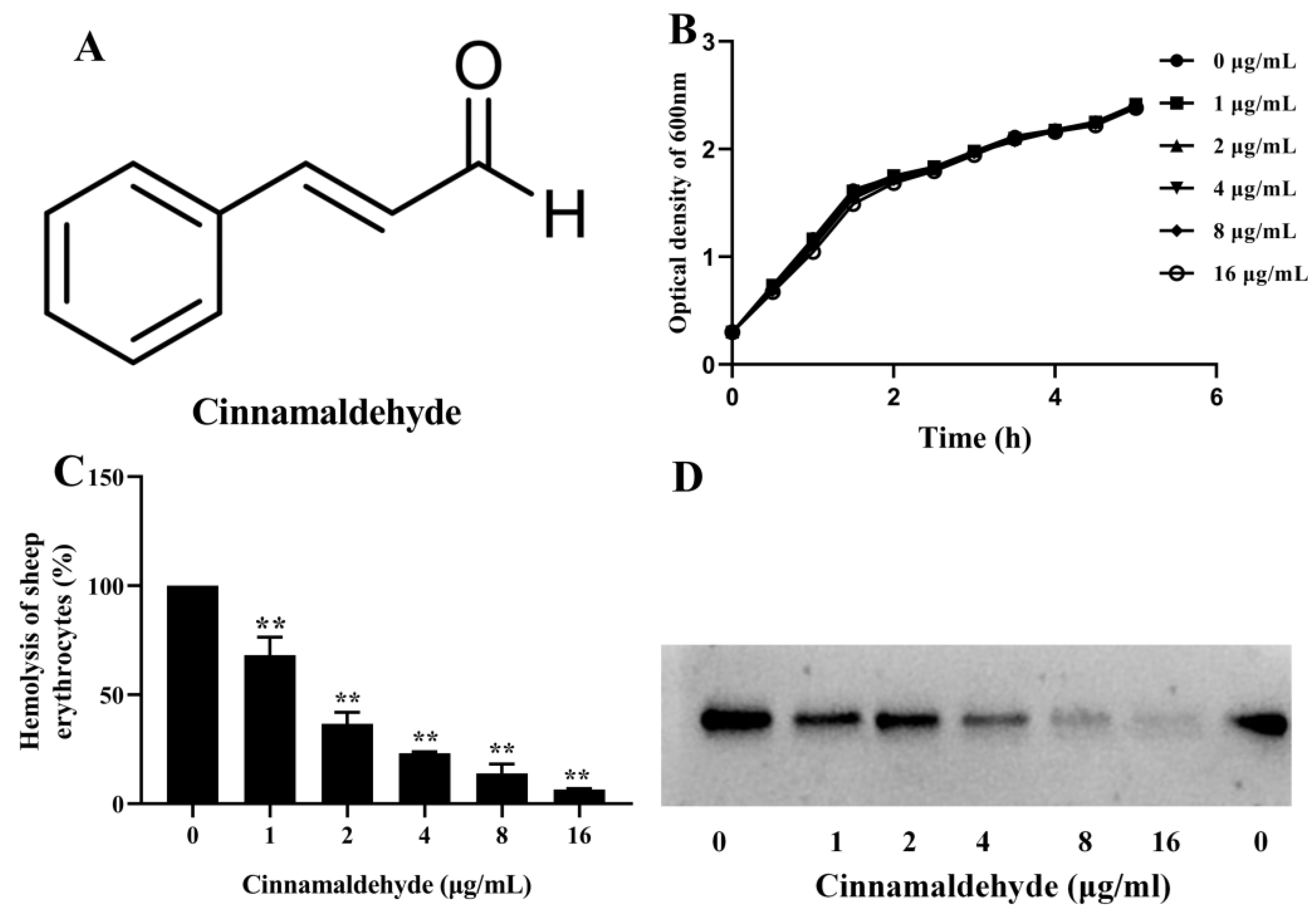
3.1. Effect of Cinnamaldehyde on A. hydrophila Growth
3.2. Cinnamaldehyde Inhibited the Hemolysis of A. hydrophila
3.3. Inhibitory Effect on Lipase Production
3.4. Inhibitory Effect on Protease Activity
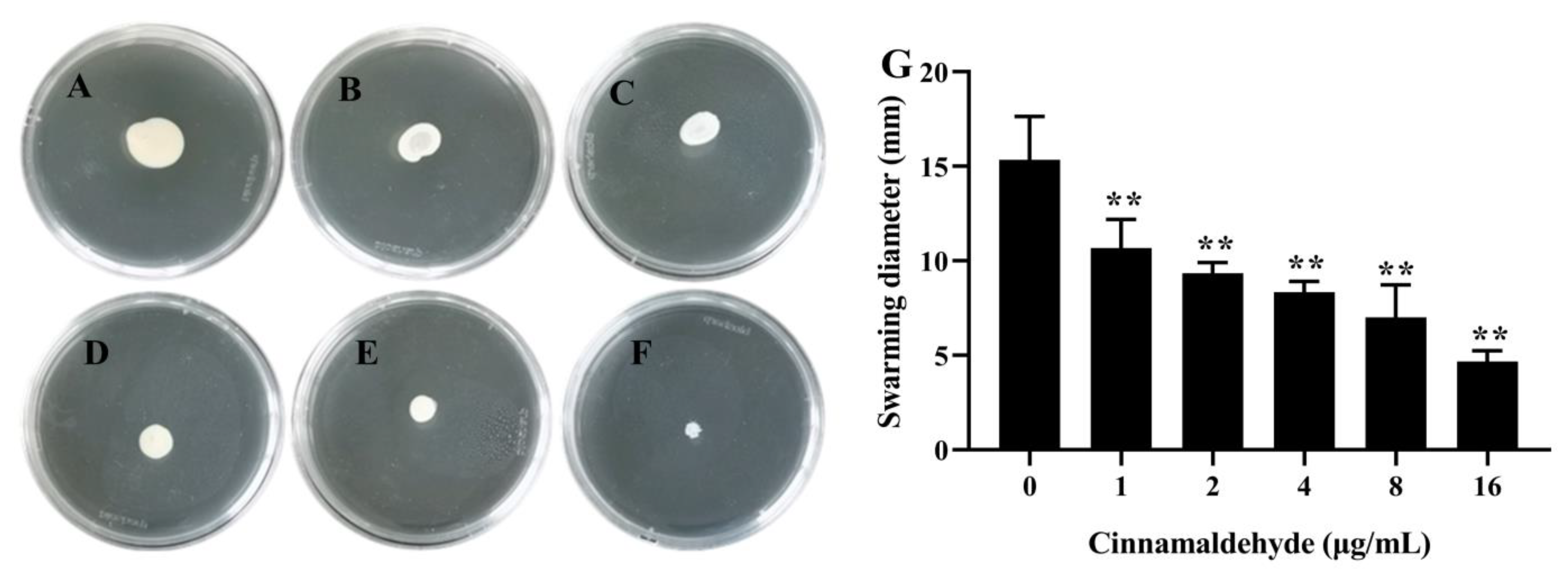
3.5. Inhibitory Effect of Cinnamaldehyde on Swarming Motility
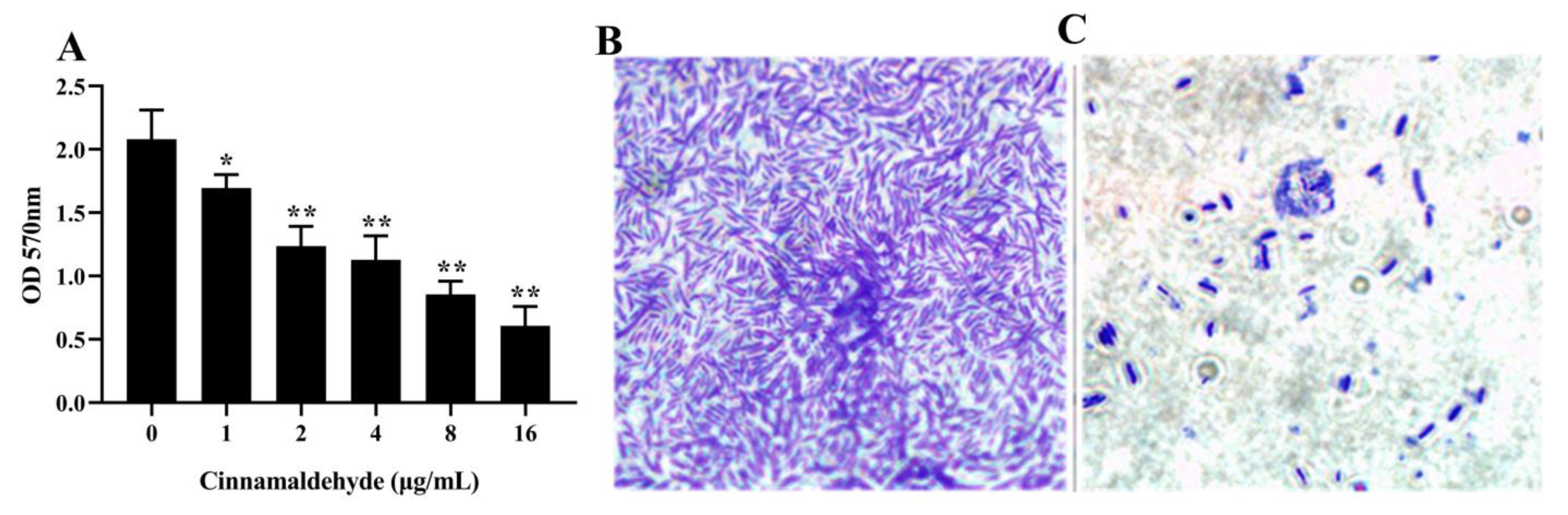
3.6. Inhibition of Biofilm Formation
3.7. Cinnamaldehyde Reduced the Transcription of Related Genes
3.8. Cinnamaldehyde Reduced AHLs Production
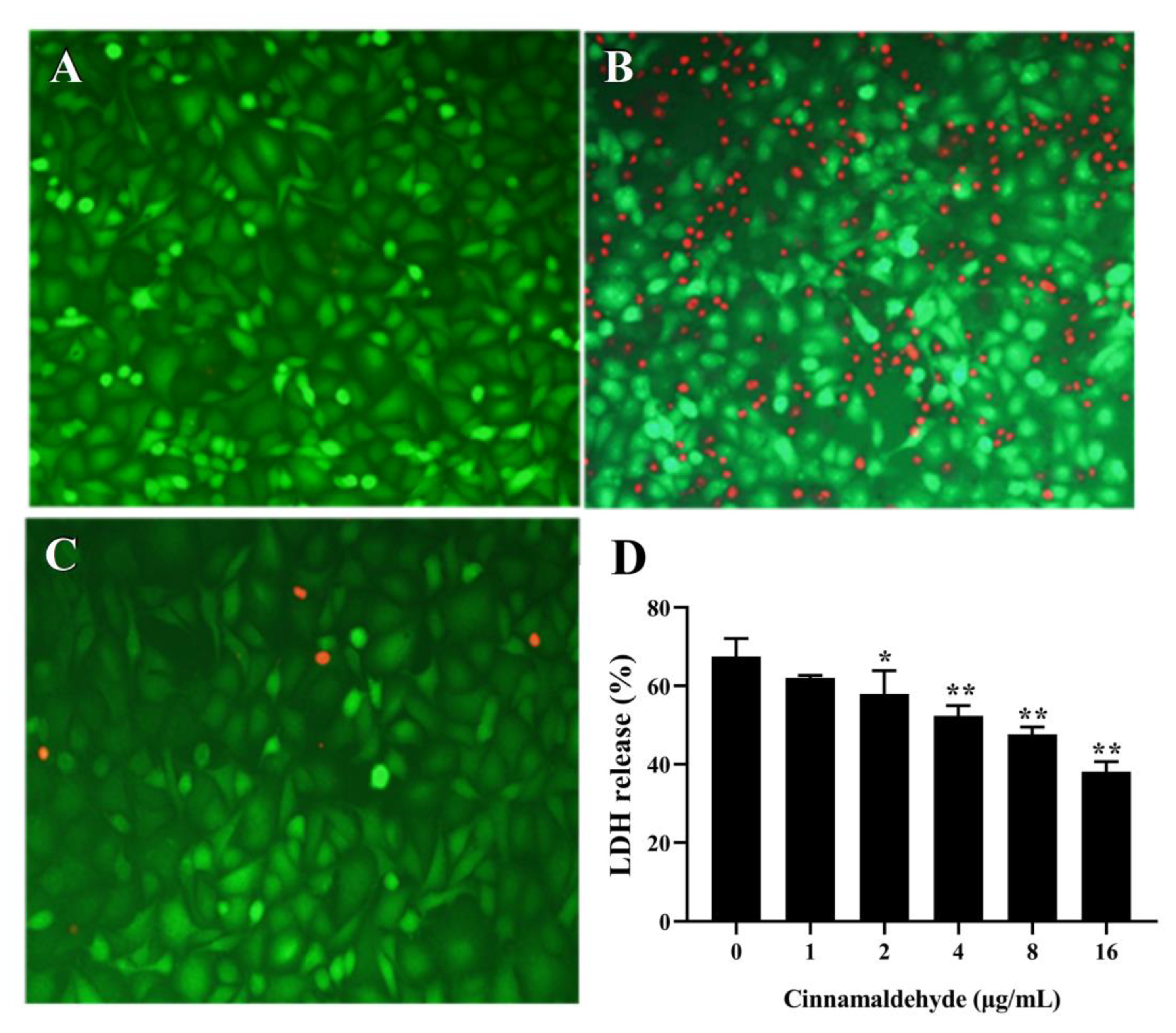
3.9. Cell Viability Results
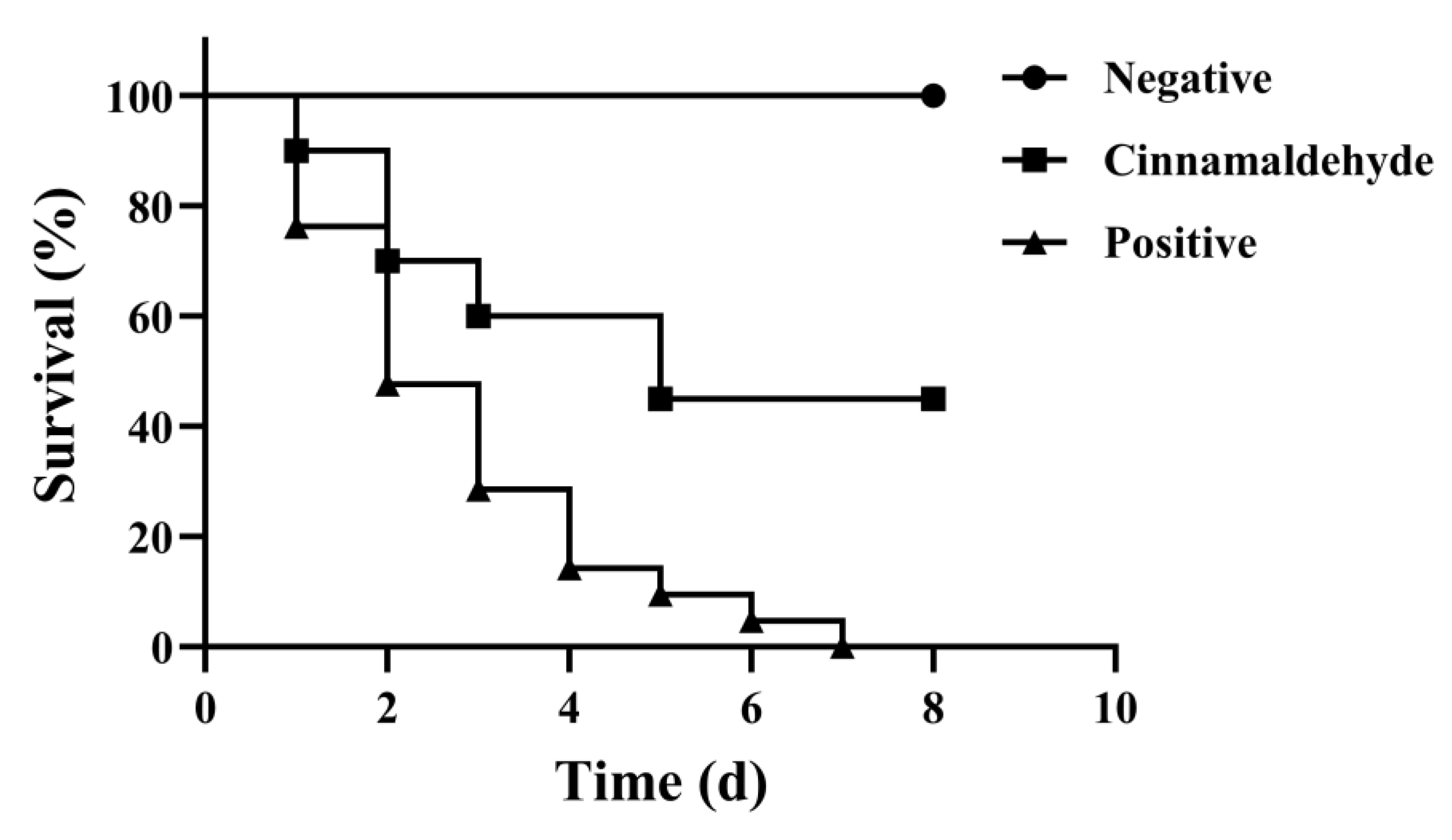
3.10. Protective Effect of Cinnamaldehyde on Crucian Carp Infected by A. hydrophila
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Patwary, Z.P.; Ali, M.M.; Faruk, M.A.R. Evaluation of the status of use of chemicals and antibiotics in freshwater aquaculture activities with special emphasis to fish health management. J. Bangladesh Agric. Univ. 1970, 6, 381–390. [Google Scholar] [CrossRef]
- Smith, P. Antibiotics in aquaculture: Reducing their use and maintaining their efficacy. In Infectious Disease in Aquaculture; Woodhead Publishing: Cambridge, UK, 2012; pp. 161–189. [Google Scholar] [CrossRef]
- Saleh, A.A. Bacterial quorum sensing and biofilm formation. Bangladesh J. Med. Microbiol. 2014, 8, 1. [Google Scholar] [CrossRef]
- Zmyslowska, I.; Korzekwa, K.; Szarek, J. Aeromonas Hydrophila in Fish Aquaculture. J. Comp. Pathol. 2009, 141, 313. [Google Scholar] [CrossRef]
- Radosavljevi, V.; Irkovi, M.; Ljubojevi, D.; Novakov, N.; Milievi, V. Detection of Aerolysin (aerA) Gene in Aeromonas hydrophila Strains Isolated from Diseased Carp. Arch. Vet. Med. 2014, 8, 1. [Google Scholar] [CrossRef]
- Mamun, M.; Nasren, S.; Abhiman, P.B.; Rathore, S.S.; Shankar, K.M. Investigation of production, formation and characterization of biofilm cells of Aeromonas hydrophila for oral vaccination of fish. J. Exp. Zool. India 2019, 22, 1115–1123. [Google Scholar]
- Chu, W. Role of the quorum-sensing system in biofilm formation and virulence of Aeromonas hydrophila. Afr. J. Microbiol. Res. 2011, 5, 5819–5825. [Google Scholar] [CrossRef]
- Haripriya, D.; Nadhiya, K.; Vijayalakshmi, K. Antioxidant Potential of Cinnamaldehyde: An In vitro study. Int. J. Pharm. Res. Bio-Sci. 2013, 2, 270–278. [Google Scholar]
- Sagar, S.; Rani, S.; Sanusree, P.S.; Sru, K.V. Exploration of Targeting Mechanisms of Cinnamon Essential Oil against Water Borne Multiple Drug Resistant Isolate Aeromonas hydrophila C4. Res. Sq. 2021, 1–19. [Google Scholar] [CrossRef]
- Dong, J.; Ding, H.; Liu, Y.; Yang, Q.; Xu, N.; Yang, Y.; Ai, X. Magnolol protects channel catfish from Aeromonas hydrophila infection via inhibiting the expression of aerolysin. Vet. Microbiol. 2017, 211, 119–123. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, L.; Liu, Y.; Xu, N.; Ai, X. Luteolin decreases the pathogenicity of Aeromonas hydrophila via inhibiting the activity of aerolysin. Virulence 2021, 12, 165–176. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.; Kannan, R.D.; Sivasubramanian, S.; Arunachalam, K.; Lin, X. Anti-quorum Sensing and Protective Efficacies of Naringin Against Aeromonas hydrophila Infection in Danio rerio. Front. Microbiol. 2020, 11, 600622. [Google Scholar] [CrossRef]
- Qing, L.; Tengteng, X.; Jiahui, Z.; Huijuan, Z.; Zunying, L. Inhibition of Aeromonas hydrophila quorum sensing by extract from Zanthoxylum bungeanum. Chin. J. Bioprocess Eng. 2020, 18, 263–268. [Google Scholar]
- Patel, B.; Kumari, S.; Banerjee, R.; Samanta, M.; Das, S. Disruption of the quorum sensing regulated pathogenic traits of the biofilm-forming fish pathogen Aeromonas hydrophila by tannic acid, a potent quorum quencher. Biofouling 2017, 33, 580–590. [Google Scholar] [CrossRef]
- Jahid, I.K.; Han, N.; Ha, S.D. Inactivation kinetics of cold oxygen plasma depend on incubation conditions of Aeromonas hydrophila biofilm on lettuce. Food Res. Int. 2014, 55, 181–189. [Google Scholar] [CrossRef]
- Vignesh, S.; Krishnaveni, G.; Devaa, J.C.W.; Muthukumar, S.; Uthandakalaipandian, R. Experimental challenge of the freshwater fish pathogen Aeromonas hydrophila Ah17 and its effect on snakehead murrel Channa striata. Aquac. Int. 2022, 30, 1221–1238. [Google Scholar] [CrossRef]
- Caruffo, M.; Navarrete, P. Antibiotics in aquaculture: Impacts and alternatives. APUA Newsletter. 2015, 33, 4–7. [Google Scholar]
- Akinyemi, A. Efficacy of Traditional Herbs as an Alternative to Antibiotics Used in Aquaculture. J. Sci. Multidiscip. Res. 2013, 5, 43–51. [Google Scholar]
- Alsalim, H.A.A.; Shawkat, M.S.; Ibrahem, M. Antibacterial Activity of Cinnamomum Zeylanicum Bark Oil and Cinnamaldehyde on some Locally Isolated Pathogenic Bacteria. World J. Pharm. Res. 2017, 6, 174–185. [Google Scholar] [CrossRef]
- Awang, A.; Taher, M.; Susanti, D. The mode of antimicrobial action of Cinnamomum burmannii’s essential oil & cinnamaldehyde. J. Teknol. 2016, 78, 41–47. [Google Scholar] [CrossRef]
- Rani, S.; Verma, S.; Singh, H.; Ram, C. Antibacterial activity and mechanism of essential oils in combination with medium-chain fatty acids against predominant bovine mastitis pathogens. Lett. Appl. Microbiol. 2022, 74, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Hua, H.; Selvaraj, J.N.; Zhao, Y.; Zhou, L.; Liu, X.; Liu, Y. Growth inhibition and morphological alterations of Fusarium verticillioides by cinnamon oil and cinnamaldehyde. Food Control 2014, 46, 343–350. [Google Scholar] [CrossRef]
- Yin, L.; Chen, J.; Wang, K.; Geng, Y.; Ouyang, P. Study the antibacterial mechanism of cinnamaldehyde against drug-resistant Aeromonas hydrophila in vitro. Microb. Pathog. 2020, 145, 104208. [Google Scholar] [CrossRef] [PubMed]
- Barbara, R.; Angéles, E.M.; María, G.J.; Giulia, G.; Alberto, C.; Andrea, P.; Ester, G. Antimicrobial Power of Organic Acids and Nature-Identical Compounds against Two Vibrio spp.: An In Vitro Study. Microorganisms 2021, 9, 966. [Google Scholar] [CrossRef]
- Jing, D.; Defu, Z.; Jianrong, L.; Yongtao, L.; Shun, Z.; Yibin, Y.; Ning, X.; Qiuhong, Y.; Xiaohui, A. Genistein Inhibits the Pathogenesis of Aeromonas hydrophila by Disrupting Quorum Sensing Mediated Biofilm Formation and Aerolysin Production Front. Pharmacology 2021, 12, 753581. [Google Scholar] [CrossRef]
- Sun, B.; Luo, H.; Jiang, H.; Wang, Z.; Jia, A. Inhibition of Quorum Sensing and Biofilm Formation of Esculetin on Aeromonas hydrophila. Front. Microbiol. 2021, 12, 737626. [Google Scholar] [CrossRef]
- Ferro, T.A.; Araujo, J.M.; Dos Santos Pinto, B.L.; Dos Santos, J.S.; Souza, E.B.; da Silva, B.L.; Colares, V.L.; Novais, T.M.; Filho, C.M.; Struve, C.; et al. Cinnamaldehyde Inhibits Staphylococcus aureus Virulence Factors and Protects against Infection in a Galleria mellonella Model. Front. Microbiol. 2016, 7, 2052. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Shi, Y.Q.; Pan, X.H.; Cao, P. Antibacterial effects of cinnamon (Cinnamomum zeylanicum) bark essential oil on Porphyromonas gingivalis. Microb. Pathog. 2018, 116, 26–32. [Google Scholar] [CrossRef]
- He, Z.; Huang, Z.; Jiang, W.; Zhou, W. Antimicrobial Activity of Cinnamaldehyde on Streptococcus mutans Biofilms. Front. Microbiol. 2019, 10, 2241. [Google Scholar] [CrossRef]
- Li, T.; Wang, D.; Liu, N.; Ma, Y.; Ding, T.; Mei, Y.; Li, J. Inhibition of quorum sensing-controlled virulence factors and biofilm formation in Pseudomonas fluorescens by cinnamaldehyde. Int. J. Food Microbiol. 2018, 269, 98–106. [Google Scholar] [CrossRef]
- Amalaradjou, M.A.; Narayanan, A.; Venkitanarayanan, K. Trans-cinnamaldehyde decreases attachment and invasion of uropathogenic Escherichia coli in urinary tract epithelial cells by modulating virulence gene expression. J. Urol. 2011, 185, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; Defoirdt, T.; Miyamoto, C.; Bossier, P.; Calenbergh, S.V.; Nelis, H.; Coenye, T. Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol. 2008, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhang, L.; Liu, Y.; Xu, N.; Zhou, S.; Yang, Q.; Yang, Y.; Ai, X. Thymol Protects Channel Catfish from Aeromonas hydrophila Infection by Inhibiting Aerolysin Expression and Biofilm Formation. Microorganisms 2020, 8, 636. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhang, X.; Shi, H.; Wang, W.; Du, W. Identification of a Novel N-Acyl Homoserine Lactone Synthase, AhyI, in Aeromonas hydrophila and Structural Basis for Its Substrate Specificity. J. Agric. Food Chem. 2020, 68, 2516–2527. [Google Scholar] [CrossRef]
- Nasuno, E.; Okano, C.; Limura, K.; Morohoshi, T.; Ikeda, T.; Kato, N. Quick detection of cell to cell communication in gram negative bacteria by colour change of polymer matrix entrapping reporter bacteria. Mater. Res. Innov. 2014, 18, S4.879–S874.883. [Google Scholar] [CrossRef]
- Guerino, B.J.; Erbice, B.A.; Carine, d.F.S.; Nunes, D.S.; Liana, d.S.F.; Lenise, d.L.S.; Felipetto, C.J.; Bernardo, B. The Use of Cinnamon Essential Oils in Aquaculture: Antibacterial, Anesthetic, Growth-Promoting, and Antioxidant Effects. Fishes 2022, 7, 133. [Google Scholar] [CrossRef]
- Abdelhamed, H.; Ozdemir, O.; Ibrahim, I.; Lawrence, M.; Karsi, A. Antibacterial activities of trans-cinnamaldehyde, caprylic acid, and β-resorcylic acid against catfish pathogens. Aquaculture 2019, 504, 334–344. [Google Scholar] [CrossRef]
- Faikoh, E.N.; Hong, Y.H.; Hu, S.Y. Liposome-encapsulated cinnamaldehyde enhances zebrafish (Danio rerio) immunity and survival when challenged with Vibrio vulnificus and Streptococcus agalactiae. Fish Shellfish Immun. 2014, 38, 15–24. [Google Scholar] [CrossRef]



| Primer | Sequence | PCR Amplicon (bp) | Accession No. |
|---|---|---|---|
| aerA-F | TCTACCACCACCTCCCTGTC | 218 | NC008570.1 |
| aerA-R | GACGAAGGTGTGGTTCCAGT | ||
| ahyI-F | GTCAGCTCCCACACGTCGTT | 202 | CP000462.1 |
| ahyI-R | GGGATGTGGAATCCCACCGT | ||
| ahyR-F | TTTACGGGTGACCTGATTGAG | 206 | CP000462.1 |
| ahyR-R | CCTGGATGTCCAACTACATCTT | ||
| 16S rRNA-F | TAATACCGCATACGCCCTAC | 164 | NR074841.1 |
| 16S rRNA-R | ACCGTGTCTCAGTTCCAGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zhou, S.; Yang, Q.; Liu, Y.; Yang, Y.; Xu, N.; Ai, X.; Dong, J. Cinnamaldehyde Decreases the Pathogenesis of Aeromonas hydrophila by Inhibiting Quorum Sensing and Biofilm Formation. Fishes 2023, 8, 122. https://doi.org/10.3390/fishes8030122
Li S, Zhou S, Yang Q, Liu Y, Yang Y, Xu N, Ai X, Dong J. Cinnamaldehyde Decreases the Pathogenesis of Aeromonas hydrophila by Inhibiting Quorum Sensing and Biofilm Formation. Fishes. 2023; 8(3):122. https://doi.org/10.3390/fishes8030122
Chicago/Turabian StyleLi, Shengping, Shun Zhou, Qiuhong Yang, Yongtao Liu, Yibin Yang, Ning Xu, Xiaohui Ai, and Jing Dong. 2023. "Cinnamaldehyde Decreases the Pathogenesis of Aeromonas hydrophila by Inhibiting Quorum Sensing and Biofilm Formation" Fishes 8, no. 3: 122. https://doi.org/10.3390/fishes8030122
APA StyleLi, S., Zhou, S., Yang, Q., Liu, Y., Yang, Y., Xu, N., Ai, X., & Dong, J. (2023). Cinnamaldehyde Decreases the Pathogenesis of Aeromonas hydrophila by Inhibiting Quorum Sensing and Biofilm Formation. Fishes, 8(3), 122. https://doi.org/10.3390/fishes8030122





