Abstract
In this study, a linoleic and linolenic acid were incubated with petroleum ether extract, ethyl acetate extract, acetone extract (AE) and aqueous extract of Astragalus membranaceus. The phenolic content and total antioxidant capacity (T-AOC) were determined in the extracts of Astragalus membranaceus (EAms) above. Results showed that EAms decreased the levels of malonaldehyde, conjugated diene, and peroxide value levels in material above. Of all of EAms, AE showed the strongest T-AOC and inhibitory effect on the lipid oxidation. Next, fish feeds were incubated with graded levels of AE. The results showed that AE inhibited lipid oxidation in fish feed. The appropriate dosage for reducing lipid oxidation was 6.74 g AE kg−1 feeds. The effect of EAms on the lipid oxidation may be closely associated with their phenolic content. Then, juvenile Jian carp (Cyprinus carpio var. Jian, 10.2 ± 0.3 g) were fed with diets containing graded levels of AE (0.0, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, and 7.0 g kg−1) for 60 days. Current data displayed that dietary AE increased the growth performance of fish. The optimum dosage for growth promotion was 5.15 g AE kg−1 diet. This result of AE may be ascribed to its enhancing effect on the activity of digestive and absorptive enzymes and antioxidant capacity in digestive organs of fish. Our present study indicated that EAm holds promise as a natural antioxidant for fish and their feed.
Keywords:
Astragalus membranaceus; lipid oxidation; growth performance; digestion; absorption; antioxidant Key Contribution:
A. Extracts of Astragalus membranaceus can depress lipid oxidation in fish feed. B. Extracts of Astragalus membranaceus can improve fish growth performance and antioxidant capacity.
1. Introduction
Fish feed is distinguished by its high lipid content, which notably includes a substantial proportion of unsaturated fatty acids [1,2]. In aquatic feeds, the presence of an abundance of redox-active metal ions and reactive oxygen species (ROS) leads to the rapid oxidation of these unsaturated fatty acids. This oxidation process results in the formation of various oxidative byproducts, including peroxides (PO), conjugated dienes (CD), and malondialdehyde (MDA) [3]. It is recognized that lipid oxidation can degrade nutritional components, produce toxic metabolites, and reduce the shelf life of the feed [4]. Moreover, oxidative deterioration of feed may impair fish growth, decrease disease resistance, and increase mortality rates [5]. It is well known that the antioxidants can quench lipid oxidation in fish feed [6]. Antioxidants are crucial components in the formulation of animal feeds. Currently, the feed industry predominantly utilizes synthetic antioxidants, such as ethoxyquin (EQ), butylhydroxyanisole (BHA), and dibutylhydroxytoluene (BHT). However, these additives have not been shown to have significant benefits for the growth and health of fish [6]. Recent studies from our laboratory have indicated that EQ and BHT are effective in curbing lipid oxidation in fish feed [7]. Yet, there are concerns as several reports suggest potential carcinogenicity and toxicity of these synthetic antioxidants in animal models [8]. Considering their adverse effects on both animal and human health, it is imperative to enforce stringent regulations on the usage of these synthetic antioxidants in the food industry [9]. Otherwise, it would lead to residues and enrichment in fish products, causing food safety risks and endangering human health by consumption [10,11,12]. Meanwhile, the disadvantages associated with synthetic antioxidants will lead to increased market demand for antioxidants from natural ingredients, and it is of great significance to develop new natural feed antioxidants.
In Astragalus membranaceus, a traditional Chinese medicine herb, the main bioactive chemical components are phenols, flavonoids, saponins, and polysaccharides [13]. Recently, our laboratory reported that supplementation of the extract of Astragalus membranaceus (EAm) decreased the generation of ROS in fish erythrocytes induced by the hydroxyl radical (•OH) [14]. Moreover, study in vitro showed that Astragalus mongholicus extract has the effects of scavenging lipid free radicals detected by electron spin resonance (ESR) [15]. Similarly, in the liver of juvenile Pangasianodon hypophthalmus, dietary inclusion of Astragalus membranaceus extract decreased MDA levels, which could be a biomarker of lipid peroxidation [16]. Meanwhile, dietary supplementation of Astragalus membranaceus extract not only enhanced fish growth performance and feed utilization but also improved digestive ability and antioxidant capacity [16]. So, it can be concluded that EAm may be used as a potential natural antioxidant in fish feed. However, there are limited reports on the use of EAm in fish feed.
Jian carp (Cyprinus carpio var. Jian) is one of the most important freshwater farmed and high-yield fish in China because of its fast growth, strong resistance again disease, and strong adaptability [17]. At the same time, its high nutritional value, cheap price, and delicious taste, are favored by consumers [18]. On the basis of former research, EAm might has great application potential as a natural antioxidant element in fish, but the effects of EAm on Jian carp has not been evaluated. Therefore, this study evaluated the mitigation effect of EAm on lipid oxidation in feed, as well as its effects on growth performance, digestion and absorption capability, and antioxidant capacity in Jian carp, providing reference for the application of EAm in aquaculture species.
2. Materials and Methods
2.1. Chemical Reagent
In this research, key reagents of analytical reagent (AR) grade were carefully selected from reputable sources. Chengdu Kelong Chemical Reagent Factory, located in Chengdu of China, supplied essential chemicals such as petroleum ether, ethyl acetate, Tween 20, and acetone. These reagents were instrumental in our experimental procedures. Specifically, for extracting active compounds from Astragalus membranaceus, water were utilized to isolate water-soluble substances, while petroleum ether, ethyl acetate and acetone were effective in extracting water-insoluble substances [19]. Moreover, to ensure high accuracy in our analysis, linoleic acid and linolenic acid of exceptional purity (exceeding 97% and 95%, respectively) were procured from Shanghai Biochemical Reagent Co., Ltd., a renowned supplier based in Shanghai, China. All other chemicals used in the study conformed to the stringent requirements of analytical reagent grade, ensuring the reliability and precision of our experimental results.
2.2. Preparation of EAm
The root of Astragalus membranaceus utilized in this study was obtained from the Chengdu Pharmaceuticals market located in Chengdu, China. The roots, once dried, were processed into fine powder; ensuring particles did not exceed 0.32 mm in size, using a traditional Chinese medicine mill (Model RHP-2000A, Ronghao, Zhejiang, China). For the extraction process, in line with the methodology outlined by Wojcikowski et al. [20], 50 g of this powdered root underwent extraction. This involved the use of 500 mL each of petroleum ether, ethyl acetate, acetone, and water, respectively. The procedure was carried out at a controlled temperature of 20 °C over 8 h, employing an agitator (Model OS40-S, Dalong, Beijing, China) for each solvent. The extraction process for each solvent was meticulously repeated three times to ensure consistency. Post-extraction, the filtrates were subjected to evaporation under reduced pressure using a rotary evaporator (Model RE-52CS, Jinye, Shanghai, China), continuing until a constant dry mass was achieved, as referenced in studies [14,21]. Consequently, this method ensured the complete volatilization of organic solvents in the ethyl acetate extract (EAE), petroleum ether extract (PEE), and acetone extract (AE). The resulting extracts, obtained using water, ethyl acetate, petroleum ether, and acetone, were then securely stored in airtight containers, shielded from light, and maintained at a temperature of −80 °C until further analysis was conducted.
2.3. Measurement of Phenolic Content and Total Antioxidant Capacity (T-AOC)
The quantification of the phenolic compounds in the EAms was conducted based on the protocol established by Yuan et al. [22]. For this analysis, 100 μL of the extract samples were blended with 2 mL of a 2% solution of Na2CO3. This mixture was then left to stand at ambient temperature for 2 min. Following this incubation period, 100 μL of a 50% solution of Folin–Ciocalteau reagent was introduced to the mixture. Subsequently, the samples were left to settle at ambient temperature for an additional period of 30 min without any disturbance. Following this incubation phase, absorbance values were recorded at a 720 nm wavelength. The phenolic composition of the extracts was quantified in terms of gallic acid equivalents. This quantification was carried out through four individual replicates for each sample to ensure accuracy and reproducibility. In assessing the total antioxidant capacity of EAms, the method described by Serpen et al. [23] was employed based on ABTS rapid oxidation. Trolox, a standard antioxidant, was utilized as a reference for calibrating the antioxidant capacity of the samples. This calibration allowed for the expression of the samples’ antioxidant ability in terms of Trolox equivalent antioxidant capacity (TEAC).
2.4. Measurement of Lipid Oxidation in Linoleic Acid and Linolenic Acid Emulsion
To investigate the antioxidant properties of EAms on the emulsion of linoleic acid and linolenic acid, our methodology was adapted from Yuan et al. and Li et al. [22,24]. We prepared the emulsion with equal volumes (0.1 mL) of both acids, combined with 9.9 mL of a phosphate buffer (pH 7.0, 0.02 M) and 50 μL Tween 20, followed by homogenization using a FJ200-SH homogenizer (Shanghai, China) for two 10 s periods at 21,000× g [24]. EAm concentrations, including a control (0 mg mL−1) and 1.0 mg mL−1, were integrated into the emulsion. After 8-day incubation at 45 °C, the levels of MDA, CD, and PO were quantified using the Maqsood and Benjakul method [25]. For this, the emulsion was combined with 2.5 mL of TBA solution, heated, cooled, and centrifuged. Absorbance readings were carried out of the supernatant at 532 nm for MDA. The absorbance of CD was determined by UV-1601 spectrophotometer (Shimadzu, Kyoto, Japan) at 234 nm. The PO measurement involved an emulsion sample with ethanol, ammonium thiocyanate, and ferrous chloride solution, followed by an absorbance reading at 500 nm after 3 min.
2.5. Measurement of Lipid Oxidation in Fish Feed
In this experimental study, AE demonstrated superior total antioxidant capacity (T-AOC) and effectiveness in counteracting oxidative processes in both linoleic and linolenic acids. Given these findings, AE was chosen as the optimal EAm for evaluating the lipid oxidation markers in fish feed. For the preparation of experimental diets, we adhered to a rigorous protocol established by our research team [26]. A series of ten diets was meticulously formulated, incorporating increasing concentrations of AE, starting from a baseline of 0.0 g kg−1 (serving as the control) and escalating up to 9.0 g kg−1 in 1.0 g kg−1 increments. To maintain nutritional integrity, any augmentation in AE levels was counterbalanced by a proportional reduction in the cellulose content of the diet. As delineated in Table 1, the basic composition of the diet included 34.32% crude protein and 6.84% crude lipid. The processing method for this feed mirrored the emulsification process earlier described for linoleic and linolenic acids. To quantify the lipid oxidation in the fish feed, we employed established analytical methods to measure the levels of malondialdehyde (MDA), conjugated diene (CD), and peroxide (PO), with the results expressed as a percentage relative to the control group.

Table 1.
Composition and nutrient content of the basal diet.
2.6. Feeding Trial
Juvenile Jian carp were obtained from a fish farm in Neijiang, China. In the laboratory, their habitat was meticulously replicated to mirror their natural environment, maintaining a temperature of (22.0 ± 1) °C, with daily changes of dechlorinated water and a natural light cycle [26,27]. The baseline diet was provided for the fish. A total of 640 juvenile Jian carp, each weighing an average of 10.2 ± 0.3 g, were distributed randomly into eight experimental groups. Each group was allocated four tanks, with each tank housing 20 fish. The experimental diets fed to these carp were carefully prepared with incremental concentrations of AE, namely 0.0, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, and 7.0 g per kilogram of feed. Over a period of 60 days, each group of fish in the study received eight daily feedings. This feeding regime was meticulously monitored to achieve full satiation without the risk of overfeeding, as per the guidelines in the referenced study [28]. To accurately assess various growth parameters, the fish were weighed and counted both at the onset and conclusion of the trial. These measurements facilitated the calculation of several key indicators: survival rate (SR), feed intake (FI), weight gain (WG), feed efficiency (FE), and specific growth rate (SGR).
The methodology for collecting samples was in line with the standardized procedures developed by our research team [29]. Following the completion of the experimental phase, the fish were gently sedated using a 50 mg L−1 benzocaine solution to minimize stress. Once the fish was sedated and killed, and key organs such as the hepatopancreas and intestine were carefully extracted for further study. To preserve the integrity of these tissues for subsequent analyses, they were immediately frozen and stored at an ultra-low temperature, specifically −80 °C.
2.7. Biochemical Analysis
Tissue specimens were processed by homogenization in a solution comprising nine parts (w/v) of chilled physiological saline. This homogenate then underwent centrifugation at 3200× g at a temperature of 4 °C for duration of 20 min. The resulting supernatant was preserved for the analysis of various enzymes, including trypsin, lipase, alpha-amylase (referred to as amylase), alkaline phosphatase (AKP), and Na+/K+-ATPase, as delineated in our preceding study [29]. Additionally, assessments for anti-superoxide anion (ASA) capacity and reduced glutathione (GSH) level were conducted using the same method. Parallel, enzyme activities such as catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR), and glutathione peroxidase (GPx) in the intestinal or hepatopancreatic tissues were analyzed following the methodologies established by Jiang et al. [30]. MDA measurement was consistent with those in feed as described by Maqsood and Benjakul [25]. Protein content was quantified according to the method of Bradford [31]. The above indexes read the color intensity at different wavelengths to calculate enzyme activities or contents.
2.8. Statistical Analysis
The study’s results are displayed as mean values ± standard deviation (S.D), with in vitro studies involving three replicates (n = 3) and animal experiments comprising four replicates (n = 4). For the statistical evaluation of the data, a one-way analysis of variance (ANOVA) was utilized, employing SPSS software, version 15.0. Prior to conducting the ANOVA, the data underwent preliminary tests for consistency and normality, specifically through Levene’s test for homogeneity and the Shapiro–Wilk test for normal distribution. To identify significant variances between the groups post-analysis, Duncan’s multiple range tests were applied for detailed post hoc comparison.
3. Results
3.1. Phenolic Content and Total Antioxidant Capacity in EAms
We determined glycosides, flavonoids and phenolic content in EAms. The correlation analysis results show that the phenolic compounds have the most effective antioxidant activity. Thus, only phenolic compounds were listed in this study. According to Table 2, AE had a significantly higher phenolic content than other EAms (p < 0.05). The phenolic content in EAE was substantially lower than in AE (p < 0.05). AQE phenolic content was notably lower compared to other extracts (p < 0.05), while PEE had a higher phenolic content than AQE. The content of phenolic of PEE was higher than AQE (p < 0.05). Regarding T-AOC, AE exhibited higher levels than other extracts (p < 0.05), followed by EAE, PEE, and AQE. Figure 1 presents the power regression analysis of phenolic content in EAms against T-AOC, revealing a positive correlation between T-AOC and phenolic content in EAms.

Table 2.
The total antioxidant capacity (T-AOC) and phenolic content of petroleum ether extract (PEE), acetone extract (AE), ethyl acetate extract (EAE), and aqueous extract (AQE) of Astragalus membranaceus.
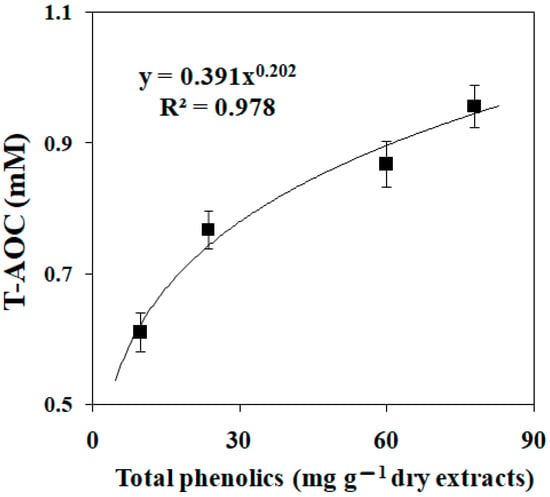
Figure 1.
Power regression of the total antioxidant capacity (T-AOC) of Astragalus membranaceus extracts containing different levels of phenolics. The data were the means ± S.D. of 3 replicates.
3.2. Effects of EAm on the Lipid Oxidation in Linoleic Acid and Linolenic Acid Emulsion
Table 3 presents a correlation analysis between EAms and the concentrations of MDA, CD, and PO in a linoleic acid emulsion. It was observed that the levels of PO were significantly reduced when treated with AE and EAE, with PEE also demonstrating a reduction, albeit to a lesser extent (p < 0.05). A similar trend was noted for CD concentrations under various EAm treatments. In terms of reducing MDA content, AE was the most effective, closely followed by EAE and PEE. Conversely, the application of AQE resulted in the highest recorded levels of PO, CD, and MDA, suggesting its relative ineffectiveness in curtailing the oxidation of linoleic acid (p < 0.05).

Table 3.
The peroxide value (PO), malonaldehyde (MDA), and conjugated diene (CD) levels in a linoleic acid emulsion treated with 1.0 mg mL−1 of ethyl acetate extract (EAE), petroleum ether extract (PEE), aqueous extract (AQE), and acetone extract (AE) of Astragalus membranaceus.
Table 4 outlines the effects of EAms on the levels of MDA, CD, and PO in a linolenic acid emulsion. The results indicated a significant reduction in PO concentrations with AE treatment, while EAE and PEE treatments also contributed to decreases, although to a smaller degree (p < 0.05). The influence of AE and EAE was notable in lowering CD levels, with PEE showing a similar but more modest impact (p < 0.05). This trend was mirrored in the reduction of MDA content across the different EAm treatments. Among the extracts tested, AQE was associated with the highest concentrations of PO, CD, and MDA, suggesting its limited efficacy in reducing the oxidation of linolenic acid (p < 0.05).

Table 4.
The peroxide value (PO), malonaldehyde (MDA), and conjugated diene (CD) levels in a linolenic acid emulsion treated with 1.0 mg mL−1 of ethyl acetate extract (EAE), petroleum ether extract (PEE), aqueous extract (AQE), and acetone extract (AE) of Astragalus membranaceus.
Figure 2 depicts the association between the phenolic content present in EAms and the concentrations of PO, MDA, and CD in linolenic and linoleic acid emulsions. The data indicate a direct relationship where increased phenolic content in EAms correlates with higher levels of PO, CD, and MDA.


Figure 2.
The correlations of phenolic content in the extracts of Astragalus membranaceus with the levels of peroxide value (PO), conjugated diene (CD), and malondialdehyde (MDA) in linoleic acid emulsion (A) and linolenic acid emulsion (B). The data were the means ± S.D. of 3 replicates.
3.3. The Effects of AE on the Lipid Oxidation in Fish Feed
Figure 3 details the impact of AE on the levels of PO, MDA, and CD in fish feed. It was observed that the inclusion of AE in the diet led to a significant reduction in the concentrations of these compounds. This reduction was most notable with AE amounts of up to 6.0 g kg−1, as evidenced by a decrease in PO, CD, and MDA levels (p < 0.05). Beyond this concentration of AE in the diet, the downward trend in these compounds’ levels reached a plateau, with no significant changes observed at higher concentrations of AE (p > 0.05). Broken line analysis indicated that the optimal AE inclusion levels for minimizing PO, CD, and MDA in fish feed were 6.09 g kg−1, 6.74 g kg−1, and 6.29 g kg−1 of the diet, respectively.

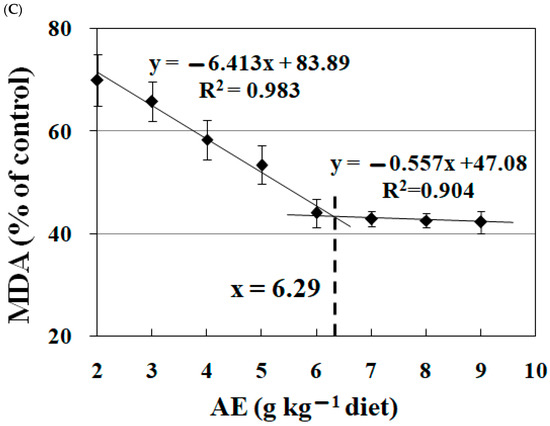
Figure 3.
Broken-line analysis of peroxide value (PO, (A)), conjugated diene (CD, (B)), and malondialdehyde (MDA, (C)) levels in fish feed treated with graded levels of acetone extract (AE) of Astragalus membranaceus. The date were the means ± S.D. of 3 replicates.
3.4. Effects of Dietary AE on Fish Growth Performance
Table 5 shows that varying AE levels in the diet did not significantly impact fish FE and SR (p > 0.05). Increasing dietary AE to 3.0 g kg−1 significantly enhanced WG (p < 0.05), with no further significant changes beyond this concentration (p > 0.05). Similar trends were observed for FBW, SGR, and FI. Polynomial regression analysis identified 5.15 g kg−1 as the optimal AE inclusion level for WG, as depicted in Figure 4. The maximum effective AE level in the diet was determined to be 10.30 g kg−1.

Table 5.
Initial body weight (IBW), final body weight (FBW), weight gain (WG), specific growth rate (SGR), feed intake (FI), feed efficiency (FE), and survival ratio (SR) of Jian carp fed diets containing graded levels of acetone extract (AE) of Astragalus membranaceus for 60 days.

Figure 4.
Polynomial regression analysis of weight gain (WG) for Jian carp fed diets containing graded levels of acetone extract (AE) of Astragalus membranaceus for 60 days. The data were means ± S.D. of 4 replicates, with 20 fish in each replicate.
3.5. Effects of Dietary AE on the Biochemical Parameters in Hepatopancreas of Jian Carp
According to the data in Table 6, increasing AE up to 3.0 g kg−1 in the diet led to a notable rise in trypsin activity, a change that was statistically significant (p < 0.05). Beyond this level of AE, the increase in trypsin activity stabilized, showing no significant improvement with higher AE concentrations (p > 0.05). This pattern was also observed in SOD and CAT activities. ASA capacity and lipase activity in hepatopancreas were higher in fish feed with AE supplementation of 4.0 and 2.0 g kg−1 diet, respectively. Enzyme amylase and GPx similarly showed significant increases in their activities at AE concentrations of 3.0 g kg−1 and 2.0 g kg−1 of the diet (p < 0.05), respectively, showing no significant improvement with higher AE concentrations (p > 0.05). Conversely, MDA content in the intestine was reduced when the diet included 6.0 and 7.0 g kg−1 AE.

Table 6.
The activities of trypsin, lipase, amylase, superoxide dismutase (SOD), anti-superoxide anion (ASA), glutathione peroxidase (GPx), catalase (CAT), and malondialdehyde level (MDA) in hepatopancreas of Jian carp fed diets containing different levels of acetone extract (AE) of Astragalus membranaceus for 60 days.
3.6. Effects of Dietary AE on the Biochemical Parameters in Intestine of Jian Carp
Table 7 presents data showing that the activities of trypsin and Na+/K+-ATPase, along with the GSH content in the fish intestine, were enhanced as AE levels in the diet increased, up to a maximum of 6.0 g kg−1. Amylase activity showed significant improvement at AE concentrations of 3.0 g kg−1 (p < 0.05), but this enhancement stabilized, with no further significant changes at higher AE levels (p > 0.05). ASA capacity and GR activity exhibited similar trends. Notably, lipase activity rose significantly increasing concomitant with AE levels up to 4.0 g kg−1 (p < 0.05), remained constant at an AE content of 6.0 g kg−1 (p > 0.05), and then decreased with further increases in AE concentration. AKP activity demonstrated a similar trend. Conversely, MDA content in the intestine was reduced when the diet included 6.0 g kg−1 of AE.

Table 7.
The activities of amylase, lipase, trypsin, alkaline phosphatase (AKP), Na+/K+-ATPase, glutathione reductase (GR), and anti-superoxide anion (ASA) as well as the contents of reduced glutathione (GSH) and malondialdehyde (MDA) in intestine of Jian carp fed diets containing different levels of acetone extract (AE) of Astragalus membranaceus for 60 days.
4. Discussion
4.1. EAm Inhibited Lipid Oxidation in Fish Feed
PO, CD, and MDA represent different stages of lipid oxidation in fish feed, occurring in the early, middle, and later phases, respectively, as noted in previous studies [24]. This research found that AE, an extract of Astragalus membranaceus, effectively reduced these oxidative products in fish feed. This aligns with previous findings where AE was shown to decrease MDA levels in fish erythrocytes in vitro [14]. However, the interaction between EAm and lipid oxidative products in fish feed has not been extensively studied before. Our findings suggest that the EAm can mitigate lipid oxidation in fish feed, positioning it as a potential natural antioxidant. Utilizing a broken-line model analysis for PO, CD, and MDA, we identified the optimal AE supplementation levels in fish feed as 6.74 g kg−1.
In the realm of aquaculture nutrition, the oxidative stability of fish feed is a subject of considerable importance, primarily due to the inherent nature of its fatty acid composition. Predominantly, the presence of unsaturated fatty acids within the feed is a critical aspect in this context. The process of autoxidation of these unsaturated fats stands as the primary mechanism triggering lipid oxidation in fish feeds, a phenomenon well-documented in scholarly research [26]. Among these unsaturated fatty acids, certain types, such as linoleic acid (18:2n-6) and linolenic acid (18:3n-3), are recognized for their essentiality in the dietary requirements of freshwater fish species [32]. Our investigation delved into the role of EAm in mitigating this oxidation process. This study specifically highlights that EAm exhibits a notable efficacy in lowering the concentrations of various oxidative markers, including PO, CD, and MDA, within emulsions composed of linoleic and linolenic acids. This reduction points towards the potential of EAm in impeding the lipid oxidation cycle, particularly in unsaturated fatty acids. The action of EAm appears to extend beyond merely halting the initial phase of oxidation; it seemingly disrupts the subsequent secondary phase as well, thereby suggesting a comprehensive impact on the lipid oxidation pathway. Among the various extracts tested, AE of Astragalus membranaceus stood out as the most effective in reducing lipid oxidation in fatty acid emulsions. This finding is consistent with previous research, which demonstrated that the ethanol extract of Astragalus membranaceus effectively inhibited lipid oxidation in vitro [15].
ROS are crucial in the lipid oxidation of unsaturated fatty acids [7]. T-AOC measuring free radical scavenging activity, indicates antioxidative capacity in both hydrophilic and lipophilic substances [33,34]. In this study, EAm demonstrated strong T-AOC similar to trolox that was determined by the ABTS assay. Previous research from our laboratory showed that EAm reduced ROS levels in fish erythrocytes, particularly against hydroxyl radicals (•OH) [14]. In vitro studies also revealed EAm effectiveness in scavenging lipid free radicals, confirmed by electron spin resonance (ESR) analysis [15]. These results align with previous reports, suggesting that EAm could prevent lipid oxidation in fish feed unsaturated fatty acids by enhancing free radical scavenging activity.
The advantageous influence of EAm on mitigating lipid oxidation in fish feed might be intricately linked to its chemical constituents. In our research, a pronounced correlation was observed between the phenolic content of EAms and the reduced levels of PO, CD, and MDA in unsaturated fatty acids. This is in alignment with existing literature which suggests that phenolic compounds play a protective role against the oxidation of the free fatty acid fraction [35]. The hydroxyl groups present in these phenolic compounds are essential for their antioxidative functionality. They not only contribute hydrogen atoms to neutralize lipid free radicals, but also facilitate the formation of stable lipid derivatives and antioxidative free radicals, enhancing the overall antioxidant defense mechanism [36]. In the processes of the free radical chain reaction of lipid oxidation, phenolic compounds participate in inhibiting two crucial steps by reacting with ROO• and inhibiting formation of hydroperoxides, as well as reacting with RO• and inhibiting formation of aldehyde [37]. This result indicated that phenolic compounds in EAms could depress lipid oxidation in unsaturated fatty acids. Meanwhile, in the present study, the T-AOC of EAms was positively correlated with the total phenol contents. Similarly, strong relationships were observed between phenolic content in the extract of Astragalus membranaceus flowers and their antioxidant capacity [38]. This result suggested that phenolic compounds in EAms enhanced free radical scavenging activity. It has been reported that there are three possible antioxidant mechanisms for phenolic compounds at the molecular level, namely, inactivation of free radicals, chelation of free metals, as well as avoiding the reactions of free radical generation [39]. These results confirmed that the antioxidant activity of EAm in fish feed might be caused by the phenolic compounds.
4.2. Dietary EAm Supplementation Improved the Growth Performance in Fish
In this research, the addition of AE to the diet was observed to enhance FI, WG, and SGR in Jian carp, suggesting that EAm supplementation can positively impact the growth of these fish. Through polynomial regression analysis focusing on WG, it was determined that the ideal amount of AE supplementation for juvenile Jian carp is 5.15 g per kg of diet. This finding aligns with previous studies reporting the beneficial effects of dietary Astragalus membranaceus on the growth of other fish species, such as bluegill sunfish (Lepomis macrochirus) and yellow perch (Perca flavescens) [40,41]. Notably, Elabd et al. identified an enhancement in growth performance attributed to the up-regulation of insulin-like growth factor-1, a key molecular biomarker associated with growth [41]. However, it is important to note that there have been relatively few studies exploring the specific growth-promoting effects of EAm on fish.
The growth performance of fish is intricately linked to their ability to digest nutrients, a process largely governed by the efficacy of digestive enzymes [29]. Such enzymes, including amylase, trypsin, chymotrypsin, and lipase are primarily synthesized in the exocrine pancreas of fish and subsequently secreted into the intestine to aid digestion [42,43]. In this research, it was observed that the supplementation of the diet with AE led to an increase in the activities of digestive enzymes such as trypsin, lipase, and amylase. This enhancement suggests that the dietary inclusion of EAm potentially improves the digestive capability of fish, facilitating better nutrient assimilation. Our results were shown to be in conformity with the report that dietary Astragalus membranaceus nanoparticles enhanced digestive ability, which was manifested by increased lipase and amylase activities in Nile tilapia (Oreochromis niloticus) [44].
The growth performance in fish is not only dependent on nutrient digestion but also significantly influenced by the efficiency of nutrient absorption. The efficacy of absorption is greatly influenced by brush border membrane enzymes [29]. Na+/K+-ATPase, a key enzyme in this process, significantly contributes to the generation of potential energy from the sodium gradient, which is vital for the cellular uptake of essential nutrients like glucose, phosphate, and amino acids [45]. Additionally, AKP is integral for the absorption of food molecules and is a measure of the absorptive capacity in fish [46]. In our investigation, we found that diet supplementation with AE increased the activity levels of both AKP and Na+/K+-ATPase, indicating an improvement in nutrient absorption in fish. This enhancement is supported by previous studies, which showed an increase in AKP activity in shrimp following the introduction of Astragalus membranaceus in their diet [47].
4.3. Dietary EAm Improved the Antioxidant Capacity in Digestive and Absorptive Organs of Fish
In aquatic species, antioxidants play a crucial role in maintaining the health and functionality of tissues and organs [48]. This is particularly evident in fish, where the structural integrity of the intestines is essential for effective digestion and nutrient absorption [29]. ROS, produced during aerobic metabolic activities and potentially sourced externally, contribute to cellular damage, negatively impacting various cellular components like lipids and proteins, thereby potentially impairing cellular functions [49]. Lipid peroxidation, indicated by elevated levels of MDA, is a primary marker of such damage, especially relevant in fish due to their high PUFA content [48]. Our research indicates that incorporating AE into the diet significantly lowers MDA levels in the fish’s hepatopancreas and intestine, implying that EAm supplementation in diets can mitigate lipid peroxidation in these organs. This is consistent with earlier studies, which have showned reduced liver MDA levels in juvenile Pangasianodon hypophthalmus fed diets enriched with Astragalus membranaceus extract [16].
Moreover, an increase in ROS production, particularly •OH and superoxide anion (O2•–), was identified as a catalyst for initiating lipid oxidation [30]. This escalation also compromises the structural stability of proteins and lipids due to the excess generation of various free radicals, such as O2•– and •OH. Such oxidative stress leads to molecular damage, impacting cellular functions and health [50]. This research found that supplementation of AE in the diet enhanced ASA capacity in the hepatopancreas and intestine of fish, suggesting an increase in O2•– scavenging ability in fish digestive and absorptive organs. This aligns with previous findings indicating that EAm decreased the O2•– levels in fish erythrocytes under •OH stress [14]. An in vitro study revealed that Astragalus membranaceus exhibits antioxidant effects by scavenging O2•– [51]. Similarly, extract of Astragalus mongholicus demonstrated a dose-dependent effect in scavenging •OH in vitro [15]. Additionally, oral administration of Astragalus mongholicus was shown to decrease ROS levels in rat brains [15]. Abuelsaad’s research indicated that oral Astragalus polysaccharides reduced ROS production in the intestinal tissues of infected mice [52]. These effects of EAm could be related to the chemical structure of its phenolic compounds, which are known for their free radical-scavenging abilities, as reported by Hidalgo and Almajano [53]. It has also been demonstrated that phenol can protect against ROS in rainbow trout gill cells [54].
The antioxidative mechanisms within fish, encompassing both non-enzymatic and enzymatic components, have evolved to effectively counteract or repair the damage caused by ROS to fish tissues and overall organism health [55]. Within these antioxidative enzyme systems, SOD plays a pivotal role as the primary enzyme that interacts with O2•–, converting it into hydrogen peroxide (H2O2) and dioxygen [48]. Subsequently, H2O2 is detoxified by enzymes such as GPx and CAT [56]. In this particular study, the addition of AE to the diet was found to elevate the activities of GPx, CAT, and SOD in the hepatopancreas, suggesting that dietary EAm supplementation enhances the antioxidative capacity in fish. This observation aligns with prior research which demonstrated that diets enriched with Astragalus membranaceus extract boosted the activities of CAT, GPx, and SOD in the liver of juvenile Pangasianodon hypophthalmus [16]. The beneficial effects of EAm may be correlated with the phenolic compound. In Cat fish (Clarias batrachus), the increased activities of CAT and SOD in liver were response to dietary supplementation of phenolic compounds [57]. Moreover, a mixture of Crataegus hupehensis, Angelica sinensis, and Astragalus membranaceus supplementation showed significant up-regulation of SOD and CAT genes in the intestine of Nile Tilapia [58].
Maintaining robust glutathione antioxidant status is key in controlling cell damage which induced by free radicals [59]. The glutathione-dependent antioxidant system, crucial in preventing radical-induced cellular damage, includes GR, glutathione-S-transferase (GST), and GSH [60]. GR plays a vital role in regenerating reduced glutathione from its oxidized form, using NADPH as the electron donor [61]. In the present study, dietary AE supplementation increased activity of GR in fish intestine. These results were in conformity with our laboratory report that EAm restored the GR activity in fish erythrocytes under •OH exposure [14]. In this study, dietary AE supplementation improved GSH content in fish intestine. These results were in conformity with the report that phenolic compounds increased the GSH level in the liver of Cat fish [57]. Therefore, our present study indicated that EAm improved enzymatic and non-enzymatic antioxidant capacity so as to enhance the radical-scavenging ability in fish intestine. However, the mechanism of EAm regulated fish antioxidant capacity needs to be further explored.
5. Conclusions
Hence, it can be deduced that EAm plays an important role in minimizing lipid oxidation in fish feed by safeguarding unsaturated fatty acids. Through broken-line analysis, the ideal dietary levels of AE supplementation in fish feed were determined to be 6.74 g kg−1. The antioxidant properties of EAm are intrinsically linked to their phenolic content. Additionally, incorporating EAm into the diet was found to enhance fish growth. Polynomial regression analysis revealed that the optimal concentration of AE supplementation for juvenile Jian carp was 5.15 g kg−1 diet. Moreover, dietary EAm supplementation boosted the digestive, absorptive and antioxidant capabilities of the fish’s digestion and absorption organs, thereby preserving their structure and function, enhancing nutrient absorption, and consequently improving growth performance. The antioxidant efficiency of EAm is intimately associated with its phenolic constituents. This study suggests that EAm holds promise as a natural antioxidant for fish and their feed.
Author Contributions
Conceptualization, J.X. and H.L.; methodology, J.X., G.C., M.W. and Q.Y.; formal analysis, G.C. and M.W.; validation, J.X., G.C., Q.Y. and H.L.; data curation, J.X. and M.W.; resources, H.L.; writing—original draft preparation, J.X., G.C., Q.Y. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Sichuan Science and Technology Program [grant number 2018JY0214], the Patent Project of Neijiang Normal University [grant number Z2019027], and the Talent Program of Neijiang Normal University [grant number R2019015].
Institutional Review Board Statement
All procedures were approved by the Neijiang Normal University Institutional Animal Care and Use Committee (Approval Code: JM2016-11; Approval Date: 1 November 2023).
Data Availability Statement
The data used to generate the results in this manuscript can be made available if requested from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Godwin, A.; Prabhu, H.R. Lipid peroxidation of fish oils. Indian J. Clin. Biochem. 2006, 21, 202–204. [Google Scholar] [CrossRef]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Guo, X.Y.; Chen, S.N.; Cao, J.Y.; Zhou, J.Y.; Chen, Y.Z.; Jamali, M.A.; Zhang, Y.W. Hydrolysis and oxidation of protein and lipids in dry-salted grass carp (Ctenopharyngodon idella) as affected by partial substitution of NaCl with KCl and amino acids. RSC Adv. 2019, 9, 39545–39560. [Google Scholar] [CrossRef]
- Filipe, D.; Gonçalves, M.; Fernandes, H.; Oliva-Teles, A.; Peres, H.; Belo, I.; Salgado, J.M. Shelf-Life performance of fish feed supplemented with bioactive extracts from fermented olive mill and winery by-products. Foods 2023, 12, 305. [Google Scholar] [CrossRef]
- Andrews, J.T.; Giesen, A.F.; Scott, F.R. Antioxidants manage effects of oxidation on feeds, feed ingredients. Glob. Aquac. Advocate 2004, 12, 66–68. [Google Scholar]
- Hamre, K.; Kolås, K.; Sandnes, K. Protection of fish feed, made directly from marine raw materials, with natural antioxidants. Food Chem. 2010, 119, 270–278. [Google Scholar] [CrossRef]
- Li, H.; Zhou, X.; Gao, P.; Li, Q.; Li, H.; Huang, R.; Wu, M. Inhibition of lipid oxidation in foods and feeds and hydroxyl radical-treated fish erythrocytes: A comparative study of Ginkgo biloba leaves extracts and synthetic antioxidants. Anim. Nutr. 2016, 2, 234–241. [Google Scholar] [CrossRef]
- Ito, N.; Fukushima, S.; Tsuda, H. Carcinogenicity and modification of the carcinogenic response by BHA, BHT, and other antioxidants. Crit. Rev. Toxicol. 1985, 15, 109–150. [Google Scholar] [CrossRef]
- EFSA. Safety and efficacy of a feed additive consisting of ethoxyquin (6-ethoxy-1, 2-dihydro-2, 2, 4-trimethylquinoline) for all animal species (FEFANA asbl). EFSA J. 2022, 20, e07166. [Google Scholar]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Santos, M.J.M.C.; Silva, L.K.R.; Pereira, L.C.L.; Santos, I.A.; Silva Lannes, S.C.; Silva, M.V. Natural antioxidants used in meat products: A brief review. Meat Sci. 2019, 148, 181–188. [Google Scholar] [CrossRef]
- Singh, B.K.; Tiwari, S.; Dubey, N.K. Essential oils and their nanoformulations as green preservatives to boost food safety against mycotoxin contamination of food commodities: A review. J. Sci. Food Agric. 2021, 101, 4879–4890. [Google Scholar] [CrossRef]
- Agyemang, K.; Han, L.; Liu, E.; Zhang, Y.; Wang, T.; Gao, X. Recent advances in Astragalus membranaceus anti-diabetic research: Pharmacological effects of its phytochemical constituents. Evid.-Based Compl. Alt. 2013, 2013, 654643. [Google Scholar] [CrossRef]
- Li, H.; Zhou, X.; Wu, M.; Deng, M.; Wang, C.; Hou, J.; Mou, P. The cytotoxicity and protective effects of Astragalus membranaceus extracts and butylated hydroxyanisole on hydroxyl radical-induced apoptosis in fish erythrocytes. Anim. Nutr. 2016, 2, 376–382. [Google Scholar] [CrossRef]
- Aldarmaa, J.; Liu, Z.; Long, J.; Mo, X.; Ma, J.; Liu, J. Anti-convulsant effect and mechanism of Astragalus mongholicus extract in vitro and in vivo: Protection against oxidative damage and mitochondrial dysfunction. Neurochem. Res. 2010, 35, 33–41. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.; Ahmed, H.A.; Shukry, M.; Chaklader, M.R.; Saleh, R.M.; Khallaf, M.A. Astragalus membranaceus extract (AME) enhances growth, digestive enzymes, antioxidant capacity, and immunity of Pangasianodon hypophthalmus juveniles. Fishes 2022, 7, 319. [Google Scholar] [CrossRef]
- Mirghaed, A.T.; Fayaz, S.; Hoseini, S.M. Effects of dietary 1, 8-cineole supplementation on serum stress and antioxidant markers of common carp (Cyprinus carpio) acutely exposed to ambient ammonia. Aquaculture 2019, 509, 8–15. [Google Scholar] [CrossRef]
- Li, S.; Ji, H.; Zhang, B.; Zhou, J.; Yu, H. Defatted black soldier fly (Hermetia illucens) larvae meal in diets for juvenile Jian carp (Cyprinus carpio var. Jian): Growth performance, antioxidant enzyme activities, digestive enzyme activities, intestine and hepatopancreas histological structure. Aquaculture 2017, 477, 62–70. [Google Scholar] [CrossRef]
- Ning, R.; Li, Y.; Wang, C.; Ji, M.; Shi, Z. Research on antimicrobial activities of crude extracts from Artemesia apiacea Hance. Jiangsu Agric. Sci. 2007, 3, 61–63. [Google Scholar]
- Wojcikowski, K.; Stevenson, L.; Leach, D.; Wohlmuth, H.; Gobe, G. Antioxidant capacity of 55 medicinal herbs traditionally used to treat the urinary system: A comparison using a sequential three-solvent extraction process. J. Altern. Complem. Med. 2007, 13, 103–110. [Google Scholar] [CrossRef]
- Dalar, A.; Türker, M.; Zabaras, D.; Konczak, I. Phenolic composition, antioxidant and enzyme inhibitory activities of Eryngium bornmuelleri leaf. Plant Food. Hum. Nutr. 2014, 69, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.V.; Bone, D.E.; Carrington, M.F. Antioxidant activity of dulse (Palmaria palmata) extract evaluated in vitro. Food Chem. 2005, 91, 485e94. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V.; Fogliano, V. Total antioxidant capacities of raw and cooked meats. Meat Sci. 2012, 90, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tang, S.; Du, W.; Jiang, J.; Peng, P.; Yuan, P.; Liao, Y.; Long, J.; Zhou, S. The effects of ethoxyquin and Angelica sinensis extracts on lipid oxidation in fish feeds and growth, digestive and absorptive capacities and antioxidant status in juvenile red carp (Cyprinus carpio var. xingguonensis): A comparative study. Fish Physiol. Biochem. 2019, 45, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, S.; Benjakul, S. Comparative studies of four different phenolic compounds on in vitro antioxidative activity and the preventive effect on lipid oxidation of fish oil emulsion and fish mince. Food Chem. 2010, 119, 123–132. [Google Scholar] [CrossRef]
- Li, H.; Yang, D.; Li, Z.; He, M.; Li, F.; Jiang, J.; Tang, S.; Peng, P.; Du, W.; Ma, Y.; et al. Effects of Angelica sinensis extracts on lipid oxidation in fish feeds and growth performance of juvenile Jian carp (Cyprinus carpio var. Jian). Anim. Nutr. 2019, 5, 109–114. [Google Scholar] [CrossRef]
- Chen, G.F.; Feng, L.; Kuang, S.Y.; Liu, Y.; Jiang, J.; Hu, K.; Jiang, W.D.; Li, S.H.; Tang, L.; Zhou, X.Q. Effect of dietary arginine on growth, intestinal enzyme activities and gene expression in muscle, hepatopancreas and intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Brit. J. Nutr. 2012, 108, 195–207. [Google Scholar] [CrossRef]
- Li, H.; Lu, L.; Wu, M.; Xiong, X.; Luo, L.; Ma, Y.; Liu, Y. The effects of dietary extract of mulberry leaf on growth performance, hypoxia-reoxygenation stress and biochemical parameters in various organs of fish. Aquacult. Rep. 2020, 18, 100494. [Google Scholar] [CrossRef]
- Saxena, T.B.; Zachariassen, K.E.; Jørgensen, L. Effects of ethoxyquin on the blood composition of turbot, Scophthalmus maximus L. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 2000, 127, 1–9. [Google Scholar] [CrossRef]
- Jiang, W.D.; Wu, P.; Kuang, S.Y.; Liu, Y.; Jiang, J.; Hu, K.; Li, S.H.; Tang, L.; Feng, L.; Zhou, X.Q. Myo-inositol prevents copper-induced oxidative damage and changes in antioxidant capacity in various organs and the enterocytes of juvenile Jian carp (Cyprinus carpio var. Jian). Aquat. Toxicol. 2011, 105, 543–551. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- El-Husseiny, O.M.; Abdul-Aziz, G.M.; Goda, A.S.; Suloma, A. Effect of altering linoleic acid and linolenic acid dietary levels and ratios on the performance and tissue fatty acid profiles of Nile tilapia Oreochromis niloticus fry. Aquacult. Int. 2010, 18, 1105–1119. [Google Scholar] [CrossRef]
- Wei, X.B.; Liu, H.Q.; Sun, X.; Fu, F.; Zhang, X.; Wang, J.; An, J.; Ding, H. Hydroxysafflor yellow A protects rat brains against ischemia-reperfusion injury by antioxidant action. Neurosci. Lett. 2005, 386, 58–62. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Duan, X.; Feng, X.; Yang, Y. The interaction effects of coke oven emissions exposure and metabolic enzyme Gene variants on total antioxidant capacity of workers. Environ. Toxicol. Pharmacol. 2019, 70, 103197. [Google Scholar] [CrossRef]
- Servili, M.; Montedoro, G. Contribution of phenolic compounds to virgin olive oil quality. Eur. J. Lipid Sci. Technol. 2002, 104, 602–613. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Frankel, E.N. Natural phenolic antioxidants and their impact on health. In Antioxidant Food Supplements in Human Health; Academic Press: Cambridge, MA, USA, 1999; pp. 385–392. [Google Scholar]
- Li, Y.; Guo, S.; Zhu, Y.; Yan, H.; Qian, D.; Wang, H.; Yu, J.; Duan, J. Flowers of Astragalus membranaceus var. mongholicus as a novel high potential by-product: Phytochemical characterization and antioxidant activity. Molecules 2019, 24, 434. [Google Scholar]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Elabd, H.; Wang, H.P.; Shaheen, A.; Yao, H.; Abbass, A. Astragalus membranaceus (AM) enhances growth performance and antioxidant stress profiles in bluegill sunfish (Lepomis macrochirus). Fish Physiol. Biochem. 2016, 42, 955–966. [Google Scholar] [CrossRef]
- Elabd, H.; Wang, H.P.; Shaheen, A.; Yao, H.; Abbass, A. Feeding Glycyrrhiza glabra (liquorice) and Astragalus membranaceus (AM) alters innate immune and physiological responses in yellow perch (Perca flavescens). Fish Shellfish Immun. 2016, 54, 374–384. [Google Scholar] [CrossRef]
- Gilloteaux, J.; Kashouty, R.; Yono, N. The perinuclear space of pancreatic acinar cells and the synthetic pathway of zymogen in Scorpaena scrofa L.: Ultrastructural aspects. Tissue Cell 2008, 40, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Zambonino Infante, J.L.; Cahu, C.L. Ontogeny of the gastrointestinal tract of marine fish larvae. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Elabd, H.; Wang, H.P.; Shaheen, A.; Matter, A. Astragalus membranaceus nanoparticles markedly improve immune and anti-oxidative responses; and protection against Aeromonas veronii in Nile tilapia Oreochromis niloticus. Fish Shellfish Immun. 2020, 97, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Geering, K. Subunit assembly and functional maturation of Na,K-ATPase. J. Membr. Biol. 1990, 115, 109–121. [Google Scholar] [CrossRef]
- Suzer, C.; Aktülün, S.; Çoban, D.; Okan Kamacı, H.; Saka, Ş.; Fırat, K.; Alpbaz, A. Digestive enzyme activities in larvae of sharpsnout seabream (Diplodus puntazzo). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 148, 470–477. [Google Scholar] [CrossRef]
- Angela, C.; Wang, W.; Lyu, H.; Zhou, Y.; Huang, X. The effect of dietary supplementation of Astragalus membranaceus and Bupleurum chinense on the growth performance, immune-related enzyme activities and genes expression in white shrimp, Litopenaeus vannamei. Fish Shellfish Immun. 2020, 107, 379–384. [Google Scholar] [CrossRef]
- Feng, L.; Zhao, S.; Chen, G.; Jiang, W.; Liu, Y.; Jiang, J.; Hu, K.; Li, S.; Zhou, X. Antioxidant status of serum, muscle, intestine and hepatopancreas for fish fed graded levels of biotin. Fish Physiol. Biochem. 2013, 40, 499–510. [Google Scholar] [CrossRef]
- Gliszczyńska-Świgło, A. Antioxidant activity of water soluble vitamins in the TEAC (trolox equivalent antioxidant capacity) and the FRAP (ferric reducing antioxidant power) assays. Food Chem. 2006, 96, 131–136. [Google Scholar] [CrossRef]
- Woo, S.J.; Chung, J.K. Effects of trichlorfon on oxidative stress, neurotoxicity, and cortisol levels in common carp, Cyprinus carpio L., at different temperatures. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 229, 108698. [Google Scholar] [CrossRef]
- Yang, W.J.; Li, D.P.; Li, J.K.; Li, M.H.; Chen, Y.L.; Zhang, P.Z. Synergistic antioxidant activities of eight traditional Chinese herb pairs. Biol. Pharm. Bull. 2009, 32, 1021–1026. [Google Scholar] [CrossRef]
- Abuelsaad, A.S. Supplementation with Astragalus polysaccharides alters Aeromonas-induced tissue-specific cellular immune response. Microb. Pathog. 2014, 66, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, G.I.; Almajano, M.P. Red fruits: Extraction of antioxidants, phenolic content, and radical scavenging determination: A review. Antioxidants 2017, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.J.; Walker, P.A.; Hogstrand, C. Dietary phenolic antioxidants, caffeic acid and Trolox, protect rainbow trout gill cells from nitric oxide-induced apoptosis. Aquat. Toxicol. 2006, 80, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant defenses in fish: Biotic and abiotic factors. Rev. Fish Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Ni, M.; Liu, M.; Lou, J.; Mi, G.; Gu, Z. Stocking density alters growth performance, serum biochemistry, digestive enzymes, immune response, and muscle quality of largemouth bass (Micropterus salmoides) in in-pond raceway system. Fish Physiol. Biochem. 2021, 47, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Charan, A.A.; Charan, A.I.; Verma, O.P.; Naushad, S.S. Profiling of Antioxidant enzymes in Cat fish (Clarias batrachus) exposed to phenolic compounds. Asian J. Bio Sci. 2015, 10, 6–14. [Google Scholar] [CrossRef]
- Abarike, E.D.; Jian, J.C.; Tang, J.F.; Cai, J.; Yu, H.; Chen, L.H. Traditional Chinese medicine enhances growth, immune response, and resistance to Streptococcus agalactiae in Nile tilapia. J. Aquat. Anim. Health 2019, 31, 46–55. [Google Scholar] [CrossRef]
- Kirshenbaum, L.A.; Singal, P.K. Increase in endogenous antioxidant enzymes protects hearts against reperfusion injury. Am. J. Physiol. 1993, 265, H484–H492. [Google Scholar] [CrossRef]
- Yim, T.K.; Wu, W.K.; Pak, W.F.; Mak, D.H.F.; Ko, K.M. Myocardial protection against ischaemia-reperfusion injury by a Polygonum multiflorum extract supplemented ‘Dang-Gui decoction for enriching blood’, a compound formulation, ex vivo. Phytother. Res. 2000, 14, 195–199. [Google Scholar] [CrossRef]
- Reed, D.J. Glutathione: Toxicological implications. Annu. Rev. Pharmacol. Toxicol. 1990, 30, 603–631. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).