Effects of a Diet of Phragmites australis instead of Triticum aestivum L. on Immune Performance and Liver Tissue Structure of Ctenopharyngodon idellus
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Feed
2.2. Feeding Management
2.3. Sample Collection and Processing
2.4. Detection of Serum Biochemical Parameters
2.5. Observation of Liver Tissue Structure
2.6. Detection of Immune Factor Activity and Content
2.7. Detection and Analysis of Immune Gene mRNA Expression Level
2.7.1. RNA Extraction and cDNA First Strand Synthesis
2.7.2. Fluorescence Quantitative PCR Analysis
2.8. Data Analysis
3. Results
3.1. Serum Biochemical Indicators
3.2. Histological Structure of Liver
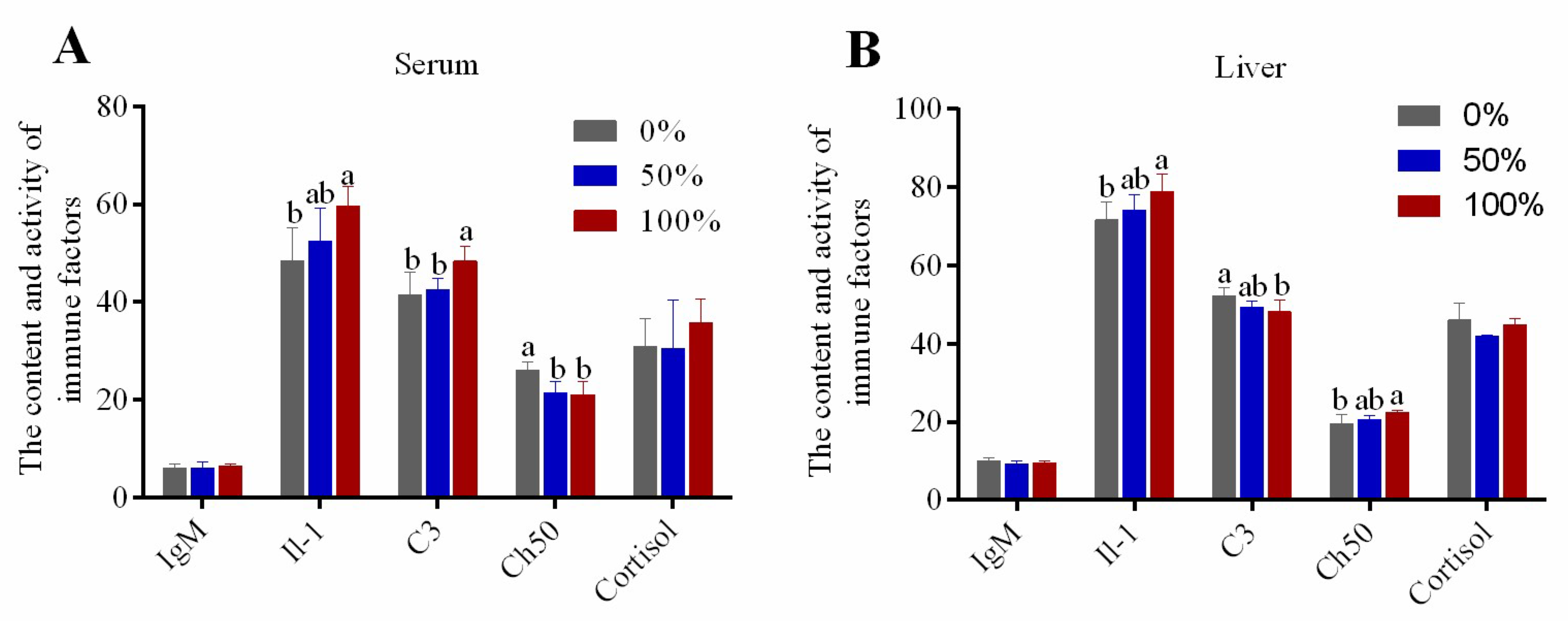
3.3. Activity and Contents of Immune Factors
3.4. The mRNA Expression Level of Immune Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Köbbing, J.F.; Thevs, N.; Zerbe, S. The Utilisation of Reed (Phragmites australis): A Review. Mires Peat 2013, 13, 1–14. [Google Scholar]
- Chen, L.; Zhang, L.; Liu, Z.; Lu, F.; Feng, G.; Yan, K.; Han, G. Physiological and Ecological Responses of Hetan and Chaotan Phragmites australis to Salt Stress. Acta Ecol. Sin. 2020, 40, 2090–2098. [Google Scholar]
- Meneses, N.C.; Baier, S.; Reidelstürz, P.; Geist, J.; Schneider, T. Modelling Heights of Sparse Aquatic Reed (Phragmites australis) Using Structure from Motion Point Clouds Derived from Rotary- And Fixed-Wing Unmanned Aerial Vehicle (Uav) Data. Limnologica 2018, 72, 10–21. [Google Scholar] [CrossRef]
- Minkina, T.; Fedorenko, G.; Nevidomskaya, D.; Fedorenko, A.; Chaplygin, V.; Mandzhieva, S. Morphological and Anatomical Changes of Phragmites australis Cav. Due to the Uptake and Accumulation of Heavy Metals from Polluted Soils. Sci. Total Environ. 2018, 636, 392–401. [Google Scholar] [CrossRef]
- Zhang, S.; Bai, J.; Wang, W.; Huang, L.; Zhang, G.; Wang, D. Heavy Metal Contents and Transfer Capacities of Phragmites australis and Suaeda salsa in the Yellow River Delta, China. Phys. Chem. Earth Parts A/B/C 2018, 104, 3–8. [Google Scholar] [CrossRef]
- Hansson, P.; Fredriksson, H. Use of Summer Harvested Common Reed (Phragmites australis) as Nutrient Source for Organic Crop Production in Sweden. Agric. Ecosyst. Environ. 2004, 102, 365–375. [Google Scholar] [CrossRef]
- Zhou, J.; Wan, R.R.; Wu, X.H.; Zhang, Y. Patterns of Long-Term Distribution of Typical Wetland Vegetation (1987–2016) and its Response to Hydrological Processes in Lake Dongting. J. Lake Sci. 2020, 6, 1723–1735. [Google Scholar]
- Sun, Z.; Mou, X.; Sun, W. Potential Effects of Tidal Flat Variations on Decomposition and Nutrient Dynamics of Phragmites australis, Suaeda salsa and Suaeda glauca Litter in Newly Created Marshes of the Yellow River Estuary, China. Ecol. Eng. 2016, 93, 175–186. [Google Scholar] [CrossRef]
- Sun, Z.; Zhou, D.; Ferreira, L.M.M.; Zhong, Q.; Lou, Y. Diet Composition, Herbage Intake and Digestibility in Inner Mongolian Cashmere Goats Grazing on Native Leymus Chinensis Plant Communities. Livest. Sci. 2008, 116, 146–155. [Google Scholar] [CrossRef]
- Kadi, S.A.; Ouendi, M.; Bannelier, C.; Berchiche, M.; Gidenne, T. Nutritive Value of Sun-Dried Common Reed (Phragmites australis) Leaves and its Effect on Performance and Carcass Characteristics of the Growing Rabbit. World Rabbit. Sci. 2018, 26, 113–121. [Google Scholar] [CrossRef]
- Xiao, T.Y.; Zhou, Z.Y.; Wang, R.H.; Li, Y.G.; Jin, S.Z.; Li, W.; Wang, H.Q. The Expression and Transmission of Immune Factors Between Generations in Maternal Ctenopharyngodon idella After Immunization with Gcrv Attenuated Vaccine. J. Fish. China 2017, 8, 1308–1318. [Google Scholar]
- Chen, L.J.; Yu, E.M.; Wang, G.J.; Jun, X.; Yu, D.G.; Li, Z.F.; Zhang, K.; Gong, W.B.; Tian, J.J. Effects of Feeding with Broad Bean on the Immune Organ Ultrastructure and Expression of Immune Genes of Grass Carp. J. China Agric. Univ. 2019, 6, 92–103. [Google Scholar]
- Zhou, W.; Rahimnejad, S.; Lu, K.; Wang, L.; Liu, W. Effects of Berberine on Growth, Liver Histology, and Expression of Lipid-Related Genes in Blunt Snout Bream (Megalobrama amblycephala) Fed High-Fat Diets. Fish Physiol. Biochem. 2019, 45, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wu, Y.; Yu, H.; Su, Y.; Ren, M.; Zhu, J.; Ge, X. Serum Biochemistry, Liver Histology and Transcriptome Profiling of Bighead Carp Aristichthys Nobilis Following Different Dietary Protein Levels. Fish Shellfish Immunol. 2019, 86, 832–839. [Google Scholar] [CrossRef]
- Jahanbakhshi, A.; Imanpoor, M.R.; Taghizadeh, V.; Shabani, A. Hematological and Serum Biochemical Indices Changes Induced by Replacing Fish Meal with Plant Protein (Sesame Oil Cake and Corn Gluten) in the Great Sturgeon (Huso huso). Comp. Clin. Pathol. 2013, 22, 1087–1092. [Google Scholar] [CrossRef]
- Guo, X.Z.; Liang, X.F.; Fang, L.; Yuan, X.C.; Zhou, Y.; Li, B. Effects of Non-Protein Energy Sources on Serum Biochemical Indices and Histology of Liver in Grass Carp (Ctenopharyngodon idella). Acta Hydrobiol. Sin. 2014, 3, 582–587. [Google Scholar]
- Jiang, D.; Wu, Y.; Huang, D.; Ren, X.; Wang, Y. Effect of Blood Glucose Level on Acute Stress Response of Grass Carp Ctenopharyngodon idella. Fish Physiol. Biochem. 2017, 43, 1433–1442. [Google Scholar] [CrossRef]
- Yang, L.; Hu, Y.; Zhu, B.; Zhong, L.; Wei, Z.H.; Tang, T.; Zhang, J.Z. Effects of Flammulina velutipes Stembase on Growth, Digestive Enzyme Activities, Intestinal Tissue Structure, Muscle Quality and Serum Biochemical Parameters of Grass Carp (Ctenopharyngodon idella). Chin. J. Anim. Nutr. 2021, 8, 4569–4579. [Google Scholar]
- Zhu, B.; Zhong, L.; Zhao, Q.; Dai, Z.; Chen, K.; Yi, H.U. Effects of Flammulina velutipes Medium Residue on Growth Performance, Intestinal Tissue Structure and Serum Biochemical, Immune and Antioxidant Indexes of Grass Carp. Chin. J. Anim. Nutr. 2020, 32, 2921–2929. [Google Scholar]
- Meton, I.; Mediavilla, D.; Caseras, A.; Canto, E.; Fernandez, F.; Baanante, I.V. Effect of Diet Composition and Ration Size on Key Enzyme Activities of Glycolysis-Gluconeogenesis, the Pentose Phosphate Pathway and Amino Acid Metabolism in Liver of Gilthead Sea Bream (Sparus aurata). Br. J. Nutr. 1999, 82, 223–232. [Google Scholar] [CrossRef]
- Peres, H.; Oliva-Teles, A. Effect of the Dietary Essential Amino Acid Pattern on Growth, Feed Utilization and Nitrogen Metabolism of European Sea Bass (Dicentrarchus labrax). Aquaculture 2007, 267, 119–128. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, G.Q.; Zhou, L.; Xu, Z.; Yang, H.; Li, X.Q.; Leng, X.J. Effects of Citric Acid on Growth Performance, Nutrient Utilization, Serum Biochemical Indices and Intestinal Tissue Morphology of Grass Carp (Ctenopharyngodon idella). Chin. J. Anim. Nutr. 2021, 8, 4580–4591. [Google Scholar]
- Chen, Y.; Ronghua, L.U.; Yang, G.; Zhang, Y.; Qin, C.; Hong, J.I.; Nie, G. University, HN, Effects of Replacing Soybean Oil with Black Soldier Fly (Hermetia illucens) Larvae Oil on the Growth Performance, Antioxidant Ability and Intestinal Microbiota of Grass Carp (Ctenopharyngodon idella). J. Fish. China 2019, 10, 2241–2255. [Google Scholar]
- Smith, N.C.; Rise, M.L.; Christian, S.L. A Comparison of the Innate and Adaptive Immune Systems in Cartilaginous Fish, Ray-Finned Fish, and Lobe-Finned Fish. Front. Immunol. 2019, 10, 2292. [Google Scholar] [CrossRef]
- Li, L.; Cardoso, J.C.R.; Félix, R.C.; Mateus, A.P.; Canário, A.V.M.; Power, D.M. Fish Lysozyme Gene Family Evolution and Divergent Function in Early Development. Dev. Comp. Immunol. 2021, 114, 103772. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Xiao, Y.; Xiao, Z.; Liu, T.; Li, J.; Li, P.; Han, F. Lysozymes in Fish. J. Agric. Food Chem. 2021, 50, 15039–15051. [Google Scholar] [CrossRef]
- Demers, N.; Bayne, C. Immediate Increase of Plasma Protein Complement C3 in Response to an Acute Stressor. Fish Shellfish Immunol. 2020, 107, 411–413. [Google Scholar] [CrossRef]
- Langevin, C.; Aleksejeva, E.; Passoni, G.; Palha, N.; Levraud, J.; Boudinot, P. The Antiviral Innate Immune Response in Fish: Evolution and Conservation of the IFN System. J. Mol. Biol. 2013, 425, 4904–4920. [Google Scholar] [CrossRef]
- Workenhe, S.T.; Rise, M.L.; Kibenge, M.J.T.; Kibenge, F.S.B. The Fight Between the Teleost Fish Immune Response and Aquatic Viruses. Mol. Immunol. 2010, 47, 2525–2536. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Guoxiang, F.U.; Dong, J.; Hao, W. Effects of Rutin on Growth Performance, Body Composition, Non-Specific Immunity and Intestinal Digestive Enzyme Activities of Grass Carp (Ctenopharyngodon idella). Chin. J. Anim. Nutr. 2019, 10, 4868–4876. [Google Scholar]
- Catoni, C.; Schaefer, H.M.; Peters, A. Fruit for Health: The Effect of Flavonoids on Humoral Immune Response and Food Selection in a Frugivorous Bird. Funct. Ecol. 2008, 22, 649–654. [Google Scholar] [CrossRef]
- Guardia, T.; Rotelli, A.E.; Juarez, A.O.; Pelzer, L.E. Anti-Inflammatory Properties of Plant Flavonoids. Effects of Rutin, Quercetin and Hesperidin on Adjuvant Arthritis in Rat. Farmaco 2001, 56, 683–687. [Google Scholar] [CrossRef] [PubMed]


| Items | Moisture | Crude Protein | Ash | Crude Fiber | Crude Fat | Gross Energy (MJ/kg) |
|---|---|---|---|---|---|---|
| Common reed | 10.93 | 14.16 | 6.43 | 39.27 | 1.6 | 17.73 |
| Wheat | 10.61 | 14.18 | 6.61 | 1.88 | 1.7 | 18.24 |
| Items | Diets | ||
|---|---|---|---|
| 0 | 50% | 100% | |
| Ingredients | |||
| Wheat | 30 | 15 | 0 |
| Common reed | 0 | 15 | 30 |
| Soybean meal | 30 | 30 | 30 |
| Fish meal | 3 | 3 | 3 |
| Rapeseed meal | 26 | 26 | 26 |
| Ca(H2PO4)2 | 2.5 | 2.5 | 2.5 |
| Carboxymethyl Cellulose | 2 | 2 | 2 |
| Choline chloride | 0.15 | 0.15 | 0.15 |
| Bentonite clay | 1.35 | 1.35 | 1.35 |
| Vitamin premix 1 | 1 | 1 | 1 |
| Mineral premix 2 | 1 | 1 | 1 |
| Soybean oil | 3 | 3 | 3 |
| Total | 100 | 100 | 100 |
| Nutrient levels 3 | |||
| Crude protein | 32.61 | 33.10 | 33.00 |
| Crude fat | 4.82 | 4.60 | 4.77 |
| Crude fiber | 4.84 | 9.24 | 13.87 |
| Crude ash | 9.95 | 11.20 | 12.45 |
| Gross energy (kJ/g) | 19.96 | 19.86 | 19.84 |
| Primers | Primer Sequence (5′-3′) | References |
|---|---|---|
| IgM-F | TGGTCATCAGGTGGCAAA | [11] |
| IgM-R | GCGGCTGTCTTCCATTCT | |
| lysozyme-F | TTCGACAGCAAAACAGGACAAC | [11] |
| lysozyme-R | GATATGATGGCAGCAATCACAGC | |
| C3-F | AATACGCCATTCCTGAGGTTTCC | [11] |
| C3-R | CTTCCACCATTTCACTGCCACTT | |
| IL-1-F | TACCGAGTCGGATGGTTCTTC | [12] |
| IL-1-R | TGTTATTAGCCACACCGGTCTC | |
| IFN-I-F | CGGCCGATACAGGATGATAAG | [12] |
| IFN-I-R | TCCTCCACCTTGGCATTGTC | |
| MHC-I-F | CCTGCTAATCCTCAAGCTGTCA | [12] |
| MHC-I-R | GCATGACACGTCACTGGAGAG | |
| Hsp70-F | GTGTCCATCCTGACCATTGA | [12] |
| Hsp70-R | ATCTGGATTGATGCTCTTGTT | |
| Actin-F | GCTATGTGGCTCTTGACTTCG | [12] |
| Actin-R | GGGCACCTGAACCTCTCATT |
| Items | 0% | 50% | 100% |
|---|---|---|---|
| TP (g/L) | 32.97 ± 3.29 | 36.50 ± 1.87 | 34.57 ± 0.35 |
| ALB (g/L) | 15.73 ± 1.11 | 17.00 ± 1.04 | 15.70 ± 0.40 |
| GLB/(g/L) | 17.23 ± 2.18 | 19.50 ± 0.90 | 18.87 ± 0.64 |
| GLU (mmol/L) | 9.85 ± 0.81 a | 6.35 ± 0.56 b | 7.68 ± 1.02 ab |
| ALT/(U/L) | 22.35 ± 2.05 c | 64.60 ± 8.34 b | 98.05 ± 1.48 a |
| AST/(U/L) | 47.00 ± 3.11 b | 84.10 ± 17.25 a | 51.45 ± 0.92 b |
| Items | 0% | 50% | 100% |
|---|---|---|---|
| The area ratio of hepatocyte vacuolization (%) 1 | 13.26 ± 1.9 a | 3.37 ± 0.52 b | 2.69 ± 0.67 b |
| The number of hepatocyte vacuolization | 74.33 ± 13.01 a | 22.33 ± 2.52 b | 32.33 ± 2.52 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Lei, C.; Li, Z.; Lei, Y.; Luo, C.; Shao, L.; Huang, C.; Yang, P. Effects of a Diet of Phragmites australis instead of Triticum aestivum L. on Immune Performance and Liver Tissue Structure of Ctenopharyngodon idellus. Fishes 2022, 7, 378. https://doi.org/10.3390/fishes7060378
Wang R, Lei C, Li Z, Lei Y, Luo C, Shao L, Huang C, Yang P. Effects of a Diet of Phragmites australis instead of Triticum aestivum L. on Immune Performance and Liver Tissue Structure of Ctenopharyngodon idellus. Fishes. 2022; 7(6):378. https://doi.org/10.3390/fishes7060378
Chicago/Turabian StyleWang, Ronghua, Chaobo Lei, Zhenyu Li, Yanju Lei, Congqiang Luo, Liye Shao, Chunhong Huang, and Pinhong Yang. 2022. "Effects of a Diet of Phragmites australis instead of Triticum aestivum L. on Immune Performance and Liver Tissue Structure of Ctenopharyngodon idellus" Fishes 7, no. 6: 378. https://doi.org/10.3390/fishes7060378
APA StyleWang, R., Lei, C., Li, Z., Lei, Y., Luo, C., Shao, L., Huang, C., & Yang, P. (2022). Effects of a Diet of Phragmites australis instead of Triticum aestivum L. on Immune Performance and Liver Tissue Structure of Ctenopharyngodon idellus. Fishes, 7(6), 378. https://doi.org/10.3390/fishes7060378