Changes in the Functional Role of the Tejo Estuary (Portugal, Europe) According to Fish Ecological Guilds
Abstract
1. Introduction
2. Materials and Methods
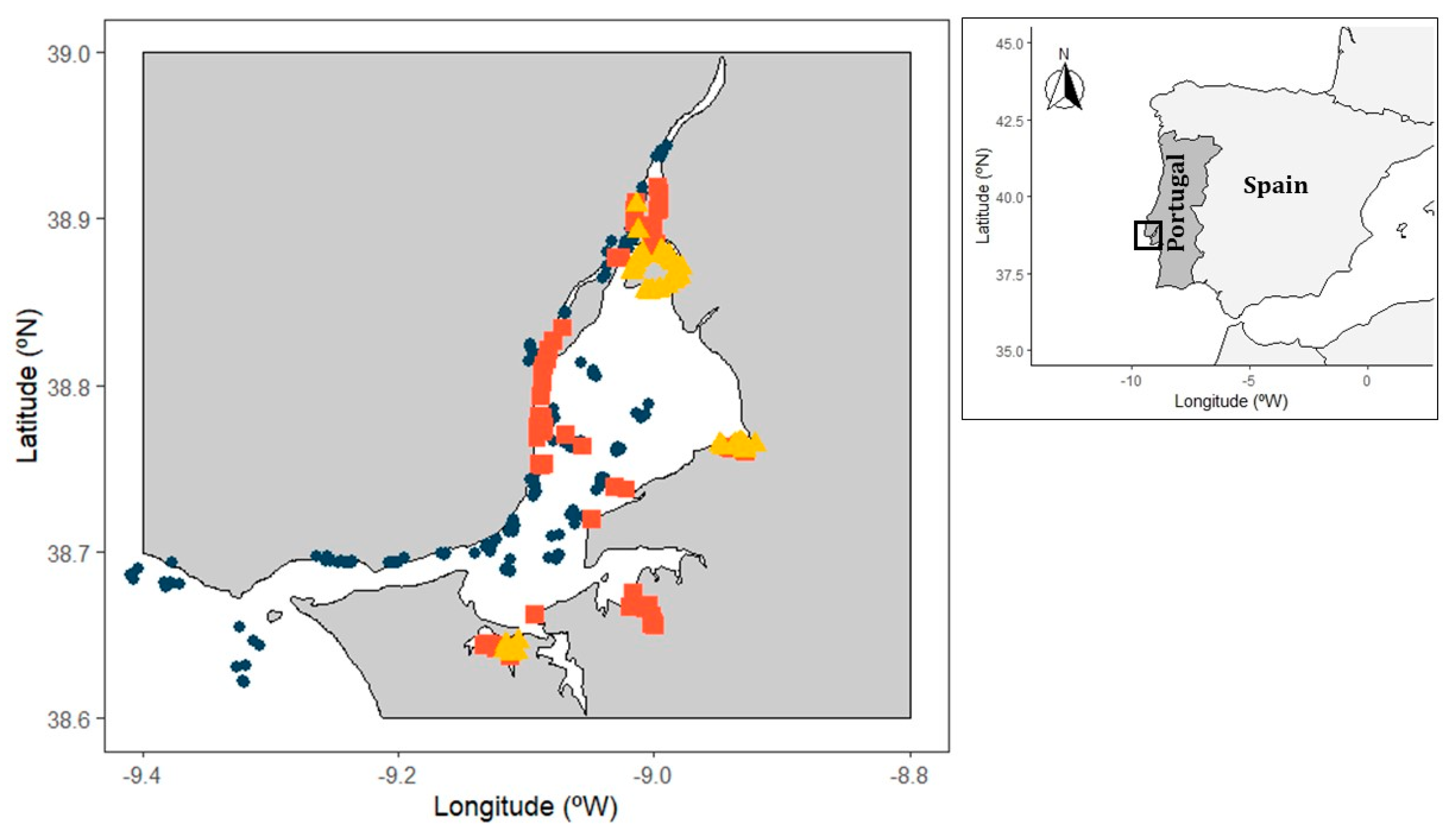
2.1. Study Area
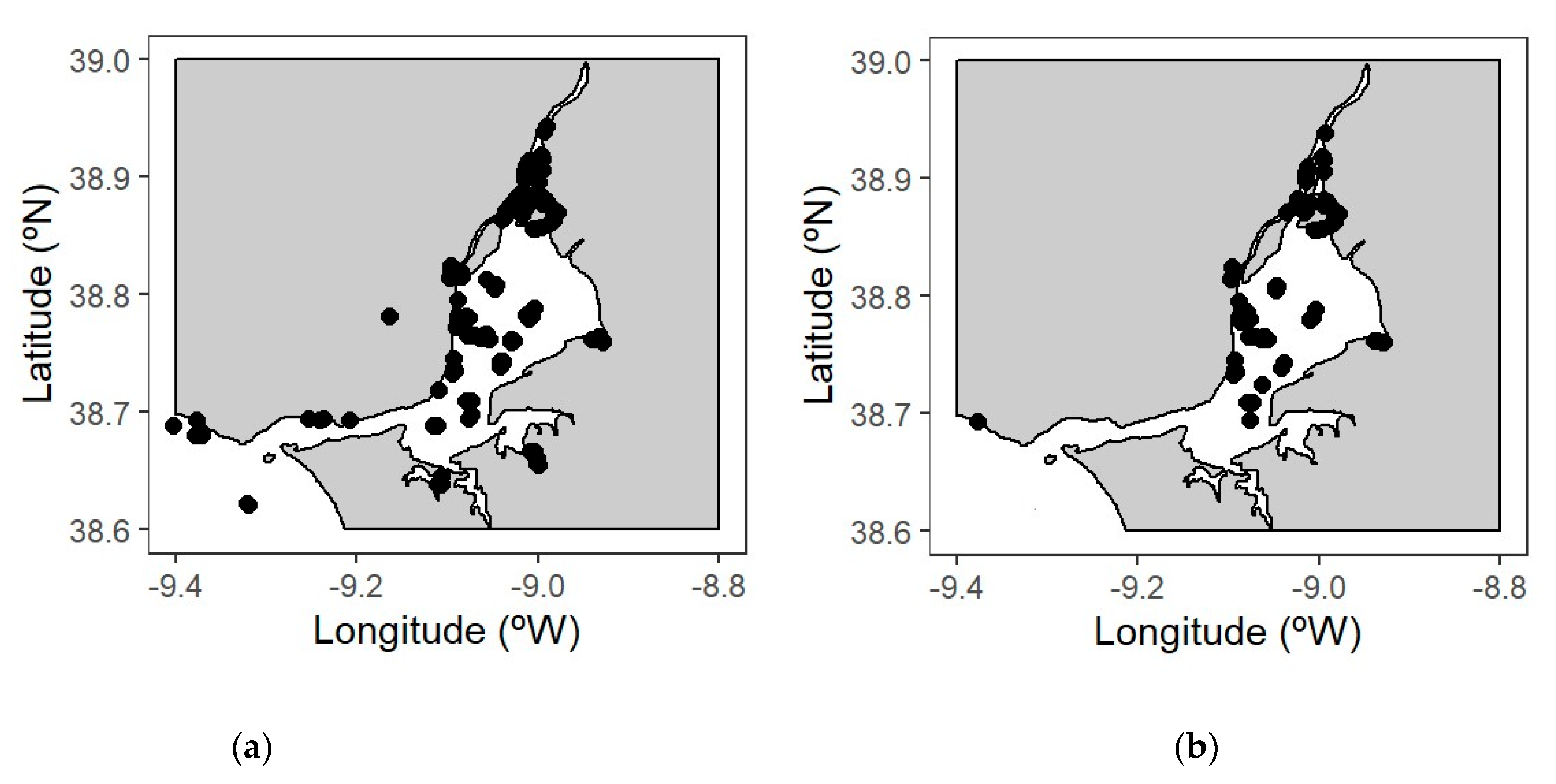
2.2. Sampling
2.3. Data Analysis
Ensemble Distribution Models
3. Results
3.1. Diversity Metrics
3.2. Ensemble Models
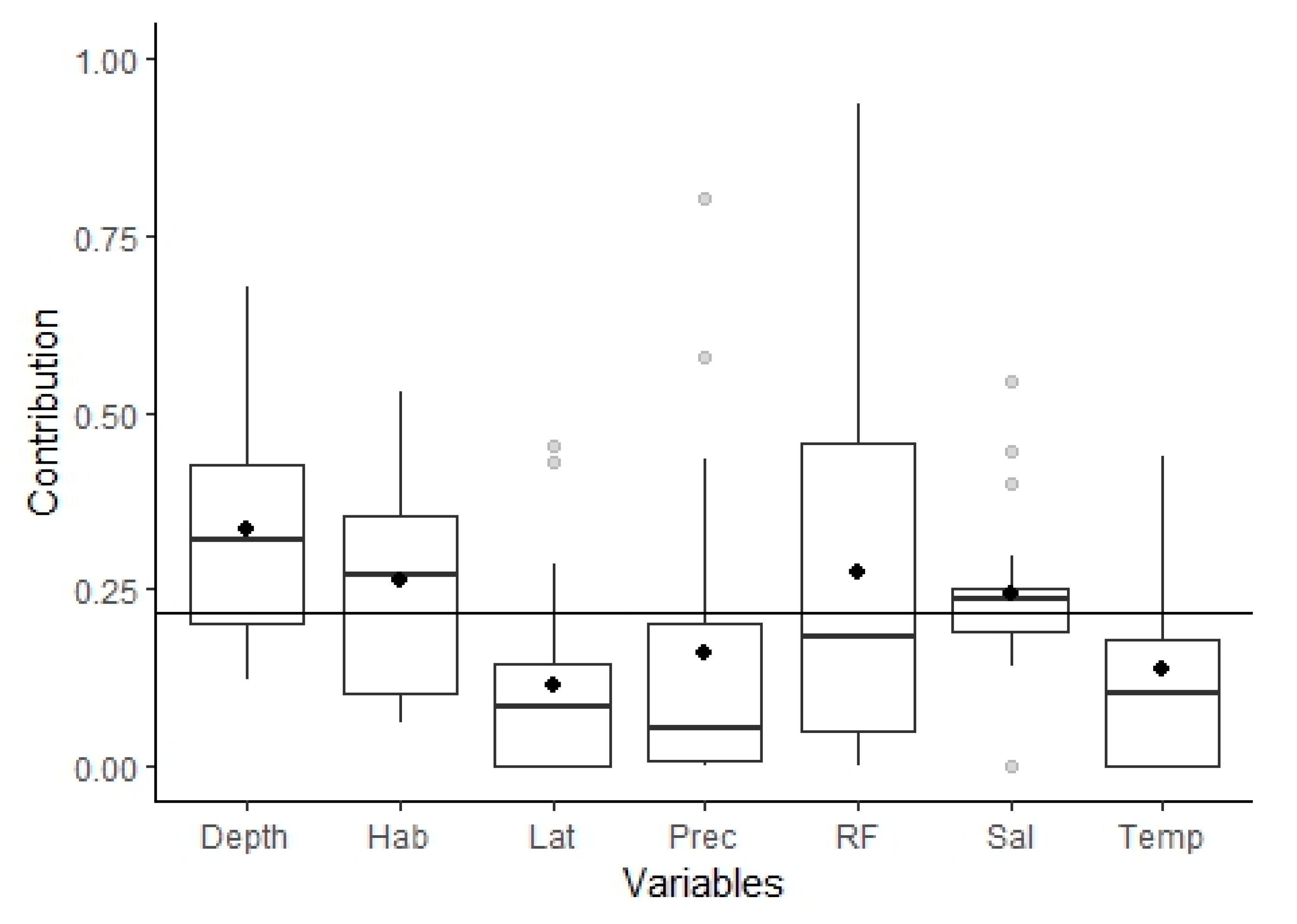
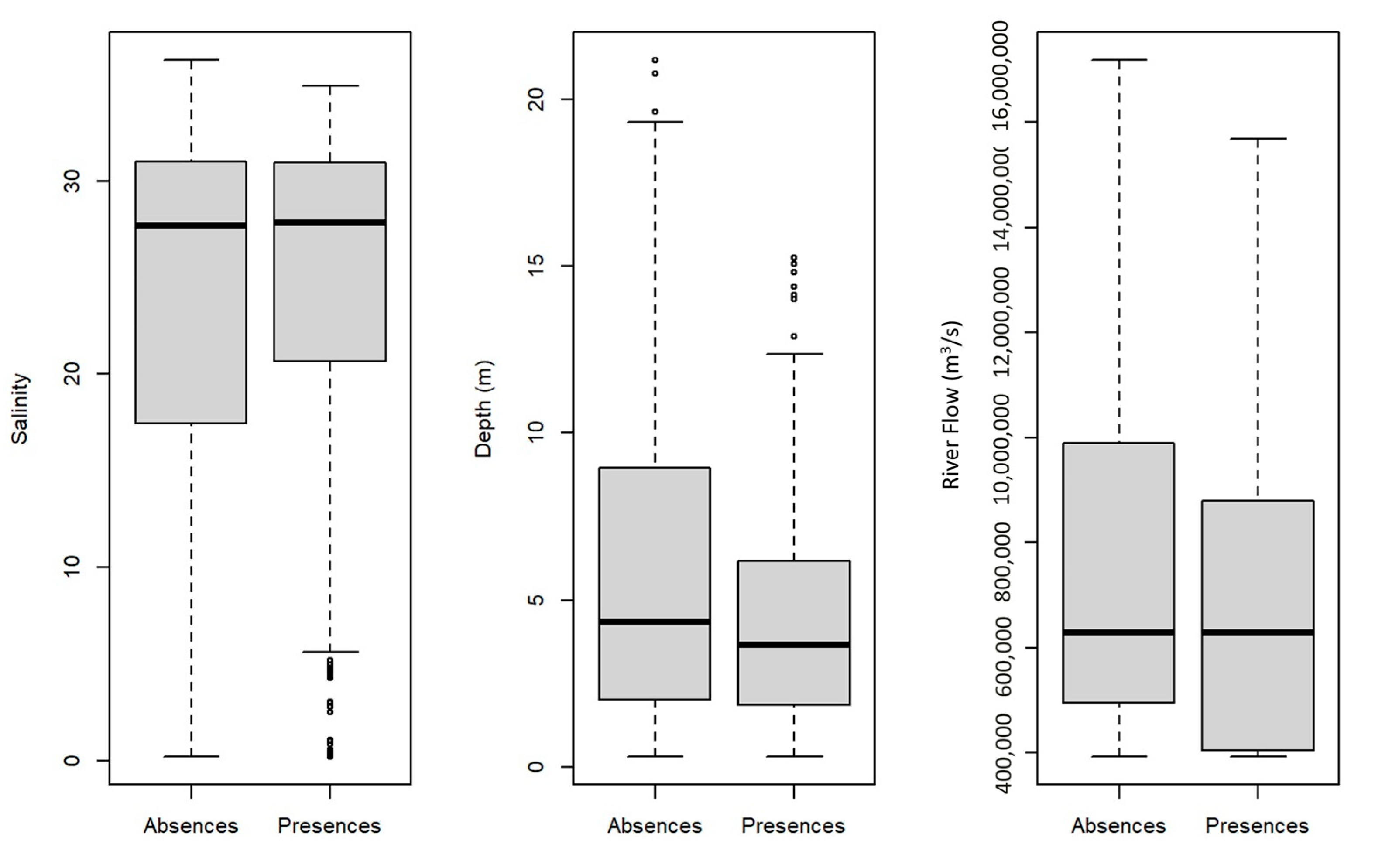
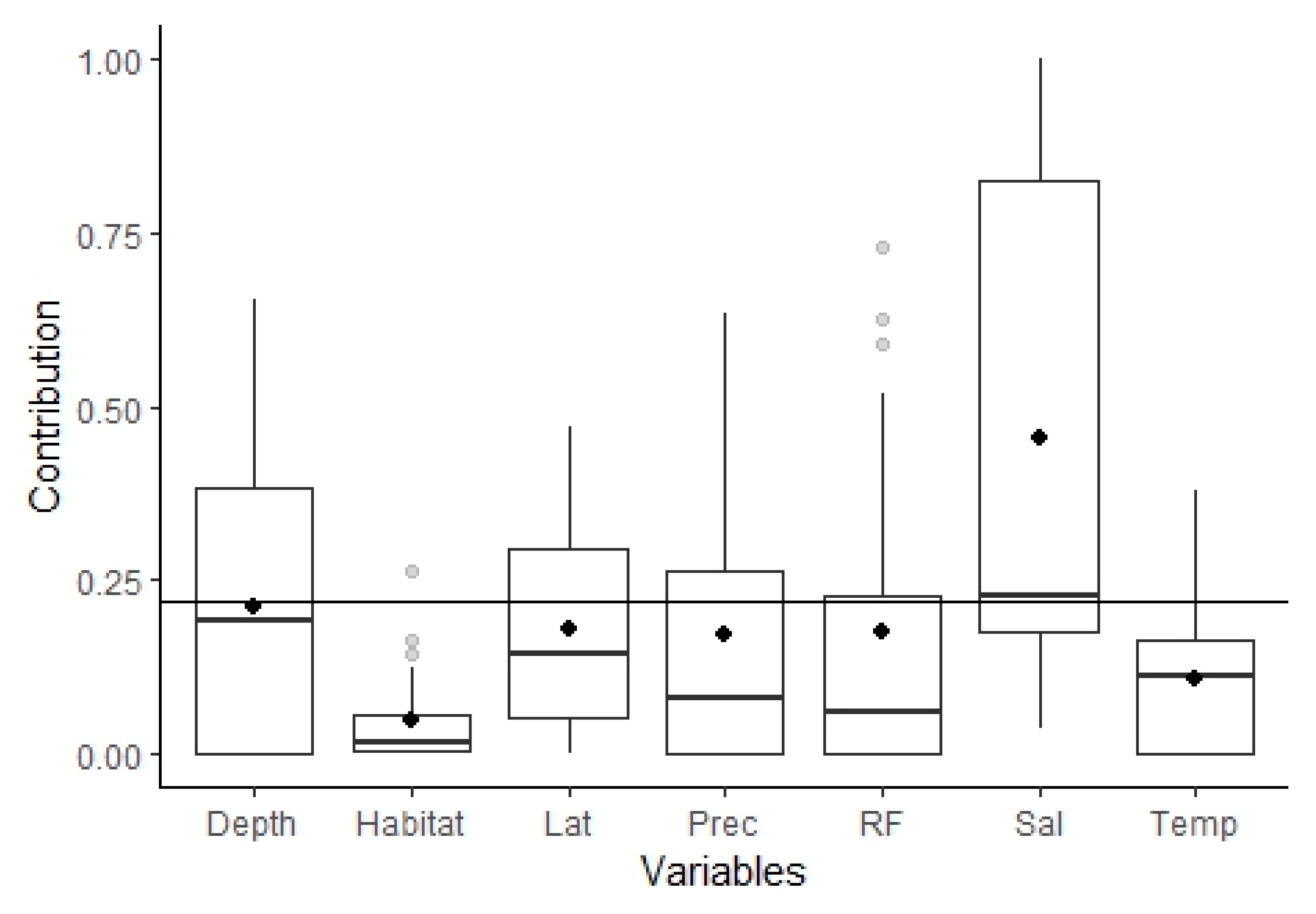
3.2.1. Model Performance and Variables Importance
3.2.2. Current and Potential Future Distributions of Ecological Guilds
4. Discussion
4.1. Fish Assemblages from the Tejo Estuary: Ecological Guild Composition and Relation with Important Environmental Variables
4.2. The Tejo Estuary Functioning in Face of Global Changes
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Ecological Guild |
|---|---|
| Anguilla anguilla | C |
| Aphia minuta | ES |
| Argyrosomus regius | MS |
| Arnoglossus imperialis | MS |
| Arnoglossus laterna | MS |
| Atherina boyeri | ES |
| Atherina presbyter | MM |
| Belone belone | MS |
| Boops boops | MS |
| Bothus podas | MS |
| Buglossidium luteum | MS |
| Callionymus lyra | MS |
| Callionymus reticulatus | MS |
| Chelidonichthys lucernus | MS |
| Chelidonichthys obscurus | MS |
| Chelon auratus | MS |
| Chelon labrosus | C |
| Chelon ramada | C |
| Conger conger | MS |
| Dicentrarchus labrax | MM |
| Dicentrarchus punctatus | MM |
| Dicologlossa cuneata | MM |
| Dicologlossa hexophthalma | MS |
| Diplodus annularis | MM |
| Diplodus bellottii | MM |
| Diplodus sargus | MM |
| Diplodus vulgaris | MM |
| Echiichthys vipera | MS |
| Engraulis encrasicolus | MS |
| Gobius niger | ES |
| Gobius paganellus | ES |
| Halobatrachus didactylus | ES |
| Hippocampus hippocampus | ES |
| Luciobarbus bocagei | F |
| Merluccius merluccius | MS |
| Mullus surmuletus | MS |
| Pagellus acarne | MS |
| Pagellus bagaravea | MS |
| Pagrus auriga | MS |
| Pagrus pagrus | MS |
| Pegusa lascaris | MS |
| Platichthys flesus | MM |
| Pollachius pollachius | MS |
| Pomastoschistus microps | ES |
| Pomastoschistus minutus | ES |
| Raja clavata | MS |
| Raja microocellata | MS |
| Raja montagui | MS |
| Raja undulata | MS |
| Sardina pilchardus | MS |
| Scomber scombrus | MS |
| Scophthalmus maximus | MM |
| Scophthalmus rhombus | MM |
| Scorpaena notata | MS |
| Solea senegalensis | MM |
| Solea solea | MM |
| Sparus aurata | MS |
| Spondyliosoma cantharus | MS |
| Sprattus sprattus | MS |
| Symphodus bailloni | MS |
| Syngnathus abaster | ES |
| Syngnathus acus | ES |
| Syngnathus typhle | ES |
| Trachurus trachurus | MS |
| Trisopterus luscus | MS |
| Zeus faber | MS |
Appendix B
| Model | Specifications |
|---|---|
| GLM | Run a stepwise GLM using linear (“simple”) terms. The statistical criteria used for selection of models of increasing fit were the Akaike Information Criterion (AIC). To select the most parsimonious model, BIOMOD uses an automatic stepwise model selection. |
| GAM | Run a generalized additive model with a spline function with a degree of smoothing of 4 (similar to a polynomial of degree 3). BIOMOD uses a cubic spline smoother, which is a collection of polynomials of degree less than or equal to 3, defined on subintervals delimited by knots. A separate polynomial, fitted for each neighborhood, enables the fitted curve to join all the points, producing a smooth linear curve. BIOMOD uses an automated stepwise process to select the most significant variables for each species. Y = s(X1, 4) + s(X2, 4) + s(X3, 4). |
| RF | Run a random forest model. Implements Breiman’s random forest algorithm (based on Breiman and Cutler’s original Fortran code) for classification and regression. It is implemented into the “random-Forest” library (Liaw and Wiener). BIOMOD uses 500 trees and extracts the importance of each selected variable. |
| CTA | Run a classification tree analysis (CTA). The optimal length of the tree is estimated using cross-validation (default = 50). BIOMOD uses a procedure running k-fold cross-validations to select the best tree, which is a trade-off between the number of leaves of the tree and the explained deviance. BIOMOD uses the rpart library to run the classification tree analysis. |
References
- Costanza, R.; d’Arge, R.; de Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’Neill, R.V.; Paruelo, J.; et al. The value of the world’s ecosystem services and natural capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Hughes, B.B.; Levey, M.D.; Fountain, M.C.; Carlisle, A.B.; Chavez, F.P.; Gleason, M.G. Climate mediates hypoxic stress on fish diversity and nursery function at the land–sea interface. Proc. Natl. Acad. Sci. USA 2015, 112, 8025–8030. [Google Scholar] [CrossRef] [PubMed]
- Henderson, C.J.; Gilby, B.L.; Schlacher, T.A.; Connolly, R.M.; Sheaves, M.; Maxwell, P.S.; Flint, N.; Borland, H.P.; Martin, T.S.H.; Olds, A.D. Low redundancy and complementarity shape ecosystem functioning in a low-diversity ecosystem. J. Anim. Ecol. 2019, 89, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, R.P.; Batista, M.I.; Henriques, S. Current limitations of global conservation to protect higher vulnerability and lower resilience fish species. Sci. Rep. 2017, 7, 7702. [Google Scholar] [CrossRef] [PubMed]
- Day, J.W., Jr.; Crump, B.C.; Kemp, W.M.; Yáñez-Arancibia, A. Estuarine Ecology; John Wiley Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Feyrer, F.; Cloern, J.E.; Brown, L.R.; Fish, M.A.; Hieb, K.A.; Baxter, R.D. Estuarine fish communities respond to climate variability over both river and ocean basins. Glob. Chang. Biol. 2015, 21, 3608–3619. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, B.; Richard, P.; Parlier, E.P.; Guillou, G.; Blanchard, G.F. Trophic ecology of mullets during their spring migration in a European saltmarsh: A stable isotope study. Estuar. Coast. Shelf Sci. 2011, 91, 502–510. [Google Scholar] [CrossRef][Green Version]
- Whitfield, A.K. Ichthyofaunal assemblages in estuaries: A South African case study. Rev. Fish Biol. Fish. 1999, 9, 151–186. [Google Scholar] [CrossRef]
- Lai, H.; Bi, S.; Yi, H.; Guo, D.; Li, H.; Wang, G.; Liu, X.; Chen, Q.; Chen, J.; Zhang, Z.; et al. Seasonal variation in the functional structure of demersal fish communities and response to the environmental changes in the Pearl River Estuary, China. Ecol. Indic. 2022, 144, 109525. [Google Scholar] [CrossRef]
- Baptista, J.; van der Linden, P.; Martinho, F.; Martins, R.; Carneiro, M.; Bento, E.G.; Pardal, M.Â. The functional composition of nearshore fish communities demonstrated by trait analysis: Response to environmental gradients. Mar. Pollut. Bull. 2021, 169, 112562. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.; Whitfield, A.K.; Potter, I.C.; Blaber, S.J.M.; Cyrus, D.P.; Nordlie, F.G.; Harrison, T.D. The guild approach to categorizing estuarine fish assemblages: A global review. Fish Fish. 2007, 8, 241–268. [Google Scholar] [CrossRef]
- Potter, I.C.; Tweedley, J.R.; Elliott, M.; Whitfield, A.K. The ways in which fish use estuaries: A refinement and expansion of the guild approach. Fish Fish. 2013, 16, 230–239. [Google Scholar] [CrossRef]
- Ferreira, V.; Le Loc’h, F.; Ménard, F.; Frédou, T.; Frédou, F.L. Composition of the fish fauna in a tropical estuary: The ecological guild approach. Sci. Mar. 2019, 83, 133. [Google Scholar] [CrossRef]
- Harrison, T.D.; Whitfield, A.K. Geographical and typological changes in fish guilds of South African estuaries. J. Fish Biol. 2008, 73, 2542–2570. [Google Scholar] [CrossRef]
- Vivier, L.; Cyrus, D.P.; Jerling, H.L. Fish community structure of the St Lucia Estuarine System under prolonged drought conditions and its potential for recovery after mouth breaching. Estuar. Coast. Shelf Sci. 2010, 86, 568–579. [Google Scholar] [CrossRef]
- Dolbeth, M.; Cardoso, P.G.; Grilo, T.F.; Bordalo, M.D.; Raffaelli, D.; Pardal, M.A. Long-term changes in the production by estuarine macrobenthos affected by multiple stressors. Estuar. Coast. Shelf Sci. 2011, 92, 10–18. [Google Scholar] [CrossRef]
- Moyle, P.B.; Kiernan, J.D.; Crain, P.K.; Quiñones, R.M. Climate change vulnerability of native and alien freshwater fishes of California: A systematic assessment approach. PLoS ONE 2013, 8, e63883. [Google Scholar] [CrossRef]
- Albouy, C.; Guilhaumon, F.; Araújo, M.B.; Mouillot, D.; Leprieur, F. Combining projected changes in species richness and composition reveals climate change impacts on coastal Mediterranean fish assemblages. Glob. Chang. Biol. 2012, 18, 2995–3003. [Google Scholar] [CrossRef]
- Martinho, F.; Leitão, R.; Neto, J.M.; Cabral, H.N.; Marques, J.C.; Pardal, M.A. The use of nursery areas by juvenile fish in a temperate estuary, Portugal. Hydrobiologia 2007, 587, 281–290. [Google Scholar] [CrossRef]
- França, S.; Costa, M.J.; Cabral, H.N. Inter- and intra-estuarine fish assemblage variability patterns along the Portuguese coast. Estuar. Coast. Shelf Sci. 2011, 91, 262–271. [Google Scholar] [CrossRef]
- Bento, E.G.; Grilo, T.F.; Nyitrai, D.; Dolbeth, M.; Pardal, M.Â.; Martinho, F. Climate influence on juvenile European sea bass (Dicentrarchus labrax L.) populations in an estuarine nursery: A decadal overview. Mar. Environ. Res. 2016, 122, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, I.; Bio, A.; Melo, W.; Avilez-Valente, P.; Pinho, J.; Cruz, M.; Gomes, A.; Vieira, J.; Bastos, L.; Veloso-Gomes, F. Hydrodynamic model ensembles for climate change projections in estuarine regions. Water 2022, 14, 1966. [Google Scholar] [CrossRef]
- Cabral, H.N.; Vasconcelos, R.; Vinagre, C.; França, S.; Fonseca, V.; Maia, A.; Reis-Santos, P.; Lopes, M.; Ruano, M.; Campos, J.; et al. Relative importance of estuarine flatfish nurseries along the Portuguese coast. J. Sea Res. 2007, 57, 209–217. [Google Scholar] [CrossRef]
- França, S.; Vasconcelos, R.P.; Fonseca, V.F.; Tanner, S.E.; Reis-Santos, P.; Costa, M.J.; Cabral, H.N. Predicting fish community properties within estuaries: Influence of habitat type and other environmental features. Estuar. Coast. Shelf Sci. 2012, 107, 22–31. [Google Scholar] [CrossRef]
- Lopes, J.F.; Lopes, C.L.; Dias, J.M. Climate change impact in the ria de aveiro lagoon ecosystem: A case study. J. Mar. Sci. Eng. 2019, 7, 352. [Google Scholar] [CrossRef]
- Iglesias, I.; Venâncio, S.; Pinho, J.L.; Avilez-Valente, P.; Vieira, J.M.P. Two models solutions for the Douro Estuary: Flood risk assessment and breakwater effects. Estuaries Coasts 2018, 42, 348–364. [Google Scholar] [CrossRef]
- Chua, V.P.; Xu, M. Impacts of sea-level rise on estuarine circulation: An idealized estuary and San Francisco Bay. J. Mar. Syst. 2014, 139, 58–67. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, T.; Voisin, N.; Copping, A. Estuarine response to river flow and sea-level rise under future climate change and human development. Estuar. Coast. Shelf Sci. 2015, 156, 19–30. [Google Scholar] [CrossRef]
- Melo, W.; Pinho, J.; Iglesias, I.; Bio, A.; Avilez-Valente, P.; Vieira, J.; Bastos, L.; Veloso-Gomes, F. Hydro- and morphodynamic impacts of sea level rise: The Minho Estuary case study. J. Mar. Sci. Eng. 2020, 8, 441. [Google Scholar] [CrossRef]
- Eastwood, P.D.; Meaden, G.J.; Carpentier, A.; Rogers, S.I. Estimating limits to the spatial extent and suitability of sole (Solea solea) nursery grounds in the Dover Strait. J. Sea Res. 2003, 50, 151–165. [Google Scholar] [CrossRef]
- Vasconcelos, R.P.; Le Pape, O.; Costa, M.J.; Cabral, H.N. Predicting estuarine use patterns of juvenile fish with Generalized Linear Models. Estuar. Coast. Shelf Sci. 2013, 120, 64–74. [Google Scholar] [CrossRef]
- França, S.; Cabral, H.N. Predicting fish species distribution in estuaries: Influence of species’ ecology in model accuracy. Estuar. Coast. Shelf Sci. 2016, 180, 11–20. [Google Scholar] [CrossRef]
- França, S.; Cabral, H.N. Distribution models of estuarine fish species: The effect of sampling bias, species ecology and threshold selection on models’ accuracy. Ecol. Inform. 2019, 51, 168–176. [Google Scholar] [CrossRef]
- Pasquaud, S.; Vasconcelos, R.P.; França, S.; Henriques, S.; Costa, M.J.; Cabral, H. Worldwide patterns of fish biodiversity in estuaries: Effect of global vs. local factors. Estuar. Coast. Shelf Sci. 2015, 154, 122–128. [Google Scholar] [CrossRef]
- Vasconcelos, R.P.; Henriques, S.; França, S.; Pasquaud, S.; Cardoso, I.; Laborde, M.; Cabral, H.N. Global patterns and predictors of fish species richness in estuaries. J. Anim. Ecol. 2015, 84, 1331–1341. [Google Scholar] [CrossRef]
- França, S.; Costa, M.J.; Cabral, H.N. Assessing habitat specific fish assemblages in estuaries along the Portuguese coast. Estuar. Coast. Shelf Sci. 2009, 83, 1–12. [Google Scholar] [CrossRef]
- Vasconcelos, R.P.; Reis-Santos, P.; Maia, A.; Fonseca, V.; França, S.; Wouters, N.; Costa, M.J.; Cabral, H.N. Nursery use patterns of commercially important marine fish species in estuarine systems along the Portuguese coast. Estuar. Coast. Shelf Sci. 2010, 86, 613–624. [Google Scholar] [CrossRef]
- Cabral, H.; Costa, M.; Salgado, J. Does the Tagus estuary fish community reflect environmental changes? Clim. Res. 2001, 18, 119–126. [Google Scholar] [CrossRef]
- Vasconcelos, R.P.; Reis-Santos, P.; Fonseca, V.; Maia, A.; Ruano, M.; França, S.; Vinagre, C.; Costa, M.J.; Cabral, H. Assessing anthropogenic pressures on estuarine fish nurseries along the Portuguese coast: A multi-metric index and conceptual approach. Sci. Total Environ. 2007, 374, 199–215. [Google Scholar] [CrossRef]
- Vaz, N.; Mateus, M.; Pinto, L.; Neves, R.; Dias, J.M. The Tagus estuary as a numerical modeling test bed: A review. Geosciences 2020, 10, 4. [Google Scholar] [CrossRef]
- Guerreiro, M.; Bustorff, A.F.; Freire, P.; Rilo, A.; Taborda, R.; Freitas, M.C.; Andrade, C.; Silva, T.; Rodrigues, M.; Bertin, X.; et al. Evolution of the hydrodynamics of the Tagus estuary (Portugal) in the 21st century. J. Integr. Coast. Zone Manag. 2015, 15, 65–80. [Google Scholar] [CrossRef]
- Dias, J.M.; Valentim, J.M.; Sousa, M.C. A numerical study of local variations in tidal regime of Tagus estuary, Portugal. PLoS ONE 2013, 8, e80450. [Google Scholar] [CrossRef]
- Belo, J.R.; Dias, M.P.; Jara, J.; Almeida, A.; Morais, F.; Silva, C.; Valadeiro, J.; Alves, J.A. Synchronous declines of wintering waders and high-tide roost area in a temperate estuary: Results of a 10-year monitoring programme. Waterbirds 2023, 45, 141–149. [Google Scholar] [CrossRef]
- Pihl, L.; Cattrijsse, A.; Codling, I.; Mathieson, S.; McLusky, D.S.; Roberts, C. Habitat Use by Fishes in Estuaries and Other Brackish Areas. In Fishes in Estuaries; Wiley: Hoboken, NJ, USA, 2002; pp. 10–53. [Google Scholar] [CrossRef]
- Franco, A.; Elliott, M.; Franzoi, P.; Torricelli, P. Life strategies of fishes in European estuaries: The functional guild approach. Mar. Ecol. Prog. Ser. 2008, 354, 219–228. [Google Scholar] [CrossRef]
- Rochette, S.; Rivot, E.; Morin, J.; Mackinson, S.; Riou, P.; Le Pape, O. Effect of nursery habitat degradation on flatfish population: Application to Solea solea in the Eastern Channel (Western Europe). J. Sea Res. 2010, 64, 34–44. [Google Scholar] [CrossRef]
- Bio, A.M.F.; Becker, P.D.; Bie, E.D.; Huybrechts, W.; Wassen, M. Prediction of plant species distribution in lowland river valleys in Belgium: Modelling species response to site conditions. Biodivers. Conserv. 2002, 11, 2189–2216. [Google Scholar] [CrossRef]
- Bombi, P.; D’Amen, M. Scaling down distribution maps from atlas data: A test of different approaches with virtual species. J. Biogeogr. 2011, 39, 640–651. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R. Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making better Maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2013, 41, 629–643. [Google Scholar] [CrossRef]
- Pearce, J.; Ferrier, S. Evaluating the predictive performance of habitat models developed using logistic regression. Ecol. Model. 2000, 133, 225–245. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Freeman, E.A.; Moisen, G.G. A comparison of the performance of threshold criteria for binary classification in terms of predicted prevalence and kappa. Ecol. Model. 2008, 217, 48–58. [Google Scholar] [CrossRef]
- Elliott, M.; Dewailly, F. The structure and components of European estuarine fish assemblages. Neth. J. Aquat. Ecol. 1995, 29, 397–417. [Google Scholar] [CrossRef]
- Whitfield, A.K.; Able, K.W.; Blaber, S.J.M.; Elliott, M.; Franco, A.; Harrison, T.D.; Potter, I.C.; Tweedley, J.R. Fish Assemblages and Functional Groups. In Fish and Fisheries in Estuaries: A Global Perspective; Wiley: Hoboken, NJ, USA, 2022; pp. 16–59. [Google Scholar] [CrossRef]
- Malavasi, S.; Fiorin, R.; Franco, A.; Franzoi, P.; Granzotto, A.; Riccato, F.; Mainardi, D. Fish assemblages of Venice Lagoon shallow waters: An analysis based on species, families and functional guilds. J. Mar. Syst. 2004, 51, 19–31. [Google Scholar] [CrossRef]
- Contente, R.F.; Stefanoni, M.F.; Spach, H.L. Feeding ecology of the Brazilian silverside Atherinella brasiliensis (Atherinopsidae) in a sub-tropical estuarine ecosystem. J. Mar. Biol. Assoc. UK 2010, 91, 1197–1205. [Google Scholar] [CrossRef]
- Macedo, M.M.; Angelini, R.; da Silva, V.E.L.; Fabré, N.N. Trophic structure of coastal meta-ecosystems in the tropical Southwestern Atlantic. Estuar. Coast. Shelf Sci. 2021, 263, 107654. [Google Scholar] [CrossRef]
- Araujo, M.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef]
- Fukuda, S.; De Baets, B.; Waegeman, W.; Verwaeren, J.; Mouton, A.M. Habitat prediction and knowledge extraction for spawning European grayling (Thymallus thymallus L.) using a broad range of species distribution models. Environ. Model. Softw. 2013, 47, 1–6. [Google Scholar] [CrossRef]
- Kindt, R. Ensemble species distribution modelling with transformed suitability values. Environ. Model. Softw. 2018, 100, 136–145. [Google Scholar] [CrossRef]
- Dorman, C.F.; Schweiger, O.; Arens, P.; Augenstein, I.; Aviron, S. Prediction uncertainty of environmental change effects on temperate European biodiversity. Ecol. Lett. 2008, 11, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Whitfield, A.K.; Cowley, P.D.; Cole, V.J. Does water depth influence size composition of estuary-associated fish? Distributions revealed using mobile acoustic-camera transects along the channel of a small shallow estuary. Mar. Freshw. Res. 2017, 68, 2163. [Google Scholar] [CrossRef]
- Scanes, E.; Scanes, P.R.; Ross, P.M. Climate change rapidly warms and acidifies Australian estuaries. Nat. Commun. 2020, 11, 1803. [Google Scholar] [CrossRef]
- Leitão, R.; Martinho, F.; Neto, J.M.; Cabral, H.; Marques, J.C.; Pardal, M.A. Feeding ecology, population structure and distribution of Pomatoschistus microps (Krøyer, 1838) and Pomatoschistus minutus (Pallas, 1770) in a temperate estuary, Portugal. Estuar. Coast. Shelf Sci. 2006, 66, 231–239. [Google Scholar] [CrossRef]
- Costa, M.J.; Vasconcelos, R.; Costa, J.L.; Cabral, H.N. River flow influence on the fish community of the Tagus estuary (Portugal). Hydrobiologia 2007, 587, 113–123. [Google Scholar] [CrossRef]
- Vinagre, C.; Salgado, J.; Cabral, H.N.; Costa, M.J. Food web structure and habitat connectivity in fish estuarine nurseries—Impact of river flow. Estuaries Coasts 2010, 34, 663–674. [Google Scholar] [CrossRef]
- Nyitrai, D.; Martinho, F.; Dolbeth, M.; Rito, J.; Pardal, M.A. Effects of local and large-scale climate patterns on estuarine resident fishes: The example of Pomatoschistus microps and Pomatoschistus minutus. Estuar. Coast. Shelf Sci. 2013, 135, 260–268. [Google Scholar] [CrossRef][Green Version]
- Fonds, M.; Buurt, G. The influence of temperature and salinity on development and survival of goby eggs (Pisces, Gobiidae). Hydrobiol. Bull. 1974, 8, 110–116. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Silva, D.; Cunha, J.; Pereira, R.; Freitas, V.; Ramos, S. Environmental influences, particularly river flow alteration, on larval fish assemblages in the Douro Estuary, Portugal. Reg. Stud. Mar. Sci. 2022, 56, 102617. [Google Scholar] [CrossRef]
- Belarmino, E.; Francisco de Nóbrega, M.; Grimm, A.M.; da Silva Copertino, M.; Vieira, J.P.; Garcia, A.M. Long-term trends in the abundance of an estuarine fish and relationships with El Niño climatic impacts and seagrass meadows reduction. Estuar. Coast. Shelf Sci. 2021, 261, 107565. [Google Scholar] [CrossRef]
- Barletta, M.; Barletta-Bergan, A.; Saint-Paul, U.; Hubold, G. The role of salinity in structuring the fish assemblages in a tropical estuary. J. Fish Biol. 2005, 66, 45–72. [Google Scholar] [CrossRef]
- Sosa-López, A.; Mouillot, D.; Ramos-Miranda, J.; Flores-Hernandez, D.; Chi, T.D. ORIGINAL ARTICLE: Fish species richness decreases with salinity in tropical coastal lagoons. J. Biogeogr. 2006, 34, 52–61. [Google Scholar] [CrossRef]
- da Silva, V.E.L.; de Assis, I.O.; Campos-Silva, J.V.; Paulino, G.V.B.; Fabré, N.N. Relative importance of habitat mosaics for fish guilds in the northeastern coast of Brazil. Reg. Stud. Mar. Sci. 2022, 50, 102145. [Google Scholar] [CrossRef]
- Nyström, M. Redundancy and response diversity of functional groups: Implications for the resilience of coral reefs. AMBIO A J. Hum. Environ. 2006, 35, 30–35. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2013: The Physical Science Basis; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Ilarri, M.; Souza, A.T.; Dias, E.; Antunes, C. Influence of climate change and extreme weather events on an estuarine fish community. Sci. Total Environ. 2022, 827, 154190. [Google Scholar] [CrossRef] [PubMed]
- Machado, P.M.; Costa, L.L.; Suciu, M.C.; Tavares, D.C.; Zalmon, I.R. Extreme storm wave influence on sandy beach macrofauna with distinct human pressures. Mar. Pollut. Bull. 2016, 107, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Leal Filho, W.; Nagy, G.J.; Martinho, F.; Saroar, M.; Erache, M.G.; Primo, A.L.; Pardal, M.A.; Li, C. Influences of climate change and variability on estuarine ecosystems: An impact study in selected European, South American and Asian countries. Int. J. Environ. Res. Public Health 2022, 19, 585. [Google Scholar] [CrossRef]
- Primo, A.L.; Azeiteiro, U.M.; Marques, S.C.; Pardal, M.Â. Impact of climate variability on ichthyoplankton communities: An example of a small temperate estuary. Estuar. Coast. Shelf Sci. 2011, 91, 484–491. [Google Scholar] [CrossRef]
- Marques, S.C.; Pardal, M.Â.; Primo, A.L.; Martinho, F.; Falcão, J.; Azeiteiro, U.; Molinero, J.C. Evidence for changes in estuarine zooplankton fostered by increased climate variance. Ecosystems 2017, 21, 56–67. [Google Scholar] [CrossRef]



| AUC | Sensitivity | Specificity | |
|---|---|---|---|
| Estuarine Species | 0.79 | 0.79 | 0.67 |
| Marine Migrants | 0.90 | 0.84 | 0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
França, S. Changes in the Functional Role of the Tejo Estuary (Portugal, Europe) According to Fish Ecological Guilds. Fishes 2023, 8, 545. https://doi.org/10.3390/fishes8110545
França S. Changes in the Functional Role of the Tejo Estuary (Portugal, Europe) According to Fish Ecological Guilds. Fishes. 2023; 8(11):545. https://doi.org/10.3390/fishes8110545
Chicago/Turabian StyleFrança, Susana. 2023. "Changes in the Functional Role of the Tejo Estuary (Portugal, Europe) According to Fish Ecological Guilds" Fishes 8, no. 11: 545. https://doi.org/10.3390/fishes8110545
APA StyleFrança, S. (2023). Changes in the Functional Role of the Tejo Estuary (Portugal, Europe) According to Fish Ecological Guilds. Fishes, 8(11), 545. https://doi.org/10.3390/fishes8110545





