Growth Performance, Feed Utilisation, Digestive and Metabolic Enzyme Activity, and Liver Morphohistology in Hybrid Tilapia (Oreochromis mossambicus × Oreochromis niloticus) Juveniles Fed with the Inclusion of Chitosan in Their Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Location
2.2. Formulation and Preparation of Experimental Diets
2.3. Chemical Analysis of Diets
2.4. Experimental Design and Rearing Conditions
2.5. Sampling
2.6. Fish Growth Performance
2.7. Digestive Enzyme Activity
2.8. Metabolic Enzyme Activity
2.9. Liver Histology
2.10. Statistical Processing
3. Results
3.1. Growth and Production Rates
3.2. Digestive Enzyme Activity
3.3. Metabolic Enzyme Activity
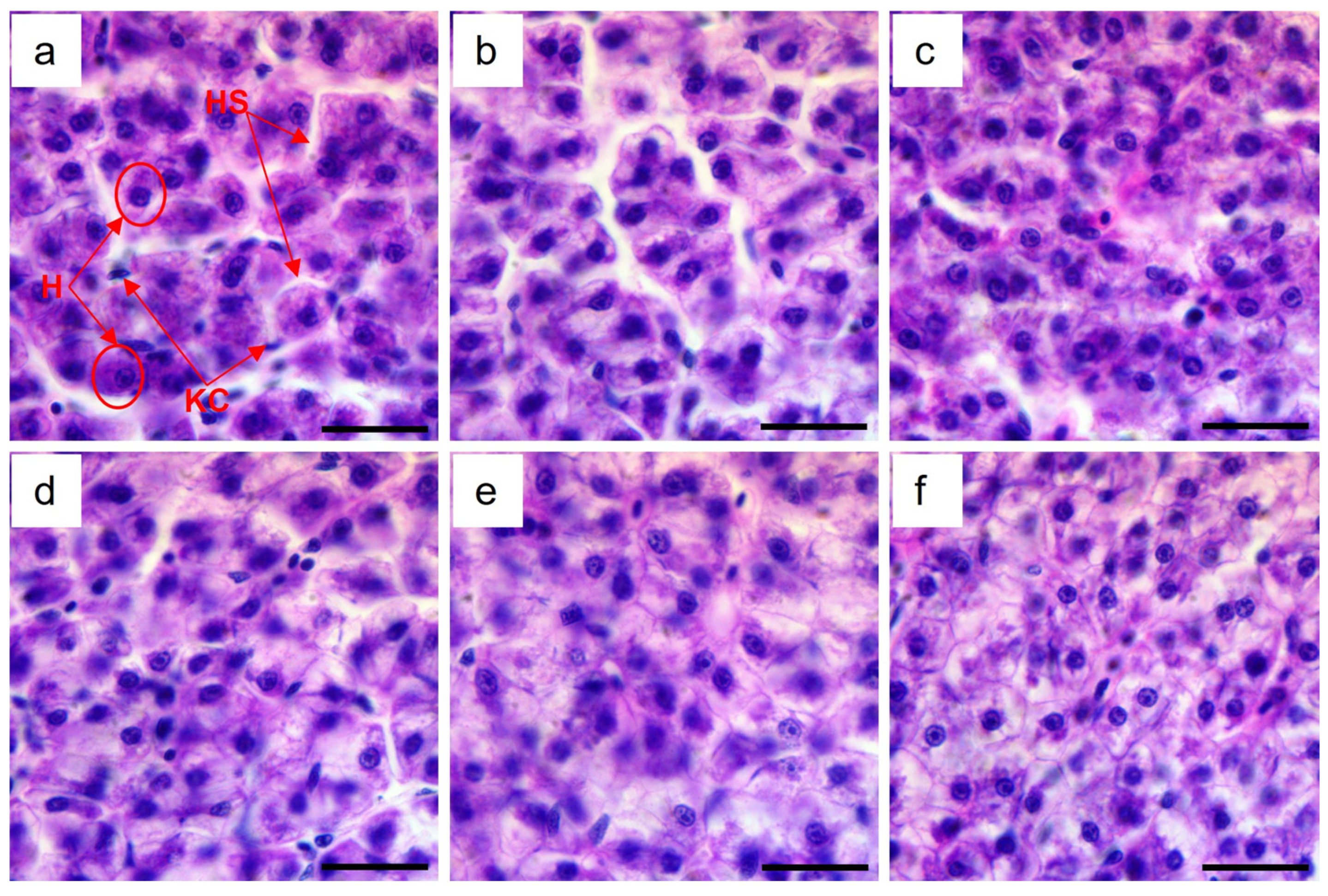
3.4. Liver Histology
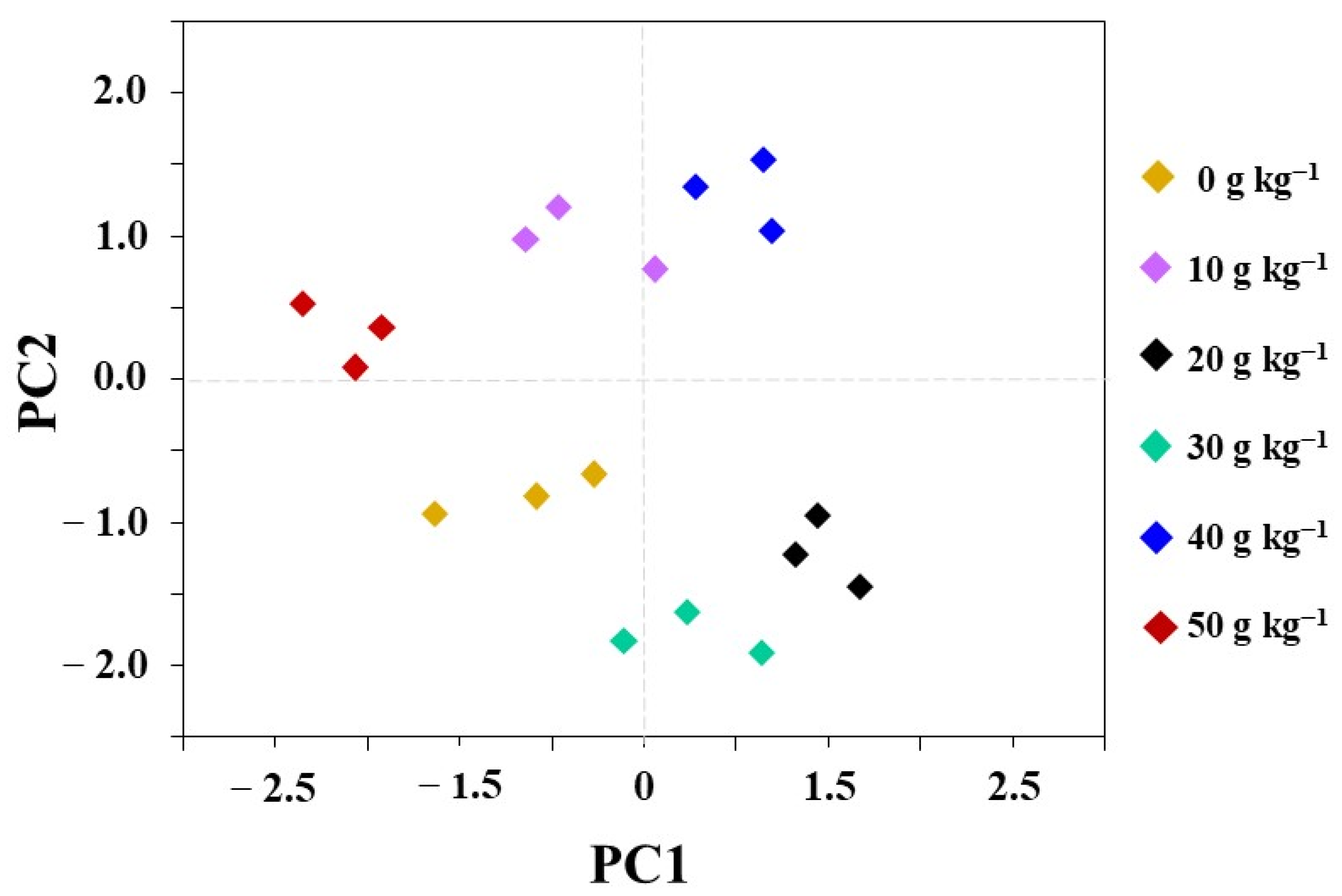
3.5. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. El Estado Mundial de la Pesca y la Acuicultura. Hacia la Transformación Azul; FAO: Rome, Italy, 2022; pp. 1–97. [Google Scholar] [CrossRef]
- Méndez-Martínez, Y.; Pacheco-Morales, G.K.; Del Barco-Ibarra, K.A.; Torres-Navarrete, Y.G.; Hernández-Vergara, M.P. Biochemical and immune response in red tilapia (Oreochromis mossambicus × O. niloticus) with dietary chitosan supplementation. Rev. Fac. Agron. 2021, 38, 1016–1034. [Google Scholar] [CrossRef]
- Méndez-Martínez, Y.; Narváez-Narváez, R.I.; Angulo, C.; Cortés-Jacinto, E.; Botello Leon, A.; Verdecia, D.; Torres-Navarrete, Y.G. Chemical composition of Tithonia diversifolia (Hemsl.) and its effect on growth performance, feed efficiency and metabolic biochemistry of juvenile hybrid tilapia, Oreochromis mossambicus × Oreochromis niloticus. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13337. [Google Scholar] [CrossRef]
- Ayyat, M.S.; Ayyat, A.M.N.; Al-Sagheer, A.A.; El-Hais, A.E.-A.M. Effect of some safe feed additives on growth performance, blood biochemistry, and bioaccumulation of aflatoxin residues of Nile tilapia fed aflatoxin-B1 contaminated diet. Aquaculture 2018, 495, 27–34. [Google Scholar] [CrossRef]
- Wu, Y.; Rashidpour, A.; Almajano, M.P.; Metón, I. Chitosan-Based Drug Delivery System: Applications in Fish Biotechnology. Polymers 2020, 12, 1177. [Google Scholar] [CrossRef]
- Saleh, M.; Essawy, E.; Shaalan, M.; Osman, S.; Ahmed, F.; El-Matbouli, M. Therapeutic Intervention with Dietary Chitosan Nanoparticles Alleviates Fish Pathological and Molecular Systemic Inflammatory Responses against Infections. Mar. Drugs 2022, 20, 425. [Google Scholar] [CrossRef]
- Udo, I.U.; Etukudo, U.; Anwana, U.I.U. Effects of chitosan and chitosan nanoparticles on water quality, growth performance, survival rate and meat quality of the African catfish, Clarias gariepinus. Nanoscience 2018, 1, 12–25. [Google Scholar] [CrossRef]
- Salam, M.A.; Rahman, M.A.; Paul, S.I.; Islam, F.; Barman, A.K.; Rahman, Z. Dietary chitosan promotes the growth, biochemical composition, gut microbiota, hematological parameters and internal organ morphology of juvenile Barbonymus gonionotus. PLoS ONE 2021, 16, e0260192. [Google Scholar] [CrossRef]
- Cheba, B.A. Chitin and chitosan: Marine biopolymers with unique properties and versatile applications. Glob. J. Biotechnol. Biochem. (GJBB) 2011, 6, 149–153. Available online: http://idosi.org/gjbb/gjbb6%283%2911/7.pdf (accessed on 10 August 2023).
- Divya, K.; Jisha, M. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Nikapitiya, C.; Dananjaya, S.H.S.; De Silva, B.C.J.; Heo, G.J.; Oh, C.; De Zoysa, M.; Lee, J. Chitosan nanoparticles: A positive immune response modulator as display in zebrafish larvae against Aeromonas hydrophila infection. Fish Shellfish Immunol. 2018, 76, 240–246. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Razek, N.A.; Abdel-Rahman, A.M. Immunostimulatory effect of dietary chitosan nanoparticles on the performance of Nile tilapia, Oreochromis niloticus (L.). Fish Shellfish Immunol. 2019, 88, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.; Gewaily, M.S.; Soliman, A.A.; Shukry, M.; Amer, A.A.; Younis, E.M.; Fadl, S.E. Marine-derived chitosan nanoparticles improved the intestinal histo-morphometrical features in association with the health and immune response of grey mullet (Liza ramada). Mar. Drugs 2020, 18, 611. [Google Scholar] [CrossRef] [PubMed]
- Akbary, P.; Younesi, A. Effect of dietary supplementation of Chitosan on growth, hematology and innate immunity of grey Mullet (Mugil cephalus). Vet. Res. Biol. Prod. 2017, 30, 194–203. [Google Scholar] [CrossRef]
- Salem, M.E. Utilization of Some Feed Additives in Improving the Nutritive Value of Marine Fish Diet. Ph.D. Dissertation, Alexandria University, Alexandria, Egypt, 2015. [Google Scholar]
- Shiau, S.Y.; Yu, Y.P. Dietary supplementation of chitin and chitosan depresses growth in tilapia, Oreochromis niloticus × O. aureus. Aquaculture 1999, 179, 439–446. [Google Scholar] [CrossRef]
- Méndez-Martínez, Y.; García-Guerrero, M.U.; Arcos-Ortega, F.G.; Martínez-Córdova, L.R.; Yamasaki-Granados, S.; Pérez-Rodríguez, J.C.; Cortés-Jacinto, E. Effect of different ratios of dietary protein-energy on growth, body proximal composition, digestive enzyme activity, and hepatopancreas histology in Macrobrachium americanum (Bate, 1868) prawn juveniles. Aquaculture 2018, 485, 1–11. [Google Scholar] [CrossRef]
- Méndez-Martínez, Y.; Ceseña, C.E.; Luna-González, A.; García-Guerrero, M.U.; Martinez-Porchas, M.; Campa-Cordova, Á.I.; Cortés-Jacinto, E. Effects of different dietary protein-energy ratios on growth, carcass amino acid and fatty acid profile of male and female Cherax quadricarinatus (von Martens, 1868) pre-adults. Aquac. Nutr. 2021, 27, 2481–2496. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 21st ed.; AOAC: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Ramanathan, G.; Ramalakshmi, P.; Gopperundevi, B.; Suresh, J.I. Production Characterization and Aqua Feed Supplementation of Astaxanthin from Halobacterium salinarium. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 56–63. Available online: https://www.ijcmas.com/vol-4-3/G (accessed on 12 August 2023).
- Anson, M.L. The estimation of pepsin, trypsin, papain and cathepsin with hemoglobin. J. Gen. Physiol. 1938, 22, 79–89. [Google Scholar] [CrossRef]
- Bernfeld, P. Amylase, alpha and beta. Methods Enzymol. 1955, 1, 149–151. Available online: https://cir.nii.ac.jp/crid/1573668924362136704 (accessed on 29 June 2023).
- Versaw, W.; Cuppett, S.L.; Winters, D.D.; Williams, L.E. An improved colorimetric assay for bacterial lipase in nonfat dry milk. J. Food Sci. 1989, 54, 232–254. [Google Scholar] [CrossRef]
- Bradford, M.M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Bergmeyer, H.U.; Bowers, G.N., Jr.; Horder, M.; Moss, D.W. Provisional recommendations on IFCC methods for the measurement of catalytic concentrations of enzymes. Part 2. IFCC method for aspartate aminotransferase. Clin. Chim. Acta 1976, 70, F19–F29. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yu, Z.; Liu, G.; Li, S.; Zhou, G.; Wang, H.; Dong, Y.; You, C.; Bai, W.; Zhou, M. Effects of Dietary Lentinus edodes Fermentation Supplementation on Digestive Enzyme Activity, Antioxidant Capacity and Morphology of the Liver and Intestine in Largemouth Bass (Micropterus salmoides) Fed High Plant Protein Diets. Fishes 2023, 8, 329. [Google Scholar] [CrossRef]
- Naiel, M.A.E.; Ismael, N.E.M.; Abd El-hameed, S.A.A.; Amer, M.S. The antioxidative and immunity roles of chitosan nanoparticle and vitamin C-supplemented diets against imidacloprid toxicity on Oreochromis niloticus. Aquaculture 2020, 523, 735219. [Google Scholar] [CrossRef]
- De Souza Filho, J.; Pires, F.S.; Grisolia, C.K.; De Sabóia Morais, S.M.T. Toxicological effects of a glyphosate-based formulation on the liver of Poecilia reticulata. Curr Top. Toxicol. 2014, 9, 81–91. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 2nd ed.; Prentice-Hall: Upper Saddle Creek, NJ, USA, 1984; pp. 236–346. [Google Scholar]
- Abdel El-Naby, F.; Naiel, M.; Al-Sagheer, A.A.; Negm, S. Dietary chitosan nanoparticles enhance the growth, production performance, and immunity in Oreochromis niloticus. Aquaculture 2019, 25, 82–89. [Google Scholar] [CrossRef]
- Najafabad, M.; Imanpoor, M.R.; Taghizadeh, V.; Alishahi, A. Effect of dietary chitosan on growth performance, hematological parameters, intestinal histology and stress resistance of Caspian kutum (Rutilus frisii kutum Kamenskii, 1901) fingerlings. Fish Physiol. Biochem. 2016, 42, 1063–1071. [Google Scholar] [CrossRef]
- Stanek, M.; Mazurkiewicz, J.; Rawski, M.; Bogucka, J.; Ziółkowska, E.; Dankowiakowska, A.; Kierończyk, B. Effect of chitosan on common carp (Cyprinus carpio) fry growth performance, feed utilization and nutriphysiological status. Aquac. Rep. 2023, 30, 101622. [Google Scholar] [CrossRef]
- Yan, J.; Guo, C.; Dawood, M.A.O.; Gao, J. Effects of dietary chitosan on growth, lipid metabolism, immune response and antioxidant-related gene expression in Misgurnus anguillicaudatus. Benef. Microbes 2017, 8, 439–449. [Google Scholar] [CrossRef]
- Fadl, S.E.; El-Gammal, G.A.; Abdo, W.S.; Barakat, M.; Sakr, O.A.; Nassef, E.; El-Sheshtawy, H.S. Evaluation of dietary chitosan effects on growth performance, immunity, body composition and histopathology of Nile tilapia (Oreochromis niloticus) as well as the resistance to Streptococcus agalactiae infection. Aquac. Res. 2020, 51, 1120–1132. [Google Scholar] [CrossRef]
- Su, P.; Han, Y.; Jiang, C.; Ma, Y.; Pan, J.; Liu, S.; Zhang, T. Effects of chitosan-oligosaccharides on growth performance, digestive enzyme and intestinal bacterial flora of tiger puffer (Takifugu rubripes Temminck et Schlegel, 1850). J. Appl. Ichthyol. 2017, 33, 458–467. [Google Scholar] [CrossRef]
- Hussain, M.A.; Sumon, T.A.; Mazumder, S.K.; Ali, M.M.; Jang, W.J.; Abualreesh, M.H.; Hasan, M.T. Essential oils and chitosan as alternatives to chemical preservatives for fish and fisheries products: A review. Food Control 2021, 129, 108244. [Google Scholar] [CrossRef]
- Zhang, B. Dietary chitosan oligosaccharides modulate the growth, intestine digestive enzymes, body composition and nonspecific immunity of loach Paramisgurnus dabryanus. Fish Shellfish Immunol. 2019, 88, 359–363. [Google Scholar] [CrossRef]
- Coutinho, F.; Castro, C.; Guerreiro, I.; Rangel, F.; Couto, A.; Serra, C.R.; Enes, P. Mealworm larvae meal in diets for meagre juveniles: Growth, nutrient digestibility and digestive enzymes activity. Aquaculture 2021, 535, 736362. [Google Scholar] [CrossRef]
- Sheikhzadeh, N.; Kouchaki, M.; Mehregan, M.; Tayefi-Nasrabadi, H.; Divband, B.; Khataminan, M.; Shabanzadeh, S. Influence of nanochitosan/zeolite composite on growth performance, digestive enzymes and serum biochemical parameters in rainbow trout (Oncorhynchus mykiss). Aquac. Res. 2017, 48, 5955–5964. [Google Scholar] [CrossRef]
- Liu, J.; Xu, W.; Liu, Y.; Wang, Y.; Zhang, J.; Wang, Z.; Ai, Q. Effects of chitosan-coated microdiet on dietary physical properties, growth performance, digestive enzyme activities, antioxidant capacity, and inflammation response of large yellow croaker (Larimichthys crocea) larvae. Aquac. Nutr. 2022, 2022, 4355182. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.E. Structure and mechanism of alkaline phosphatase. Annu. Rev. Biophys. Biomol. Struct. 1922, 21, 441–444. [Google Scholar] [CrossRef]
- Estaki, M.; DeCoffe, D.; Gibson, D.L. Interplay between intestinal alkaline phosphatase, diet, gut microbes and immunity. World J. Gastroenterol. 2014, 20, 15650. [Google Scholar] [CrossRef]
- Ray, C.S.; Singh, B.; Jena, I.; Behera, S.; Ray, S. Low alkaline phosphatase (ALP) in adult population an indicator of zinc (Zn) and magnesium (Mg) deficiency. Curr. Res. Nutr. Food Sci. J. 2017, 5, 347–352. [Google Scholar] [CrossRef]
- Abdel-Ghany, H.M.; Salem, M.E.S. Effects of dietary chitosan supplementation on farmed fish; a review. Rev. Aquac. 2020, 12, 438–452. [Google Scholar] [CrossRef]
- Dong, X.; Wang, Y.; Song, H.; Zou, X. Effects of in ovo injection of carbohydrate solution on small intestine development in domestic pigeons (Columba livia). J. Anim. Sci. 2013, 91, 3742–3749. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, Z.; Sourinejad, I.; Kazemian, H.; Rohani, S. Application of zeolites in aquaculture industry: A review. Rev. Aquac. 2018, 10, 75–95. [Google Scholar] [CrossRef]
- Mehrpak, M.; Banaee, M.; Nematdoost Haghi, B.; Noori, A. Protective effects of vitamin C and chitosan against cadmium-induced oxidative stress in the liver of common carp (Cyprinus carpio). Iran. J. Toxicol. 2015, 9, 1360–1367. Available online: https://ijt.arakmu.ac.ir/article-1-455-en.pdf (accessed on 20 June 2023).
- Ismael, N.E.M.; Abd El-Hameed, S.A.A.; Salama, A.M.; Naiel, M.A.E.; Abdel-Latif, H.M.R. The effects of dietary clinoptilolite and chitosan nanoparticles on growth, body composition, haemato-biochemical parameters, immune responses, and antioxidative status of Nile tilapia exposed to imidacloprid. Environ. Sci. Pollut. Res. Int. 2021, 28, 29535–29550. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Chang, T.C.; Liu, S.H.; Chiang, M.T. The regulatory effects of fish oil and chitosan on hepatic lipogenic signals in high-fat diet-induced obese rats. J. Food Drug Anal. 2017, 25, 919–930. [Google Scholar] [CrossRef]
- Salaah, S.M.; Dalia, M.; Gaber, H.S. Potential effects of dietary chitosan against lead-induced innate immunotoxicity and oxidative stress in Nile tilapia (Oreochromis niloticus). Egypt. J. Aquat. Res. 2022, 48, 123–129. [Google Scholar] [CrossRef]
- Thilagar, G.; Samuthirapandian, R. Chitosan from crustacean shell waste and its protective role against lead toxicity in Oreochromis mossambicus. Toxicol. Rep. 2020, 7, 296–303. [Google Scholar] [CrossRef]
- Rezende, K.F.O.; Bergami, E.; Alves, K.V.B.; Corsi, I.; Barbieri, E. Titanium dioxide nanoparticles alter routine metabolism and cause histopathological alterations in Oreochromis niloticus. B. Inst. Pesca. 2018, 44, 1–11. [Google Scholar] [CrossRef]
- Li, J.; Pan, J.; Zhang, L.; Yu, Y. Culture of hepatocytes on fructose-modified chitosan scaffolds. Biomaterials 2003, 24, 2317–2322. [Google Scholar] [CrossRef]
- Palma Leotta, M.E.; Caliri, M.N.; Cáceres Gimenez, A.R.R. Caracterización Histológica e Histoquímica de Branquia, Hígado y Riñón de Perca Criolla (Percichthys trucha, Valenciennes, 1833) Para su uso en Biomonitoreo Ambiental. Acta Microsc. 2017, 26, 32–45. Available online: https://ri.conicet.gov.ar/handle/11336/50211 (accessed on 18 August 2023).
- Park, I.K.; Yang, J.; Jeong, H.J.; Bom, H.S.; Harada, I.; Akaike, T.; Cho, C.S. Galactosylated chitosan as a synthetic extracellular matrix for hepatocytes attachment. Biomaterials 2003, 24, 2331–2337. [Google Scholar] [CrossRef] [PubMed]
| Ingredients | Chitosan Levels (g kg−1) | |||||
|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | |
| Fish meal 1 | 250 | 250 | 250 | 250 | 250 | 250 |
| Soybean meal 2 | 280 | 280 | 280 | 280 | 280 | 280 |
| Wheat flour 3 | 204 | 204 | 204 | 204 | 204 | 204 |
| Corn meal 4 | 190 | 180 | 170 | 160 | 150 | 140 |
| Chitosan 5 | - | 10 | 20 | 30 | 40 | 50 |
| Vegetable oil 6 | 10 | 10 | 10 | 10 | 10 | 10 |
| Fish oil 7 | 15 | 15 | 15 | 15 | 15 | 15 |
| Sodium alginate 8 | 20 | 20 | 20 | 20 | 20 | 20 |
| Mineral premixes 9,10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Vitamin premixes 11,12 | 20 | 20 | 20 | 20 | 20 | 20 |
| Vitamin C 13 | 1 | 1 | 1 | 1 | 1 | 1 |
| Proximal Composition (g kg−1 as Fed Basis) 1 | Chitosan Levels (g kg−1) | |||||
|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | |
| Dry matter (DM) | 946.8 | 937.7 | 940.2 | 942.8 | 938.0 | 939.9 |
| Crude protein (CP) | 334.2 | 333.5 | 333.8 | 333.2 | 333.0 | 332.8 |
| Crude lipid (CL) | 66.4 | 66.0 | 65.1 | 64.5 | 57.3 | 65.8 |
| Crude fibre (CF) | 15.7 | 16.4 | 17.1 | 18.2 | 19.3 | 20.1 |
| Ash | 114.3 | 114.9 | 115.2 | 116.1 | 117.0 | 118.4 |
| Nitrogen-free extract (NFE) | 416.2 | 406.9 | 408.9 | 410.8 | 411.4 | 402.8 |
| DE (MJ kg−1 feed) | 12.85 | 12.85 | 12.85 | 12.85 | 12.85 | 12.85 |
| CP DE−1 (mg PC MJ−1) | 26.01 | 25.95 | 25.98 | 25.93 | 25.91 | 25.9 |
| Productive Parameters | Chitosan Levels (g kg−1) | p | |||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | ||
| Initial Weight, g | 7.47 ± 0.052 | 7.62 ± 0.035 | 7.53 ± 0.040 | 7.46 ± 0.064 | 7.69 ± 0.030 | 7.41 ± 0.075 | 0.066 |
| Final Weight, g | 22.66 ± 0.133 c | 20.37 ± 0.185 d | 25.72 ± 0.202 b | 23.59 ± 0.162 c | 28.08 ± 0.150 a | 25.58 ± 0.191 b | 0.011 |
| Final Length, cm | 10.05 ± 0.017 bc | 9.49 ± 0.040 c | 10.58 ± 0.075 ab | 10.14 ± 0.029 ab | 11.14 ± 0.104 a | 10.38 ± 0.110 ab | 0.030 |
| Weight Gain, g | 15.19 ± 0.017 d | 12.75 ± 0.69 e | 18.19 ± 0.092 b | 16.23 ± 0.081 c | 20.39 ± 0.121 a | 18.27 ± 0.381 b | 0.010 |
| SGR, % | 0.96 ± 0.006 cd | 0.55 ± 0.012 d | 1.30 ± 0.017 bc | 1.11 ± 0.014 c | 1.55 ± 0.029 a | 1.34 ± 0.006 b | 0.031 |
| HIS, % | 2.03 ± 0.069 b | 2.25 ± 0.098 a | 2.38 ± 0.127 a | 1.75 ± 0.133 c | 2.15 ± 0.087 ab | 1.83 ± 0.064 c | 0.017 |
| FCF | 1.63 ± 0.023 b | 1.80 ± 0.069 a | 1.29 ± 0.052 c | 1.60 ± 0.064 b | 1.24 ± 0.035 c | 1.68 ± 0.021 b | 0.024 |
| Survival Rate, % | 98.67 ± 1.478 bc | 93.78 ± 2.182 d | 97.33 ± 1.663 c | 98.67 ± 1.490 bc | 100 ± 1.599 a | 97.33 ± 1.652 c | 0.010 |
| Enzyme Activity (U mg−1 Protein) | Chitosan Levels (g kg−1) | p | |||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | ||
| Proteases | 39.19 ± 1.091 bc | 42.99 ± 1.126 b | 53.31 ± 1.287 a | 54.05 ± 1.189 a | 54.26 ± 1.276 a | 38.38 ± 1.143 c | 0.012 |
| Lipases | 34.97 ± 0.704 c | 31.15 ± 0.566 d | 40.33 ± 0.774 a | 41.77 ± 0.762 a | 38.22 ± 0.727 b | 37.65 ± 0.768 b | 0.001 |
| Amylases | 69.41 ± 1.709 c | 76.20 ± 1.732 a | 63.97 ± 1.697 d | 72.67 ± 1.709 b | 77.89 ± 1.761 a | 77.10 ± 1.738 a | 0.010 |
| Enzyme Activity (U L−1) | Chitosan Levels (g kg−1) | p | |||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | ||
| AST | 170.37 ± 1.611 a | 109.71 ± 1.513 b | 182.22 ± 0.970 a | 74.48 ± 1.247 d | 77.49 ± 1.126 d | 93.25 ± 0.375 c | 0.022 |
| ALT | 45.33 ± 1.201 a | 45.67 ± 0.883 a | 38.00 ± 1.178 bc | 37.67 ± 1.334 bc | 39.67 ± 2.188 b | 35.67 ± 1.455 c | 0.004 |
| ALP | 89.67 ± 0.664 d | 112.00 ± 2.084 bc | 107.67 ± 1.669 c | 109.33 ± 1.767 c | 114.00 ± 1.531 ab | 118.02 ± 1.478 a | 0.015 |
| Hepatocyte | Chitosan Levels, g kg−1 | p | |||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | ||
| HA, µm2 | 66.17 ± 1.022 d | 62.68 ± 3.683 e | 79.38 ± 3.759 b | 70.75 ± 1.432 c | 88.54 ± 1.951 a | 83.55 ± 3.655 ab | 0.016 |
| CA, µm2 | 57.07 ± 1.062 c | 54.59 ± 3.406 c | 71.11 ± 3.683 ab | 62.20 ± 1.339 b | 78.71 ± 1.732 a | 74.24 ± 0.433 a | 0.015 |
| NA, µm2 | 9.09 ± 0.289 ab | 8.09 ± 0.237 c | 8.28 ± 0.092 bc | 8.55 ± 0.098 b | 9.83 ± 0.242 a | 9.31 ± 0.289 a | 0.036 |
| HR, µm2 | 6.56 ± 0.225 cd | 6.49 ± 0.219 d | 8.86 ± 0.323 a | 6.93 ± 0.087 c | 7.86 ± 0.081 b | 7.64 ± 0.225 b | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez-Martínez, Y.; Vera-Veliz, A.R.; Cortés-Jacinto, E.; Cruz-Quintana, Y.; Botello-Leon, A.; Mendoza-Carranza, P.D.; Calvo, N.S. Growth Performance, Feed Utilisation, Digestive and Metabolic Enzyme Activity, and Liver Morphohistology in Hybrid Tilapia (Oreochromis mossambicus × Oreochromis niloticus) Juveniles Fed with the Inclusion of Chitosan in Their Diet. Fishes 2023, 8, 546. https://doi.org/10.3390/fishes8110546
Méndez-Martínez Y, Vera-Veliz AR, Cortés-Jacinto E, Cruz-Quintana Y, Botello-Leon A, Mendoza-Carranza PD, Calvo NS. Growth Performance, Feed Utilisation, Digestive and Metabolic Enzyme Activity, and Liver Morphohistology in Hybrid Tilapia (Oreochromis mossambicus × Oreochromis niloticus) Juveniles Fed with the Inclusion of Chitosan in Their Diet. Fishes. 2023; 8(11):546. https://doi.org/10.3390/fishes8110546
Chicago/Turabian StyleMéndez-Martínez, Yuniel, Alan Rodrigo Vera-Veliz, Edilmar Cortés-Jacinto, Yanis Cruz-Quintana, Aroldo Botello-Leon, Pedro Daniel Mendoza-Carranza, and Natalia S. Calvo. 2023. "Growth Performance, Feed Utilisation, Digestive and Metabolic Enzyme Activity, and Liver Morphohistology in Hybrid Tilapia (Oreochromis mossambicus × Oreochromis niloticus) Juveniles Fed with the Inclusion of Chitosan in Their Diet" Fishes 8, no. 11: 546. https://doi.org/10.3390/fishes8110546
APA StyleMéndez-Martínez, Y., Vera-Veliz, A. R., Cortés-Jacinto, E., Cruz-Quintana, Y., Botello-Leon, A., Mendoza-Carranza, P. D., & Calvo, N. S. (2023). Growth Performance, Feed Utilisation, Digestive and Metabolic Enzyme Activity, and Liver Morphohistology in Hybrid Tilapia (Oreochromis mossambicus × Oreochromis niloticus) Juveniles Fed with the Inclusion of Chitosan in Their Diet. Fishes, 8(11), 546. https://doi.org/10.3390/fishes8110546











