Histopathological and Immunohistochemical Features and Expression Patterns of Cytochrome p450 1 Family Genes in Black Rockfish (Sebastes schlegelii): Exposure to 2,3,7,8-Tetrachlorodibenzo-p-dioxin and β-Naphthoflavone
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. Experimental Design
2.4. Histopathological Analysis
2.5. Immunohistochemistry (IHC)
2.6. Real-Time Polymerase Chain Reaction Analysis
2.7. Statistical Analyses
3. Results
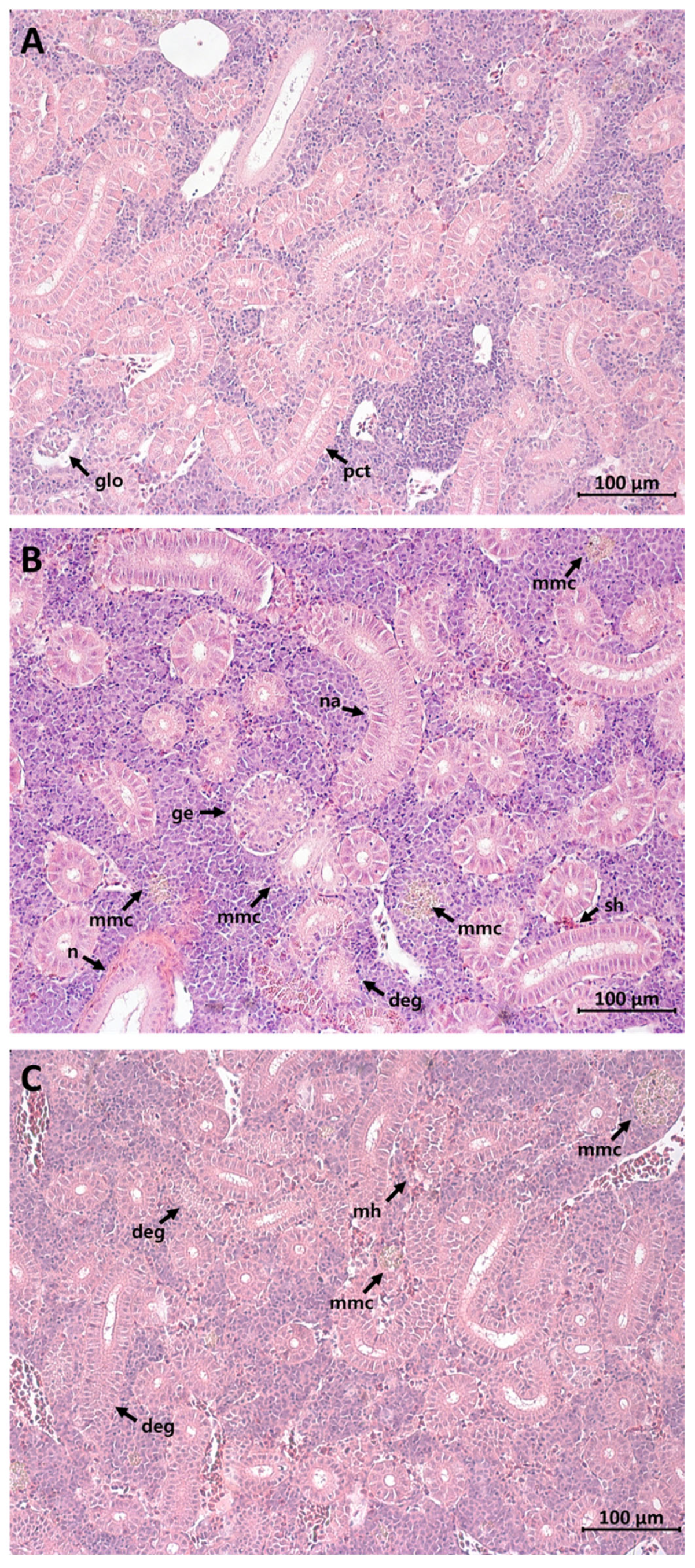
3.1. Histopathological Alterations
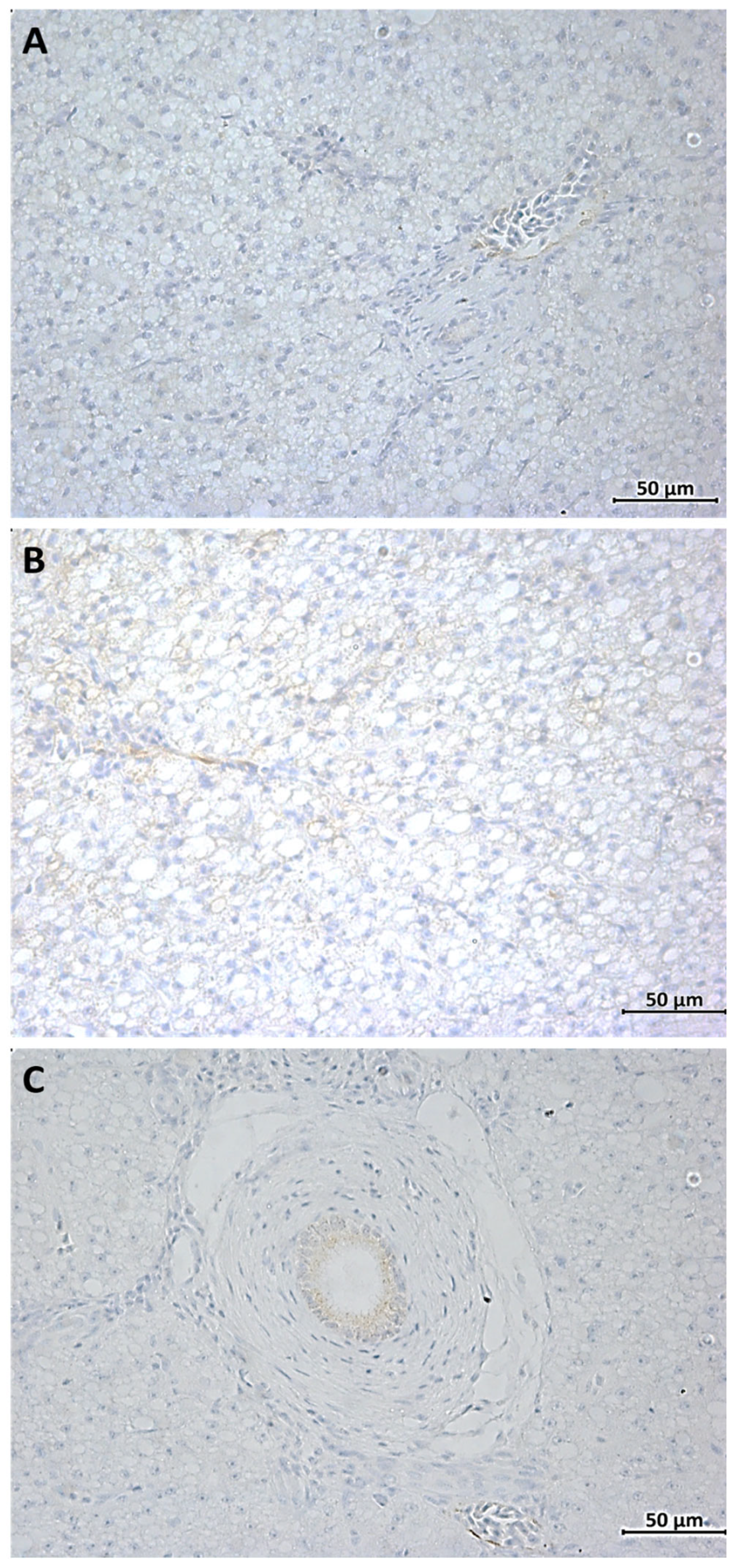
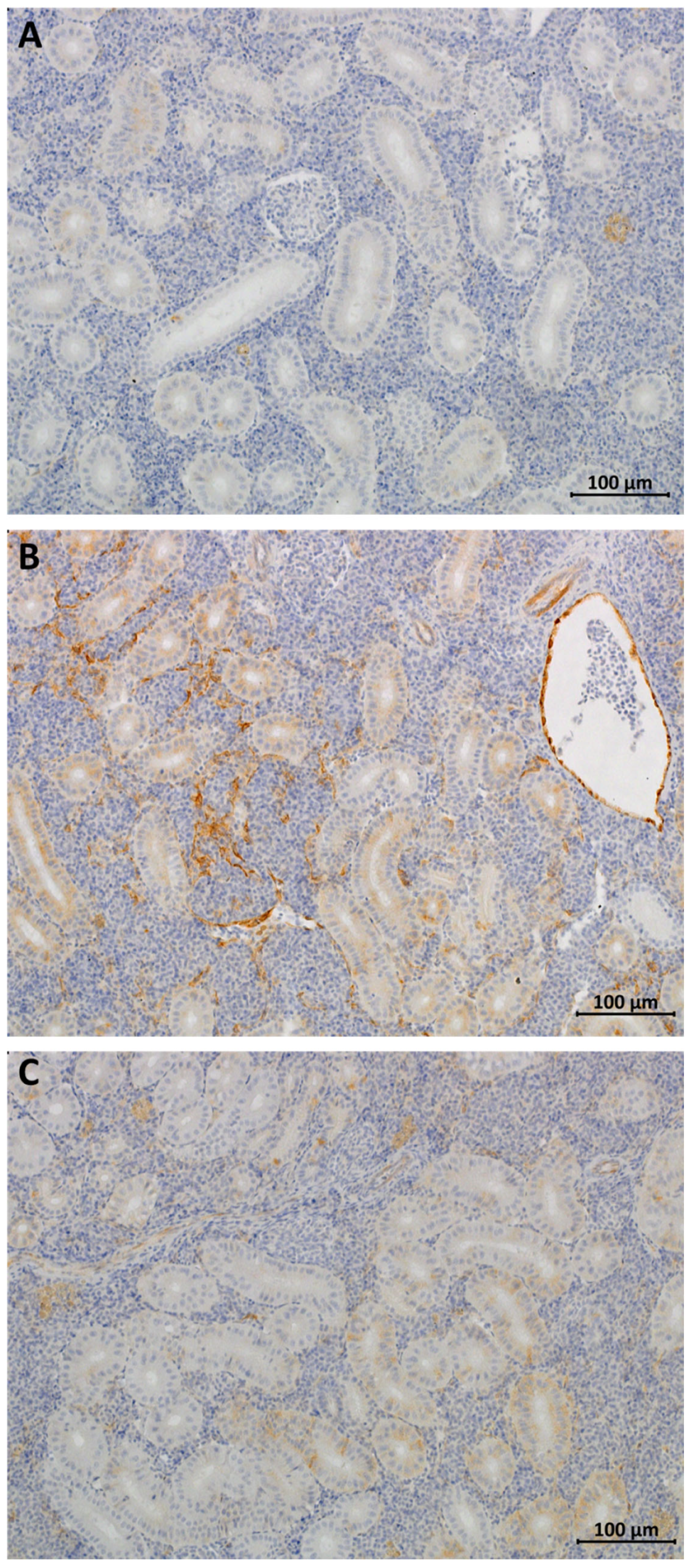
3.2. IHC Analysis
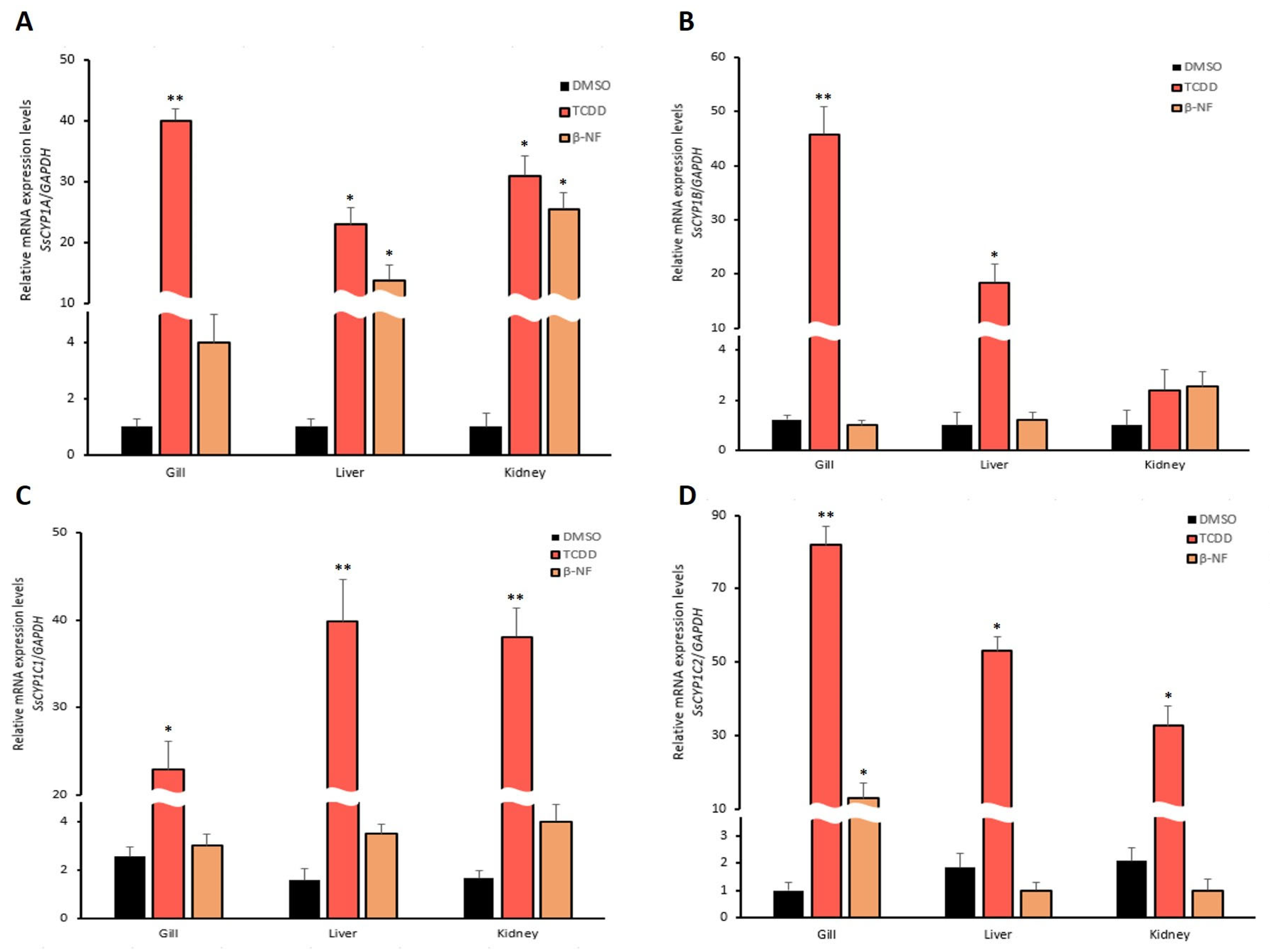
3.3. Expression of CYP1 Family Genes of mRNA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hall, C.M. Trends in ocean and coastal tourism: The end of the last frontier? Ocean. Coast. Manag. 2001, 44, 601–618. [Google Scholar] [CrossRef]
- Koch, M.; Bowes, G.; Ross, C.; Zhang, X.H. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Glob. Chang. Biol. 2013, 19, 103–132. [Google Scholar] [CrossRef]
- Liu, L.; Cheng, S.Y.; Li, J.B.; Huang, Y.F. Mitigating environmental pollution and impacts from fossil fuels: The role of alternative fuels. Energy Sources A Recovery Util. Environ. Eff. 2007, 29, 1069–1080. [Google Scholar] [CrossRef]
- York, R. Do alternative energy sources displace fossil fuels? Nat. Clim. Chang. 2012, 2, 441–443. [Google Scholar] [CrossRef]
- Shoar, F.H.; Najafi, B.; Mosavi, A. Effects of triethylene glycol mono methyl ether (TGME) as a novel oxygenated additive on emission and performance of a dual-fuel diesel engine fueled with natural gas-diesel/biodiesel. Energy Rep. 2021, 7, 1172–1189. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Hong, H.S.; Zhou, J.L.; Yu, G. Phase association of polycyclic aromatic hydrocarbons in the Minjiang River Estuary, China. Sci. Total Environ. 2004, 323, 71–86. [Google Scholar] [CrossRef]
- Feng, J.; Zhai, M.; Sun, J.; Liu, Q. Distribution and sources of polycyclic aromatic hydrocarbons (PAHs) in sediment from the upper reach of Huaihe River, East China. Environ. Sci. Pollut. Res. 2012, 19, 1097–1106. [Google Scholar] [CrossRef]
- St-Amand, A.D.; Mayer, P.M.; Blais, J.M. Seasonal trends in vegetation and atmospheric concentrations of PAHs and PBDEs near a sanitary landfill. Atmos. Environ. 2008, 42, 2948–2958. [Google Scholar] [CrossRef]
- Kong, S.; Shi, J.; Lu, B.; Qiu, W.; Zhang, B.; Peng, Y.; Zhang, B.; Bai, Z. Characterization of PAHs within PM10 fraction for ashes from coke production, iron smelt, heating station and power plant stacks in Liaoning Province, China. Atmos. Environ. 2011, 45, 3777–3785. [Google Scholar] [CrossRef]
- Hwang, H.M.; Wade, T.L. Aerial distribution, temperature-dependent seasonal variation, and sources of polycyclic aromatic hydrocarbons in pine needles from the Houston metropolitan area, Texas, USA. J. Environ. Sci. Health A 2008, 43, 1243–1251. [Google Scholar] [CrossRef]
- Pagliaccio, D.; Herbstman, J.B.; Perera, F.; Tang, D.; Goldsmith, J.; Peterson, B.S.; Rauh, V.; Margolis, A.E. Prenatal exposure to polycyclic aromatic hydrocarbons modifies the effects of early life stress on attention and thought problems in late childhood. J. Child. Psychol. Psychiatry 2020, 61, 1253–1265. [Google Scholar] [CrossRef]
- Laender, F.D.; Hammer, J.; Hendriks, A.J.; Soetaert, K.; Janssen, C.R. Combining monitoring data and modeling identifies PAHs as emerging contaminants in the Arctic. Environ. Sci. Technol. 2011, 45, 9024–9029. [Google Scholar] [CrossRef]
- Eriksson, A.N.; Rigaud, C.; Wincent, E.; Pakkanen, H.; Salonen, P.; Vehniäinen, E.R. Endogenous AhR agonist FICZ accumulates in rainbow trout (Oncorhynchus mykiss) alevins exposed to a mixture of two PAHs, retene and fluoranthene. Ecotoxicology 2022, 31, 1382–1389. [Google Scholar] [CrossRef]
- Johnson, B.T. Potential genotoxicity of sediments from the Great Lakes Environ. Toxicol. Water Qual. 1992, 7, 373–390. [Google Scholar] [CrossRef]
- Jos, A.; Segner, H.; Herradon, B.; Repetto, G.; Navas, J.M. Induction of EROD activity by 1-phenylimidazole and β-naphthoflavone in rainbow trout cultured hepatocytes: A comparative study. Toxicol. Vitr. 2007, 21, 1307–1310. [Google Scholar] [CrossRef]
- Lubet, R.A.; Heckman, B.M.; De Flora, S.L.; Steele, V.E.; Crowell, J.A.; Juliana, M.M.; Grubbs, C.J. Effects of 5,6-benzoflavone, indole-3-carbinol (I3C) and diindolylmethane (DIM) on chemically-induced mammary carcinogenesis: Is DIM a substitute for I3C? Oncol. Rep. 2011, 26, 731–736. [Google Scholar] [CrossRef]
- White, S.S.; Birnbaum, L.S. An overview of the effects of dioxins and dioxin-like compounds on vertebrates, as documented in human and ecological epidemiology. J. Environ. Sci. Health C Toxicol. Carcinog. 2009, 27, 197–211. [Google Scholar] [CrossRef]
- Schecter, A.; Birnbaum, L.; Ryan, J.J.; Constable, J.D. Dioxins: An overview. Environ. Res. 2006, 101, 419–428. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Hoogenboom, L. Risk for animal and human health related to the presence of dioxins and dioxin-like PCBs in feed and food. Efsa J. 2018, 16, e05333. [Google Scholar] [CrossRef]
- Yang, J.; Shin, D.; Park, S.; Chang, Y.; Kim, D.; Ikonomou, M.G. PCDDs, PCDFs, and PCBs concentrations in breast milk from two areas in Korea: Body burden of mothers and implications for feeding infants. Chemosphere 2002, 46, 419–428. [Google Scholar] [CrossRef]
- Pacheco, M.; Santos, M.A. Induction of Liver EROD and Erythrocytic Nuclear Abnormalities by Cyclophosphamide and PAHs in Anguilla anguilla L. Ecotoxicol. Environ. Saf. 1998, 40, 71–76. [Google Scholar] [CrossRef]
- Ahmad, I.; Maria, V.L.; Oliveira, M.; Pacheco, M.; Santos, M.A. Oxidative stress and genotoxic effects in gill and kidney of Anguilla anguilla L. exposed to chromium with or without pre-exposure to β-naphthoflavone. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2006, 608, 16–28. [Google Scholar] [CrossRef]
- Elonen, G.E.; Spehar, R.L.; Holcombe, G.W.; Johnson, R.D.; Fernandez, J.D.; Erickson, R.J.; Cook, P.M. Comparative toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin to seven freshwater fish species during early life-stage development. Environ. Toxicol. Chem. 1998, 17, 472–483. [Google Scholar] [CrossRef]
- Walker, M.K.; Hufnagle, L.C., Jr.; Clayton, M.K.; Peterson, R.E. An egg injection method for assessing early life stage mortality of polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls in rainbow trout, (Oncorhynchus mykiss). Aquat. Toxicol. 1992, 22, 15–37. [Google Scholar] [CrossRef]
- Giesy, J.P.; Jones, P.D.; Kannan, K.; Newsted, J.L.; Tillitt, D.E.; Williams, L.L. Effects of chronic dietary exposure to environmentally relevant concentrations to 2,3,7,8-tetrachlorodibenzo-p-dioxin on survival, growth, reproduction and biochemical responses of female rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2002, 59, 35–53. [Google Scholar] [CrossRef]
- Greco, L.; Serrano, R.; Blanes, M.A.; Serrano, E.; Capri, E. Bioaccumulation markers and biochemical responses in European sea bass (Dicentrarchus labrax) raised under different environmental conditions. Ecotoxicol. Environ. Saf. 2010, 73, 38–45. [Google Scholar] [CrossRef]
- Nunes, M.; Marchand, P.; Vernisseau, A.; Le Bizec, B.; Ramos, F.; Pardal, M.A. PCDD/Fs and dioxin-like PCBs in sediment and biota from the Mondego estuary (Portugal). Chemosphere 2011, 83, 1345–1352. [Google Scholar] [CrossRef]
- Zodrow, J.M.; Tanguay, R.L. 2,3,7,8-tetrachlorodibenzo-p-dioxin inhibits zebrafish caudal fin regeneration. Toxicol. Sci. 2003, 76, 151–161. [Google Scholar] [CrossRef]
- Della Torre, C.; Buonocore, F.; Frenzilli, G.; Corsolini, S.; Brunelli, A.; Guidi, P.; Kocan, A.; Mariottini, M.; Mottola, F.; Nigro, M.; et al. Influence of titanium dioxide nanoparticles on 2,3,7,8-tetrachlorodibenzo-p-dioxin bioconcentration and toxicity in the marine fish European sea bass (Dicentrarchus labrax). Environ. Pollut. 2015, 196, 185–193. [Google Scholar] [CrossRef]
- Grinwis, G.C.M.; Vethaak, A.D.; Wester, P.W.; Vos, J.G. Toxicology of environmental chemicals in the flounder (Platichthys flesus) with emphasis on the immune system: Field, semi-field (mesocosm) and laboratory studies. Toxicol. Lett. 2000, 112, 289–301. [Google Scholar] [CrossRef]
- Isin, E.M.; Guengerich, F.P. Complex reactions catalyzed by cytochrome P450 enzymes. Biochim. Biophys. Acta—Gen. Subj. 2007, 1770, 314–329. [Google Scholar] [CrossRef]
- Beedanagari, S.R.; Bebenek, I.; Bui, P.; Hankinson, O. Resveratrol inhibits dioxin-induced expression of human CYP1A1 and CYP1B1 by inhibiting recruitment of the aryl hydrocarbon receptor complex and RNA polymerase II to the regulatory regions of the corresponding genes. Toxicol. Sci. 2009, 110, 61–67. [Google Scholar] [CrossRef]
- Goldstone, J.V.; Jönsson, M.E.; Behrendt, L.; Woodin, B.R.; Jenny, M.J.; Nelson, D.R.; Stegeman, J.J. Cytochrome P450 1D1: A novel CYP1A-related gene that is not transcriptionally activated by PCB126 or TCDD. Arch. Biochem. Biophys. 2009, 482, 7–16. [Google Scholar] [CrossRef]
- Bucheli, T.D.; Fent, K. Induction of cytochrome P450 as a biomarker for environmental contamination in aquatic ecosystems. Crit. Rev. Environ. Sci. Technol. 1995, 25, 201–268. [Google Scholar] [CrossRef]
- Woo, S.J. Effects of benzo [a] pyrene exposure on black rockfish (Sebastes schlegelii): EROD activity, CYP1A protein, and immunohistochemical and histopathological alterations. Environ. Sci. Pollut. Res. 2022, 29, 4033–4043. [Google Scholar] [CrossRef]
- Kim, K.H.; Park, H.J.; Kim, J.H.; Kim, S.; Williams, D.R.; Kim, M.K.; Jung, Y.D.; Teraoka, H.; Park, H.C.; Choy, H.E.; et al. Cyp1a reporter zebrafish reveals target tissues for dioxin. Aquat. Toxicol. 2013, 134, 57–65. [Google Scholar] [CrossRef]
- Xi, D.; Zhang, X.; Lü, H.; Zhang, Z. Cannibalism in juvenile black rockfish, Sebastes schlegelii (Hilgendorf, 1880), reared under controlled conditions. Aquaculture 2017, 479, 682–689. [Google Scholar] [CrossRef]
- Statistics Korea. Fishery Production Survey. Available online: https://www.kostat.go.kr (accessed on 5 May 2022).
- Andreasen, E.A.; Hahn, M.E.; Heideman, W.; Peterson, R.E.; Tanguay, R.L. The zebrafish (Danio rerio) aryl hydrocarbon receptor type 1 is a novel vertebrate receptor. Mol. Pharmacol. 2002, 62, 234–249. [Google Scholar] [CrossRef]
- Maria, V.L.; Correia, A.C.; Santos, M.A. Anguilla anguilla L. blood and liver DNA strand breaks after beta-naphthoflavone exposure. Fresenius Environ. Bull. 2004, 13, 93–97. [Google Scholar]
- Woo, S.J. Molecular characterization of the aryl hydrocarbon receptor 2 gene in black rockfish, Sebastes schlegelii, and its expression patterns upon exposure to benzo [a] pyrene, 2,3,7,8-tetrachlorodibenzo-p-dioxin, and β-naphthoflavone. J. Appl. Toxicol. 2022, 42, 638–650. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Hylland, K. Polycyclic aromatic hydrocarbon (PAH) ecotoxicology in marine ecosystems. J. Toxicol. Environ. Health Part A 2006, 69, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Zodrow, J.M.; Stegeman, J.J.; Tanguay, R.L. Histological analysis of acute toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in zebrafish. Aquat. Toxicol. 2004, 66, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Cao, L.; Jia, R.; Yin, G. Hepatoprotective and antioxidant effects of dietary Glycyrrhiza polysaccharide against TCDD-induced hepatic injury and RT-PCR quantification of AHR2, ARNT2, CYP1A mRNA in Jian Carp (Cyprinus carpio var. Jian). J. Environ. Sci. 2017, 51, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Al-Musawi, M.T.; Ali, A.E.M.H.; Humadi, A.A.; Al-Kaisei, B.I. Nephropathy Effects of 2,3,7,8-Tetrachlorodibenzo-P-Dioxin (TCDD) Toxicity in Ova Injected Chicken. Ann. Rom. Soc. Cell Biol. 2021, 25, 4418–4429. [Google Scholar]
- Esteban, J.; Sánchez-Pérez, I.; Hamscher, G.; Miettinen, H.M.; Korkalainen, M.; Viluksela, M.; Pohjanvirta, R.; Håkansson, H. Role of aryl hydrocarbon receptor (AHR) in overall retinoid metabolism: Response comparisons to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure between wild-type and AHR knockout mice. Reprod. Toxicol. 2021, 101, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Delgado, J.B.; Behrens, A.; Segner, H.; Sarasquete, C. Tissue-specific induction of EROD activity and CYP1A protein in Sparus aurata exposed to B(a)P and TCDD. Ecotoxicol. Environ. Saf. 2008, 69, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Reimschuessel, R. A fish model of renal regeneration and development. ILAR J. 2001, 42, 285–291. [Google Scholar] [CrossRef]
- Carlson, E.A.; Li, Y.; Zelikoff, J.T. Benzo [a] pyrene-induced immunotoxicity in Japanese medaka (Oryzias latipes): Relationship between lymphoid CYP1A activity and humoral immune suppression. Toxicol. Appl. Pharmacol. 2004, 201, 40–52. [Google Scholar] [CrossRef]
- Reynaud, S.; Deschaux, P. The effects of 3-methylcholanthrene on lymphocyte proliferation in the common carp (Cyprinus carpio L.). Toxicology 2005, 211, 156–164. [Google Scholar] [CrossRef]
- Reynaud, S.; Deschaux, P. The effects of polycyclic aromatic hydrocarbons on the immune system of fish: A review. Aquat. Toxicol. 2006, 77, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Grinwis, G.C.M.; Besselink, H.T.; Van den Brandhof, E.J.; Bulder, A.S.; Engelsma, M.Y.; Kuiper, R.V.; Wester, P.W.; Vaal, M.A.; Vethaak, A.D.; Vos, J.G. Toxicity of TCDD in European flounder (Platichthys flesus) with emphasis on histopathology and cytochrome P450 1A induction in several organ systems. Aquat. Toxicol. 2000, 50, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Carlson, E.A.; Li, Y.; Zelikoff, J.T. Suppressive effects of benzo [a] pyrene upon fish immune function: Evolutionarily conserved cellular mechanisms of immunotoxicity. Mar. Environ. Res. 2004, 58, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Whyte, J.J.; Jung, R.E.; Schmitt, C.J.; Tillitt, D.E. Ethoxyresorufin-O-deethylase (EROD) activity in fish as a biomarker of chemical exposure. Crit. Rev. Toxicol. 2000, 30, 347–570. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, M.A.; Mitsuo, R.; Kaminishi, Y.; Itakura, T. Isolation of cDNA of novel cytochrome P450 1B gene, CYP1B2, from Carp (Cyprinus carpio) and its induced expression in gills. Environ. Sci. 2004, 11, 345–354. [Google Scholar] [PubMed]
- Yin, H.C.; Tseng, H.P.; Chung, H.Y.; Ko, C.Y.; Tzou, W.S.; Buhler, D.R.; Hu, C.H. Influence of TCDD on zebrafish CYP1B1 transcription during development. Toxicol. Sci. 2008, 103, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Godard, C.A.; Goldstone, J.V.; Said, M.R.; Dickerson, R.L.; Woodin, B.R.; Stegeman, J.J. The new vertebrate CYP1C family: Cloning of new subfamily members and phylogenetic analysis. Biochem. Biophys. Res. Commun. 2005, 331, 1016–1024. [Google Scholar] [CrossRef][Green Version]
- Woo, S.J.; Chung, J.K. Cytochrome P450 1 enzymes in black rockfish, Sebastes schlegelii: Molecular characterization and expression patterns after exposure to benzo [a] pyrene. Aquat. Toxicol. 2020, 226, 105566. [Google Scholar] [CrossRef]
- Kubota, A.; Stegeman, J.J.; Woodin, B.R.; Iwanaga, T.; Harano, R.; Peterson, R.E.; Hiraga, K.; Teraoka, H. Role of zebrafish cytochrome P450 CYP1C genes in the reduced mesencephalic vein blood flow caused by activation of AHR2. Toxicol. Appl. Pharmacol. 2011, 253, 244–252. [Google Scholar] [CrossRef]
- Bugiak, B.; Weber, L.P. Hepatic and vascular mRNA expression in adult zebrafish (Danio rerio) following exposure to benzo-a-pyrene and 2,3,7,8-tetrachlorodibenzo-p-dioxin. Aquat. Toxicol. 2009, 95, 299–306. [Google Scholar] [CrossRef]
- Zanette, J.; Jenny, M.J.; Goldstone, J.V.; Woodin, B.R.; Watka, L.A.; Bainy, A.C.; Stegeman, J.J. New cytochrome P450 1B1, 1C2 and 1D1 genes in the killifish Fundulus heteroclitus: Basal expression and response of five killifish CYP1s to the AHR agonist PCB126. Aquat. Toxicol. 2009, 93, 234–243. [Google Scholar] [CrossRef] [PubMed]

| Components | Value |
|---|---|
| Temperature (°C) | 18 ± 1.0 |
| pH | 8.2 ± 0.5 |
| Salinity (‰) | 33.1 ± 0.5 |
| Light photoperiod (h) | 12 |
| Dark photoperiod (h) | 12 |
| Dissolved oxygen (mg/L) | 7.6 ± 0.3 |
| Chemical oxygen demand (mg/L) | 1.2 ± 0.1 |
| Ammonia (µg/L) | 11.3 ± 0.5 |
| Nitrite (µg/L) | 1.5 ± 0.2 |
| Nitrate (µg/L) | 10.1 ± 1.0 |
| Gene | Accession Number | Sequence (5′−3′) | Product Size (bp) | Reference |
|---|---|---|---|---|
| GAPDH | KF430617.1 | F: AGTACGACTCCACTCACGGC | 112 | This study |
| R: TTGGAAGGGTCCTTCTCGTG | ||||
| CYP1A | MK331129.1 | F: GACACCTGCGTCTTCATCAATCA | 118 | [38] |
| R: GCTTGATGACTTCGGTGCCATC | ||||
| CYP1B | MK331130.1 | F: GCACCATCAGGGACATGACA | 133 | |
| R: AGTGTGTCTTGACTTGCTCCAA | ||||
| CYP1C1 | MK331131.1 | F: CGCGCTTCCTGGATGGAAAC | 111 | |
| R: CTTCCACCTTGGCGATCTGG | ||||
| CYP1C2 | MK331132.1 | F: GGCTCCCTAGCAAGGATCTCA | 126 | |
| R: GCATTGGTGGATAGAATCGCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, S.-J.; Joo, M.-S.; Kim, S.-S.; Yoo, H.-K.; Park, J.-J. Histopathological and Immunohistochemical Features and Expression Patterns of Cytochrome p450 1 Family Genes in Black Rockfish (Sebastes schlegelii): Exposure to 2,3,7,8-Tetrachlorodibenzo-p-dioxin and β-Naphthoflavone. Fishes 2023, 8, 583. https://doi.org/10.3390/fishes8120583
Woo S-J, Joo M-S, Kim S-S, Yoo H-K, Park J-J. Histopathological and Immunohistochemical Features and Expression Patterns of Cytochrome p450 1 Family Genes in Black Rockfish (Sebastes schlegelii): Exposure to 2,3,7,8-Tetrachlorodibenzo-p-dioxin and β-Naphthoflavone. Fishes. 2023; 8(12):583. https://doi.org/10.3390/fishes8120583
Chicago/Turabian StyleWoo, Soo-Ji, Min-Soo Joo, So-Sun Kim, Hae-Kyun Yoo, and Jung-Jun Park. 2023. "Histopathological and Immunohistochemical Features and Expression Patterns of Cytochrome p450 1 Family Genes in Black Rockfish (Sebastes schlegelii): Exposure to 2,3,7,8-Tetrachlorodibenzo-p-dioxin and β-Naphthoflavone" Fishes 8, no. 12: 583. https://doi.org/10.3390/fishes8120583
APA StyleWoo, S.-J., Joo, M.-S., Kim, S.-S., Yoo, H.-K., & Park, J.-J. (2023). Histopathological and Immunohistochemical Features and Expression Patterns of Cytochrome p450 1 Family Genes in Black Rockfish (Sebastes schlegelii): Exposure to 2,3,7,8-Tetrachlorodibenzo-p-dioxin and β-Naphthoflavone. Fishes, 8(12), 583. https://doi.org/10.3390/fishes8120583






