Discarded but Not Dismissed: A Comprehensive Study of the Feeding Habits of the Brown Comber (Serranus hepatus, (Linneaus 1758)) in the Gulf of Cádiz (NE Atlantic)
Abstract
1. Introduction
2. Materials and Methods
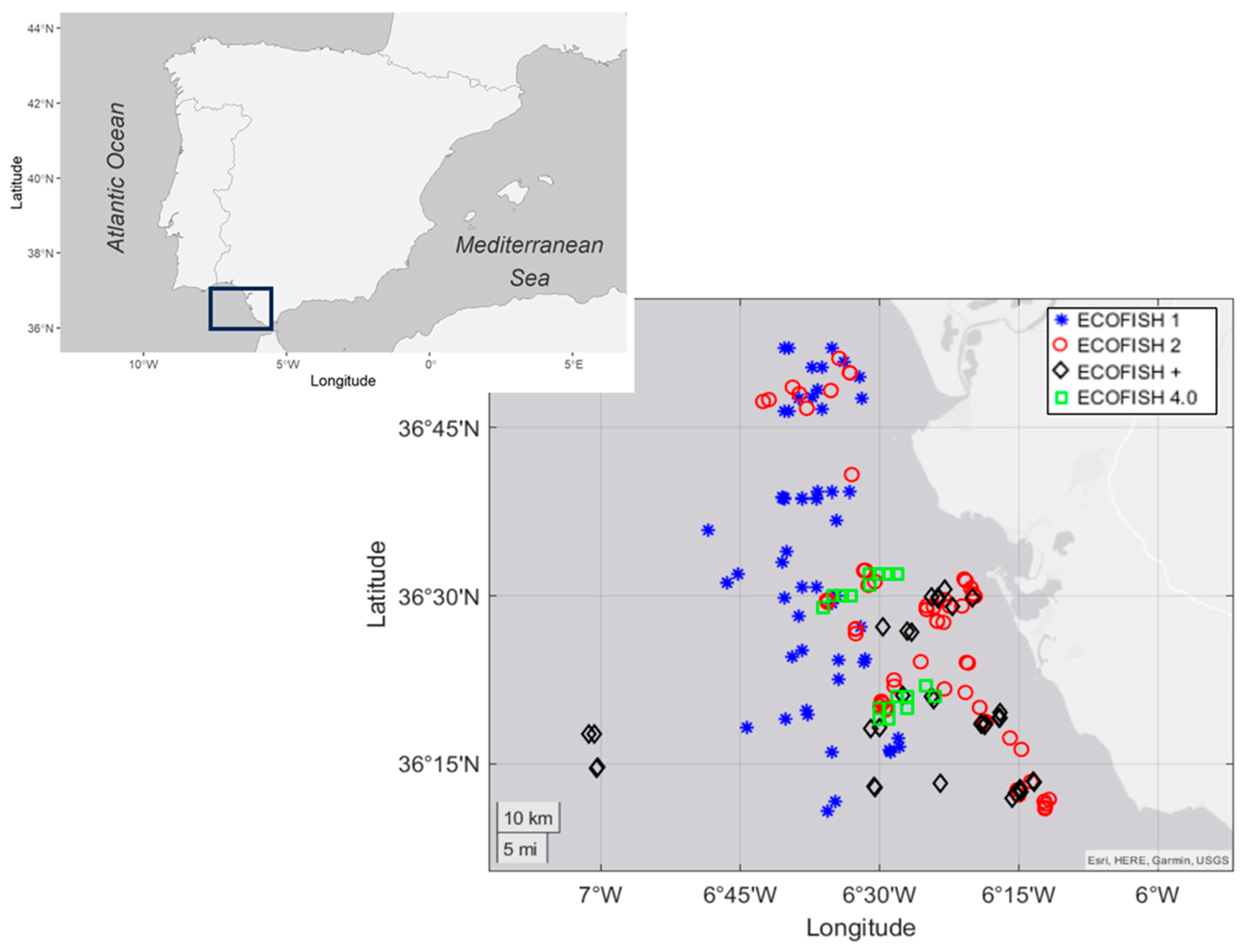
2.1. Study Area and Sampling Details
2.2. Feeding Indices
2.3. Data Analysis
3. Results
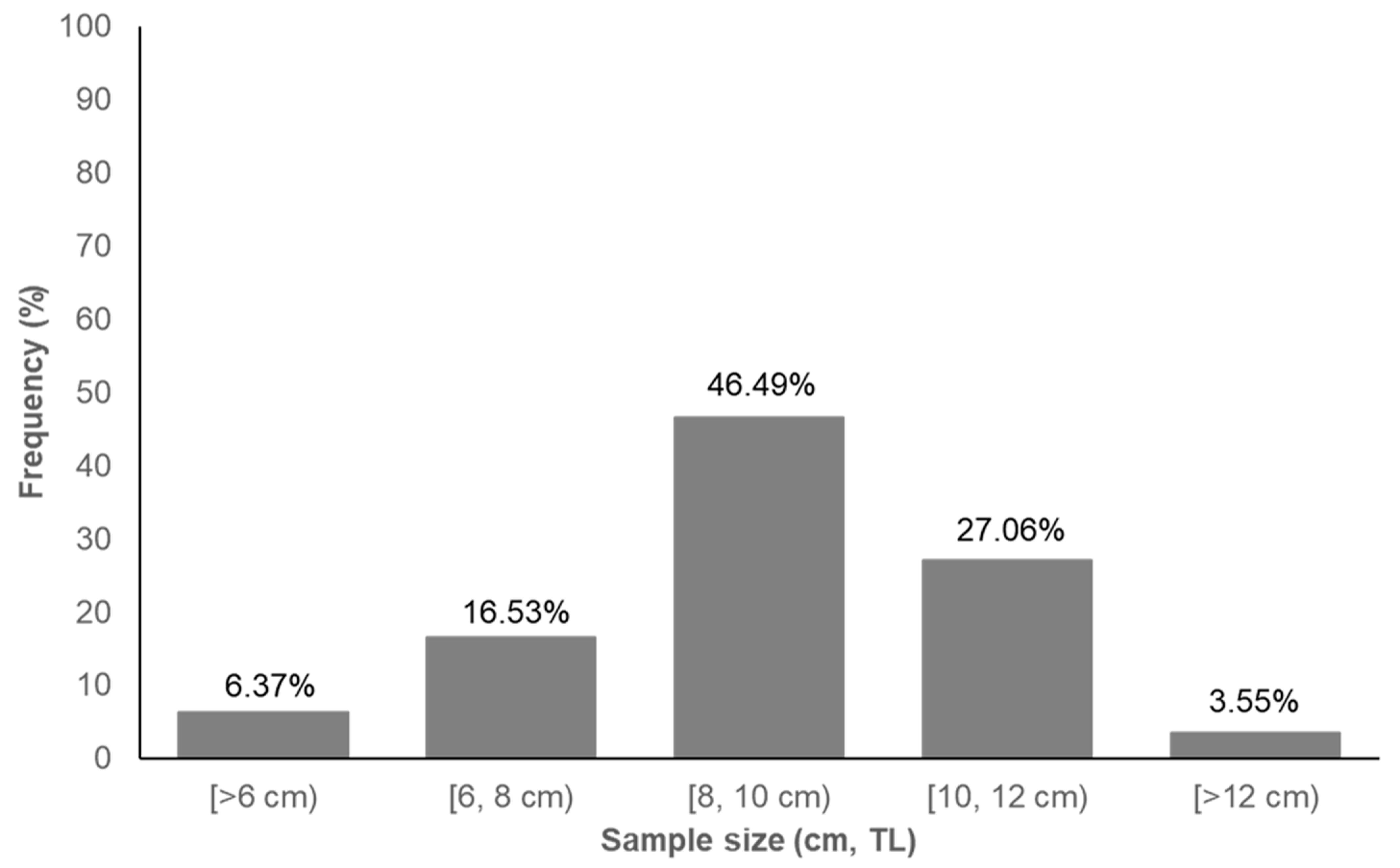
3.1. Fish Abundance and Fish Size Variability
3.2. Prey Content and Feeding Index
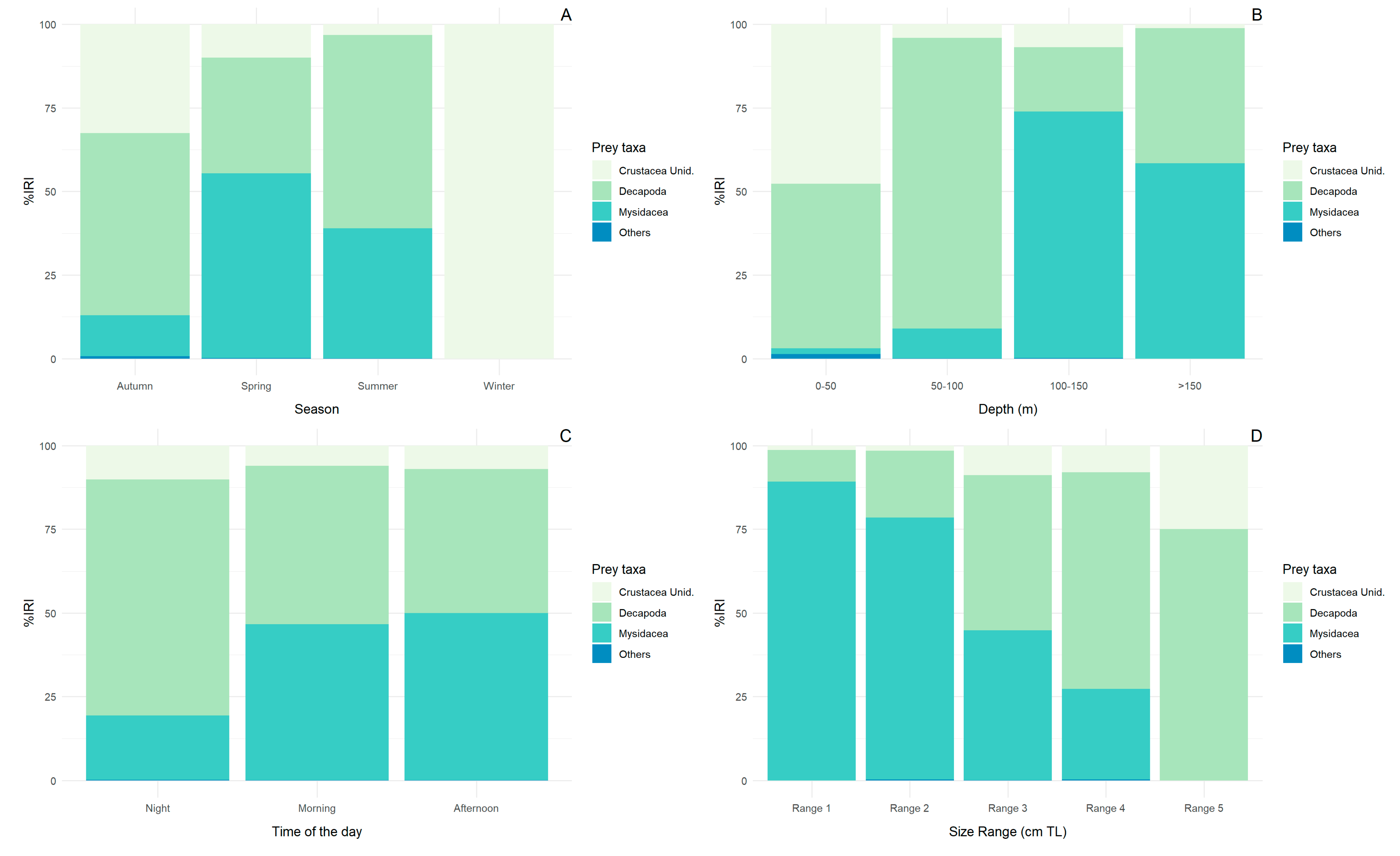
3.3. Seasonal, Depth Range, Time of Day, and Size Diet Variability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jennings, S.; Kaiser, M.J.; Reynolds, J.D. Marine Fisheries Ecology; Blackwell Science: Oxford, UK, 2001; Volume 417. [Google Scholar]
- Ulltang, Ø. Stock assessment and biological knowledge: Can prediction uncertainty be reduced? ICES J. Mar. Sci. 1996, 53, 659–675. [Google Scholar] [CrossRef][Green Version]
- Dulčić, J.; Matić-Skoko, S.; Paladin, A.; Kraljević, M. Age, growth and mortality of brown comber, Serranus hepatus (Linnaeus, 1758) (Pisces: Serranidae), in the eastern Adriatic (Croatian coast). J. Appl. Ichthyol. 2007, 23, 195–197. [Google Scholar] [CrossRef]
- Bilecenoglu, M. Growth and feeding habits of the brown comber, Serranus hepatus (Linnaeus, 1758) in Izmir Bay, Aegean Sea. Acta Adriática 2009, 50, 105–110. [Google Scholar]
- Yapici, S.; Filiz, H.; Ozkan, O. Age, growth, reproduction and feeding habits of brown comber, Serranus hepatus (L., 1758) in eastern Aegean Sea. Biharean Biol. 2012, 6, 99–107. [Google Scholar]
- Smith, C.L. FAO Species Identification Sheets for Fishery Purposes; Eastern Central Atlantic; Fishing Areas 34, 47 (In Part). Fischer, W., Bianchi, G., Scott, W.B., Eds.; Department of Fisheries and Oceans Canada and FAO, Huntsman Marine Laboratory Brandy Cove: St. Andrews, NB, Canada, 1981; Volume 4, pp. 53–54. [Google Scholar]
- Smith, C.L. Check-List of the Fishes of the Eastern Tropical Atlantic (CLOFETA); Quero, J.C., Hureay, J.C., Karrer, C., Post, A., Saldanha, L., Eds.; JNICT/SEI/UNESCO: París, France, 1990; Volume 2, pp. 695–706. [Google Scholar]
- Dalgiç, G.; Gümüş, A.; Zengin, M. First record of brown comber Serranus hepatus (Linnaeus, 1758) for the Black Sea. Turk. J. Zool. 2013, 37, 523–524. [Google Scholar] [CrossRef]
- Bell, J.D.; Harmelin-Vivien, M.L. Fish fauna of French Mediterranean Posidonia oceanica seagrass meadows. Tethys 1983, 11, 1–14. [Google Scholar]
- Tortonese, E. Fishes of the North-Eastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.L., Hureau, J.C., Nielsen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1986; Volume II, pp. 780–792. [Google Scholar]
- Golani, D.; Oztürk, B.; Başusta, N. Fishes of the Eastern Mediterranean; Turkish Marine Research Foundation: Istanbul, Turkey, 2006; Volume 24, 259p. [Google Scholar]
- Despoti, S.; Stergiou, K.I.; Machias, A.; Vassilopoulou, V.; Tsagarakis, K.; Valavanis, V.; Giannoulaki, M. Assessing the spatial distribution of five non-commercial fish species in the Aegean Sea (Greece, eastern Mediterranean Sea) based on discards data. Reg. Stud. Mar. Sci. 2021, 44, 101736. [Google Scholar] [CrossRef]
- D’Ancona, U. Osservazioni sull’organizzazione della gonade ermafrodita di alcuni Serranidi. Nova Thallassia 1949, 1, 3–15. [Google Scholar]
- Reinboth, R. Morphologische und funktionelle Zweigeschlechtlichkeit bei marinen Teleostiern (Serranidae, Sparidae, Centracanthidae, Labridae). Zool. Jahrb. 1962, 69, 405–480. [Google Scholar]
- Labropoulou, M.; Eleftheriou, A. The foraging ecology of two pairs of congeneric demersal fish species: Importance of morphological characteristics in prey selection. J. Fish Biol. 1997, 50, 324–340. [Google Scholar] [CrossRef]
- Labropoulou, M.; Tserpes, G.; Tsimenidis, N. Age, growth and feeding habits of the brown comber Serranus hepatus (Linnaeus, 1758) on the Cretan shelf. Estuar. Coast. Shelf Sci. 1998, 46, 723–732. [Google Scholar] [CrossRef]
- Programa Pleamar. Available online: https://www.programapleamar.es/ecofish-estrategias-para-una-pesqueria-sostenible-en-la-zepa-del-golfo-de-cadiz-avanzando-hacia-una (accessed on 18 October 2023).
- Torres, M.A. Modelización Ecológica del Golfo de Cádiz: Relaciones Tróficas, Análisis de la Estructura de la Comunidad e Impacto de la Pesca en el Ecosistema. Ph.D. Thesis, Universidad de Cádiz, Cádiz, Spain, 2013. [Google Scholar]
- Ferry, L.A.; Cailliet, G.M. Sample size and data analysis: Are we characterizing and comparing diet properly? In GUTSHOP ’96: Feeding Ecology and Nutrition in Fish Symposium Proceedings; Mackinlay, D., Shearer, K., Eds.; American Fisheries Society: Bethesda, MD, USA, 1996; pp. 71–80. [Google Scholar]
- Matić-Skoko, S.; Tutman, P.; Bojanić Varezić, D.; Skaramuca, D.; Đikić, D.; Lisičić, D.; Skaramuca, B. Food preferences of the Mediterranean moray eel, Muraena helena (Pisces: Muraenidae), in the southern Adriatic Sea. Mar. Biol. Res. 2014, 10, 807–815. [Google Scholar] [CrossRef]
- MacKinlay, D.; Shearer, K. Gutshop’96: Feeding Ecology and Nutrition in Fish: Symposium Proceedings; American Fisheries Society: San Francisco, CA, USA, 1996; p. 214. [Google Scholar]
- Scenna, L.B.; García De La Rosa, S.B.; Díaz de Astarloa, J.M. Trophic ecology of the Patagonian skate, Bathyraja macloviana, on the Argentine continental shelf. ICES J. Mar. Sci. 2006, 63, 867–874. [Google Scholar] [CrossRef][Green Version]
- Hayward, P.J.; Ryland, J.S. (Eds.) Handbook of the Marine Fauna of North-West Europe; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Ketchen, K.S. Stratified subsampling for determining age distributions. Trans. Am. Fish. Soc. 1950, 79, 205–212. [Google Scholar] [CrossRef]
- Castro-Gutiérrez, J.; Madera-Santana, S.; Rodríguez-García, C.; Domínguez-Bustos, Á.R.; Sarmiento-Carbajal, J.; Gonçalves Neto, J.B.; Cabrera-Castro, R. Exploring Morphometric Frontiers: A Comprehensive Study of Otolith Growth Patterns in Brown Comber [Serranus hepatus (Linnaeus, 1758)]. J. Fish Biol. 2023, 1–8. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N.; Somerfield, P.J.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 3rd ed.; Primer-E Ltd.: Plymouth, UK, 2014; 256p. [Google Scholar]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 326–349. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analysis of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Warton, D.I.; Wright, T.W.; Wang, Y. Distance-based multivariate analyses confound location and dispersion effects. Methods Ecol. Evol. 2012, 3, 89–101. [Google Scholar] [CrossRef]
- Bowen, S.H. Quantitative description of the diet. In Fisheries Techniques; Nielsen, L.A., Johnson, D.L., Eds.; American Fisheries Society: Bethesda, MD, USA, 1983; pp. 325–336. [Google Scholar]
- Moreno-Lopez, A.; Tuset, V.M.; Gonzalez, J.A.; García-Díaz, M.M. Feeding hábitats of Serranus scriba (osteichthyes: Serranidae) in the marine reserve of Lanzarote (Canary Islands). Bol. Mus. Munic. Funchal 2002, 53, 5–17. [Google Scholar]
- Morato, T.; Serrao, S.; Andrade, J.P. Feeding habits, seasonal and ontogenetic diet shift of blacktail comber, Serranus atricauda (Pisces: Serranidae), from the Azores, north-eastern Atlantic. Fish. Res. 2000, 49, 51–59. [Google Scholar] [CrossRef]
- Putman, R. Community Ecology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Cabral, H.N.; Lopes, M.; Loeper, R. Trophic niche overlap between flatfishes in a nursery area on the Portuguese coast. Sci. Mar. 2002, 66, 293–300. [Google Scholar] [CrossRef]
- Carpentieri, P.; Colloca, F.; Ardizzone, G.D. Day–night variations in the demersal nekton assemblage on the Mediterranean shelf-break. Estuar. Coast. Shelf Sci. 2005, 63, 577–588. [Google Scholar] [CrossRef]
- Iwasa, Y. Vertical Migration of Zooplankton: A Game Between Predator and Prey. Am. Nat. 1982, 120, 171–180. [Google Scholar] [CrossRef]
- Eggers, D.M. Factors in interpreting data obtained by diel sampling of fish stomachs. J. Fish. Response 1977, 34, 290–294. [Google Scholar] [CrossRef]
- Carpentieri, P.; Colloca, F.; Cardinale, M.; Belluscio, A.; Ardizzone, G.D. Feeding habits of European hake (Merluccius merluccius) in the central Mediterranean Sea. Fish. Bull. 2005, 103, 411–416. Available online: http://hdl.handle.net/1834/26234 (accessed on 25 October 2023).

| Prey Category | %F | %N | %W | IRI | %IRI |
|---|---|---|---|---|---|
| Crustaceans | |||||
| Mysidacea (unidentified) | 30.35 | 74.21 | 15.67 | 2728.01 | 71.33 |
| Euphasiacea (unidentified) | 0.39 | 0.81 | 0.34 | 0.45 | 0.012 |
| Amphipoda (unidentified) | 1.69 | 0.53 | 0.14 | 1.13 | 0.030 |
| Copepoda (unidentified) | 0.13 | 0.03 | 0,001 | 0.0041 | 0.0001 |
| Caridea (unidentified) | 12.84 | 3.72 | 19.23 | 294.60 | 7.70 |
| Alpheus glaber | 8.69 | 2.19 | 19.45 | 188.01 | 4.92 |
| Parapenaeus longirostris | 0.13 | 0.03 | 0.52 | 0.07 | 0.0019 |
| Processa canaliculata | 0.78 | 0.22 | 0.74 | 0.75 | 0.020 |
| Plesionika heterocarpus | 0.13 | 0.03 | 0.71 | 0.097 | 0.003 |
| Brachyura (unidentified) | 11.15 | 2.87 | 7.13 | 111.60 | 2.92 |
| Liocarcinus sp. | 0.26 | 0.06 | 0.34 | 0.10 | 0.0027 |
| Goneplax rhomboides | 4.15 | 1.25 | 8.04 | 38.55 | 1.01 |
| Galatheidae | 4.02 | 1.09 | 0.98 | 8.32 | 0.22 |
| Anomura (unidentified) | 0.13 | 0.03 | 0.05 | 0.01 | 0.0003 |
| Thalassinidea (unidentified) | 0.26 | 0.06 | 0.05 | 0.03 | 0.0008 |
| Decapoda (unidentified) | 1.30 | 0.47 | 1.13 | 2.08 | 0.054 |
| Crustacea (unidentified) | 25.42 | 6.40 | 11.16 | 446.44 | 11.67 |
| Insects | |||||
| Diptera (unidentified) | 0.13 | 0.03 | 0.001 | 0.004 | 0.0001 |
| Molluscs | |||||
| Sepiolida | 0.26 | 0.06 | 3.30 | 0.87 | 0.023 |
| Teuthida | 0.13 | 0.03 | 0.53 | 0.07 | 0.0019 |
| Cephalopoda (unidentified) | 0.26 | 0.06 | 0.64 | 0.18 | 0.0047 |
| Teleosts | |||||
| Gobiidae | 0.26 | 0.06 | 1.05 | 0.29 | 0.0075 |
| Fishes (unidentified) | 1.69 | 0.47 | 1.10 | 2.64 | 0.069 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madera-Santana, S.; Rodríguez-García, C.; Castro-Gutiérrez, J.; Domínguez-Bustos, Á.R.; Cabrera-Castro, R. Discarded but Not Dismissed: A Comprehensive Study of the Feeding Habits of the Brown Comber (Serranus hepatus, (Linneaus 1758)) in the Gulf of Cádiz (NE Atlantic). Fishes 2023, 8, 541. https://doi.org/10.3390/fishes8110541
Madera-Santana S, Rodríguez-García C, Castro-Gutiérrez J, Domínguez-Bustos ÁR, Cabrera-Castro R. Discarded but Not Dismissed: A Comprehensive Study of the Feeding Habits of the Brown Comber (Serranus hepatus, (Linneaus 1758)) in the Gulf of Cádiz (NE Atlantic). Fishes. 2023; 8(11):541. https://doi.org/10.3390/fishes8110541
Chicago/Turabian StyleMadera-Santana, Sara, Carlos Rodríguez-García, Jairo Castro-Gutiérrez, Ángel Rafael Domínguez-Bustos, and Remedios Cabrera-Castro. 2023. "Discarded but Not Dismissed: A Comprehensive Study of the Feeding Habits of the Brown Comber (Serranus hepatus, (Linneaus 1758)) in the Gulf of Cádiz (NE Atlantic)" Fishes 8, no. 11: 541. https://doi.org/10.3390/fishes8110541
APA StyleMadera-Santana, S., Rodríguez-García, C., Castro-Gutiérrez, J., Domínguez-Bustos, Á. R., & Cabrera-Castro, R. (2023). Discarded but Not Dismissed: A Comprehensive Study of the Feeding Habits of the Brown Comber (Serranus hepatus, (Linneaus 1758)) in the Gulf of Cádiz (NE Atlantic). Fishes, 8(11), 541. https://doi.org/10.3390/fishes8110541







