A Case of Mycobacteriosis in Cultured Japanese Seabass (Lateolabrax japonicus) in Southern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Case History
2.2. Parasitological and Microbiological Examination
2.3. Phenotype Testing
2.4. Molecular Identification
2.5. Histopathologic Examination
2.6. Challenge Experiment
2.7. Antimicrobial Susceptibility Testing
2.8. Statistical Analysis
3. Results
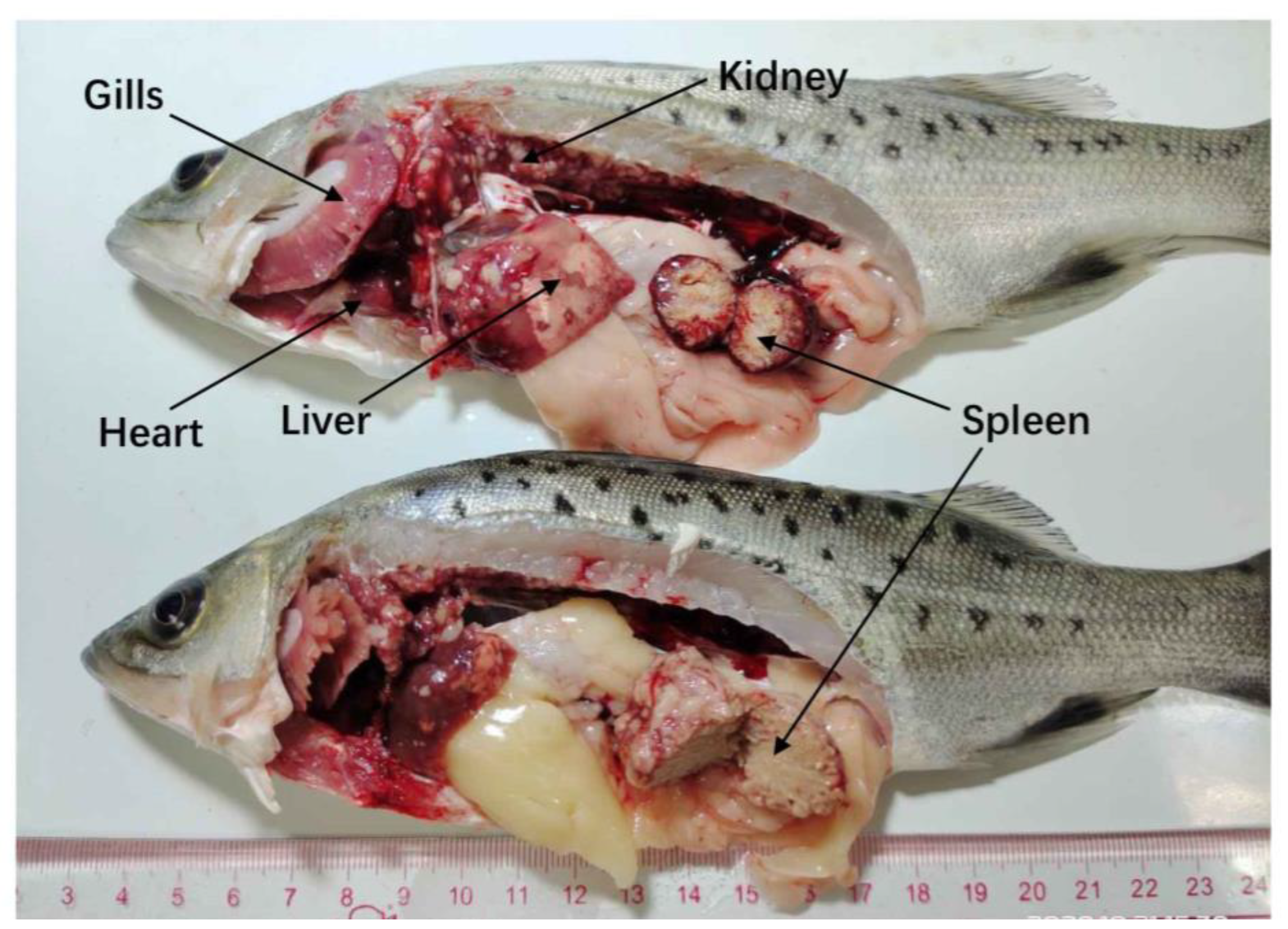
3.1. Disease Characterization and Clinical Signs
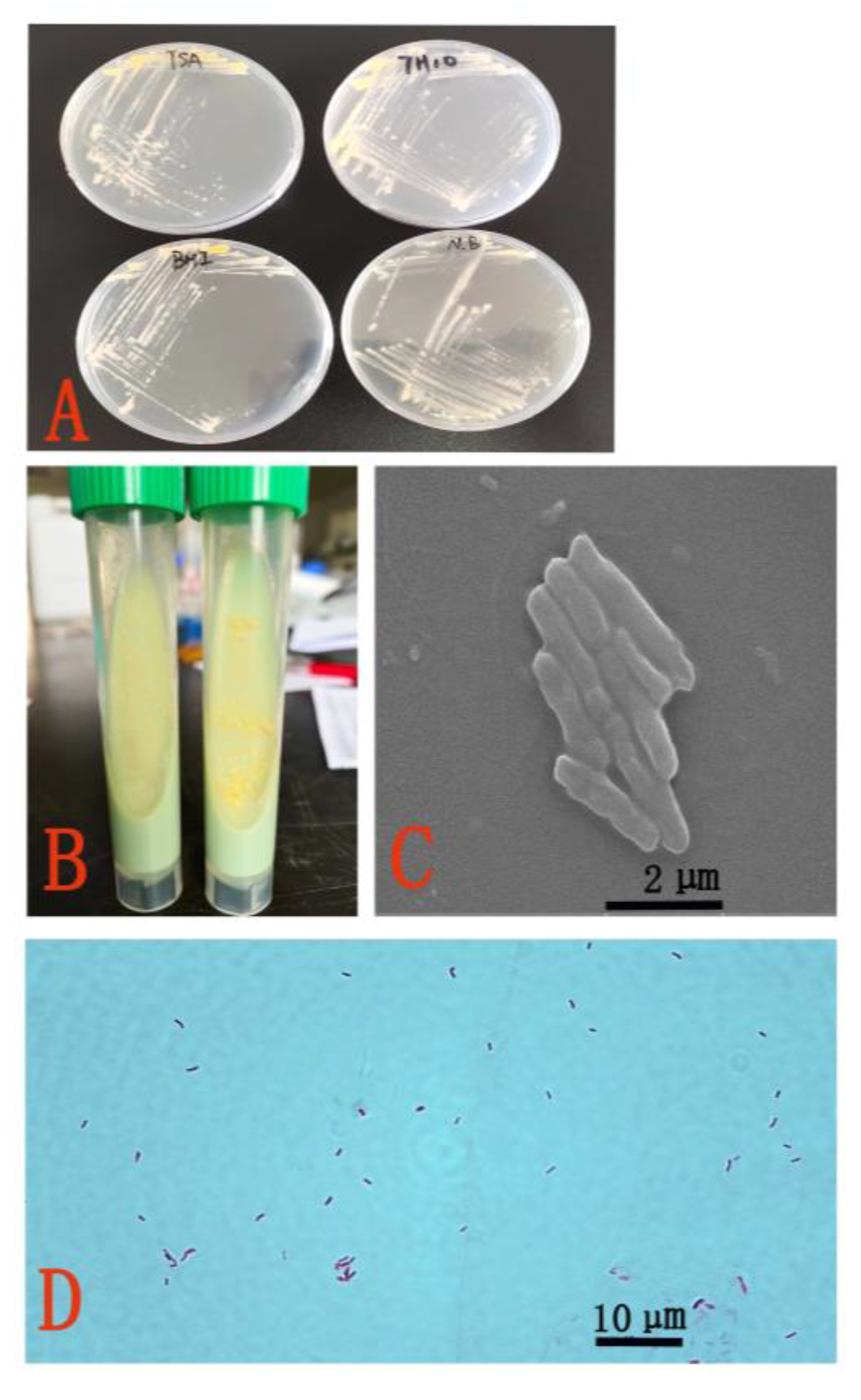
3.2. Morphological and Biochemical Characteristics
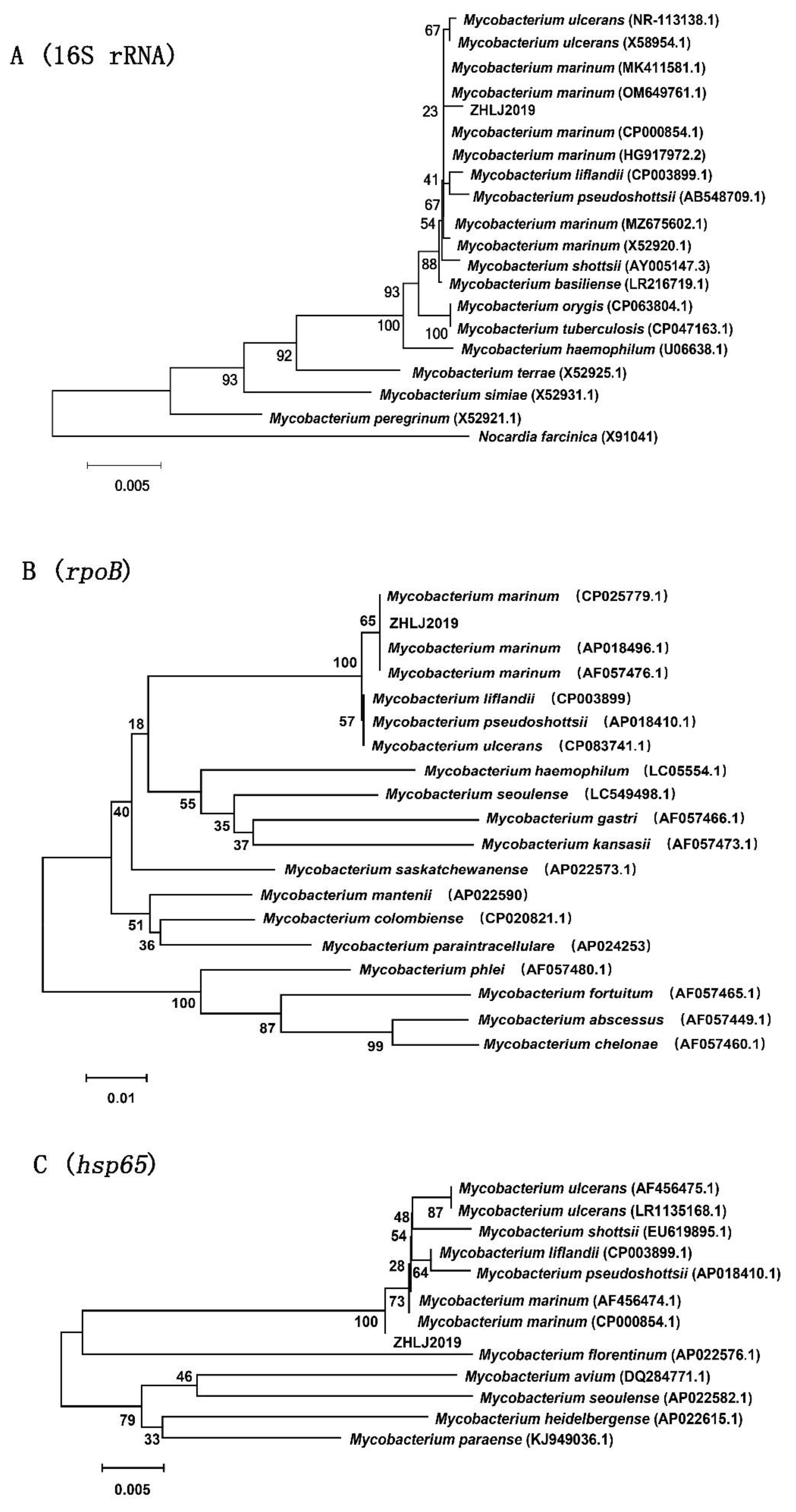
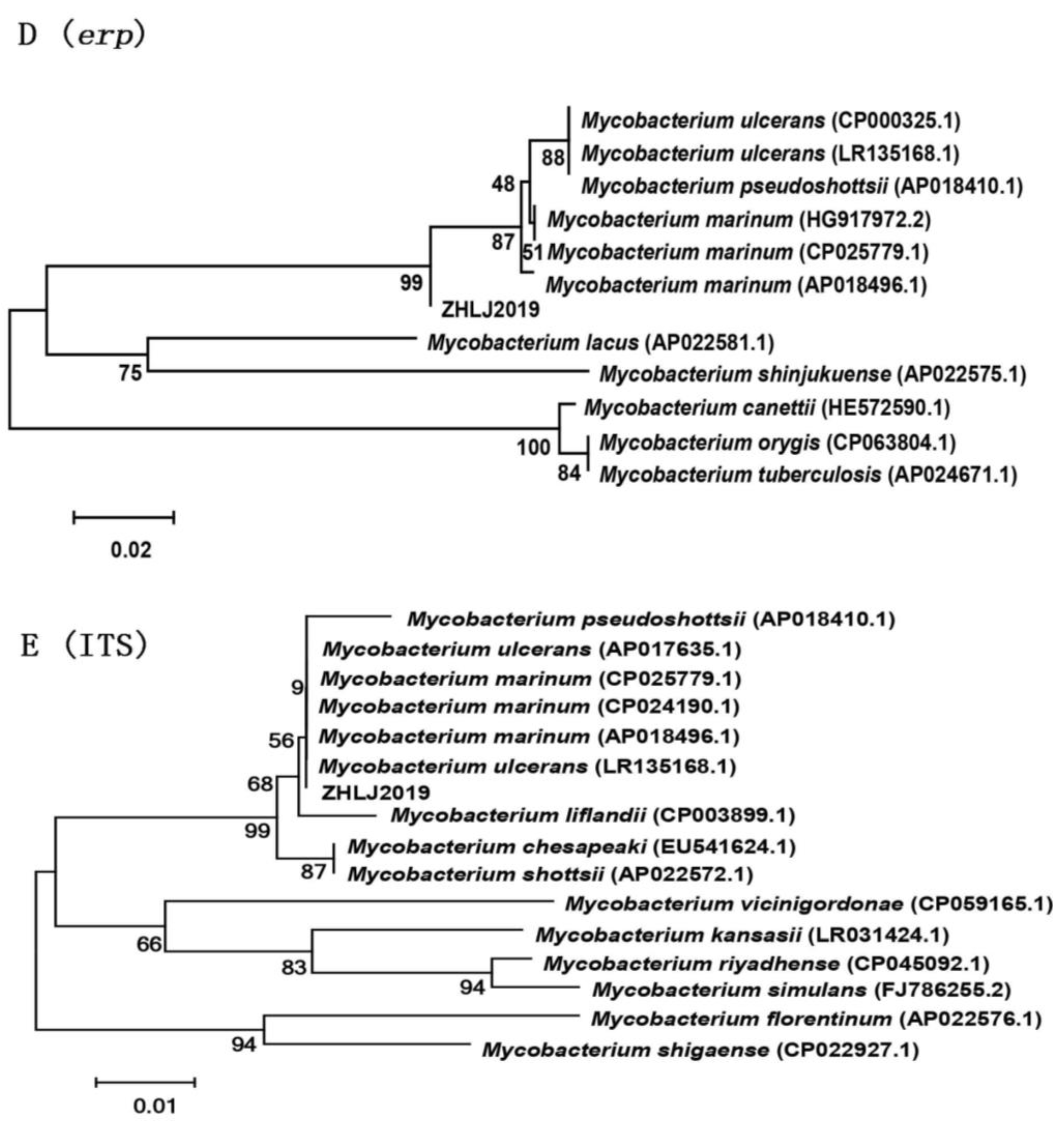
3.3. Molecular Analysis
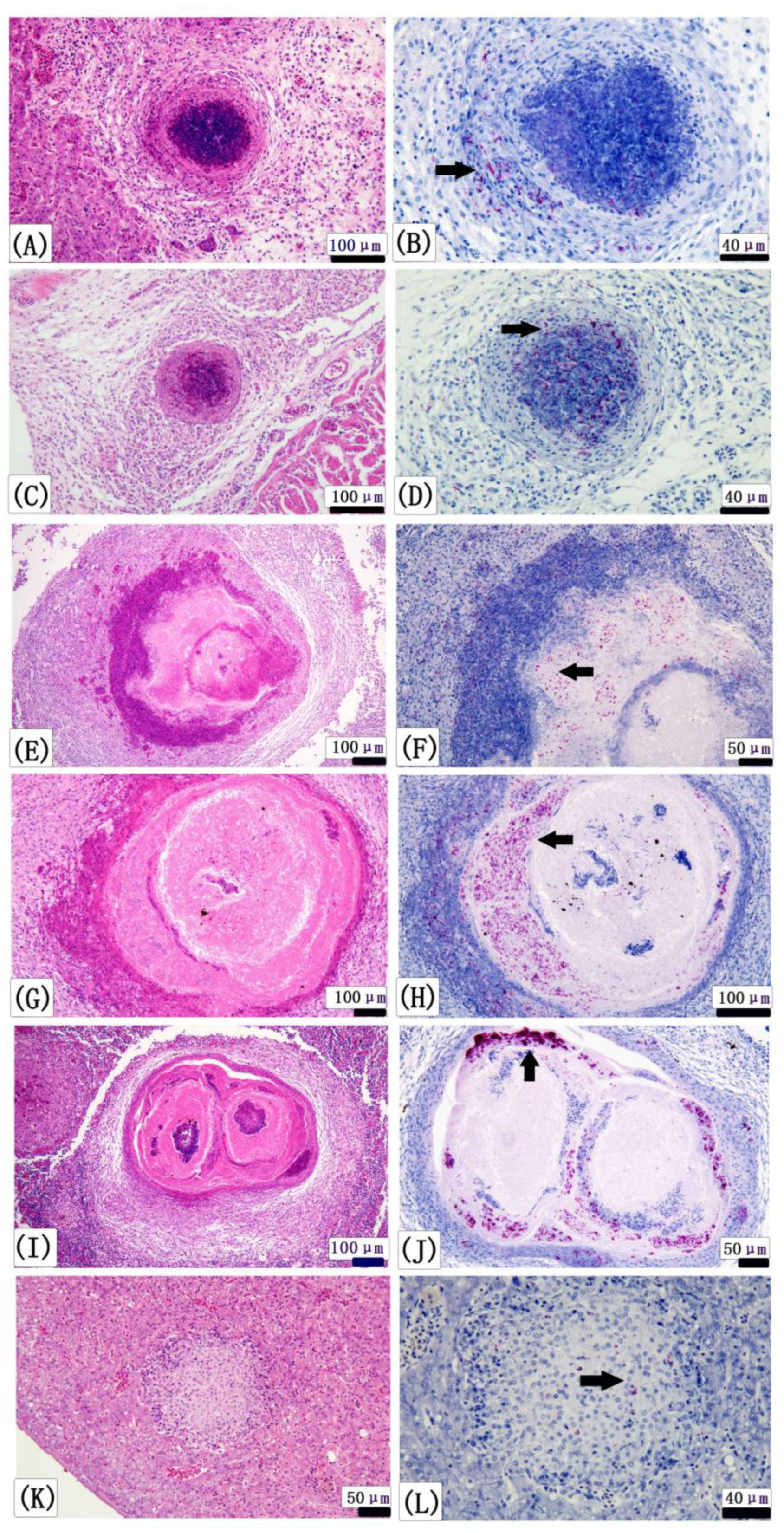
3.4. Histopathology
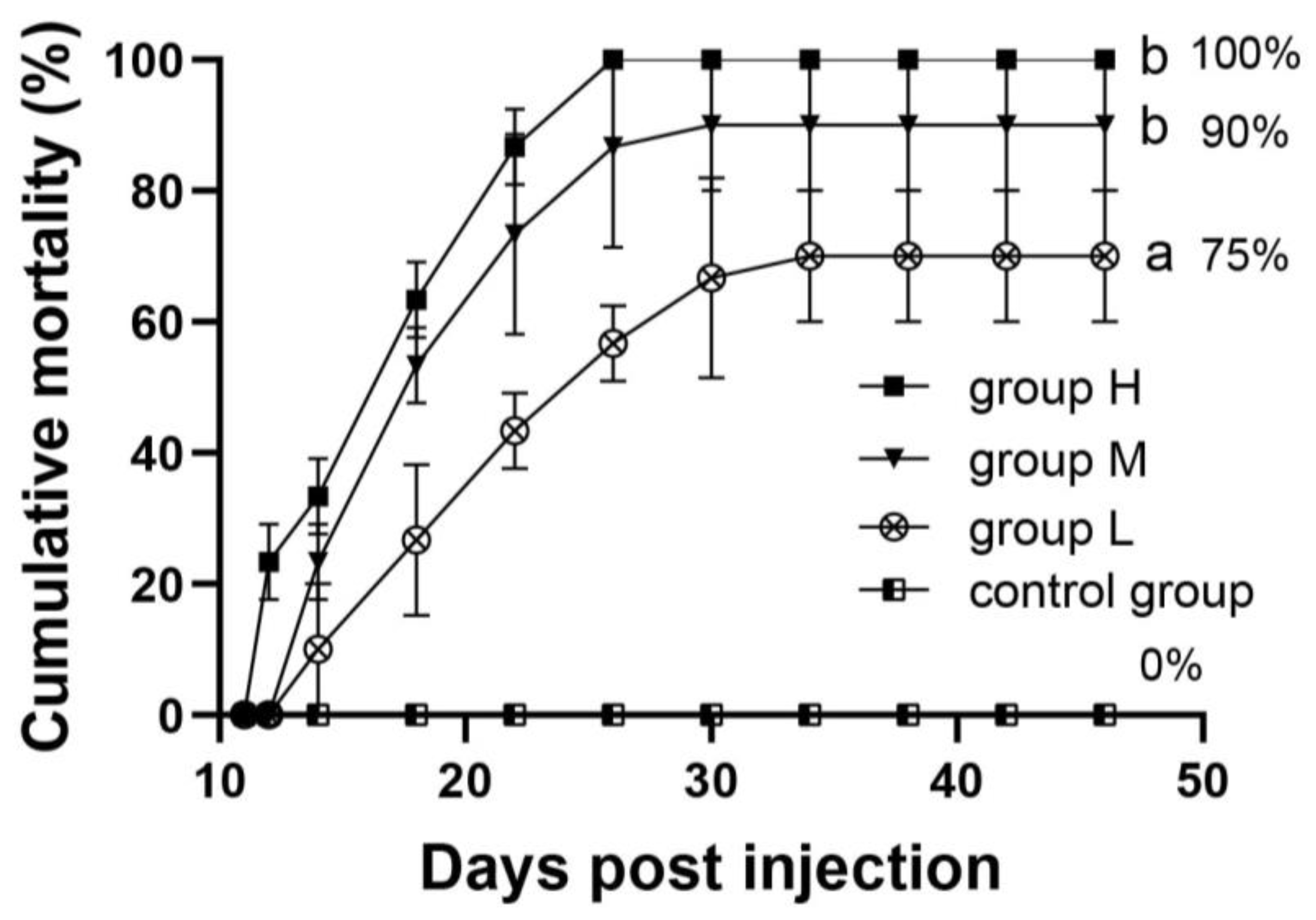
3.5. Challenge Experiment
3.6. Antibiotic Sensitivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chinabut, S. Mycobacteriosis and nocardiosis. In Fish Diseases and Disorders; Woo, P.T.K., Bruno, D.W., Eds.; CABI Publishing: New York, NY, USA, 1999; Volume 3, pp. 319–340. [Google Scholar]
- Decostere, A.; Hermans, K.; Haesebrouck, F. Piscine mycobacteriosis: A literature review covering the agent and the disease it causes in fish and humans. Vet. Microbiol. 2004, 99, 159–166. [Google Scholar] [CrossRef]
- Gauthier, D.T.; Rhodes, M.W.; Vogelbein, W.K.; Kator, H.; Ottinger, C.A. Experimental mycobacteriosis in striped bass Morone saxatilis. Dis. Aquat. Organ. 2003, 54, 105–117. [Google Scholar] [CrossRef]
- Bruno, D.W.; Griffiths, J.; Mitchell, C.G.; Wood, B.P.; Fletcher, Z.J.; Drobniewski, F.A.; Hastings, T.S. Pathology attributed to Mycobacterium chelonae infection among farmed and laboratory infected Atlantic salmon Salmo salar. Dis. Aquat. Organ. 1998, 33, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Landsell, W.; Dixon, B.; Smith, N.; Benjamin, L. Isolation of several Mycobacterium species from fish. J. Aquat. Anim. Health. 1993, 5, 73–76. [Google Scholar] [CrossRef]
- Rhodes, M.W.; Kator, H.; Kotob, S.; van Berkum, P.; Kaattari, I.; Vogelbein, W.; Quinn, F.; Floyd, M.M.; Butler, W.R.; Ottinger, C.A. Mycobacterium shottsii sp. nov., a slowly growing species isolated from Chesapeake Bay striped bass (Morone saxatilis). Int. J. Syst. Evol. Microbiol. 2003, 53, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Teska, J.D. Isolation of Mycobacterium abscessus from Japan medaka. J. Aquat. Anim. Health 1997, 9, 234–238. [Google Scholar] [CrossRef]
- Tortoli, E.; Bartoloni, A.; Bozzetta, E.; Burrini, C.; Lacchini, C.; Mantella, A.; Pavlik, I. Identification of the newly described Mycobacterium poriferae from tuberculous lesions of snakehead fish (Channa striatus). Comp. Immunol. Microbiol. Infect. Dis. 1996, 19, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, D.T.; Rhodes, M.W. Mycobacteriosis in fishes: A review. Vet. J. 2009, 180, 33–47. [Google Scholar] [CrossRef]
- Keller, C.; Wenker, C.; Jermann, T.; Hirschi, R.; Schildger, B.; Meier, R.; Schmidt-Posthaus, H. Piscine mycobacteriosis—Involvement of bacterial species and reflection in pathology. Mycobakteriose bei Fischen—Eine Studie unter Berücksichtigung der beteiligten Bakterienarten und Pathologie. Schweiz. Arch. Tierh. 2018, 160, 385–393. [Google Scholar] [CrossRef]
- Petrini, B. Mycobacterium marinum: Ubiquitous agent of water-borne granulomatous skin infections. Eur. J. Clin. Microbiol. 2006, 25, 609–613. [Google Scholar] [CrossRef]
- Swetter, S.M.; Kindel, S.E.; Smoller, B.R. Cutaneous nodules of Mycobacterium chelonae in an immunosuppressed patient with preexisting pulmonary colonization. J. Am. Acad. Dermatol. 1993, 28, 352–355. [Google Scholar] [CrossRef]
- Pozniak, A.; Bull, T. Recently recognized mycobacteria of clinical significance. J. Infect. 1999, 38, 157–161. [Google Scholar] [CrossRef]
- Gebo, K.A.; Srinivasan, A.; Perl, T.M.; Ross, T.; Groth, A.; Merz, W.G. Pseudo-outbreak of Mycobacterium fortuitum on a human immunodeficiency virus ward: Transient respiratory tract colonization from a contaminated ice machine. Clin. Infect. Dis. 2002, 35, 32–38. [Google Scholar] [CrossRef]
- Abalain-Colloc, M.L.; Guillerm, D.; Saläun, M.; Gouriou, S.; Vincent, V.; Picard, B. Mycobacterium szulgai isolated from a patient, a tropical fish and aquarium water. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 768–769. [Google Scholar] [CrossRef]
- Xu, H.G.; Dong, X.J.; Zuo, R.T.; Mai, K.S.; Ai, Q.H. Response of juvenile Japanese seabass (Lateolabrax japonicus) to different dietary fatty acid profiles: Growth performance, tissue lipid accumulation, liver histology andflesh texture. Aquaculture 2016, 461, 40–47. [Google Scholar] [CrossRef]
- Fisheries Bureau of the Ministry of Agriculture and Rural Affairs. China Fisheries Statistical Yearbook 2020; China Agricultural press: Beijing, China, 2020; p. 22. [Google Scholar]
- Iida-Aoyama, N.; Harada, T.; Kawai, T.; Yokoyama, H.; Kawatsu, K. Development of a Real-Time PCR Assay for Detection of Kudoa iwatai (Myxosporea: Multivalvulida) in Japanese Seabass (Lateolabrax japonicus). J. Food Prot. 2018, 81, 1346–1350. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, L.; Ye, B.Q.; Huang, S.Q.; Wong, S.M.; Yue, G.H. Characterization of a novel disease resistance gene rtp3 and its association with VNN disease resistance in Asian seabass. Fish Shellfish Immunol. 2017, 61, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Peterson, T.S.; Ferguson, J.A.; Watral, V.G.; Mutoji, K.N.; Kent, M.L. Paramecium caudatum enhances transmission and infectivity of Mycobacterium marinum and M. chelonae in zebrafish Danio rerio. Dis. Aquat. Organ. 2013, 106, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Bhatty, M.A.; Turner, D.P.; Chamberlain, S.T. Mycobacterium marinum hand infection: Case reports and review of literature. Br. J. Plast. Surg. 2000, 53, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Lewis, F.M.; Marsh, B.J.; von Reyn, C.F. Fish tank exposure and cutaneous infections due to Mycobacterium marinum: Tuberculin skin testing, treatment, and prevention. Clin. Infect. Dis. 2003, 37, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Kusuda, R. Efficacy of rifampicin, streptomycin, and erythromycin against experimental mycobacterium infection in cultured yellowtail. Nippon. Suisan. Gakk. 1990, 56, 51–53. [Google Scholar] [CrossRef]
- Jacobs, J.M.; Stine, C.B.; Baya, A.M.; Kent, M.L. A review of mycobacteriosis in marine fish. J. Fish. Dis. 2009, 32, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Cai, S.H.; Jian, J.C.; Liu, G.F.; Xu, L.W. Co-infection of infectious spleen and kidney necrosis virus and francisella sp. in farmed pearl gentian grouper (♀Epinephelus fuscoguttatus ×♂E. lanceolatus) in china—A case report. Aquaculture 2020, 526, 735409. [Google Scholar] [CrossRef]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics Stackebrandt; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley and Sons.: New York, NY, USA, 1991; pp. 115–175. [Google Scholar]
- Kim, B.J.; Lee, S.H.; Lyu, M.A.; Kim, S.J.; Bai, G.H.; Chae, G.T.; Kim, E.C.; Cha, C.Y.; Kook, Y.H. Identification of mycobacterial species by comparative sequence analysis of the RNA polymerase gene (rpoB). J. Clin. Microbiol. 1999, 37, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, S.H.; Shim, T.S.; Kim, M.N.; Bai, G.H.; Park, Y.G.; Lee, S.H.; Chae, G.T.; Cha, C.Y.; Kook, Y.H.; et al. Differentiation of Mycobacterium species by analysis of the heat-shock protein 65 gene (hsp65). Int. J. Syst. Evol. Microbiol. 2005, 55, 1649–1656. [Google Scholar] [CrossRef]
- De Mendonça-Lima, L.; Picardeau, M.; Raynaud, C.; Rauzier, J.; Goguet de la Salmonière, Y.O.; Barker, L.; Bigi, F.; Cataldi, A.; Gicquel, B.; Reyrat, J.M. Erp, an extracellularprotein family specific to mycobacteria. Microbiology 2001, 147, 2315–2320. [Google Scholar] [CrossRef]
- Whipps, C.M.; Watral, V.G.; Kent, M.L. Characterization of a Mycobacterium sp. in rockfish, Sebastes alutus (Gilbert) and Sebastes reedi (Westrheim & Tsuyuki), using rDNA sequences. J. Fish Dis. 2003, 26, 241–245. [Google Scholar] [CrossRef]
- Colorni, A.; Avtalion, R.; Knibb, W.; Berger, E.; Colorni, B.; Timan, B. Histopathology of sea bass (Dicentrarchus labrax) experimentally infected with mycobacterium marinum and treated with streptomycin and garlic (allium sativum) extract. Aquaculture 1998, 160, 1–17. [Google Scholar] [CrossRef]
- Hashish, E.; Merwad, A.; Elgaml, S.; Amer, A.; Kamal, H.; Elsadek, A.; Marei, A.; Sitohy, M. Mycobacterium marinum infection in fish and man: Epidemiology, pathophysiology and management; a review. Vet. Quart. 2018, 38, 35–46. [Google Scholar] [CrossRef]
- Rallis, E.; Koumantaki-Mathioudaki, E. Treatment of Mycobacterium marinum cutaneous infections. Expert Opin. Pharmacother. 2007, 8, 2965–2978. [Google Scholar] [CrossRef]
- Adékambi, T.; Colson, P.; Drancourt, M. rpoB-based identification of nonpigmented and late-pigmenting rapidly growing mycobacteria. J. Clin. Microbiol. 2003, 41, 5699–5708. [Google Scholar] [CrossRef] [PubMed]
- Devulder, G.; De Montclos, M.P.; Flandrois, J.P. A multigenic approach to phylogenetic analysis using the genus Mycobacterium as a model. Int. J. Syst. Evol. Microbiol. 2005, 55, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Clarridge, J.E. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin. Microbiol. Rev. 2004, 17, 840–862. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, B.J.; Kim, J.H.; Park, K.H.; Kook, Y.H. Differentiation of borrelia burgdorferi sensu lato on the basis of RNA polymerase gene (rpoB) sequences. J. Clin. Microbiol. 2000, 38, 2557–2562. [Google Scholar] [CrossRef]
- López-Marín, L.M.; Lanéelle, M.A.; Promé, D.; Daffé, M. Structures of the glycopeptidolipid antigens of two animal pathogens: Mycobacterium senegalense and Mycobacterium porcinum. Eur. J. Biochem. 1993, 215, 859–866. [Google Scholar] [CrossRef]
- Lee, H.; Bang, H.E.; Bai, G.H.; Cho, S.N. Novel polymorphic region of the rpoB gene containing Mycobacterium species-specific sequences and its use in identification of mycobacteria. J. Clin. Microbiol. 2003, 41, 2213–2218. [Google Scholar] [CrossRef]
- Roth, A.; Fischer, M.; Hamid, M.E.; Michalke, S.; Ludwig, W.; Mauch, H. Differentiation of phylogenetically related slowly growing mycobacteria based on 16S-23S rRNA gene internal transcribed spacer sequences. J. Clin. Microbiol. 1998, 36, 139–147. [Google Scholar] [CrossRef]
- Kaattari, I.M.; Rhodes, M.W.; Kaattari, S.L.; Shotts, E.B. The evolving story of Mycobacterium tuberculosis clade members detected in fish. J. Fish. Dis. 2006, 29, 509–520. [Google Scholar] [CrossRef]
- Luo, Z.; Li, J.; Zhang, Z.G.; Hao, S.; Bai, X.H.; You, H.Z.; Mo, Z.L.; Feng, S.M. Mycobacterium marinum is the causative agent of splenic and renal granulomas in half-smooth tongue sole (Cynoglossus semilaevis Günther) in China. Aquaculture 2018, 490, 203–207. [Google Scholar] [CrossRef]
- Gauthier, D.T.; Vogelbein, W.K.; Ottinger, C.A. Ultrastructure of Mycobacterium marinum granuloma in striped bass (Morone saxatilis). Dis. Aquat. Organ. 2004, 62, 121–132. [Google Scholar] [CrossRef]
- Weerakhun, S.; Aoki, N.; Kurata, O.; Hatai, K.; Nibe, H.; Hirae, T. Mycobacterium marinum infection in cultured yellowtail seriola quinqueradiata in Japan. Fish Pathology. 2007, 42, 79–84. [Google Scholar] [CrossRef][Green Version]
- Zerihun, M.A.; Colquhoun, D.J.; Poppe, T.T. Experimental mycobacteriosis in Atlantic cod, Gadus morhua L. J. Fish. Dis. 2012, 35, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lara, P.; Martínez-Porchas, M.; Gollas-Galván, T.; Hernández-López, J.; Robles-Porchas, G.R. Granulomatosis in fish aquaculture: A mini review. Rev. Aquacult. 2021, 13, 259–268. [Google Scholar] [CrossRef]
- Bozzetta, E.; Varello, K.; Giorgi, I.; Fioravanti, M.L.; Pezzolato, M.; Zanoni, R.G.; Prearo, M. Mycobacterium marinum infection in a hybrid striped bass farm in Italy. J. Fish Dis. 2010, 33, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Novotny, L.; Halouzka, R.; Matlova, L.; Vavra, O.; Bartosova, L.; Slany, M.; Pavlik, I. Morphology and distribution of granulomatous inflammation in freshwater ornamental fish infected with mycobacteria. J. Fish. Dis. 2010, 33, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Gjurčević, E.; Kužir, S.; Žmak, L.; Obrovac, M.; Gudan Kurilj, A.; Savoca, S.; Matanović, K. A case of mycobacteriosis in farmed pikeperch (Sander lucioperca) cultured in a recirculating aquaculture system. Aquac. Res. 2020, 51, 4824–4827. [Google Scholar] [CrossRef]
- Jacobs, J.M.; Rhodes, M.R.; Baya, A.; Reimschuessel, R.; Townsend, H.; Harrell, R.M. Influence of nutritional state on the progression and severity of mycobacteriosis in striped bass Morone saxatilis. Dis. Aquat. Organ. 2009, 87, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, R.P.; McDowell, T.; Groff, J. Mycobacteriosis in cultured striped bass from California. J. Wildl. Dis. 1987, 23, 391–395. [Google Scholar] [CrossRef]
- Brown-Elliott, B.A.; Nash, K.A.; Wallace, R.J. Antimicrobial Susceptibility Testing, Drug Resistance Mechanisms, and Therapy of Infections with Nontuberculous Mycobacteria. Clin. Microbiol. Rev. 2012, 25, 545–582. [Google Scholar] [CrossRef]
- van Ingen, J.; Boeree, M.J.; van Soolingen, D.; Mouton, J.W. Resistance mechanismsand drug susceptibility testing of nontuberculous mycobacteria. Drug Resist. Updates 2012, 15, 149–161. [Google Scholar] [CrossRef]
- Lahey, T. Invasive Mycobacterium marinum infections. Emerg Infect Dis. 2003, 9, 1496–1498. [Google Scholar] [CrossRef] [PubMed]
- Aubry, A.; Chosidow, O.; Caumes, E.; Robert, J.; Cambau, E. Sixty-three cases of Mycobacterium marinum infection: Clinical features, treatment, and antibiotic susceptibility of causative isolates. Arch. Intern. Med. 2002, 162, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.M.; Xu, H.S.; Wang, H.S.; Zhang, C.P.; Zong, W.K.; Wu, Q.X. Outbreak of a cutaneous Mycobacterium marinum infection in Jiangsu Haian. Chin. Diagn. Microbiol. Infect. Dis. 2011, 71, 267–272. [Google Scholar] [CrossRef]
- Harris, D.M.; Keating, M.R. Mycobacterium marinum: Current recommended pharmacologic therapy. J. Hand Surg. Am. 2009, 34, 1734–1735. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.G.; Stout, J.E. Twenty-eight cases of Mycobacterium marinum infection: Retrospective case series and literature review. Infection 2015, 43, 655–662. [Google Scholar] [CrossRef]
| Gene | Primer Names/Sequence (5′-3′) | Product Size (bp) | References | PCR Conditions |
|---|---|---|---|---|
| 16S rRNA | 27F/AGAGTTTGATCCTGGCTCAG | 1435 | [26] | 95 °C for 30 s, 51 °C for 30 s, and 72 °C for 90 s for 35 cycles |
| 1492R/GGTTACCTTGTTACGACTT | ||||
| rpoB | MF/ CGACCACTTCGGCAACCG | 350 | [27] | 95 °C for 30 s, 61 °C for 30 s, and 72 °C for 90 s for 35 cycles |
| MR/ TCGATCGGGCACATCCGG | ||||
| hsp65 | Hsp-F/ATCGCCAAGGAGATCGAGCT | 643 | [28] | 95 °C for 30 s, 59 °C for 30 s, and 72 °C for 90 s for 35 cycles |
| Hsp-R/AAGGTGCCGCGGATCTTGTT | ||||
| erp | Erp-8/GTGCCGAACCGACGCCGACG | 230 | [29] | 95 °C for 30 s, 69 °C for 30 s, and 72 °C for 90 s for 35 cycles |
| Erp-9/GGCACCGGCGGCAGGTTGATCCCG | ||||
| ITS | MycoITS1F/GACGAAGTCGTAACAAGG | 265 | [30] | 95 °C for 30 s, 53 °C for 30 s, and 72 °C for 90 s for 35 cycles |
| MycoITS1R/ATGCTCGCAACCACTATCCA |
| Item | ZHLJ2019 | M. marinum ATCC 927 |
|---|---|---|
| Nitrate reduction | − | − |
| Nitrite reduction | + | + |
| Urease | + | + |
| Growth on 5% sodium chloride | − | − |
| Aromatic sulfatase | + | + |
| Nicotinic acid | + | + |
| Pyrazinamidase | + | + |
| thiophene-2-carboxylic acid | + | + |
| Glucose agar | + | + |
| MacConkey | − | − |
| 25 °C, growth | + | + |
| 37 °C, growth | + | + |
| 40 °C, growth | − | − |
| Tween-80 hydrolysis (5 day) | + | + |
| pigmentation | + | + |
| Antibiotic | Concentrations (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| C | 1 | 2 | 4 | 8 | 16 | 32 | 64 | |
| Minocycline | + | + | + | − | − | − | − | − |
| Ethambutol | + | + | + | + | − | − | − | − |
| Levofloxacin | + | + | + | + | − | − | − | − |
| Rifampin | + | + | − | − | − | − | − | − |
| Isoniazid | + | + | + | − | − | − | − | − |
| Roxithromycin | + | + | + | − | − | − | − | − |
| Streptomycin | + | + | + | + | + | − | − | − |
| Doxycycline hydrochloride | + | + | + | + | − | − | − | − |
| Kanamycin Sulfate | + | + | + | + | − | − | − | − |
| Prothionamide | + | + | + | + | − | − | − | − |
| C | 4 | 8 | 16 | 32 | 64 | 128 | 256 | |
| P-aminosalicylic acid | + | + | + | + | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Xu, L.; Yang, S.; Cai, S.; Jian, J.; Huang, Y. A Case of Mycobacteriosis in Cultured Japanese Seabass (Lateolabrax japonicus) in Southern China. Fishes 2023, 8, 33. https://doi.org/10.3390/fishes8010033
Huang Z, Xu L, Yang S, Cai S, Jian J, Huang Y. A Case of Mycobacteriosis in Cultured Japanese Seabass (Lateolabrax japonicus) in Southern China. Fishes. 2023; 8(1):33. https://doi.org/10.3390/fishes8010033
Chicago/Turabian StyleHuang, Zengchao, Liwen Xu, Shiping Yang, Shuanghu Cai, Jichang Jian, and Yucong Huang. 2023. "A Case of Mycobacteriosis in Cultured Japanese Seabass (Lateolabrax japonicus) in Southern China" Fishes 8, no. 1: 33. https://doi.org/10.3390/fishes8010033
APA StyleHuang, Z., Xu, L., Yang, S., Cai, S., Jian, J., & Huang, Y. (2023). A Case of Mycobacteriosis in Cultured Japanese Seabass (Lateolabrax japonicus) in Southern China. Fishes, 8(1), 33. https://doi.org/10.3390/fishes8010033







