Effects of Short-Term Intermittent Fasting on Growth Performance, Fatty Acids Profile, Glycolysis and Cholesterol Synthesis Gene Expression in European Seabass Dicentrarchus labrax
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Growth Performance and Proximate Composition
3.2. Biochemical Parameters
3.3. Fatty Acids (FA) Profiles
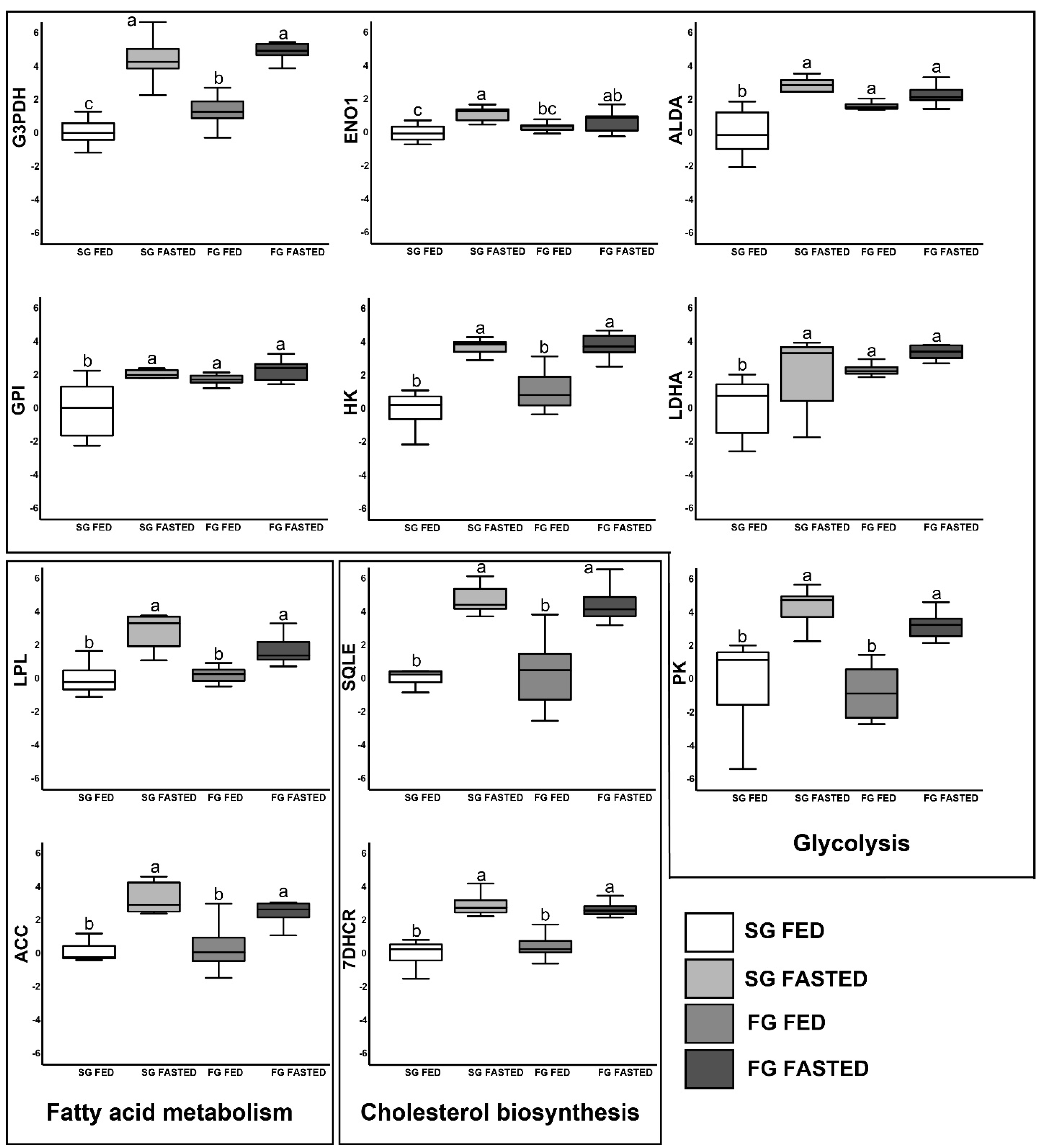
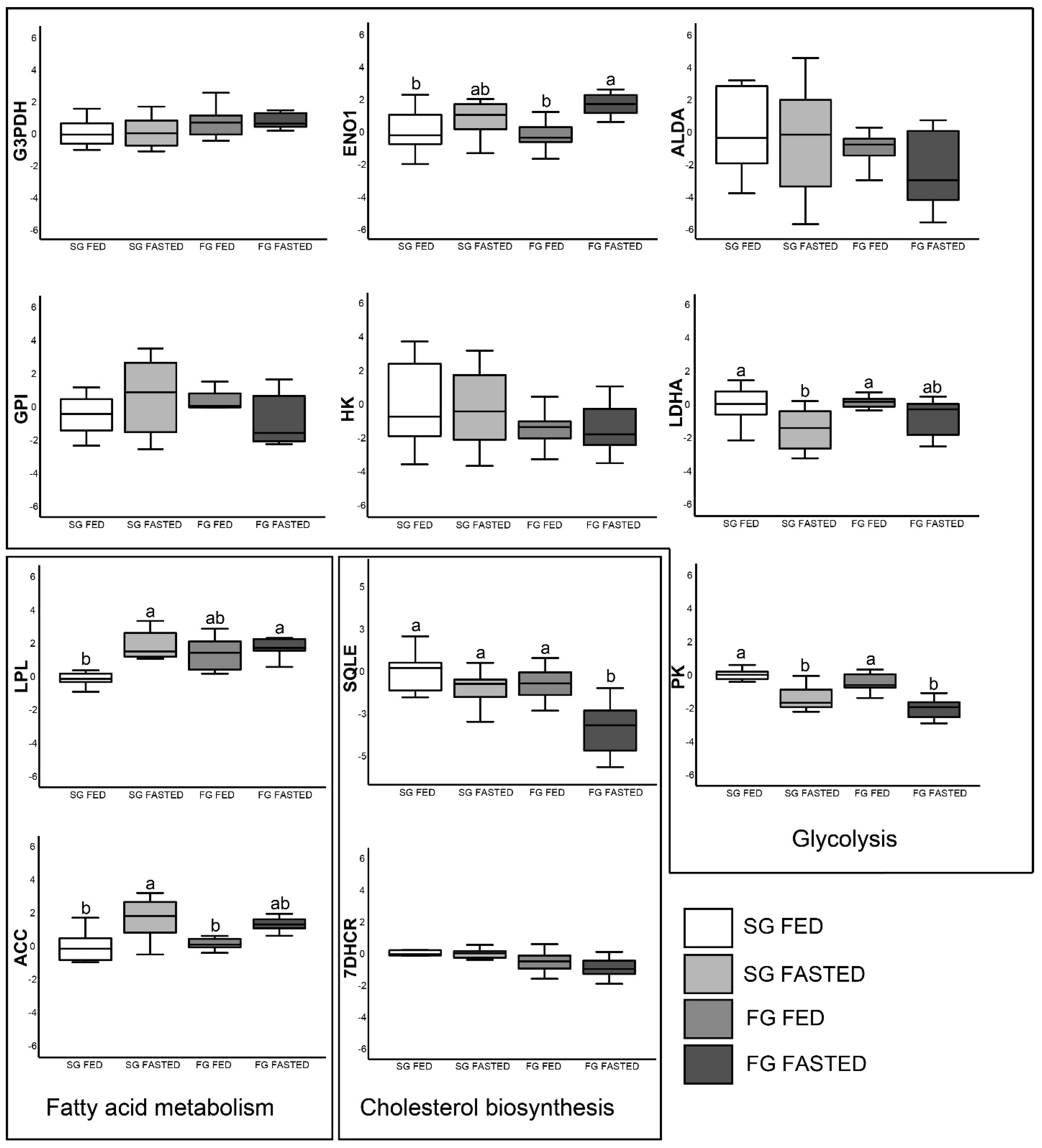
3.4. Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, S.J.; Xie, X.J.; Cao, Z.D. Effect of Fasting on Resting Metabolic Rate and Postprandial Metabolic Response in Silurus Meridionalis. J. Fish Biol. 2005, 67, 279–285. [Google Scholar] [CrossRef]
- Ng, W.K.; Lu, K.S.; Hashim, R.; Ali, A. Effects of Feeding Rate on Growth, Feed Utilizationand Body Composition of a Tropical Bagrid Catfish. Aquac. Int. 2000, 8, 19–29. [Google Scholar] [CrossRef]
- Chatzifotis, S.; Papadaki, M.; Despoti, S.; Roufidou, C.; Antonopoulou, E. Effect of Starvation and Re-Feeding on Reproductive Indices, Body Weight, Plasma Metabolites and Oxidative Enzymes of Sea Bass (Dicentrarchus labrax). Aquaculture 2011, 316, 53–59. [Google Scholar] [CrossRef]
- Rubio, V.C.; Sánchez, E.; Cerdá-Reverter, J.M. Compensatory Feeding in the Sea Bass after Fasting and Physical Stress. Aquaculture 2010, 298, 332–337. [Google Scholar] [CrossRef]
- McKenzie, D.J.; Vergnet, A.; Chatain, B.; Vandeputte, M.; Desmarais, E.; Steffensen, J.F.; Guinand, B. Physiological Mechanisms Underlying Individual Variation in Tolerance of Food Deprivation in Juvenile European Sea Bass, Dicentrarchus labrax. J. Exp. Biol. 2014, 217, 3283–3292. [Google Scholar] [CrossRef]
- Adaklı, A.; Taşbozan, O. The Effects of Different Cycles of Starvation and Refeeding on Growth and Body Composition on European Sea Bass (Dicentrarchus labrax). Turkish J. Fish. Aquat. Sci. 2015, 15, 425–433. [Google Scholar] [CrossRef]
- Daulé, S.; Vandeputte, M.; Vergnet, A.; Guinand, B.; Grima, L.; Chatain, B. Effect of Selection for Fasting Tolerance on Feed Intake, Growth and Feed Efficiency in the European Sea Bass Dicentrarchus labrax. Aquaculture 2014, 420–421, S42–S49. [Google Scholar] [CrossRef]
- Hornick, J.L.; Van Eenaeme, C.; Gérard, O.; Dufrasne, I.; Istasse, L. Mechanisms of Reduced and Compensatory Growth. Domest. Anim. Endocrinol. 2000, 19, 121–132. [Google Scholar] [CrossRef]
- Ali, M.; Nicieza, A.; Wootton, R.J. Compensatory Growth in Fishes: A Response to Growth Depression. Fish Fish. 2003, 4, 147–190. [Google Scholar] [CrossRef]
- Nikki, J.; Pirhonen, J.; Jobling, M.; Karjalainen, J. Compensatory Growth in Juvenile Rainbow Trout, Oncorhynchus Mykiss (Walbaum), Held Individually. Aquaculture 2004, 235, 285–296. [Google Scholar] [CrossRef]
- Stefansson, S.O.; Imsland, A.K.; Handeland, S.O. Food-Deprivation, Compensatory Growth and Hydro-Mineral Balance in Atlantic Salmon (Salmo salar) Post-Smolts in Sea Water. Aquaculture 2009, 290, 243–249. [Google Scholar] [CrossRef]
- Nicieza, A.G.; Metcalfe, N.B. Growth Compensation in Juvenile Atlantic Salmon: Responses to Depressed Temperature and Food Availability. Ecology 1997, 78, 2385–2400. [Google Scholar] [CrossRef]
- Ali, T.E.S.; Martínez-Llorens, S.; Moñino, A.V.; Cerdá, M.J.; Tomás-Vidal, A. Effects of Weekly Feeding Frequency and Previous Ration Restriction on the Compensatory Growth and Body Composition of Nile Tilapia Fingerlings. Egypt. J. Aquat. Res. 2016, 42, 357–363. [Google Scholar] [CrossRef]
- Bavčević, L.; Klanjšček, T.; Karamarko, V.; Aničić, I.; Legović, T. Compensatory Growth in Gilthead Sea Bream (Sparus aurata) Compensates Weight, but Not Length. Aquaculture 2010, 301, 57–63. [Google Scholar] [CrossRef]
- Peres, H.; Santos, S.; Oliva-Teles, A. Lack of Compensatory Growth Response in Gilthead Seabream (Sparus aurata) Juveniles Following Starvation and Subsequent Refeeding. Aquaculture 2011, 318, 384–388. [Google Scholar] [CrossRef]
- Zhu, X.; Xie, S.; Zou, Z.; Lei, W.; Cui, Y.; Yang, Y.; Wootton, R.J. Compensatory Growth and Food Consumption in Gibel Carp, Carassius Auratus Gibelio, and Chinese Longsnout Catfish, Leiocassis longirostris, Experiencing Cycles of Feed Deprivation and Re-Feeding. Aquaculture 2004, 241, 235–247. [Google Scholar] [CrossRef]
- Canosa, L.F.; Bertucci, J.I. Nutrient Regulation of Somatic Growth in Teleost Fish. The Interaction between Somatic Growth, Feeding and Metabolism. Mol. Cell. Endocrinol. 2020, 518, 111029. [Google Scholar] [CrossRef]
- Jezierska, B.; Hazel, J.R.; Gerking, S.D. Lipid Mobilization during Starvation in the Rainbow Trout, Salmo gairdneri Richardson, with Attention to Fatty Acids. J. Fish Biol. 1982, 21, 681–692. [Google Scholar] [CrossRef]
- Johnston, I.A.; Bower, N.I.; Macqueen, D.J. Growth and the Regulation of Myotomal Muscle Mass in Teleost Fish. J. Exp. Biol. 2011, 214, 1617–1628. [Google Scholar] [CrossRef]
- Fuller, S.A.; Beck, B.H.; McEntire, M.E.; Peatman, E.; Abernathy, J.; Bahler, J. Heritability of Growth Traits and Correlation with Hepatic Gene Expression among Hybrid Striped Bass Exhibiting Extremes in Performance. Cogent Biol. 2018, 4, 1453319. [Google Scholar] [CrossRef]
- Skiba-Cassy, S.; Lansard, M.; Panserat, S.; Médale, F. Rainbow Trout Genetically Selected for Greater Muscle Fat Content Display Increased Activation of Liver TOR Signaling and Lipogenic Gene Expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Rescan, P.Y.; Montfort, J.; Rallière, C.; Le Cam, A.; Esquerré, D.; Hugot, K. Dynamic Gene Expression in Fish Muscle during Recovery Growth Induced by a Fasting-Refeeding Schedule. BMC Genom. 2007, 8, 438. [Google Scholar] [CrossRef] [PubMed]
- Rimoldi, S.; Benedito-Palos, L.; Terova, G.; Pérez-Sánchez, J. Wide-Targeted Gene Expression Infers Tissue-Specific Molecular Signatures of Lipid Metabolism in Fed and Fasted Fish. Rev. Fish Biol. Fish 2016, 26, 93–108. [Google Scholar] [CrossRef]
- Terova, G.; Rimoldi, S.; Chini, V.; Gornati, R.; Bernardini, G.; Saroglia, M. Cloning and Expression Analysis of Insulin-like Growth Factor I and II in Liver and Muscle of Sea Bass (Dicentrarchus labrax, L.) during Long-Term Fasting and Refeeding. J. Fish Biol. 2007, 70, 219–233. [Google Scholar] [CrossRef]
- Bjørndal, T.; Guillen, J.; Rad, F. Are Farmed European Seabass (Dicentrarchus labrax) Prices in European Union Markets Affected by Turkish Exports of Farmed European Seabass? Aquac. Econ. Manag. 2019, 23, 341–357. [Google Scholar] [CrossRef]
- FEAP. European Aquaculture Production Report; IEMed: Barcelona, Spain, 2021; Volume 2020. [Google Scholar]
- Carvalho, N.; Guillen, J. Aquaculture in the Mediterranean; IEMed: Barcelona, Spain, 2021. [Google Scholar]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Hellenic Aquaculture Producers Organisation. Greek Aquaculture: Annual Report 2022; HAPO: Athens, Greece, 2023. [Google Scholar]
- Yılmaz, H.A.; Eroldogan, O.T. Combined Effects of Cycled Starvation and Feeding Frequency on Growth and Oxygen Consumption of Gilthead Sea Bream, Sparus aurata. J. World Aquac. Soc. 2011, 42, 522–529. [Google Scholar] [CrossRef]
- Mccarthy, I.D.; Carter, C.G.; Houlihan, D.F. The Effect of Feeding Hierarchy on Individual Variability in Daily Feeding of Rainbow Trout, Oncorhynchus Mykiss (Walbaum). J. Fish Biol. 1992, 41, 257–263. [Google Scholar] [CrossRef]
- Talbot, C.; Corneillie, S.; Korsøen, Ø. Pattern of Feed Intake in Four Species of Fish under Commercial Farming Conditions: Implications for Feeding Management. Aquac. Res. 1999, 30, 509–518. [Google Scholar] [CrossRef]
- Lovell, T. Fish Nutrition and Feeding Experiments. In Nutrition and Feeding of Fish; Springer: New York, NY USA, 1998; pp. 123–134. [Google Scholar]
- Cho, S.H.; Lee, S.M.; Park, B.H.; Ji, S.C.; Lee, J.; Bae, J.; Oh, S.Y. Compensatory Growth of Juvenile Olive Flounder, Paralichthys olivaceus L., and Changes in Proximate Composition and Body Condition Indexes during Fasting and after Refeeding in Summer Season. J. World Aquac. Soc. 2006, 37, 168–174. [Google Scholar] [CrossRef]
- Cho, S.H.; Lee, S.M.; Park, B.H.; Ji, S.C.; Choi, C.Y.; Lee, J.H.; Kim, Y.C.; Lee, J.H.; Oh, S.Y. Effect of Daily Feeding Ratio on Growth and Body Composition of Subadult Olive Flounder, Paralichthys olivaceus, Fed an Extruded Diet during the Summer Season. J. World Aquac. Soc. 2007, 38, 68–73. [Google Scholar] [CrossRef]
- Kim, Y.O.; Oh, S.Y.; Lee, W.S. Feeding Ratio Affects Growth, Body Composition, and Blood Chemistry of Mandarin Fish (Siniperca scherzeri) in Recirculating Aquaculture System. Fish. Aquat. Sci. 2021, 24, 219–227. [Google Scholar] [CrossRef]
- Kim, K.D.; Kang, Y.J.; Kim, K.W.; Kim, K.M. Effects of Feeding Rate on Growth and Body Composition of Juvenile Flounder, Paralichthys olivaceus. J. World Aquac. Soc. 2007, 38, 169–173. [Google Scholar] [CrossRef]
- Tian, X.; Qin, J.G. A Single Phase of Food Deprivation Provoked Compensatory Growth in Barramundi Lates Calcarifer. Aquaculture 2003, 224, 169–179. [Google Scholar] [CrossRef]
- Rueda, F.M.; Martinez, F.J.; Zamora, S.; Kentouri, M.; Divanach, P. Effect of Fasting and Refeeding on Growth and Body Composition of Red Porgy, Pagrus pagrus L. Aquac. Res. 1998, 29, 447–452. [Google Scholar] [CrossRef]
- Gaylord, I.G.; Gatlin, D.M. Assessment of Compensatory Growth in Channel Catfish Ictalurus punctatus R. and Associated Changes in Body Condition Indices. J. World Aquac. Soc. 2007, 31, 326–336. [Google Scholar] [CrossRef]
- Jobling, M. Fish Bioenergetics; Chapman & Hall: London, UK, 1994. [Google Scholar]
- Mattila, J.; Koskela, J.; Pirhonen, J. The Effect of the Length of Repeated Feed Deprivation between Single Meals on Compensatory Growth of Pikeperch Sander lucioperca. Aquaculture 2009, 296, 65–70. [Google Scholar] [CrossRef]
- Tian, X.; Fang, J.; Dong, S. Effects of Starvation and Recovery on the Growth, Metabolism and Energy Budget of Juvenile Tongue Sole (Cynoglossus semilaevis). Aquaculture 2010, 310, 122–129. [Google Scholar] [CrossRef]
- Urbinati, E.C.; Sarmiento, S.J.; Takahashi, L.S. Short-Term Cycles of Feed Deprivation and Refeeding Promote Full Compensatory Growth in the Amazon Fish Matrinxã (Brycon amazonicus). Aquaculture 2014, 433, 430–433. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Sun, W.; Chen, J.; Gao, Q.; Shuai, K.; Leng, X. Effects of Different Feeding Rates of Extruded and Pelleted Feeds on Growth and Nutrient Retention in Channel Catfish (Ictalurus punctatus). Aquac. Int. 2017, 25, 1361–1372. [Google Scholar] [CrossRef]
- Tocher, D.R. Metabolism and Functions of Lipids and Fatty Acids in Teleost Fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Peruzzi, S.; Varsamos, S.; Chatain, B.; Fauvel, C.; Menu, B.; Falguière, J.C.; Sévère, A.; Flik, G. Haematological and Physiological Characteristics of Diploid and Triploid Sea Bass, Dicentrarchus labrax L. Aquaculture 2005, 244, 359–367. [Google Scholar] [CrossRef][Green Version]
- Peres, H.; Santos, S.; Oliva-Teles, A. Blood Chemistry Profile as Indicator of Nutritional Status in European Seabass (Dicentrarchus labrax). Fish Physiol. Biochem. 2014, 40, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Svetina, A.; Matašin, Ž.; Tofant, A.; Vučemilo, M.; Fijan, N. Haematology and Some Blood Chemical Parameters of Young Carp till the Age of Three Years. Acta Vet. Hung. 2002, 50, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.J.; Slater, M.J.; Bögner, M.; Zeytin, S.; Kunzmann, A. Extreme Ambient Temperature Effects in European Seabass, Dicentrarchus Labrax: Growth Performance and Hemato-Biochemical Parameters. Aquaculture 2020, 522, 735093. [Google Scholar] [CrossRef]
- Sinha, A.K.; Dasan, A.F.; Rasoloniriana, R.; Pipralia, N.; Blust, R.; De Boeck, G. Hypo-Osmotic Stress-Induced Physiological and Ion-Osmoregulatory Responses in European Sea Bass (Dicentrarchus labrax) Are Modulated Differentially by Nutritional Status. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 181, 87–99. [Google Scholar] [CrossRef]
- Caruso, G.; Denaro, M.G.; Caruso, R.; Mancari, F.; Genovese, L.; Maricchiolo, G. Response to Short Term Starvation of Growth, Haematological, Biochemical and Non-Specific Immune Parameters in European Sea Bass (Dicentrarchus labrax) and Blackspot Sea Bream (Pagellus bogaraveo). Mar. Environ. Res. 2011, 72, 46–52. [Google Scholar] [CrossRef]
- Somero, G.N.; Childress, J.J. A Violation of the Metabolism-Size Scaling Paradigm: Activities of Glycolytic Enzymes in Muscle Increase in Larger-Size Fish. Physiol. Zool. 1980, 53, 322–337. [Google Scholar] [CrossRef]
- Churova, M.V.; Shulgina, N.; Kuritsyn, A.; Krupnova, M.Y.; Nemova, N.N. Muscle-Specific Gene Expression and Metabolic Enzyme Activities in Atlantic Salmon Salmo salar L. Fry Reared under Different Photoperiod Regimes. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2020, 239, 110330. [Google Scholar] [CrossRef]
- Pribyl, A.L.; Hyde, J.R.; Robertson, L.; Vetter, R. Defining an Ideal Temperature Range for the Northern Subpopulation of Pacific Sardine, Sardinops Sagax Caeruleus. Environ. Biol. Fishes 2016, 99, 275–291. [Google Scholar] [CrossRef]
- Mittakos, I.; Ayala, M.D.; López-Albors, O.; Grigorakis, K.; Lenas, D.; Kakali, F.; Nathanailides, C. Muscle Cellularity, Enzyme Activities, and Nucleic Acid Content in Meagre (Argyrosomus regius). Can. J. Zool. 2012, 90, 1270–1277. [Google Scholar] [CrossRef]
- Bar, N.; Volkoff, H. Adaptation of the Physiological, Endocrine, and Digestive System Functions to Prolonged Food Deprivation in Fish. In Comparative Physiology of Fasting, Starvation, and Food Limitation; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2012; pp. 69–89. [Google Scholar]
- Gaudreau, C.M.; Le François, N.R.; Tveiten, H.; Blier, P.U. Evaluation of the Physiological Relationship Between Oxidative Stress and Metabolic Characters: Insights from Hybridization Between Anarhichas Minor × A. Lupus. Open Fish Sci. J. 2014, 6, 107–114. [Google Scholar] [CrossRef][Green Version]
- Sherwood, G.D.; Pazzia, I.; Moeser, A.; Hontela, A.; Rasmussen, J.B. Shifting Gears: Enzymatic Evidence for the Energetic Advantage of Switching Diet in Wild-Living Fish. Can. J. Fish. Aquat. Sci. 2002, 59, 229–241. [Google Scholar] [CrossRef]
- Robledo, D.; Rubiolo, J.A.; Cabaleiro, S.; Martínez, P.; Bouza, C. Differential Gene Expression and SNP Association between Fast- and Slow-Growing Turbot (Scophthalmus maximus). Sci. Rep. 2017, 7, 12105. [Google Scholar] [CrossRef] [PubMed]
- Danzmann, R.G.; Kocmarek, A.L.; Norman, J.D.; Rexroad, C.E.; Palti, Y. Transcriptome Profiling in Fast versus Slow-Growing Rainbow Trout across Seasonal Gradients. BMC Genom. 2016, 17, 60. [Google Scholar] [CrossRef]
- Polakof, S.; Panserat, S.; Soengas, J.L.; Moon, T.W. Glucose Metabolism in Fish: A Review. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2012, 182, 1015–1045. [Google Scholar] [CrossRef]
- Song, Y.; Alami-Durante, H.; Skiba-Cassy, S.; Marandel, L.; Panserat, S. Higher Glycolytic Capacities in Muscle of Carnivorous Rainbow Trout Juveniles after High Dietary Carbohydrate Stimulus at First Feeding. Nutr. Metab. 2019, 16, 77. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Vatner, D.F.; Shulman, G.I. Regulation of Hepatic Glucose Metabolism in Health and Disease. Nat. Rev. Endocrinol. 2017, 13, 572–587. [Google Scholar] [CrossRef]
- Hemre, G.-I.; Mommsen, T.P.; Krogdahl, Å. Carbohydrates in Fish Nutrition: Effects on Growth, Glucose Metabolism and Hepatic Enzymes. Aquac. Nutr. 2002, 8, 175–194. [Google Scholar] [CrossRef]
- Ma, J.-L.; Qiang, J.; Tao, Y.-F.; Bao, J.-W.; Zhu, H.-J.; Li, L.-G.; Xu, P. Multi-Omics Analysis Reveals the Glycolipid Metabolism Response Mechanism in the Liver of Genetically Improved Farmed Tilapia (GIFT, Oreochromis niloticus) under Hypoxia Stress. BMC Genom. 2021, 22, 105. [Google Scholar] [CrossRef]
- Kondo, H.; Suda, S.; Kawana, Y.; Hirono, I.; Nagasaka, R.; Kaneko, G.; Ushio, H.; Watabe, S. Effects of Feed Restriction on the Expression Profiles of the Glucose and Fatty Acid Metabolism-Related Genes in Rainbow Trout Oncorhynchus Mykiss Muscle. Fish. Sci. 2012, 78, 1205–1211. [Google Scholar] [CrossRef]
- Abernathy, J.; Panserat, S.; Welker, T.; Plagne-Juan, E.; Sakhrani, D.; Higgs, D.A.; Audouin, F.; Devlin, R.H.; Overturf, K. Food Shortage Causes Differential Effects on Body Composition and Tissue-Specific Gene Expression in Salmon Modified for Increased Growth Hormone Production. Mar. Biotechnol. 2015, 17, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Leaver, M.J.; Villeneuve, L.A.N.; Obach, A.; Jensen, L.; Bron, J.E.; Tocher, D.R.; Taggart, J.B. Functional Genomics Reveals Increases in Cholesterol Biosynthetic Genes and Highly Unsaturated Fatty Acid Biosynthesis after Dietary Substitution of Fish Oil with Vegetable Oils in Atlantic Salmon (Salmo salar). BMC Genom. 2008, 9, 299. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Solares, A.; Xue, X.; Parrish, C.C.; Foroutani, M.B.; Taylor, R.G.; Rise, M.L. Changes in the Liver Transcriptome of Farmed Atlantic Salmon (Salmo salar) Fed Experimental Diets Based on Terrestrial Alternatives to Fish Meal and Fish Oil. BMC Genom. 2018, 19, 796. [Google Scholar] [CrossRef]
- Lin, G.; Thevasagayam, N.M.; Wan, Z.Y.; Ye, B.Q.; Yue, G.H. Transcriptome Analysis Identified Genes for Growth and Omega-3/-6 Ratio in Saline Tilapia. Front. Genet. 2019, 10, 244. [Google Scholar] [CrossRef]
- Nathanailides, C.; Stickland, N.C. Activity of Cytochrome c Oxidase and Lactate Dehydrogenase in Muscle Tissue of Slow Growing (Lower Modal Group) and Fast Growing (Upper Modal Group) Atlantic Salmon. J. Fish Biol. 1996, 48, 549–551. [Google Scholar] [CrossRef]
- Churova, M.V.; Meshcheryakova, O.V.; Nemova, N.N.; Shatunovskii, M.I. The Correlation between Fish Growth and Several Biochemical Characteristics with Reference to the Steelhead Parasalmo Mykiss Walb. Biol. Bull. 2010, 37, 236–245. [Google Scholar] [CrossRef]
- Blier, P.U.; Lemieux, H.; Devlin, R.H. Is the Growth Rate of Fish Set by Digestive Enzymes or Metabolic Capacity of the Tissues? Insight from Transgenic Coho Salmon. Aquaculture 2002, 209, 379–384. [Google Scholar] [CrossRef]
| Pathway | Gene Name | NCBI Acc. No. | Primer Name | Primer Sequence (5′–3′) | Tm (°C) | Product Length (bp) |
|---|---|---|---|---|---|---|
| Glucolysis | l-lactate dehydrogenase A chain | CBXY010014016 | LDHAF | TTGGCCTTAACTCAGCCTGT | 60 | 86 |
| LDHAR | ATACAGTACACAGAGTATAT | 58.5 | ||||
| Glucolysis | Hexokinase I | CBXY010013088 | HKF | GATGAGTGCTGCTCCTTTCC | 60 | 96 |
| HKR | GTCTCTGTCTAGTTTCTCTG | 61 | ||||
| Glucolysis | Glucose-6-phosphate isomerase | CBXY010012918 | GPIF | CTCACACAGGACCCCAACTT | 60 | 87 |
| GPIR | TTGTTGAATCTCTCTTTGTCA | 59 | ||||
| Glucolysis | Enolase 1 | CBXY010009925 | ENO1F | AGATCGTCATTGGCATGGAT | 60 | 85 |
| ENO1R | AGGGGAGATGTAGCGGCTGG | 60 | ||||
| Glucolysis | Glyceraldehyde-3-phosphate dehydrogenase | CBXY010005389 | G3PDHF | TGTAACCCAGCACTCCCTTC | 60 | 84 |
| G3PDHR | GTGGACCTGACATGCCGTCT | 61 | ||||
| Glucolysis | Aldolase A | CBXY010008418 | ALDAF | CTGTCCGACCACCATGTCTA | 60 | 80 |
| ALDAR | GATCTCCTGGTTGCTGTACT | 60 | ||||
| Glucolysis | Pyruvate kinase | KF857578 | PKF | GGCGTTCAGAATTTTGAGGA | 60 | 109 |
| PKR | TTGCAGCGTCCAATCATCAT | 60 | ||||
| Fatty acid metabolism | Lipoprotein lipase | AM411614 | LPLF | GTAACGGGGATGTTCGAGAG | 59 | 89 |
| LPLR | CTGGTTGGCGCGGGTCAGCC | 59 | ||||
| Fatty acid metabolism | Acetyl-CoA carboxylase | CBXY010003615 | ACCF | AGTACCTGCACAGCCAGGAT | 60 | 83 |
| ACCR | GCAAGTTGACATCAGCCACC | 60 | ||||
| Cholesterol biosynthesis | Squalene epoxidase | CABK01002385 | SQLEF | GAATCGACCGTGATGGAAAG | 60 | 112 |
| SQLER | AGGGTCTGGATGCCCATCTG | 60 | ||||
| Cholesterol biosynthesis | 7-dehydrocholesterol reductase | CBXY010012845 | 7DHCRF | TCGGCCACATACTCCCATAC | 60 | 116 |
| 7DHCRR | GGTAAGGCACAGTGTCTGTG | 60 | ||||
| Reference gene | Beta-actin | AY148350 | ACTBF | ATCAAGATCATTGCCCCACCT | 63 | 92 |
| ACTBR | TCATACTCCTGCTTGCTGA | 59 | ||||
| Reference gene | Elongation factor 1 | AJ866727 | EF1F | CGCTCTGTGGAAGTTTGAGA | 59 | 102 |
| EF1R | GATCAGCACAGCGCAGTCAG | 61 |
| SG | FG | T | G | |||
|---|---|---|---|---|---|---|
| Fed | Fasted | Fed | Fasted | |||
| Final Weight (g) | 73.08 ± 4.12 | 84.43 ± 5.00 | 163.88 ± 1.80 | 164.57 ± 2.30 | ns | *** |
| Final Length (cm) | 20.26 ± 0.09 | 19.77 ± 0.09 | 23.18 ± 0.07 | 22.68 ± 0.08 | ns | *** |
| WGR (%) | 42.1 ± 2.06 | 39.70 ± 1.79 | 47.53 ± 0.60 | 47.20 ± 0.30 | ns | * |
| SGR (% day−1) | 0.86 ± 0.04 | 0.83 ± 0.04 | 0.97 ± 0.01 | 0.97 ± 0.005 | ns | ** |
| CF | 1.04 ± 0.06 | 1.18 ± 0.07 | 1.35 ± 0.03 | 1.41 ± 0.03 | ns | *** |
| HSI (%) | 0.48 ± 0.14 | 0.51 ± 0.09 | 0.76 ± 0.09 | 0.58 ± 0.08 | ns | ns |
| VSI (%) | 2.56 ± 0.15 | 2.72 ± 0.22 | 2.50 ± 0.10 | 2.64 ± 0.18 | ns | ns |
| ISI (%) | 1.56 ± 0.16 | 0.9 ± 0.11 | 1.41 ± 0.06 | 1.05 ± 0.06 | * | ns |
| SSI (%) | 0.12 ± 0.01 | 0.10 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.01 | ns | ns |
| IFR (%) | 1.65 ± 0.67 | 2.91 ± 0.67 | 5.5 ± 0.45 | 5.37 ± 0.63 | ns | *** |
| Moisture (%) | 68.15 ± 1.71 | 60.73 ± 2.68 | 50.53 ± 1.13 | 60.60 ± 1.35 | ns | *** |
| Crude protein (%) | 54.77 ± 2.54 | 49.33 ± 2.86 | 41.37 ± 0.98 | 41.06 ± 3.92 | ns | *** |
| Crude lipid (%) | 25.72 ± 2.61 | 30.86 ± 2.60 | 32.36 ± 0.84 | 33.03 ± 1.28 | ns | * |
| Ash (%) | 14.51 ± 1.04 | 11.39 ± 1.41 | 7.51 ± 0.30 | 11.21 ± 0.96 | ns | *** |
| Gross energy (KJ/g) | 22.40 ± 0.57 | 23.38 ± 0.72 | 21.94 ± 0.28 | 24.14 ± 0.53 | ns | ns |
| SG | FG | T | G | |||
|---|---|---|---|---|---|---|
| Fed | Fasted | Fed | Fasted | |||
| Glucose (mg/dL) | 16.9 ± 1.70 | 21.1 ± 3.20 | 27.6 ± 1.10 | 26.4 ± 3.40 | ns | ns |
| Triglycerides (mg/dL) | 238.0 ± 50.70 | 110.0 ± 47.5 | 117.3 ± 21.01 | 78.1 ± 14 | * | ns |
| Cholesterol (mg/dL) | 216.6 ± 20.20 | 161.3 ± 12 | 189.7 ± 23.20 | 157.8 ± 9.10 | * | ns |
| NEFA (mmol/L) | 8.5 ± 1.90 | 7.9 ± 1.80 | 5.9 ± 0.40 | 3.7 ± 0.40 | ns | ** |
| LDH (U/L) | 1348.9 ± 64.60 | 554.6 ± 134.10 | 415.7 ± 127.30 | 541.3 ± 165 | ns | ns |
| SG | FG | T | G | |||
|---|---|---|---|---|---|---|
| Fed | Fasted | Fed | Fasted | |||
| 14:0 | 3.5 ± 0.18 | 3.07 ± 0.15 | 2.76 ± 0.11 | 2.59 ± 0.08 | ns | *** |
| 16:0 | 17.05 ± 0.36 | 16.83 ± 0.21 | 17.64 ± 0.69 | 16.34 ± 0.51 | ns | ns |
| 16:1n-7 | 5.21 ± 0.20 | 4.98 ± 0.13 | 5.02 ± 0.20 | 4.55 ± 0.17 | ns | ns |
| 18:0 | 4.04 ± 0.11 | 3.87 ± 0.07 | 3.73 ± 0.33 | 3.7 ± 0.12 | ns | ns |
| 18:1n-9 | 25.71 ± 1.03 | 28.57 ± 1.31 | 29.69 ± 2.57 | 31.24 ± 1.05 | ns | ns |
| 18:1n-7 | 3.16 ± 0.08 | 3 ± 0.06 | 2.94 ± 0.11 | 2.82 ± 0.08 | ns | ns |
| 18:2n-6 | 9.55 ± 0.45 | 10.53 ± 0.52 | 11.8 ± 0.50 | 11.52 ± 0.41 | ns | ** |
| 18:3n-3 | 2.08 ± 0.17 | 2.46 ± 0.19 | 2.93 ± 0.13 | 2.87 ± 0.10 | ns | *** |
| 18:4n-3 | 0.86 ± 0.03 | 0.76 ± 0.02 | 0.73 ± 0.03 | 0.66 ± 0.02 | ns | *** |
| 20:1n-9 | 3.03 ± 0.10 | 2.72 ± 0.12 | 2.64 ± 0.12 | 2.65 ± 0.08 | ns | * |
| 20:2n-6 | 0.73 ± 0.02 | 0.66 ± 0.02 | 0.69 ± 0.02 | 0.76 ± 0.03 | ns | * |
| 20:4n-6 | 0.13 ± 0.01 | 0.1 ± 0.02 | 0.11 ± 0.02 | 0.12 ± 0.02 | ns | ns |
| 20:5n-3 | 5.31 ± 0.33 | 4.28 ± 0.20 | 3.77 ± 0.16 | 3.44 ± 0.12 | ns | *** |
| 22:1n-11 | 1.68 ± 0.09 | 1.45 ± 0.10 | 1.09 ± 0.11 | 1.19 ± 0.04 | ns | *** |
| 22:5n-3 | 1.35 ± 0.08 | 1.06 ± 0.05 | 0.98 ± 0.08 | 0.89 ± 0.03 | ns | *** |
| 22:6n-3 | 7.36 ± 0.46 | 5.46 ± 0.41 | 4.56 ± 0.17 | 4.17 ± 0.13 | ns | *** |
| SFA | 33.45 ± 0.96 | 31.39 ± 0.49 | 31.4 ± 1.37 | 30.89 ± 0.77 | ns | ns |
| MUFA | 41.36 ± 0.6 | 43.1 ± 0.81 | 43.37 ± 2.07 | 44.67 ± 0.93 | ns | ns |
| Σn-3 | 13.42 ± 0.90 | 12.89 ± 0.99 | 11.07 ± 0.63 | 10.3 ± 0.56 | ns | * |
| Σn-3 HUFA | 10.12 ± 0.93 | 9.37 ± 1.15 | 7.15 ± 0.61 | 6.54 ± 0.62 | ns | ** |
| Σn-6 | 11.08 ± 0.45 | 11.89 ± 0.48 | 13.27 ± 0.49 | 12.99 ± 0.37 | ns | ** |
| EPA + DHA | 8.14 ± 0.95 | 7.68 ± 1.09 | 5.61 ± 0.61 | 5.12 ± 0.59 | ns | * |
| SFA/MUFA | 0.81 ± 0.03 | 0.73 ± 0.02 | 0.76 ± 0.10 | 0.7 ± 0.03 | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntantali, O.; Malandrakis, E.E.; Abbink, W.; Bastiaansen, J.; Chatzoglou, E.; Karapanagiotidis, I.T.; Golomazou, E.; Panagiotaki, P. Effects of Short-Term Intermittent Fasting on Growth Performance, Fatty Acids Profile, Glycolysis and Cholesterol Synthesis Gene Expression in European Seabass Dicentrarchus labrax. Fishes 2023, 8, 582. https://doi.org/10.3390/fishes8120582
Ntantali O, Malandrakis EE, Abbink W, Bastiaansen J, Chatzoglou E, Karapanagiotidis IT, Golomazou E, Panagiotaki P. Effects of Short-Term Intermittent Fasting on Growth Performance, Fatty Acids Profile, Glycolysis and Cholesterol Synthesis Gene Expression in European Seabass Dicentrarchus labrax. Fishes. 2023; 8(12):582. https://doi.org/10.3390/fishes8120582
Chicago/Turabian StyleNtantali, Olga, Emmanouil E. Malandrakis, Wout Abbink, John Bastiaansen, Evanthia Chatzoglou, Ioannis T. Karapanagiotidis, Eleni Golomazou, and Panagiota Panagiotaki. 2023. "Effects of Short-Term Intermittent Fasting on Growth Performance, Fatty Acids Profile, Glycolysis and Cholesterol Synthesis Gene Expression in European Seabass Dicentrarchus labrax" Fishes 8, no. 12: 582. https://doi.org/10.3390/fishes8120582
APA StyleNtantali, O., Malandrakis, E. E., Abbink, W., Bastiaansen, J., Chatzoglou, E., Karapanagiotidis, I. T., Golomazou, E., & Panagiotaki, P. (2023). Effects of Short-Term Intermittent Fasting on Growth Performance, Fatty Acids Profile, Glycolysis and Cholesterol Synthesis Gene Expression in European Seabass Dicentrarchus labrax. Fishes, 8(12), 582. https://doi.org/10.3390/fishes8120582









