1. Introduction
The population status of the European eel (
Anguilla anguilla) is of significant concern, with continuing International Council for the Exploration of the Sea (ICES) advice to reduce human impacts to as close to zero as possible, and the species listed as Critically Endangered on the International Union for Conservation of Nature’s Red List [
1,
2]. As a facultative catadromite, juveniles hatched in the sea migrate to continental waters, and a significant proportion enter freshwater where they grow for ca. 3–15 years, then ultimately undertake an oceanic spawning migration as maturing adults [
3,
4,
5]. Artificial structures such as weirs and dams, which are numerous in our waterways (e.g., an estimated 1.2 million across Europe [
6] and 16,000 in the UK alone [
7]), can impede upstream migration and prevent eel from accessing freshwater habitats [
8,
9]. Accordingly, restoring connectivity at barriers is a conservation priority for the European eel, legislated for under the European Union eel recovery plan (EU Council Regulation No 1100/2007) [
10]).
The installation of fish passage facilities is a widespread and proven approach to mitigate the negative effects of barriers on fluvial connectivity for fish [
11,
12]. Eel have poor burst and sustained swimming capabilities compared to other anadromous and potadromous fishes, and multispecies technical passes often function poorly for them [
8]. Eel-specific upstream passes are, therefore, designed to create lower velocity routes and usually seek to exploit the anguilliform climbing behaviour typical of the juvenile life stages [
13]. There are a wide variety of designs in use, ranging from the simple addition of artificial climbing substrate to the barrier face to technical up-and-over passes which provide a migration route that completely circumvents the structure [
14]. Various climbing materials have been developed specifically, while others are adapted from use in other industries (e.g., drainage, horticulture). The most commonly used substrates in England are bristle boards and eel tiles (studs) [
15]. Bristle boards, which comprise tufts of stiff synthetic fibres on a backing board, are deployed both horizontally, usually lining the channel of up-and-over type passes, and vertically, mounted parallel to the wing wall of a weir with the bristles protruding towards the wall. Both installation types have been shown effective at passing pigmented glass eel through to yellow eel life stages [
16,
17,
18]. Narrow spacing of the bristle clusters (15–20 mm) is most suited to small eel because they require substantial support during climbing [
19,
20]. Studded substrates are considered a more robust alternative to bristles in high-velocity environments and are generally less prone to clogging by debris [
15]. Studs arranged in staggered rows (quincunx) are the most effective [
21], with higher-density arrangements most suited to small size classes [
22]. In trials on a model crump weir, the addition of studded substrate oriented horizontally achieved a 46% improvement in passage efficiency for yellow eel (424 +/− 76 mm total length) [
23] and 67% for pigmented glass eel [
22].
The positive response of juvenile eel to rheotactic cues is well documented and fundamental to the pass design [
24,
25]. The optimum flow rate through the pass represents a balance between sufficient flow to attract migrating eel and stimulate them to ascend, while maintaining velocities comfortably within swimming capabilities [
12,
13]. In up-and-over type passes, water is pumped to the crest where it splits to either flow down the pass length as conveyance flow or down the opposite side of the pass crest to wash ascended eel back into the watercourse upstream of the structure [
14]. The relatively high-cost inputs for such passes, particularly in the set-up and maintenance of electrical pumps, can be reduced by using river flow, as within gravity-fed passes. These are placed within the watercourse, often attached to the sides of weirs, with the upstream exit submerged to allow water to naturally flow through them [
15]. Whereas pumped passes offer the advantage of high consistency in the conveyance flow rate, it is necessary for gravity-fed passes to operate over a broad range of ambient water levels and resultant flow rates. In a wide-reaching review of facilities in Europe and North America, passes typically operated at flow levels equivalent to 0.14–1.1 L s
−1 per m width of pass [
26]. However, such measurements of total discharge only partially describe the hydrodynamics within the pass, with factors such as longitudinal slope and substrate properties exerting a strong influence on localised flow velocities and turbulence [
27,
28]. Despite the key role of flow in the functioning of eel passes, few studies have tested pass designs under different flow scenarios and/or conducted flow velocity measurements (but see [
16,
23]).
The design of a fishway should aim to facilitate passage without inducing delay, stress, disease or injury and without demanding additional energy expenditure [
29]. Steeper longitudinal gradients require a higher energy expenditure per metre of the pass, but there is an obvious trade-off with pass length; a steeper pass provides opportunity to employ a shorter pass to surmount an equivalent elevation [
30]. Several studies have compared eel passage efficiency on substrates with various longitudinal slopes (25–70°), and in general, the shallower slopes were associated with the highest passage rates [
18,
19,
21]. Far less attention has been given to the lateral slope. The majority of constructed passes currently operating in England are rectangular in their cross-section and aligned flat on the horizontal plane, i.e., with no lateral slope [
15]. Variations in lateral slope, created by tilting the pass channel or substrate bed in a flat-bottomed pass, or using a V- or U-shaped cross-section, can achieve a greater diversity of flow velocities and enhance passage [
21]. It is hypothesised that the hydrodynamic heterogeneity created will provide suitable ascent conditions for a broad range of eel size classes within the same facility [
13]. Although not widely tested for eel, a 15° lateral slope is currently recommended within New Zealand’s fish passage guidance for small structures with the aim of benefiting a range of native species including
Galaxias spp., red fin bullies (
Gobiomorphus huttoni) and long-finned and short-finned eel (
Anguilla dieffenbachia and
Anguilla australis), all of which are poor swimmers and employ climbing when ascending structures [
31,
32]. Within the context of gravity-fed passes, the integration of a lateral slope may facilitate their effective operation over a wider range of flow rates.
The aim of this study was to quantify the effect of substrate, lateral slope and flow rate on the efficacy of passage facilities for enhancing the upstream migration of juvenile European eel. A novel pass design, which incorporates two lateral slopes to create a symmetrical V-shaped channel, was trialled alongside the traditional flat-channelled equivalent. The passes were furnished with either bristles or studs and tested under a range of flow rates. The experimental set-up emulated gravity-fed passes, for which there are currently large knowledge gaps regarding their optimum operating criteria and design.
2. Materials and Methods
2.1. Experimental Setup
Trials were conducted from May to August 2020 at an outdoor research facility in Essex, UK. Four test passes were constructed within two filming tents, to exclude ambient light, and each pass comprised a high-density polyethylene ramp (2 m length), a header tank (0.7 m × 0.4 m × 0.35 m) and a footer tank (0.6 × 1 m) (
Figure 1). Two ramps were furnished with bristle climbing substrate (nylon bristles, 22 mm spacing in staggered rows, manufactured by Aquatic Control Engineering, Rampton, UK) and two with stud substrate (17 mm spacing in staggered rows, manufactured by Berry Escott Engineering, Bridgwater, UK). Within each substrate type, one ramp had a flat lateral slope (0.2 m width), and one was V-shaped in cross-section (0.24 m horizontal width, 15° lateral slopes). For each pass, water was recirculated from the footer tank to the header tank using a separate submersible pump (120 L min
−1) and inline valve, and from there it flowed over the narrow crest and down the ramp, simulating a gravity-fed design. The pump intake was located underneath the pass, approximately 0.8 m away from the downstream entrance to minimise its influence on flow patterns at the entrance. The flow volume delivered down the pass was regulated using a ball valve fitted inline to the pipework (25 mm diameter) between the pump and the header tank to create five flow levels: 0.2, 0.3, 0.4, 0.5 and 0.6 L s
−1. The flow was measured at the start and end of each trial using an ultrasonic clamp-on pipe flow meter (DMTFP, Dynaflox, Shanghai, China) and the actual flow rates differed from the desired flow rates by a maximum of 0.02 L s
−1. No additional attraction flow was provided to the downstream entrance of the ramps.
All test ramps were set with a longitudinal slope of 30°. The footer tanks were filled to a water depth of 0.35 m, and the ramps were positioned so that the lower 0.25 m length of the ramp was submerged. A modified keep net (0.5 m length, 0.4 m width, 0.4 height) was placed over the lower 0.25 m of each ramp and secured with a shock cord to retain test subjects in the vicinity of the pass entrance.
The water temperature was maintained using a water heater and aquarium chiller, both automatically controlled (D-D Dual Temp Controller, D-D, Ilford, UK). The actual temperature was logged every 15 min throughout the experimental period (Tiny Tag, Gemini dataloggers, Chichester, UK) (mean = 19.82 ± 0.59 °C, ± SD). The water in the footer tanks was continuously aerated during the trials and replaced by 50% daily with dechlorinated water.
Upstream eel migration occurs predominantly during darkness [
3]. To enable observation of eel behaviour during the night-time trials, test subjects were marked with fluorescent visible implant elastomer (VIE) (Northwest Marine Technology Inc., Anacortes, WA, USA), which has been shown to cause no adverse effect on juvenile eel behaviour (59–158 mm total length) [
33]. The test ramps were lit from above with blue-black light (peak wavelength = 365 nm), which is outside the spectral sensitivity of European eel (kmax = 482 nm) [
34,
35]. Two cameras (HDCC500, Abus, Wetter, Germany and SDN-550, Samsung, Yeongtong-gu, South Korea) were mounted above each pass, parallel to the ramps, to record eel activity in two marked sections: (1) from 0.25 to 0.5 m upstream from the pass entrance and (2) the crest (
Figure 1).
2.2. Fish Capture and Marking
Actively migrating juvenile eel were sourced by the Environment Agency close to the tidal limit of the Chelmer and Blackwater Navigation at Beeleigh, Essex, UK (51.743° N, 0.662° E) using a small pumped pass and trap. Captured eel were transported to the research facility in aerated river water and held outside in enclosed aerated tanks (500 L) of dechlorinated tap water maintained at 17 ± 1 °C using a heater, cooler and temperature control system, as above. A transparent panel (0.15 m diameter) in the lid of each tank enabled natural light to enter. The eel were acclimated to holding conditions for a minimum of 48 h and fed defrosted frozen bloodworm, artemia and daphnia every other day, followed a few hours later by a 70% water change. Eel were not fed on the day they undertook trials.
On the morning of a trial, test subjects were collected from the holding tank by random sweeps of a hand net at all heights in the water column. Prior to marking, eel were anaesthetised using an alcoholic solution of eugenol (10%) administered by an anaesthetic bath (3.5 mL L
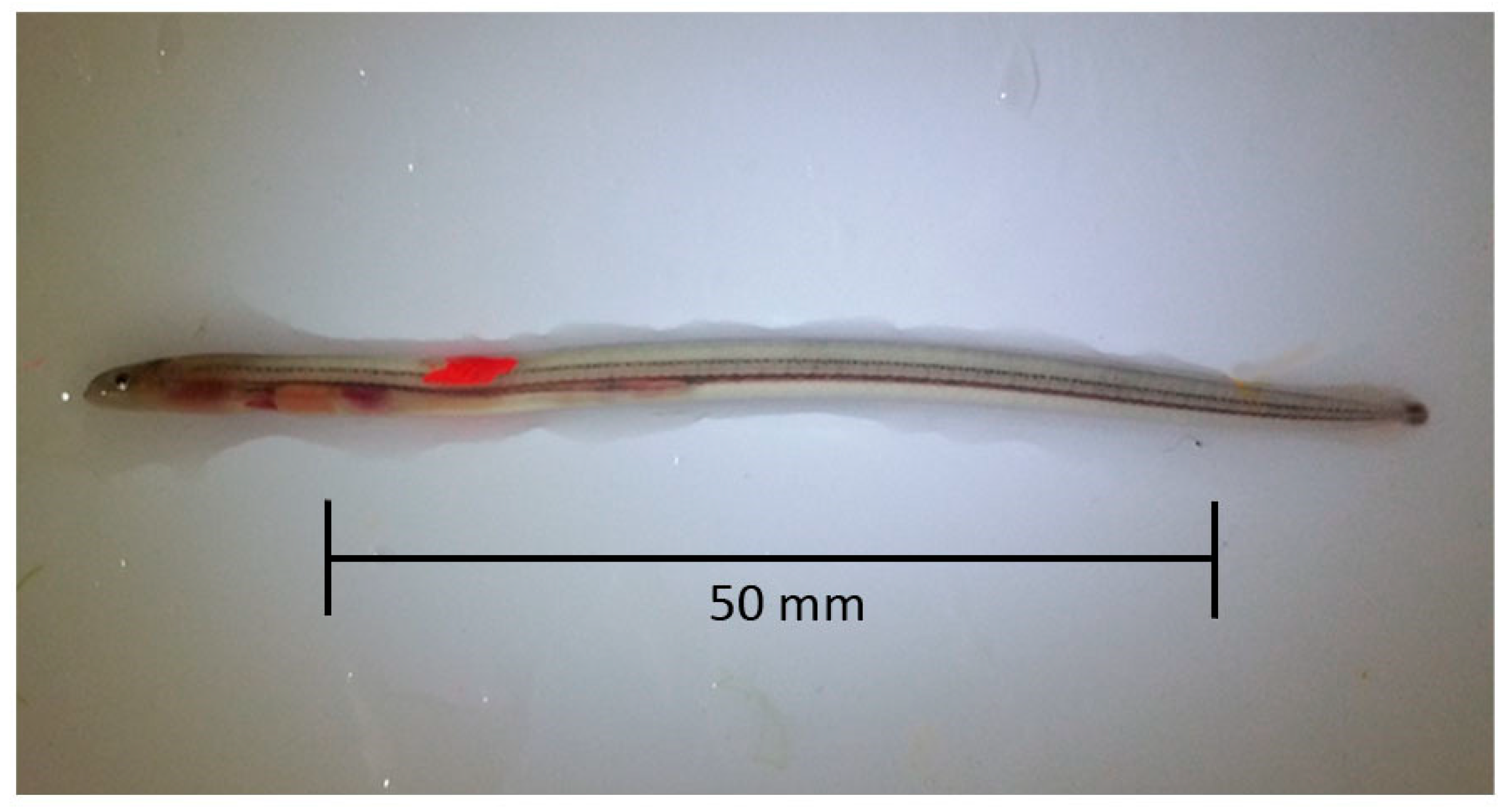
−1) and measured (total length, mm). Only individuals of length 60–80 mm were retained for marking; all others were placed in a recovery tank (500 L) and subsequently released close to the site of capture. VIE of either fluorescent pink or orange was injected subcutaneously anterior to the dorsal fin to create a mark of approximately 3 mm in length (
Figure 2). After recovery, eel were transferred to perforated holding tubes (200 mm length, 100 mm diameter), 20 individuals per tube, and placed in the appropriate footer tank. Procedures were subject to ethical approval by the Zoological Society of London Ethics Committee and conducted under Home Office licence (PPL 7008909). No eel died during anaesthesia or recovery. The mean length of test subjects was 73 mm ± 4.94 (S.D.).
2.3. Experimental Protocol
Trials were conducted in darkness (20:00–02:00), and each lasted 2 h with up to two trials per ramp per night. At the start of each trial, 20 eel were transferred in their holding tube and emptied into the net cage at the base of the test ramp. Video footage was recorded continuously at 25 fps (HD Analogue Recorder HDCC9001, Abus, Wetter, Germany).
The four substrate/slope treatments were assigned to the ramps and retained throughout the study. The five flow rates were randomly assigned on a daily basis but remained consistent throughout one night’s trials. A total of 100 trials were conducted, with five replicates for each substrate/slope type and flow rate combination. At the end of each trial, the recirculatory pumps were stopped and eel present on the ramps were manually washed into the holding nets. The header tank and holding net on each ramp were then emptied of eel. Individuals were used only once and subsequently released to the wild close to the site of capture.
2.4. Data Processing and Analysis
Video footage of the lower pass section (from 0.25 to 0.5 m) was analysed using Tracker 6.1.1 (
https://physlets.org/tracker/index.html, accessed on 14 January 2022), which enabled the manual marking of individual eel and the extraction of the XY cartesian coordinates of their fluorescent VIE mark at a time (t). The large time input required for footage processing did not permit analysis of the entire ascent ramp. Occasionally, individuals climbed onto the vertical walls of the pass and ascended out of the flow; these attempts were excluded. The calibration scale was set using fluorescent distance marks on the passes. The footage was processed at a step rate of 5 frames, which, based on preliminary explorations, represented the best balance of processing time versus the risk of missing the eel. Trials affected by occasional power failures, technical issues with the filming equipment and pump failures were excluded from analysis, yielding a total of 71 trials with a minimum of 3 replicates per substrate/slope type and flow rate combination (
Table S1).
The metrics derived from the eel tracks within the 0.25 m–0.5 m pass section were as follows: (1) No. of attempts per trial, where each attempt commenced when an eel crossed the 0.25 m mark and ended either when it ascended beyond the 0.5 m mark or descended below the 0.25 m mark. Eel were not individually recognisable in the footage, so individual attempt data were not obtainable; (2) Success of attempt—an attempt was deemed successful when the eel reached the 0.5 m distance mark, even if it was visibly washed down or volitionally descended after reaching this point; (3) Track duration (s)—calculated for each trajectory; and (4) Distance ascended/time—calculated for each marked step of the trajectory, where the direction of movement was up the pass, i.e., this excluded steps where the eel remained stationary or descended. Descent movements could not be reliably tracked because they often occurred very quickly, for example, when an individual was washed down in the flow. For the pass crest, the footage was watched back at 3× speed to extract the time at which the first eel in each trial successfully passed over the crest into the header tank. Crest ascent times were compared between substrate types using a Wilcoxon rank sum test. Two analysts processed all the footage in a random order with two trials repeated by both to gauge the consistency of the approach.
Generalised linear models (GLMs) with a binomial error distribution were used to investigate the influence of the substrate type (bristles or studs), lateral slope (flat or V-profile) and flow rate (numerical) on the success of ascent attempts (1 or 0 binary response). GLMs with a gamma error distribution and log link function were used to investigate the influence of the substrate type, lateral slope and flow rate on the distance ascended/time for each step (numerical response). The maximal models with third-order interaction terms were fitted, and a stepwise deletion with likelihood ratio tests was performed to arrive at the most parsimonious model with the lowest AIC value [
36]. The suitability of the error structures was evaluated using plots of standardised residuals against plots of the fitted values. All analyses were conducted in R version 4.3.0. The number of degrees of freedom is denoted by DF and the standard deviation by SD throughout.
3. Results
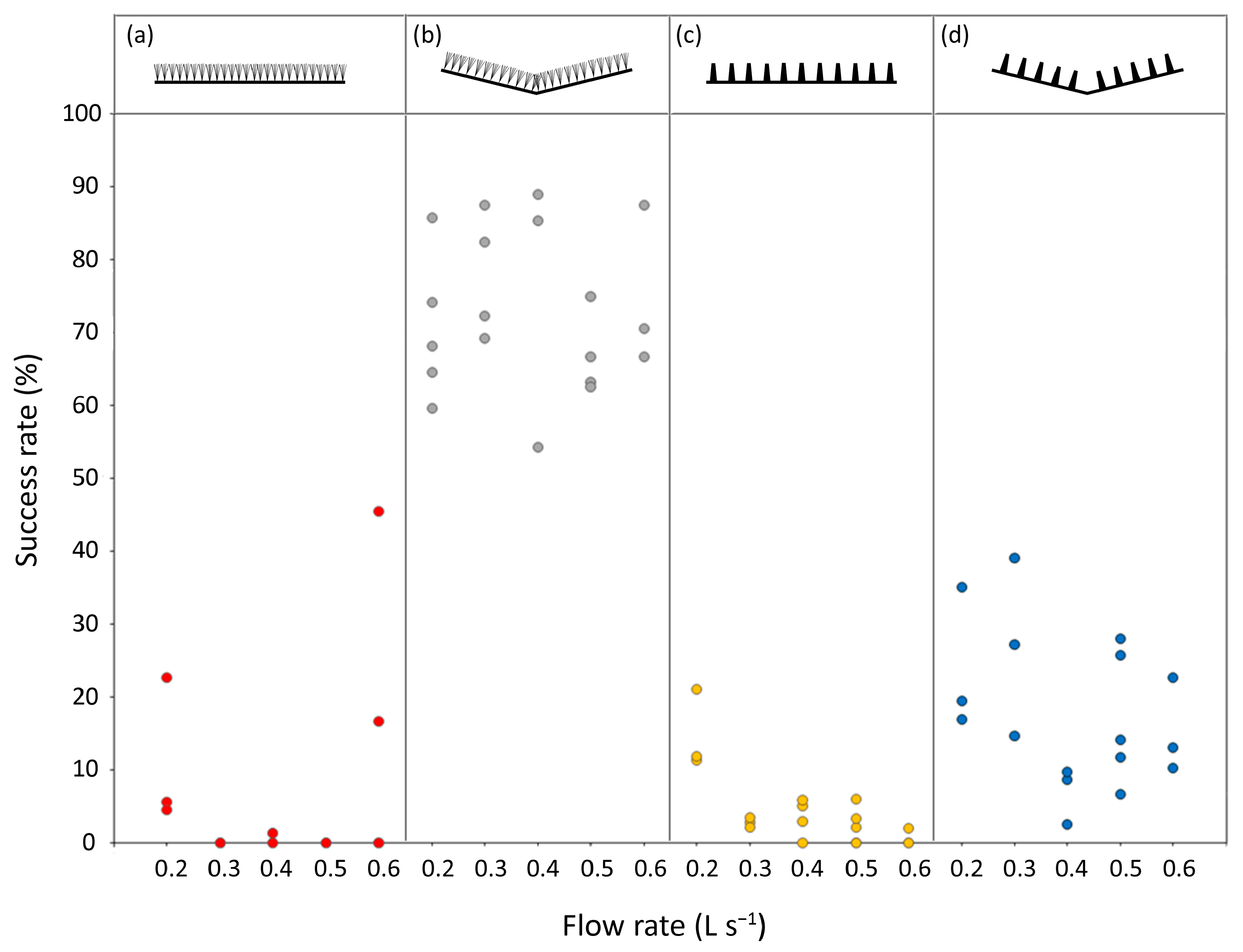
A total of 4032 eel ascent attempts were tracked across the 71 trials analysed. Of these, 615 were successful for the 0.25–0.5 m pass section, with the overall percentage of successful attempts ranging from 0 to 89% across the trials (
Figure 3,
Table S1). In the best fitting model, lateral slope was the strongest predictor of an ascent attempt being successful (deviance = 18.6%,
p < 0.01), followed by the substrate type (deviance = 8.5%,
p < 0.05) and the interaction between them (deviance = 3.0%,
p < 0.01). The flow rate and its interactions with lateral slope and substrate type were weak significant predictors and collectively explained 0.9% of the deviance (
Table S2). The laterally sloped ‘V’ passes, particularly the V bristle pass, were associated with the highest percentages of success (
Figure 3).
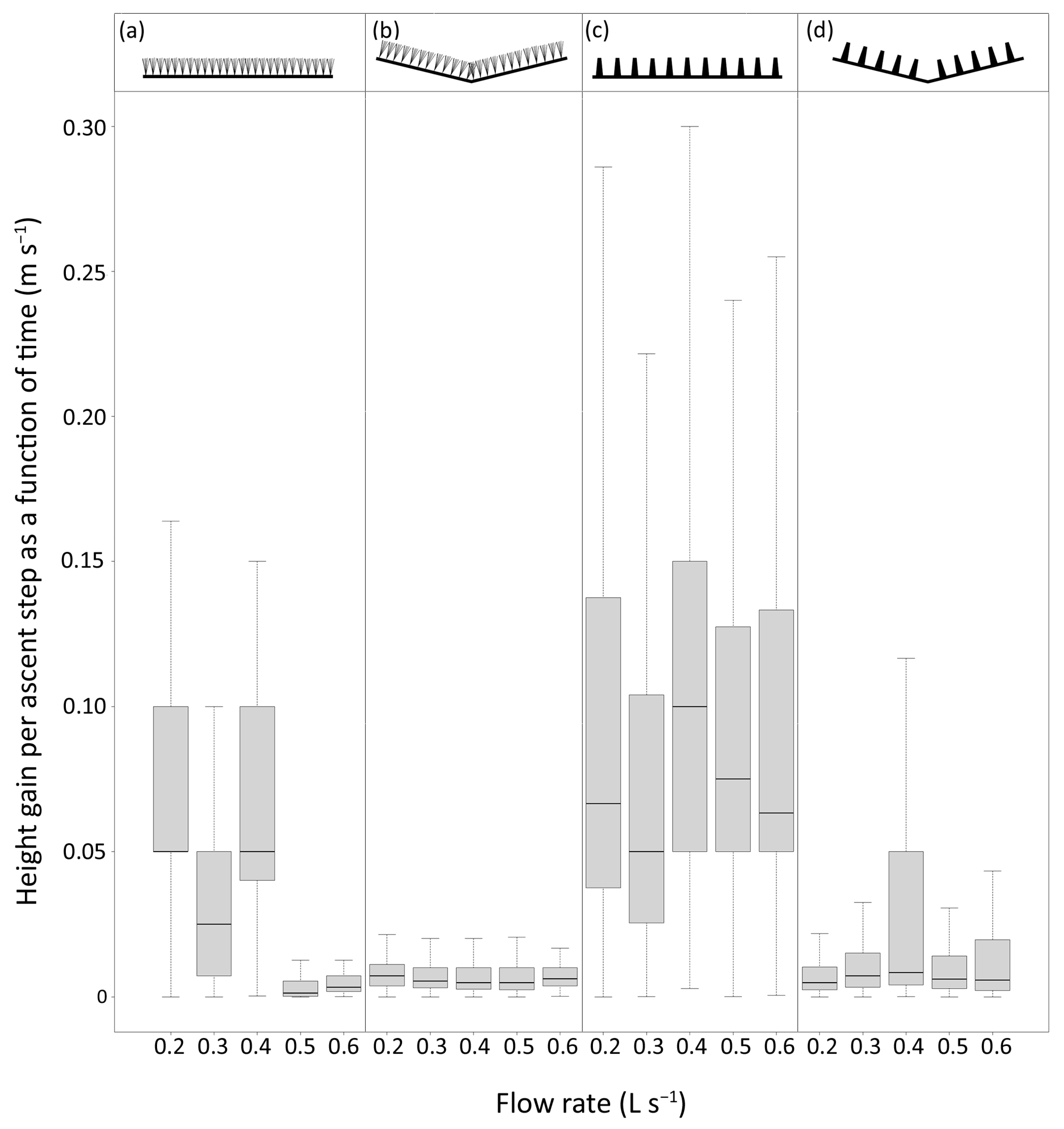
The duration of ascent attempts in the 0.25–0.5 m pass section ranged from less than one second to 108 min, although 86% of attempts lasted less than 1 min (median = 2 s). The rate at which eel advanced up the passes ranged from 3.4 × 10
−6 to 0.7 m s
−1 (median = 0.008 m s
−1). In the best-fitting model, the lateral slope was the strongest predictor of the ascent step rate (deviance = 30.7%,
p < 0.01), followed by the substrate type (deviance = 2.3%,
p < 0.01) and the flow rate (deviance = 1.7%,
p < 0.01). Interaction terms collectively explained 1% of the deviance (
Table S2). The non-laterally sloped (i.e., flat) passes were associated with the fastest ascent rates (
Figure 4).
Successful ascent of the entire 2 m ramp was observed in just 25 of the 71 trials, all within the laterally slopped passes; 72% in the V-shaped bristle pass and 28% in the V-shaped stud pass. The time between eel release and the first successful ascent of the crest ranged from 17 min to 1 h 58 min (median = 44 min) and did not vary between the two V-profile pass types (W = 37, p = 0.12).
4. Discussion
Eel passes are a widely used management tool to help restore fluvial connectivity for migrating eel and allow access to important freshwater habitats upstream of barriers [
25,
37]. Gravity-fed as opposed to pumped passes are a valuable, generally lower-cost option in scenarios where there is no electrical supply and/or high-frequency maintenance is not feasible. However, there is a current dearth of information on the optimum design and operating flows for such passes. Under the range of flows used in the current study (0.2–0.6 L s
−1), the V-shaped bristle pass was consistently the most effective for passing juvenile eel (60–80 mm, total length), while the overall effect of flow rate was minimal and non-linear.
The lateral slope followed by substrate type had the most influence on all the metrics of eel pass performance measured in the current study. Ascent of the full 2 m long ramp by at least one individual within the 2 h trial period was only observed for the V-shaped passes, with 2.5 times more successes for the bristle as opposed to the stud substrate. Despite numerous tracked attempts and successful ascents of the lower section of the ramp for all the substrate/slope combinations, the proportion of successful attempts was consistently highest for the V-shaped bristle pass followed by the V-shaped stud pass. The life stage of eel used was relatively small, a size class that typically dominates in areas close to the tidal limit of rivers [
38,
39]. Bristles are known to be effective for small eel, which rely on a combination of climbing and swimming to ascend structures [
21,
40,
41]. The stud substrate tested, with narrow spacing between studs, has been previously shown suitable for similarly sized small eel (mean length 72 ± 3.9 mm, ± S.D.) [
22], although a comparison of studded versus bristle substrates in eel 60–110 mm indicated that studs were both more attractive and yielded a higher successful passage rate once a climb was initiated [
18]. However, direct comparison of the current results with previous studies is limited by differences in the specifications of materials used, flow rates and the development phase of the study subjects. Very little focus has been directed towards pass optimisation for the early unpigmented glass eel life phase, in no small part due to the logistical difficulties in tracking such small and transparent individuals. Here we demonstrated how the use of VIE marking and appropriate lighting and filming techniques can be applied to help resolve this bias. At elevated flow rates, as may be associated with gravity-fed passes, incorporating a lateral slope is a far more important design criterion than the selection of bristles or studs. In comparison, both of the laterally flat passes performed poorly, irrespective of the substrate.
The flow rates tested in the current study were selected to recreate conditions in gravity-fed passes, which, due to changes in ambient water level, are subject to a far greater range of operating flows than pumped passes. For small eel (60–110 mm, total length), it has been shown that effective passage in bristle and stud passes can be achieved with flows as low as 0.07 L s
−1 [
18], while high velocities result in individuals being repeatedly washed down the ramp [
26]. The inability of any eel in the current study to ascend the full 2 m ramp in either of the laterally flat passes, even in a flow of 0.2 L s
−1, supports the idea that the presence of a low-velocity wetted margin is a crucial design feature for eel passes targeting small-size classes [
13,
42]. Although not tested in the current study, V-shaped passes would be expected to offer an advantage over single-sided laterally sloped passes because two wetted margins are created, one on either side of the central flow plume. Observations of ascending eel on the V-shaped passes in the video footage confirmed that successful ascents were generally in this zone. Further, the most common cause of failure during ascents was when an individual ascending within the wetted perimeter moved towards and entered the central flow plume and was subsequently washed down.
The absence of a strong relationship between the flow rate and the measured passage metrics suggested that, first, the minimum flow rate of 0.2 L s
−1 was too high for the effective operation of the laterally flat passes for this early life stage of the eel, and second, the presence of the wetted perimeter in the V-shaped passes effectively decoupled the influence of the flow. Based on these findings, V-shaped passes would be expected to provide effective passage at even higher flows than those tested, so long as the large wetted perimeter is maintained. Under the tested flow rates of ≤0.6 L s
−1, the central flow plume did not extend to the edges of the pass, which also incidentally rendered the overall pass width to some extent irrelevant. The positive effect of the V-shape on passage would presumably be lost at the point where the increased flow caused the margins of the central flow plume to extend to the vertical walls of the pass, increasing water depth across the pass to a level that small individuals would be forced to swim rather than climb. Juvenile eel of the size studied can burst swim at 0.35–0.5 m s
−1 for a few seconds, but the modelled sustained (30–40 min) swimming speeds are in the order of 0.2–0.3 m s
−1 [
43,
44]. The rapidity of the ascent steps was highest for the laterally flat passes, and an examination of the footage revealed this was attributable to the high incidence of burst swimming behaviour, which was usually immediately followed by washdown. One outlier group in the laterally flat bristle pass under the 0.6 L s
−1 flow rate exhibited an unexpectedly high success rate in the tracked section, although all eel were subsequently washed down. The reasons for this are unclear but may be due to exceptionally motivated individuals in this group despite efforts to avoid sampling bias among the wild-caught fish. Occasionally, eel on the laterally flat passes would rest within the flow by wrapping around a bristle cluster or stud before making another burst swim attempt. In the V-shaped passes, the ascent steps were generally slower because eel tended to climb rather than swim. Eel were also frequently observed resting in these passes, usually within the wetted perimeter or moving just outside of it towards the vertical walls of the ramp.