Abstract
Based on morphological and molecular data, Brotulella n. gen. is proposed to accommodate the dactylogyrid monogeneans Brotulella laurafernandae n. sp. (type species) and Brotulella luisahelenae n. sp. on the gill filaments of the Pacific bearded brotula Brotula clarkae Hubbs, 1944 (Ophidiiformes: Ophidiidae) from Puerto Pizarro in the Tumbes region (northern Peru). Species of the new genus are distinguished from all other dactylogyrids by the combination of the following features: (1) anchors with a stocking-shaped sheath associated with the distal end of superficial and deep roots, (2) tandem gonads, (3) a vas deferens looping left intestinal caecum, (4) a distally twisted male copulatory organ (MCO) with a delicate membranous accessory piece articulated to the shaft of the MCO, (5) a U-shaped ovary, (6) an almost sigmoid seminal vesicle, and (7) two prostatic reservoirs with thick muscular walls. A 28S ribosomal DNA-based phylogenetic analysis (Maximum likelihood and Bayesian inference) of sequences of two new species of Brotulella n. gen. from the Southeastern Pacific Ocean, along with sequences from closely related genera of the marine Dactylogyridae, supports the establishment of the new genus for the dactylogyrid parasites on the gills of the Pacific bearded brotula.
Keywords:
Brotulella n. gen.; Brotulella laurafernandae n. sp.; Brotulella luisahelenae n. sp.; cusk-eels; gill ectoparasites; taxonomy; South America Key Contribution:
The manuscript introduces a new genus, Brotulella n. gen., to accommodate two newly discovered dactylogyrid monogeneans, Brotulella laurafernandae n. sp. and Brotulella luisahelenae n. sp., found on the gill filaments of the Pacific bearded brotula, Brotula clarkae. These parasites are distinguished by unique morphological features. Additionally, a phylogenetic analysis based on 28S ribosomal DNA sequences supports the establishment of this new genus, shedding light on the biodiversity of these marine parasites in the Southeastern Pacific Ocean.
1. Introduction
Peru, as other Neotropical countries, harbors a rich fauna of fishes, especially a large number of bony fishes []. Regarding its marine ichthyology diversity, a total of 698 fish species have been reported belonging to 388 genera and 138 families [], most of them recorded from the northern sea, being one of the richest marine ecosystems in Peru []. Several parasitological surveys performed in northern Peru have found a rich fauna of the Dactylogyridea Bychowsky, 1937 from marine fishes, especially in serranid and sciaenid fishes [,].
Dactylogyridae Bychowsky, 1933, a diverse family of monogenean parasites, represents a significant component of parasitic fauna among fishes worldwide []. This group exhibits remarkable diversity in morphology, life cycles, and host specificity, making it imperative to comprehend their taxonomy and ecological roles []. Understanding the intricate relationships between Dactylogyridae species and their fish hosts is crucial for several reasons. Firstly, some of these parasites can induce pathogenic effects on their hosts, impacting fish health, growth, and even leading to mortality in some cases []. Secondly, their prevalence and abundance within aquatic ecosystems can serve as indicators of ecosystem health and environmental changes []. Moreover, due to their high host specificity and site selection within fish gills or skin, Dactylogyridae species can offer insights into fish migration patterns and habitat preferences [].
The Pacific bearded brotula Brotula clarkae Hubbs, 1944 (Ophidiiformes: Ophidiidae) is a benthopelagic fish endemic to the Eastern Tropical Pacific [,,], and is found deep in mud and in broken shell bottoms []. This species is distributed from the Gulf of California (USA) to Paita (Peru) [,,]. Although numerous studies have focused on the reproductive biology of the Pacific bearded brotula [,,], the parasitic fauna associated with Br. clarkae remains unknown to date. During a study on gill ectoparasites of marine fishes from Peru, two species belonging to the Dactylogyridae Bychowsky, 1937 were found on the Pacific bearded brotula. In this study, both species are examined based on morphological and molecular data.
2. Materials and Methods
2.1. Specimen Collection and Morphological Analyses
Two specimens of Br. clarkae were obtained from local fishermen from the Puerto Pizarro resort (3°29′ S, 80°24′ W), Tumbes, Peru, during a field expedition in February 2019. The hosts were dissected immediately, and the gill arches were removed and placed in vials containing sea water (60 °C). Each vial was vigorously shaken, and formalin was added to obtain a 4% solution. Some monogeneans were fixed directly in 70% ethanol and subsequently preserved in 90% ethanol until use. The anterior and posterior parts of these specimens were cut and used for morphological identification, whereas the middle parts were used in molecular procedures. In the laboratory, the contents of each vial were examined under a dissecting microscope and monogeneans were removed from the gills or sediment using small probes. Some specimens were stained with Gomori’s trichrome, clarified in clove oil, and mounted in Canada balsam. Other specimens were mounted in Gray & Wess medium [] for the study of sclerotised structures. Specimens were examined and photographed using a compound OlympusTM BX51 photomicroscope equipped with normal light and differential interference contrast microscopy (DIC) optics (Olympus Corporation, Tokyo, Japan). Drawings were made with the aid of a drawing tube. Measurements are in micrometers (µm), unless otherwise indicated, using straight-line distances between extreme points of the structures measured and are expressed as the range followed by the mean and number (n) of structures measured in parentheses. Body length represents the length of the body proper with the haptor. Numbering of haptoral-hook pairs followed the system of Mizelle [] and Mizelle & Price []. Fishes were identified according to Chirichigno & Vélez []. The abbreviation B. for the parasite (Brotulella n. gen.) and Br. for the host (Brotulla) are used to avoid doubt as to the genera. The type-material was deposited in the Helminthological Collection in the Museum of Natural History at the San Marcos University (MUSM-HEL), Lima, Peru.
2.2. Molecular Characterization and Phylogenetic Analyses
Genomic DNA were isolated from one specimen of each new species of parasite using the Qiagen QIAamp DNA Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s protocol. Partial 28S rRNA gene was amplified by polymerase chain reaction (PCR) using the primers C1 (5′–ACCCG CTGAA TTTAA GCAT–3′) and D2 (5′–TGGTC CGTGT TTCAA GAC–3′) [,,]. The thermocycling profile, as described by Mendoza-Palmero et al. [], consisted of an initial denaturation set at 95 °C for 3 min, followed by 34 cycles of amplification (94 °C for 30 s, 56 °C for 30 s and 72 °C for 1:30 min), with a final extension hold at 72 degrees C. for 4 min. Samples were sequenced using an ABI 3730 DNA analyzer of the RPT01A subunit for DNA sequencing of the Technological Platforms Network at the Oswaldo Cruz Foundation, Rio de Janeiro, Brazil. The resulting fragments were assembled into contiguous sequences in Geneious []. These sequences were deposited in GenBank SUB13983437 and SUB13983462. A total of 36 species, representing 18 genera of the Dactylogyridae infecting 27 marine fish host species, were included in the phylogenetic analyses. Additionally, 3 species of the Diplectanidae were utilized as an outgroup for comparison (see Table 1). Alignment and editing of sequences were performed using Clustal W in MEGA version 7.0 []. The Mega 7.0 software was used for construction of genetic distances using Kimura two parameters []. The online PhyML 3.0 software [] was used to reconstruct phylogenies based on the maximum likelihood (ML) approach. The model of nucleotide evolution, general time reversible (GTR), was selected with Smart Model Selection (SMS), run in PhyML [], using the Bayesian information criterion. Node support was computed by the approximate likelihood-ratio test for branches (aLRT) [] and by nonparametric bootstrap percentages (ML-BP) with 1000 pseudoreplicates. Bayesian phylogenetic inference (BI) was carried out using MrBayes version 3.2.6 [] in XSEDE using the CIPRES Science Gateway [], with the GTR + G model. Markov chain Monte Carlo samplings for each matrix were performed for 10,000,000 generations with four simultaneous chains in two runs. Branch supports in Bayesian trees by Bayesian posterior probabilities (BPP) were assessed from trees that were sampled every 100 generations, after removal of a burn-in fraction of 25%. The sequences from GenBank that were included for the phylogenetic analyses are listed in Table 1 as well as sequences for out-groups.

Table 1.
List of dactylogyrid species included in the phylogenetic analyses. Sequences obtained for the present study are in bold.
3. Results
3.1. Order Dactylogyridea Bychowsky, 1937
- Dactylogyridae Bychowsky, 1933
- Brotulella n. gen. Cruces, Chero & Luque
- Diagnosis
- Body divided into cephalic region, trunk, peduncle, and haptor. Tegument thin, smooth. Head organs, cephalic lobes present; cephalic glands unicellular, anterior and posterolateral to pharynx. Eyespots absent; accessory chromatic granules absent. Mouth subterminal, midventral; pharynx muscular, glandular; oesophagus short, intestine bifurcated; intestinal caeca confluent posteriorly to testis, without diverticula. Common genital pore midventral, near level of intestinal bifurcation. Haptor armed with 14 hooks with ancyrocephaline distribution sensu Mizelle [], 2 pairs of anchors, 2 haptoral bars, lacking haptoral reservoirs; anchors dissimilar, distal end of superficial and deep roots with stocking-shaped sheath; ventral bar bowed; dorsal bar with anterior and posterior broad process; hooks with undilated shanks and upright blunt thumb. Gonads tandem, intercaecal. Testis posterior to ovary. Vas deferens looping left intestinal caecum. Seminal vesicle almost sigmoid. Prostatic reservoirs two, with thick muscular walls. Male copulatory organ (MCO) tubular, sclerotized, with twisted distal end; base cylindrical; accessory piece delicate, membranous, articulated to shaft of MCO. Ovary U-shaped; oviduct, uterus not observed. Vagina sclerotized, emptying to seminal receptacle, vaginal aperture dextrolateral. Vitelline follicles dense, coextensive with intestinal caeca. Parasites of gills of marine fishes.
- Type species by original designation: Brotulella laurafernandae n. sp. from the gills of the Pacific bearded brotula Brotula clarkae Hubbs, 1944 (Ophidiiformes: Ophidiidae). Other species: Brotulella luisahelenae n. sp. from B. clarkae.
- Etymology: The genus name refers to the genus name of the fish host (Brotula). The diminutive -ella is appended to the genus name and should be treated as female.
- Remarks: Brotulella n. g. is characterized by the combination of the following features: (1) anchors with a stocking-shaped sheath associated with the distal end of superficial and deep roots, (2) tandem gonads, (3) a distally twisted MCO with a delicate membranous accessory piece articulated to the shaft of the MCO, (4) a U-shaped ovary, (5) an almost sigmoid seminal vesicle, and (6) two prostatic reservoirs with thick muscular walls. Brotulella n. g. most closely resembles species of the genus Platycephalotrema Kritsky & Nitta, 2019, which comprises nine species that infect the gill lamellae of the flathead fishes (Platycephalidae). Species of both genera share (1) tandem gonads, (2) a vas deferens looping left intestinal caecum, (3) two prostatic reservoirs, (4) a sclerotized vagina, (5) dextral vaginal aperture, (6) seven pairs of hooks, (7) and tubular MCO with complex distal end. However, Brotulella n. g. differs from Platycephalotrema by its species having anchors unequal (anchors equal in Platycephalotrema spp.); a stocking-shaped sheath associated with the distal end of superficial and deep roots of the anchors (absent in Platycephalotrema spp.); a MCO with accessory piece (absent in Platycephalotrema spp.); hooks with upright blunt thumb (protruding blunt thumb in Platycephalotrema spp.); a U-shaped ovary (ovary entire in Platycephalotrema spp.); a short vaginal vestibule (large vaginal vestibule in Platycephalotrema spp.); prostatic reservoirs with thick muscular walls (prostatic reservoirs without thick muscular walls in Platycephalotrema spp.); and absence of eyespots (four eyespots in Platycephalotrema spp.).
- Species of Brotulella n. g. resembles species of Ligophorus Euzet & Suriano, 1977, which includes species parasitizing mullets (Mugilidae). Members of both genera share the following features: (1) gonads in tandem, (2) a tubular sclerotized and uncoiled MCO, (3) an ovary with U-shaped, and (4) a sclerotized vagina. However, species of these genera differ by having anchors with a stocking-shaped sheath associated with the distal end of superficial and deep roots (absent in Ligophorus spp.), a vas deferens looping the left intestinal caecum (vas deferens not looping the intestinal caecum in Ligophorus spp.), two prostatic reservoirs with thick muscular walls (one pyriform prostatic reservoir without thick muscular wall in Ligophorus spp.) and by having a ventral bar bowed (V-shaped ventral bar in Ligophorus spp.).
- Species of the new genus slightly resemble those of Bravohollisia Bychowsky & Nagibina, 1970 and Caballeria Bychowsky & Nagibina, 1970 by having tandem gonads, a tubular sclerotized and uncoiled MCO, and a bowed ventral bar. However, species of Brotulella n. g. differ from species of these genera by having a MCO with accessory piece (absent in Bravohollisia and Caballeria species) and by the absence of haptoral glands (present in Bravohollisia and Caballeria species). Species of the new genus slightly resembles species of Mexicana Caballero & Bravo-Hollis, 1959, parasitic on haemulid fishes, by having tandem gonads and an almost U-shaped ovary but differ from these species by having two prostatic reservoirs (one prostatic reservoir in Mexicana spp.), a testis not bipartite (principally bipartite posteriorly in Mexicana spp.) and absence of eyespots (four eyespots in Mexicana spp.).
- Finally, species of Brotulella n. gen. resemble species of the Boegeriella Mendoza-Palmero & Hsiao, 2020 and Nanayella Acosta, Mendoza-Palmero, da Silva & Scholz, 2019 by the shape of the ovary (U-shaped). However, species of Brotulella n. gen. differ from Boegeriella spp. by the morphology of the MCO (tubular with twisted distal end in Brotulella n. gen. vs coiled in Boegeriella spp.). Species of the new genus can be distinguished from species of Nanayella by having hooks of similar size (hook of dissimilar size in Nanayella).
3.1.1. Brotulella laurafernandae n. sp. Cruces, Chero & Luque
Body elongates (Figure 1), 486–702 (603, n = 13) long; greatest width 68–106 (89, n = 13) usually at level of testis. Cephalic region moderately broad; cephalic lobes moderately developed; cephalic glands bilateral, paired, at pre and postpharyngeal level. Pharynx spherical, greatest width 23–30 (27, n = 6). Peduncle short to elongated. Haptor subhexagonal, 59–84 (72, n = 13) long, 78–113 (100, n = 13) wide; group of inconspicuous secretory gland-cells lying on peduncle. Anchors with fine conspicuous alae; ventral anchor 59–63 (61, n = 7) long, with rounded deep root, robust superficial root, slightly arced shaft, elongated point, base 19–24 (21, n = 7) wide; dorsal anchor 65–69 (66, n = 7) long, with rounded deep root, elongated superficial root, slightly arced shaft, elongated point, base 20–23 (21, n = 7) wide. Ventral bar 44–57 (50, n = 11) long, rod-shaped, posteromedially bilobed, with expanded lateral ends. Dorsal bar 44–56 (51; n = 11) long, with mask-shaped, short anterior processes, curved posterior processes. Hooks 14, similar, 12–15 (13, n = 8) long, each with protruded obtuse thumb, uniform shank, and delicate point; filamentous hook (FH) loop around shank length. MCO 36–46 (41, n = 12) long, tapered, accessory piece with medial and proximal expansions; base of MCO with cylinder-shaped. Testis large, intercaecal, ovate, not lobulated, 77–100 (89, n = 6) long, 42–58 (52, n = 6) wide; vas deferens dilate to form big seminal vesicle in left side of trunk, posterolateral to MCO; anterior prostatic reservoir ovate, dorsal to MCO, posterior prostatic reservoir with tadpole-shaped, dextrolateral to MCO. Ovary 32–55 (45, n = 6) long, 26–40 (34, n = 6) wide; oviduct, oötype and uterus not observed. Vaginal vestibule infundibuliform, sclerotized, lying horizontal on right side of body anterior to ovary; vaginal duct running posteriorly to join large subspherical seminal receptacle. Vitelline follicles throughout trunk, lateral fields of follicles confluent posterior to MCO and posterior to testis. Eggs not observed.
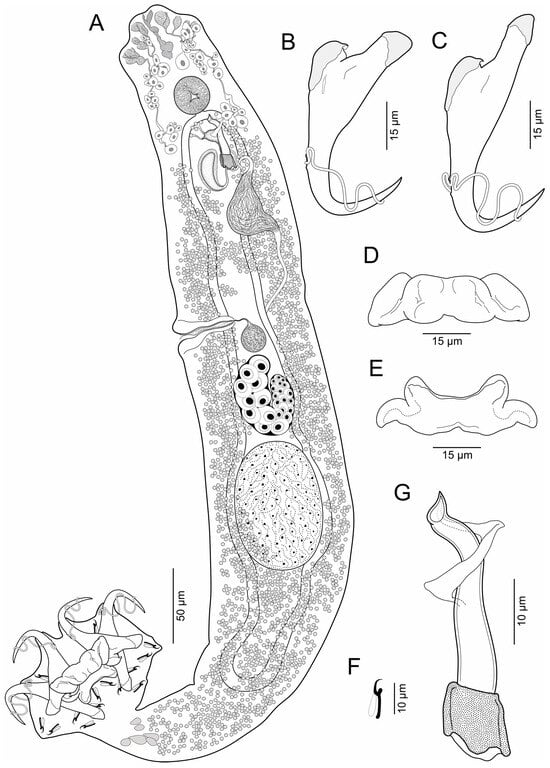
Figure 1.
Brotulella laurafernandae n. sp. from the Pacific bearded brotula Brotula clarkae Hubbs, 1944 (Ophidiiformes: Ophidiidae). (A) Whole mount (composite, ventral view) (B) Ventral anchor (C) Dorsal anchor (D) Ventral bar (E) Dorsal bar (F) Hook (G) MCO.
- Type host: Brotula clarkae Hubbs, 1944 (Ophidiiformes: Ophidiidae), Pacific bearded brotula.
- Type locality: Puerto Pizarro resort (3°29′ S, 80°24′ W), Tumbes, Peru.
- Site in host: Gills.
- Type specimens: Holotype (MUSM-HEL 5132), 12 paratypes (MUSM-HEL 5133a–l), 1 hologenophore (MUSM 5133m).
- ZooBank registration: The Life Science Identifier (LSID) for Brotulella laurafernandae n. sp. is urn:lsid:zoobank.org:act:A1C5B22A-E8FF-4A70-B574-1B9A8B8082CC.
- Representative DNA sequence: Sequence was deposited in GenBank under the accession number OR860318 for the 28S rDNA with 720 bp.
- Etymology: The new species is named in honor of Laura Fernanda do Amarante Luque, daughter of the senior author.
- Remarks: Brotulella laurafernandae n. sp. is the type species of the genus.
3.1.2. Brotulella luisahelenae n. sp. Cruces, Chero & Luque
- Body elongates (Figure 2), 625–930 (769, n = 12) long; greatest width 111–153 (132, n = 12) usually at level of seminal vesicle. Cephalic region broad; cephalic lobes poorly developed; cephalic glands bilateral, paired at pre and postpharyngeal level. Pharynx spherical, in greatest width 29–44 (35, n = 8). Peduncle short to elongated. Haptor subquadrangular, 78–109 (94, n = 12) long, 120–165 (139, n = 12) wide; group of inconspicuous secretory gland-cells lying on peduncle. Anchors with fine conspicuous alae; ventral anchor 67–76 (70, n = 7) long, with rounded deep root, robust superficial root, slightly arced shaft, elongated point with furrow on external surface, base 22–26 (24, n = 7) wide; dorsal anchor 80–89 (88; n = 7) long, with almost rounded deep root, elongated superficial root, slightly arced shaft, elongated point with furrow on external surface, base 20–24 (22, n = 7) wide. Ventral bar 60–78 (69, n = 8) long, broadly V-shaped, with moderately enlarged lateral ends. Dorsal bar 56–73 (66, n = 8) long, with almost cat face-shaped, short anterior processes, oblique posterior processes. Hooks 14, similar, 12–15 (14, n = 7) long, each with protruded obtuse thumb, uniform shank, and delicate point; filamentous hook (FH) loop around shank length. MCO 88–107 (96, n = 12) long, tapered, accessory piece with proximal expansion; base of MCO with almost trapezium-shaped. Testis large, intercaecal, subtriangular, not lobulated, 91–129 (108, n = 5) long, 75–97 (88, n = 5) wide; vas deferens dilating to form big seminal vesicle in left side of trunk, posterolateral to MCO; ventral prostatic reservoir elongated, ventral to MCO, dorsal prostatic reservoir well-developed, dextrolateral to MCO. Ovary 42–61 (54, n = 5) long, 58–73 (65, n = 5) wide; oviduct, oötype and uterus not observed. Vaginal vestibule infundibuliform, sclerotized, lying horizontal on right side of body anterior to ovary; vaginal duct running posteriorly to join big subspherical seminal receptacle. Vitelline follicles throughout trunk, lateral fields of follicles confluent posterior to MCO and posterior to testis. Eggs not observed.
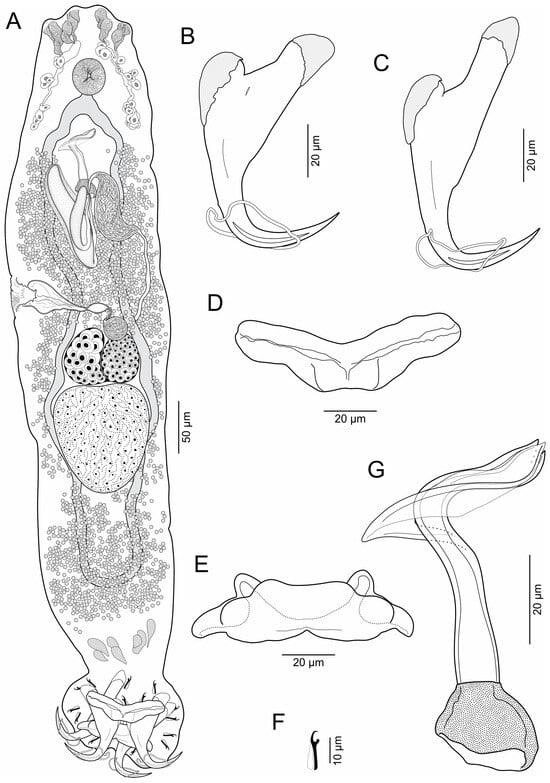 Figure 2. Brotulella luisahelenae n. sp. from the Pacific bearded brotula Brotula clarkae Hubbs, 1944 (Ophidiiformes: Ophidiidae). (A) Whole mount (composite, ventral view) (B) Ventral anchor (C) Dorsal anchor (D) Ventral bar (E) Dorsal bar (F) Hook (G) MCO.
Figure 2. Brotulella luisahelenae n. sp. from the Pacific bearded brotula Brotula clarkae Hubbs, 1944 (Ophidiiformes: Ophidiidae). (A) Whole mount (composite, ventral view) (B) Ventral anchor (C) Dorsal anchor (D) Ventral bar (E) Dorsal bar (F) Hook (G) MCO. - Type host: Brotula clarkae Hubbs, 1944 (Ophidiiformes: Ophidiidae), Pacific bearded brotula.
- Type locality: Puerto Pizarro resort (3°29′ S, 80°24′ W), Tumbes, Peru.
- Site in host: Gills.
- Type specimens: Holotype (MUSM-HEL 5134), 12 paratypes (MUSM-HEL 5135a–k), 1 hologenophore (MUSM 5135l).
- ZooBank registration: The Life Science Identifier (LSID) for Brotulella luisahelenae n. sp. is urn:lsid:zoobank.org:act:6E75215A-471D-490E-809C-4968C7901A34.
- Representative DNA sequence: Sequence was deposited in GenBank under the accession number OR860321 for the 28S rRNA with 720 bp.
- Etymology: The new species is named in honor of Luisa Helena do Amarante Luque, daughter of the senior author.
- Remarks: Brotulella luisahelenae n. sp. can be distinguished from Brotulella laurafernandae n. sp. by its MCO, which have an almost trapezium-shaped base and a blanket-shaped membrane with proximal expansion. In addition, B. luisahelenae n. sp. is typified by having the points of the ventral anchors with a furrow on external surface.
3.2. Phylogenetic Relationships
The alignment of all analyzed species comprised 769 characters, with 229 being constant and 478 being parsimony-informative variables. The Bayesian analyses yielded a mean estimated marginal likelihood of −11,962.2929, accompanied by a median value of −11,961.93. The effective sample size for all parameters exceeded 100, indicating a substantial number of effectively independent samples. Moreover, this sample size was considerably larger for the majority of parameters, underscoring the robust nature of the samples. ML and BI analyses generated phylogenetic trees with similar general topology, with little variation in node support values (Figure 3). The sequences obtained from the new species differed in 10 bases. The smallest distance was found between the sequences of Brotulella laurafernandae n. sp. and Brotulella luisahelenae n. sp. (0.01%). The distance between the two new species and the sister genera are: 0.12% for Platycephalotrema bassense and 0.15% for Haliotrema johnstoni and P. platycephali. The species Brotulella laurafernandae n. sp. and Brotulella luisahelenae n. sp. formed a strongly supported clade with Bayesian posterior probabilities (BPP = 0.97) and ML (aLRT = 0.99; ML-BP = 1000) values. This clade also constituted a moderately supported monophyletic group alongside Platycephalotrema bassense, Haliotrema johnstoni and P. platycephali (BPP = 0.97; aLRT = 0.66; ML-BP = 603) (Figure 3). These species are positioned within a significantly supported major clade referred to as the ‘Haliotrema’ group as per Kmentová et al. []). This clade encompasses other species such as those belonging to Bravohollisia Bychowsky & Nagibina, 1970, Caballeria Bychowsky & Nagibina, 1970, Lethrinitrema Lim & Justine, 2011, Pseudohaliotrema Yamaguti, 1953 and Tetrancistrum Goto & Kikuchi, 1917.
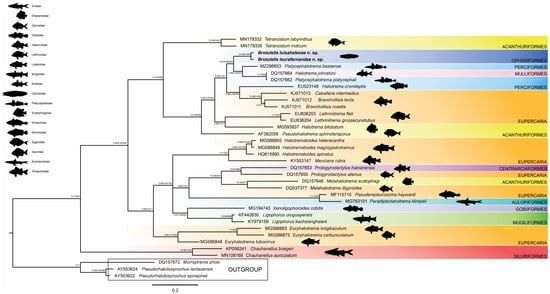
Figure 3.
Phylogenetic tree based on 28S region for B. laurafernandae n. sp. and B. luisahelenae n. sp. (Dactylogyridae) inferred by using the Bayesian inference (BI) and maximum likelihood (ML) methods (aLRT and bootstrap replicates). The nodal support is described at the left by bayesean posterior probability, aRLT and bootstrap replicates to each node represented. The scale bar represents the number of substitutions per site. The symbol of one asterisk (*) indicates low nodal support.
4. Discussion
In the present study, Brotulella n. gen. is proposed to accommodate two marine dactylogyrids, B. laurafernandae n. sp. and B. luisahelenae n. sp., infecting the gill filaments of the Pacific bearded brotula in the Southeast Pacific off Peru, based on the presence of a combination of morphological characteristics which include anchors with a stocking-shaped sheath associated with the distal end of superficial and deep roots, tandem gonads, a distally twisted MCO with an accessory piece articulated to the shaft of the MCO, a U-shaped ovary, and two prostatic reservoirs with thick muscular walls. Moreover, the phylogenetic position of the species of Brotulella n. gen. support and justify the erection of the new genus. Our partial sequences of the 28S rDNA of both new species of Brotulella n. gen. from the Southeast Pacific Ocean cluster within a strongly supported clade that includes Platycephalotrema bassense (Hughes, 1928) Kritsky & Nitta, 2019 from Australia, along with Haliotrema johnstoni Bychowsky & Nagibina, 1970, and P. platycephali (Yin & Sproston, 1948) Kritsky & Nitta, 2019 from China. Species of Brotulella n. gen. and the two Platycephalotrema species share some morphological characteristics such as two seminal vesicles and tandem gonads.
Haliotrema johnstoni, a dactylogyrid infecting the dark-barred goatfish Upeneus luzonius Jordan & Seale, 1907 (Mullidae), is nested within Platycephalotrema. The genetic similarity between H. johnstoni and the two Platycephalotrema species observed in the present analysis appears to be consistent with the morphological data, i.e., a dorsal bar with bifurcating ends in H. johnstoni and Platycephalotrema species, as mentioned by Kmentová et al. []. Unfortunately, we were unable to access the type material of H. johnstoni to corroborate this hypothesis. Furthermore, as mentioned by Kmentová et al. [] the presence of a MCO with an accessory piece and the fact that H. johnstoni infect a different host repertoire provide evidence that the relationship between H. johnstoni and Platycephalotrema species warrants further investigation.
Brotulas, Brotula spp. (Ophidiidae), are principally benthopelagic fishes that occur in the Atlantic and Pacific Oceans []. Previously, only one of the six valid species of Brotula [] has been reported as host for monogeneans []. Haliotrema brotulae Yamaguti, 1968 and H. spiculare are the only species of monogeneans described from these hosts []. Both species have some morphological similarities with the new species described here, i.e., a distally twisted MCO, lacking accessory piece, an almost sigmoid seminal vesicle, and two prostatic reservoirs, these species could eventually be removed from Haliotrema Johnston & Tiegs, 1922 and transferred to the new genus. However, our endeavor to assess the type material was thwarted by complications related to gaining access to the collection, consequently impeding an exhaustive investigation. Additionally, there are no available sequences of the 28S rRNA gene of H. brotulae and H. spiculare to test their phylogenetic relationship with species of Brotulella n. gen. Hence, a meticulous study based on the examination of both type and novel specimens of H. brotulae and H. spiculare, in conjunction with genetic data, is required to confirm their taxonomic classification.
The taxonomy of monogeneans has long been a subject of scientific interest, and the utilization of alternative tools to address taxonomic uncertainties enhances our understanding of monogenean diversity [,,]. The application of molecular tools has proven invaluable in resolving taxonomic ambiguities within monogeneans [,]. Through the analysis of DNA sequences and the construction of phylogenetic trees, researchers have gained insights into the evolutionary history and genetic diversity of monogenean species [,,,]. Additionally, comparative genomics has provided a deeper understanding of the genomic characteristics and evolutionary adaptations of these parasites []. Furthermore, the integration of alternative tools, such as transcriptomics and proteomics, can offer valuable insights into the functional genomics of monogeneans [].
The knowledge of the marine monogenean parasite fauna of Peruvian fishes has increased remarkably in the last years [,]. Most of these taxonomic studies were solely based on morphological data. Only four studies provided molecular data on monogeneans of the Diclidophoridae (3 species), Hexabothriidae (2 species) and Monocotylidae (1 species) families []. Thus, the present study provides the first sequence of the partial rRNA gene 28S region of marine dactylogyrids from Peru.
Nineteen marine dactylogyrid monogenean species from the genera Bicentenariella Cruces, Chero, Sáez & Luque, 2021 (5 spp.), Haliotrema Johnston & Tiegs, 1922 (3 spp.), Haliotrematoides Kritsky, Yang & Sun, 2009 (1 species), Euryhaliotrema (4 spp.) Kritsky & Boeger, 2002, Mexicana Caballero & Bravo-Hollis, 1959 (1 species), Pronotogrammella Cruces, Chero, Sáez & Luque, 2020 (3 spp.), and Tylosuricola Unnithan, 1964 (1 species) have been described or reported infecting the gills of eleven fish species from Peru []. From these, three species are known for parasitizing the gills of fishes captured in central Peru []. The other sixteen species of dactylogyrids infect fishes in northern Peru. The two new species described here increase the number of dactylogyrid species that infect fishes from Peru to 21.
5. Conclusions
In this study, a new genus, Brotulella n. gen., is proposed to accommodate two newly discovered species of monogenean parasites found on the gills of the Pacific bearded brotula, Brotula clarkae. These species in the new genus are distinguished by unique morphological features, including specific characteristics of their anchors, gonads, male copulatory organ, ovary, seminal vesicle, and prostatic reservoirs. The phylogenetic analysis based on 28S ribosomal DNA sequences supports the establishment of the new genus and provides insights into the biodiversity of these parasites in the Southeastern Pacific Ocean.
Author Contributions
C.L.C., J.D.C. and J.L.L. conceived and designed the study; J.D.C. and C.L.C. carried out the field work; C.L.C., R.S. (Raquel Simões) and A.M.J. performed molecular analyses. Additional analyses were performed by C.L.C., J.D.C., R.S. (Raquel Simões), R.S. (Ruperto Severino) and J.L.L.; J.D.C. and C.L.C. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
C.L.C. was supported by a student fellowship from the Coordenação de Aperfeiçoamento de Pessoal do Ensino Superior, Brazil (CAPES)—Finance Code 001. J.L.L. was supported by a Researcher fellowship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil (CNPq).
Institutional Review Board Statement
This study did not consider experiments with live animals. All fishes were obtained from commercial catches, and none of the species are subject to conservation measures.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors are grateful to the following people who helped with the collection of fishes in Peru: Milagros K. Carrillo, Alexander Reyes, and Cynthia E. Rodríguez, all from the National University Federico Villarreal (UNFV).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Marín, A.; Gozzer-Wuest, R.; Grillo-Nuñez, J.; Alavarez-Jaque, I.B.; Riveros, J.C. DNA barcoding reveals overlooked shark and bony fish species in landing reports of small-scale fisheries from northern Peru. Mar. Fish. Sci. 2022, 35, 307–314. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. Available online: https://www.fishbase.se/search.php (accessed on 24 May 2023).
- Nature & Culture International. Available online: https://www.natureandculture.org/directory/marine-reserve-peru/ (accessed on 24 May 2023).
- Chero, J.D.; Cruces, C.L.; Sáez, G.; Luque, J.L. Proposal of Cynoscionella n. g. (Monogenea: Diplectanidae), with description of a new species from the gills of Cynoscion phoxocephalus (Actinopterygii: Sciaenidae) in Peru and reassignment of two species of Diplectanum Monticelli, 1903. Syst. Paraitology 2022, 100, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Cruces, C.L.; Chero, J.D.; Sáez, G.; Luque, J.L. Redescription of Haliotrematoides mediohamides (Monogenea: Dactylogyridae), a gill parasite of the Pacific porgy Calamus brachysomus (Perciformes: Sparidae) from the Eastern Pacific Ocean. Syst. Parasitol. 2022, 93, e933960. [Google Scholar]
- Thatcher, V.E. Aquatic Biodiversity in Latin America: Amazon Fish Parasites, 2nd ed.; Pensoft: Sofia, Bulgaria, 2006; 508p. [Google Scholar]
- Tancredo, K.R.; Martins, M.L. Three previous recorded species of Dactylogyrus Diesing, 1850 (Monogenea: Dactylogyridae) infecting cultured Carassius auratus in southern Brazil. J. Parasit. Dis. 2019, 43, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Mbokane, E.M.; Matla, M.M.; Theron, J.; Luus-Powell, W.J. Seasonal dynamics and occurrences of three Dactylogyrus species on the gills of three cyprinids at Nwanedi–Luphephe dams in Limpopo province, South Africa. Afr. Zool. 2015, 50, 119–125. [Google Scholar] [CrossRef]
- Allen, G.R.; Robertson, D.R. Fishes of the Tropical Eastern; Pacific University of Hawaii Press: Honolulu, HI, USA, 1994; 332p. [Google Scholar]
- Nielsen, J.G.; Cohen, D.M.; Markle, D.F.; Robins, C.R. Ophidiiform fishes of the world (Order Ophidiiformes). An annotated and illustrated catalogue of pearlfishes, cusk-eels, brotulas and other ophidiiform fishes known to date. FAO Fish. Synop. 1999, 125, 178. [Google Scholar]
- Herrera, M.; Clarke, T.; Naranjo-Elizondo, B.; Espinoza, M.; Wehrtmann, I.S. Size at maturity of the Pacific bearded brotula (Ophidiidae: Brotula clarkae): A commercially exploited species in the Pacific of Costa Rica. Lat. Am. J. Aquat. Res. 2016, 44, 657–661. [Google Scholar] [CrossRef]
- Ambrose, D.A. Ophidiidae: Cusk-eels. In The Early Stages of Fishes in the California Current Region; Moser, H.G., Ed.; California Cooperative Oceanic Fisheries Investigations (CalCOFI): San Diego, CA, USA, 1996; 1505p. [Google Scholar]
- Chirichigno, N.; Cornejo, R.M. Catálogo Comentado de los Peces Marinos del Perú; Publicación Especial del Instituto del Mar, Instituto del Mar del Perú: Callao, Peru, 2001; 314p. [Google Scholar]
- Lea, R.N.; Allen, M.J.; Power, W. Records of the Pacific bearded brotula, Brotula clarkae, fromSouthern California. Bull. South. Calif. Acad. Sci. 2009, 108, 163–167. [Google Scholar]
- Acevedo, J.; Angulo, W.; Ramírez, M.; Zapata, L.A. Reproduction of the fish Brotula clarkae (Pisces: Ophidiidae) in the Colombian Pacific. Rev. Biol. Trop. 2007, 55, 957–967. [Google Scholar] [CrossRef]
- Chávez-Cevallos, J.M.; Caballero-Vergara, J.A. Análisis del contenido gastrointestinal de la corvina deroca (Brotula clarkae, Hubbs, 1994) desembarcadosen la Playa de Tarqui, Cantón Manta, Provincia de Manabí. Ph.D. Thesis, Universidad Laica Eloy Alfaro de Manabí, Manta, Ecuador, 2008; 84p. [Google Scholar]
- Humason, G.L. Animal Tissue Techniques, 4th ed.; W. H. Freeman and Co.: San Francisco, CA, USA, 1997; 661p. [Google Scholar]
- Mizelle, J.D. New species of trematodes from the gills of Illinois fishes. Am. Midl. Nat. 1936, 17, 785–806. [Google Scholar] [CrossRef]
- Mizelle, J.D.; Price, C.E. Additional haptoral hooks in the genus Dactylogyrus. J. Parasitol. 1963, 19, 785–806. [Google Scholar] [CrossRef]
- Chirichigno, N.; Vélez, M. Clave Para Identificar los peces Marinos del Perú, 2nd ed.; Publicación Especial del Instituto del Mar, Instituto del Mar del Perú: Callao, Peru, 1998; 500p. [Google Scholar]
- Littlewood, D.T.J.; Curini-Galletti, M.; Herniou, E.A. The interrelationships of Proseriata (Platyhelminthes: Seriata) tested with molecules and morphology. Mol. Phylogenet. Evol. 2000, 16, 449–466. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, A.E.; Olson, P.D.; Littlewood, D.T.J. Utility of complete large and small subunit rRNA genes in resolving the phylogeny of the Neodermata (Platyhelminthes): Implications and a review of the cercomer theory. Biol. J. Linn. Soc. 2003, 78, 155–171. [Google Scholar] [CrossRef]
- Olson, P.D.; Cribb, T.H.; Tkach, V.V.; Bray, R.A.; Littlewood, D.T.J. Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). Int. J. Parasitol. 2003, 33, 733–755. [Google Scholar] [CrossRef]
- Mendoza-Palmero, C.A.; Blasco-Costa, I.; Scholz, T. Molecular phylogeny of Neotropical monogeneans (Platyhelminthes: Monogenea) from catfishes (Siluriformes). Parasites Vectors 2015, 8, 164. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum likelihood phylogenies: Assessing the performance ofPhyML3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Lefort, V.; Longueville, J.E.; Gascuel, O. SMS: Smart Model Selection in PhyML. Mol. Biol. Evol. 2017, 34, 2422–2424. [Google Scholar] [CrossRef]
- Anisimova, M.; Gascuel, O. Approximate likelihood-ratio test for branches: A fast, accurate, and powerful alternative. Syst. Biol. 2006, 55, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.; Teslenko, M. Draft MrBayes Version 3.2 Manual: Tutorials and Model Summaries. Available online: https://nbisweden.github.io/MrBayes/manual.html (accessed on 6 April 2023).
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. The CIPRES science gateway: A community resource for phylogenetic analyses. In Proceedings of the 2011 TeraGrid Conference: Extreme Digital Discovery, Salt Lake City, UT, USA, 18–21 July 2011; pp. 1–8. [Google Scholar]
- Kmentová, N.; Cruz-Laufer, A.J.; Pariselle, A.; Smeets, K.; Artois, T.; Vanhove, M.P.M. Dactylogyridae 2022: A meta-analysis of phylogenetic studies and generic diagnoses of parasitic flatworms using published genetic and morphological data. Int. J. Parasitol. 2022, 52, 427–457. [Google Scholar] [CrossRef] [PubMed]
- Yamaguti, S. Monogenetic Trematodes of Hawaiian Fishes; University of Hawaii Press: Honolulu, HI, USA, 1968; 287p. [Google Scholar]
- Justine, J.-L.; Lambert, A.; Mattei, X. Spermatozoon ultrastructure and phylogenetic relationships in the monogeneans (Platyhelminthes). Int. J. Parasitol. 1985, 15, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Perkins, E.M.; Donnellan, S.C.; Bertozzi, T.; Chisholm, L.A.; Whittington, I.D. Looks can deceive: Molecular phylogeny of a family of flatworm ectoparasites (Monogenea: Capsalidae) does not reflect current morphological classification. Mol. Phylogenet. Evol. 2009, 52, 705–714. [Google Scholar] [CrossRef]
- Justine, J.-L.; Poddubnaya, L.G. Spermiogenesis and spermatozoon ultrastructure in basal polyopisthocotylean monogeneans, Hexabothriidae and Chimaericolidae, and their significance for the phylogeny of the Monogenea. Parasite 2018, 25, 7. [Google Scholar] [CrossRef]
- Mollaret, I.; Jamieson, B.G.M.; Adlard, R.D.; Hugall, A.; Lecointre, G.; Chombard, C.; Justine, J.-L. Phylogenetic analysis of the Monogenea and their relationships with Digenea and Eucestoda inferred from 28S rDNA sequences. Mol. Biochem. Parasitol. 1997, 90, 433–438. [Google Scholar] [CrossRef]
- Brabec, J.; Salomaki, E.D.; Kolísko, M.; Scholz, T.; Kutchta, R. The evolution of endoparasitism and complex life cycles in parasitic platyhelminths. Curr. Biol. 2023, 33, 4269–4275. [Google Scholar] [CrossRef]
- Mendoza-Palmero, C.A.; Blasco-Costa, I.; Hernández-Mena, D.; de León, G.P. Parasciadicleithrum octofasciatum n. gen., n. sp. (Monogenoidea: Dactylogyridae), parasite of Rocio octofasciata (Regan) (Cichlidae: Perciformes) from Mexico characterised by morphological and molecular evidence. Parasitol. Int. 2017, 66, 152–162. [Google Scholar] [CrossRef]
- Franceschini, L.; Acosta, A.A.; Zago, A.C.; Müller, M.I.; da Silva, R.J. Trinigyrus spp. (Monogenea: Dactylogyridae) from Brazilian catfishes: New species, molecular data and new morphological contributions to the genus. J. Helminthol. 2020, 94, e126. [Google Scholar] [CrossRef]
- Mendoza-Palmero, C.; Acosta, A.; Scholz, T. Molecular phylogeny of Cosmetocleithrum Kritsky, Thatcher & Boeger, 1986 (Monogenoidea: Dactylogyridae), gill parasites of Neotropical catfishes (Siluriformes). J. Helminthol. 2022, 96, E56. [Google Scholar]
- Ciccheto, J.R.M.; Razzolini, E.L.; de Buron, I.; Boeger, W.A. Position of Polyclithrum within Gyrodactylidae (Monogenoidea): Incongruences between morphological and molecular phylogenies. Syst. Parasitol. 2023, 100, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.E.; Sepulveda, F.A.; González, M. Parapedocotyle prolatili gen. n. et sp. n., a representative of a new subfamily of the Diclidophoridae (Monogenea), a gill parasite of Prolatilus jugularis (Teleostei: Pinguipedidae) from Chile. Folia Parasitol. 2014, 61, 543–548. [Google Scholar] [CrossRef]
- Cohen, S.C.; Justo, M.C.N.; Kohn, A. South American Monogenoidea Parasites of Fishes, Amphibians and Reptiles; Oficina de Livros: Rio de Janeiro, Brazil, 2013; 663p. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).