Abstract
The current study addresses the influence of Moringa oleifera leaves nanoparticles (MO-NPs) on growth, biochemical, immunological, and hepatic antioxidant alterations induced by zinc oxide nanoparticles toxicity in Nile tilapia (O. niloticus). Fish (N = 180) were divided into four groups with replicates. The first one was set as a control group and the second group was fed an MO-NPs-enriched diet (2.5 g/kg diet). The third group was exposed to 8 mg/L ZnO-NPs, while the forth group was exposed to 8 mg/L ZnO-NPs and fed on MO-NPs (2.5 g/kg diet) for 2 months. Exposure of O. niloticus to 8 mg/L ZnO-NPs induced the following consequences: a sharp decrease in the growth parameters; a marked increment in the biochemical biomarkers (glucose, cortisol, and liver enzymes (ALT, AST, ALP); a significant increase in serum renal products, urea and creatinine, cholesterol, and LDH levels. Nonetheless, the dietary MO-NPs supplementation for 2 months significantly alleviated the ZnO-NPs toxicity and significantly enhanced the growth indices, plus normalizing the physio-biochemical levels in the exposed group to ZnO-NPs toxicity to reach the levels of the control group. The MO-NPs markedly improved hepatic antioxidant biomarkers, MDA, and TAC, while, decreasing SOD, CAT, and GSH levels to be near the control values. Moreover, supplemented fish in MO-NPs (2.5 g/kg diet) and exposed to ZnO-NPs provided a remarkable increase in the immune profile (respiratory burst (RB) activity, lysozyme, and total immunoglobulins (IgM)) compared to the ZnO-NPs-intoxicated group. Based on the findings of the study, the exposed O. niloticus to ZnO-NPs were immune-antioxidant-depressed, besides showing growth retardation, and physio-biochemical alterations. On the other hand, a supplemented diet with MO-NPs is a novel approach to ameliorate ZnO-NPs toxicity for sustaining aquaculture and correspondingly protecting human health.
1. Introduction
Fish is a prime source of high-quality protein, vitamins, and other substantial elements like selenium and iodine, which is not found in meat or other crops [1]. Focusing on cultured fish species worldwide, they are mostly exposed to various toxicants mixed in their aquatic environment owing to intensive aquaculture [2,3], including toxicity with nanomaterials [4]. In spite of the wide and safe applications of nanoparticles [5], even in the aquaculture industry as a dietary and an aqueous supplement [6,7,8,9], their toxicity commonly occurs in fish [10]. Among NPs, ZnO-NPs are the third most produced ones following TiO2 and SiO2 [11]. The major sources for the entrance of NPs into the aquatic environment include unintentional sources, anthropogenic sources, and the usage of engineered nanoparticles causing adverse effects on all aquatic organisms [12]. The toxicity by ZnO-NPs can induce bioaccumulation in fish tissues, oxidative stress, and sub-acute toxicity in Cyprinus carpio [13,14,15,16]. Moreover, they induce oxidative damage in zebrafish and O. niloticus [17,18], and hepatic and gill damage in Oreochromis mossambicus [19]. Therefore, bioaccumulation of ZnO-NPs in fish fillets induces direct toxicity to all fish consumers, mainly human.
Plant extracts achieve a successful way in the aquaculture industry as dietary supplements in promoting growth and immunity, and against NPs toxicity [20]. Specifically, the plant-based NPs are characterized by their porous nature and easy penetration efficacy to fish cells boosting the immune-antioxidant status [21,22]. Among all herbal plants, Moringa oleifera (MO) belongs to the family of Moringaceae, native to India, and it is widely distributed worldwide, especially in African and Asian countries [23,24]. It is commonly known as drumstick tree or horseradish tree (Gopalakrishnan et al., 2016). Every part of M. oleifera is a storehouse of substantial nutrients. The extract of MO leaves are rich in potassium; iron; phosphorous; vitamins A, C, E, and D; flavonoids; ascorbic acid; β-carotene; oxidase; and catalase [25,26,27]. Additionally, they provide 17 times more calcium than milk and are utilized to treat malnutrition. About six spoonful’s of leaf powder can meet a woman’s daily iron and calcium requirements during pregnancy [27]. The pods are fibrous and are valuable for the therapy of digestive problems and for thwarting colon cancer [28]. In addition, MO has long been used in herbal medicine by Indians and Africans and can be used to cure more than 300 diseases [28]. The presence of phytochemicals in MO leaves makes them a good medicinal agent. MO has an effect on diabetes, where MO scavenges the ROS released from mitochondria, thereby protecting the beta cells and in turn controlling hyperglycemia [29,30].
Moringa with its antioxidants can lessen the reactive oxygen species, thereby protecting the brain from cerebral ischemia [31,32]. MO is used to treat dementia, as it has been shown to be a promoter of spatial memory. Additionally, MO can be used as an anti-neoproliferative agent, thereby inhibiting the growth of cancer cells. Therefore, it can be used as an anticancer agent. MO powder can be used as a substitute for iron tablets, hence as a treatment for anemia [27].
Other uses of MO leaves in medicine are that leaves are utilized for the therapy of various diseases as it has many privileges such as hypotensive, antibiotic, hypocholesterolemic, and anti-inflammatory [33,34]. Previous studies indicate that MO has a hepatoprotective effect [26,35] and has a potent immune-antioxidant activity to protect against toxicity in O. niloticus [36]. Thus far, just a few studies have assessed the potential effect of neutraceuticals against ZnO-NPs toxicity [22,37]. Therefore, the current study is a new pioneer trial to assess the promoting effect of plant (moringa leaves)-based NPs against the toxicological impacts induced by ZnO-NPs plus their promising influence on growth, physiological, immunological, hepato-renal, and antioxidant response in O. niloticus.
2. Materials and Methods
2.1. Chemicals
Zinc oxide was purchased from Lab Grade—Sigma Company. It was used to synthesize zinc oxide nanoparticles (ZnO-NPs) at the Faculty of Nanotechnology, Cairo University, Egypt, according to [38]. Fresh leaves from the Moringa oleifera (MO) plant were picked up from trees grown on sandy soil in El-Sharkia Governorate, Egypt. The collected leaves were purified, washed with distilled water, and dried at room temperature for 3 weeks, after which the leaves were powdered into a coarse form.
Biochemical kits for cortisol, glucose, lactate dehydrogenase (LDH), total proteins, albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), cholesterol, urea, creatinine, malondialdehyde (MDA), total antioxidant capacity (TAC), superoxide dismutase (SOD), reduced glutathione (GSH), and catalase (CAT) were purchased from Bio-Diagnostic Co. (Cairo, Egypt).
2.2. Synthesis of ZnO Nanoparticles (ZnO-NPs)
ZnO-NPs were synthesized at the Faculty of Nanotechnology, Cairo University, Egypt, using the chemical precipitation method beneath the effect of ultrasound irradiation. In a normal process, zinc acetate dihydrate (Zn (CH3COO)2•2H2O, Loba Chemie, Mumbai, India) as a precursor, and an ammonia (NH3) solution of 30–33% in an aqueous solution (NH4OH, Advent chembio, Mumbai, India) as a reducing agent, had been used. The ZnO-NPs were produced by means of dissolving the right amount of zinc acetate in 100 mL of deionized water to supply 0.1 M of zinc ions. Subsequently, the zinc ions solution was subjected to ultrasonic wave irradiation using a Hielscher UP400S (four hundred W, 24 kHz, Berlin, Germany) at an amplitude of 79% and a cycle of 0.76 for 5 min at a temperature 40 °C [38].
2.3. Synthesis of Moringa oleifera Leaves Nanoparticles
Fresh Moringa oleifera (MO) leaves were harvested from trees grown on sandy soil, from El-Sharkia Governorate, Egypt. Then, the collected leaves were purified, washed with distilled water, and air-dried for 4 weeks to remove residual debris. After that, the dried leaves were powdered into a coarse form as described by Hamed and El-Sayed [26]. Then, MO leaves were synthesized into MO leaves nanoparticles (MO-NPs) by a bottom-up process for the synthesis of nanoparticles using the ball mill method. In a typical synthesis, 5 g of MO presenting a micron size was put in a stainless steel dram of ball mill instrument (photon ball mill model of 210 s, Egypt) with ceramics balls of 0.1 cm diameter and 30 g weight.
2.4. Transmission Electron Microscopy (TEM)
For TEM analysis, synthetic nanoparticles of ZnO and MO were added to deionized water and sonication for 15 min using ultrasound prop with a 50 kHz, at the amplitude of 50% and 0.59 of a cycle (Up 400s, Hielscher, Germany). Then, 10 microns of desperation were put onto a carbon-coated copper grid (CCG). Electron micrographs were taken using the JEOL model of the GEM-1010 transmission electron microscope at 70 kV.
2.5. Fish Maintenance
A total number of 180 healthy Nile tilapia, Oreochromis niloticus were obtained from the Central Laboratory of Abbassa Fish Farm, Egypt, with an average body weight of 70 ± 5 g. Fish were kept in 100 L glass aquaria containing de-chlorinated tap water under laboratory circumstances (conductivity 2000 L/cm; pH 6.5; oxygen 88–95% saturation; water temperature 25–26 °C; total hardness 150 mg/L as CaCO3; photoperiod 12:12 light/dark) for 2 weeks for acclimatization. Fish received a commercial pellet diet (3% of body weight per day) and were transferred to a fresh volume of water daily.
2.6. Experimental Design
Nile tilapia (N = 180) were allocated into four equal groups, each group with three replicates of 15 fish per aquarium, and fed on a commercial pellet diet (3% of body weight), which was given to fish up to satiation twice a day at 08:00 and 13:00 for 2 months. The 1st group was set as the control group. The 2nd group was fed on MO-NPs (2.5 g/kg diet). In the 3rd group, the ZnO-NPs were added to the water at the concentration of 8 mg/L for 2 months and this concentration was chosen according to Goda et al. [39]. The 4th group was exposed to 8 mg/L ZnO-NPs and fed on MO-NPs (2.5 g/kg diet) for 2 months. At the end of the experiment, seven fish per group were collected and anesthetized with 0.02% (v/v) benzocaine solution. Blood samples were collected from the caudal vessels of fish through two parts. The first part of the samples was allowed to clot in clean dry centrifuge tubes at room temperature and then, centrifuged at 3000× g at 4 °C for 15 min. Fish blood serum was collected for biochemical analysis. However, the second part of the blood samples was collected using a 1 mL heparinized syringe for the determination of respiratory burst activity. After that, fish were dissected and liver samples were collected for antioxidant assay.
2.7. Growth Performance Assessment
At the end of the experiment, all fish in each aquarium were counted and weighed, and the calculations were done as follows:
where W1 and W2 are the initial and final weights, and T is the experimental period (months).
Weight gain = W2 − W1
Specific growth rate (%/day) = 100 (Ln W2 − Ln W1)/T
Feed intake (g feed/fish) = Total feed consumed/fish number/aquarium
Feed conversion ratio (FCR) = Feed intake/fish weight gain.
Fish survival (%) = 100 (number of fish at the end/number of fish at the beginning)
2.8. Biochemical Assays
Serum ALP was assayed following the colorimetric enzymatic method of Tietz et al. [40]. Both AST and ALT activities were evaluated in accordance with the protocol discussed by Reitman and Frankel [41]. Cholesterol estimation was performed following the method described by Allain et al. [42]. LDH was assayed following the protocol registered by Vassault [43]. Creatinine was evaluated following the method described by Larsen [44]. Urea was assayed following Henry et al. [45]. Serum glucose and cortisol levels were measured using the protocols mentioned by Trinder [46] and Foster and Dunn [47], respectively. Serum total protein and albumin were assayed following the steps designated by Lowry et al. [48] and Doumas et al. [49], respectively. The globulin content of the same sample was counted by subtracting the value of albumin from the value of total protein.
2.9. Oxidative Stress Biomarkers in Hepatic Tissues
Liver tissue samples were homogenized in cold phosphate-buffered saline (pH 7.4, 0.1 M) with a glass/Teflon homogenizer, and then centrifuged at 3000× g at 4 °C for 15 min. After centrifugation, supernatants were collected and preserved at 20 °C until analysis. Malondialdehyde (MDA) content was assayed following Mihara and Uchiyama [50]. Superoxide dismutase (SOD) and catalase (CAT) were measured following Nishikimi et al. [51] and Aebi [52], respectively. Reduced glutathione (GSH) and total antioxidant capacity (TAC) values were evaluated after the procedures recorded by Beutler et al. [53], and Koracevic et al. [54], respectively.
2.10. Immunological Assay
Respiratory burst (RB) activity was estimated through Nitroblue Tetrazolium dye following Secombes [55], as mentioned by Abdel-Tawwab et al. [56]. Lysozyme (LYZ) was assayed through the turbidimetric method by using Micrococcus luteus as the substrate in phosphate buffer (pH, 6.2) following Ellis [57]. Total immunoglobulin (IgM) was estimated using the precipitation of polyethylene glycol of IgM, and subtraction of the values of the initial and final total protein following Siwicki and Anderson [58].
2.11. Statistical Analysis
The Kolmogorov–Smirnov test was used to test the normality of distribution of all data and the different treatments’ homogeneity of variances was assessed through the Bartlett method. Then, the one-way ANOVA data was used to assess the effects of ZnO-NPs toxicity and dietary MO leaves nanoparticles. Differences between means were checked at the 5% probability level using Duncan’s test. All the statistical analyses were carried out using the SPSS program (version 20; SPSS, Richmond, VA, USA), as described by Dytham [59].
3. Results
3.1. TEM Analysis
TEM images of ZnO and MO nanoparticles illustrate that the particles are spherical to sub-spherical in shape with clear edges without any agglomeration (Figure 1 and Figure 2).
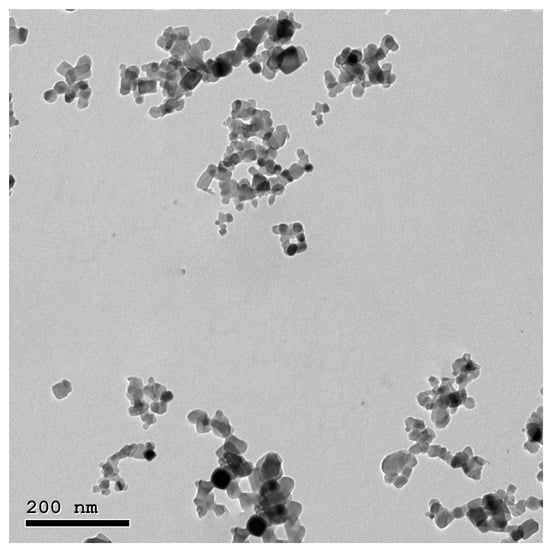
Figure 1.
TEM image of ZnO-NPs illustrates the spherical and sub-spherical shapes with clear edges without any agglomeration.
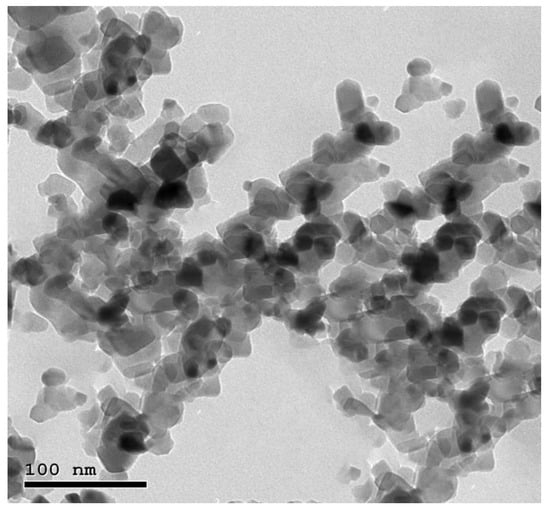
Figure 2.
TEM image of MO-NPs illustrates the spherical in shape with clear edges without any agglomeration.
3.2. Growth Parameters
Dietary MO leaves nanoparticles and ZnO-NPs exposure markedly influenced the growth parameters; however, their interaction was not affected (Table 1; p < 0.05). It was noticed that supplementing diets with MO leaves nanoparticles induced significant (p < 0.05) increments in comparison with the control group; meanwhile, ZnO-NPs exposure led to marked retardation in FBW, WG%, and SGR (3rd group). Dietary MO leaves nanoparticles significantly alleviated the ZnO-NPs toxicity and significantly enhanced the growth indices. A significant increase in feed intake was recorded with dietary MO leaves nanoparticles; meanwhile, exposing tilapia fish to ZnO-NPs toxicity significantly (p < 0.05) depressed the feed intake (Table 1). However, feeding tilapia fish with MO leaves nanoparticles along with ZnO-NPs exposure (4th group) significantly improved the feed intake compared to ZnO-NPs-exposed fish (3rd group).

Table 1.
Growth and feed utilization indices (mean ± SD) of Nile tilapia, O. niloticus exposed to ZnO-NPs with MO leaves nanoparticles administration for 2 months.
3.3. Biochemical Analyses
Exposure of tilapia fish to 8 mg/L ZnO-NPs caused marked (p < 0.05) increments in levels of serum glucose, cortisol, and liver enzymes (ALT, AST, ALP) compared to the control group (Table 2). Serum renal products, urea and creatinine, cholesterol, and LDH levels were also increased (p < 0.05) significantly in ZnO-NPs-exposed fish (Table 3). On the other hand, significant (p < 0.05) declines in serum total protein, albumin, and globulin were observed compared to the control tilapia (Table 4). In contrast, feeding tilapia fish intoxicated with ZnO-NPs on a 2.5 g/kg diet of MO leaves nanoparticles for 2 months normalized the levels of the ZnO-NPs profile to be near the levels of the control group. Nile tilapia-administrated MO leaves nanoparticles exhibited no significant difference in the tested biochemical parameters compared to the control.

Table 2.
Changes in liver enzymes and stress parameters (mean ± SD) of Nile tilapia, O. niloticus exposed to ZnO-NPs with MO leaves nanoparticles administration for 2 months.

Table 3.
Changes in kidney products and lipid profile (mean ± SD) of Nile tilapia, O. niloticus exposed to ZnO-NPs with MO leaves nanoparticles administration for 2 months.

Table 4.
Changes in total proteins (mean ± SD) of Nile tilapia, O. niloticus exposed to ZnO-NPs with MO leaves nanoparticles administration for 2 months.
3.4. Liver MDA and Antioxidant Biomarkers
Nile catfish exposed to 8 mg/L ZnO-NPs exhibited a significant (p < 0.05) increase in hepatic MDA, and TAC as presented in Table 5. And markedly decreased (p < 0.05) levels of SOD, CAT, and GSH activities of the same tissue compared to the control fish (Table 5). Co-administration of tilapia fish with MO leaves nanoparticles (2nd group) showed no significant difference in hepatic antioxidant biomarkers compared to the control group (1st group). Combined treatment with ZnO-NPs, and MO leaves nanoparticles (2.5 g/kg) resulted in a significant improvement in hepatic antioxidant biomarkers to be near the control values (Table 5).

Table 5.
Changes in liver antioxidant enzymes and malondialdehyde (MDA) levels (mean ± SD) of Nile tilapia, O. niloticus, exposed to ZnO-NPs with MO leaves nanoparticles administration for 2 months.
3.5. Immunological Assay
Data presented in Table 6 revealed that ZnO-NPs exposed fish (3rd group) experienced marked (p < 0.05) reductions in the levels of respiratory burst (RB) activity, lysozyme, and total immunoglobulins (IgM) compared to the controls. In the contrary, combined treatment with ZnO-NPs and MO leaves nanoparticles (2.5 g/kg diet) (4th group) provided a remarkable increase in the immune profile compared to ZnO-NPs-intoxicated group (Table 6).

Table 6.
Changes in immunological parameters (mean ± SD) of Nile tilapia, O. niloticus exposed to ZnO-NPs with MO leaves nanoparticles administration for 2 months.
4. Discussion
Nanoparticles toxicity is considered a major challenge for the aquaculture sector. In this regard, the application of neutraceuticals and plant-based NPs has been increasing and represents a significant tool in reaching the goal of sustainable fish health and alleviating toxicity worldwide [60,61,62] Recent studies utilize different herbals to mitigate the induced toxicity [63,64,65,66,67,68]. Herein, we are the first report to test the antitoxic role of MO-NPs to antagonize ZnO-NPs toxicity and to evaluate its promising role as a growth-promoter, an antioxidant-immune supplement, and to enhance fish physiological response.
Blood biomarkers are the key bio-monitoring tools to investigate fish performance and judge the impact of nanoparticles toxicity [63,69]. Herein, we recorded altered hematological profiles in response to ZnO-NPs toxicity. Similar outcomes were obtained by Shahzad et al. [19] that observed the occurrence of DNA damage on erythrocytes of Tilapia fish at a high dose of ZnO-NPs exposure. On the other hand, an enriched diet in MO-NPs retrieved the altered values and caused an elevated level of RBCs reflecting potent antioxidant activity and protection for cell membranes. These outcomes were supported by Hamed and El-Sayed [26] and Sarkar et al. [70] that recorded that the MO protects the RBCs membrane and strengthens its stability. Moreover, Guyton and Hall [71] mentioned that MO contains iron, which is one of the elements for the synthesis of hemoglobin. Concurrent with a recent study, Ibrahim et al. [36] verified that diet rich in MO seeds supplementation improved the blood parameters of O. niloticus upon exposure to chlorpyrifos toxicity. Proteins are included in cell architecture and play a substantial role in cell metabolism in fish. The elevated protein content was clear in the MO-NPs-supplemented diet, which reflected better utilization of protein and consequently can enhance growth performance as reported by Glencross et al. [72].
Hepatic enzymes (ALT and AST) are essential enzymes to indicate fish toxicity with NPs [73]. The exposure to ZnO-NPs elevated the key transaminase enzymes, ALT, and AST activity in the blood, reflecting oxidative damage and consequently, hepatotoxicity. These results were confirmed by elevating the antioxidant parameters. These outcomes were in line with a previous report that found elevated levels of hepatic enzymes in C. carpio upon exposure to ZnO-NPs toxicity [74]. Nevertheless, fish that received an enriched diet in MO-NPs showed a retrieve in the altered parameters. This could be dominated by the action of kaempferol and quercetin, major components of MO, which enhanced liver function [75]. A similar study by Ibrahim et al. [36] detected amelioration in hepatic enzymes upon the dietary intervention of O. niloticus with MO seeds and exposure to chlorpyrifos toxicity.
Based on the findings of the antioxidant assays, it was clear that the group exposed to ZnO-NPs demonstrated a sign of oxidative damage represented in lessening the activity of antioxidant indices (SOD, CAT, GSH, and TAC). This outcome was supported by previous studies that verified that ZnO-NPs were able to induce the production of reactive oxygen species (ROS) [76,77]. The formation of ROS triggers an oxidative damage response because of the direct impact of nanoparticles on cells [78,79], and the excess production of ROS can produce inflammatory and cytotoxic effects [80], and leads to apoptosis [81]. Furthermore, Vimercati et al. [82] elucidated that the mechanism of oxidative stress induced by ZnO-NPs toxicity in freshwater fish is via the generation of ROS, and then damage to proteins, lipids, and DNA, causing cell death. Likewise, Shahzad et al. [19] revealed the occurrence of oxidative stress upon exposure to ZnO-NPs represented in elevated levels of LPO, CAT, GSH, and SOD in the gills and liver of O. mossambicus. Moreover, Mahboub et al. [6] reported a state of oxidative damage following exposure of African catfish to ZnO-NPs as represented by a clear increase in oxidative stress parameters (GST, CAT, and GPx). On the other hand, an enriched diet with MO-NPs notably promised the fish antioxidant response via elevating their values reflecting a potent antioxidant action. This could be dominated by the richness of MO in various antioxidants, such as β-carotene, α-andɣ-tocopherol, vitamin A, and β-sitosterol, plus the presence of phenolic constituents, including flavonoids, quercetin, kaempferol, and anthocyanins, as reported by Hamed and El-Sayed [26] and Sánchez-Machado et al. [83]. Additionally, Siddhuraju and Becker [84] documented that MO is rich in vitamin C, which is characterized by many antioxidant properties via its ability to form poorly ionized but soluble complexes with toxic metals. In the same manner, Hamed and El-Sayed [26] and Ibrahim et al. [36] found a strong antioxidant response in O. niloticus exerted by MO upon exposure to pendimethalin and chlorpyrifos toxicity, respectively. Furthermore, a recent study by Hamed and Abdel Tawwab [61] utilized a natural extract, pomegranate peel, to mitigate the oxidative stress induced by silver nanoparticles toxicity in O. niloticus.
In the current experiment, the exposure to ZnO-NPs toxicity induced a clear alteration in the immune profile via lessening the measured immune parameters (RBA, lysozyme, and IgM). A recent study by Rashidian et al. [15] supported our findings, as it recorded that exposure to ZnO-NPs toxicity altered the key immunological molecules in both the serum and skin of C. carpio. On the other hand, the enriched diet in MO-NPs significantly augmented concentrations of immune biomarkers. A such enhanced immune response could be returned to modifications of cell membranes because of an enriched amount of saturated fatty acids (palmitic and stearic acid) and monounsaturated fatty acids (oleic acid) in MO, as mentioned by DePablo and De Cienfuegos [85]. Another study supported our findings and recorded that MO is rich in vitamins A, C, and K, which strengthen the immune response by stimulating the production of immunoglobulin [23]. Moreover, the presence of amino acids in MO is essential for the formation of immunoglobulin [86]. Concurrently, Ibrahim et al. [36] found enhanced immunity of O. niloticus via elevating levels of lysozyme and IgM, following dietary supplement with MO seeds and exposure to chlorpyrifos toxicity.
Taken together, we highlighted for the first time the protective role of dietary MO-NPs as an antidotal natural supplement to combat fish alterations on growth, biochemical, physiological, hepato-renal, and immune-antioxidant induced by ZnO-NPs toxicity in O. niloticus.
5. Conclusions
The current study demonstrated that ZnO-NPs exposure induces growth suppression, hepato-renal toxicity, protein depletion, immunotoxicity, and oxidative injury in O. niloticus. Dietary MO-NPs supplementation can act as a growth promoter and immune-antioxidant stimulator owing to attenuating the ZnO-NPs-induced toxic impacts via stimulating growth, lessening the FCR, enhancing immune-antioxidative response, and modulating hepatic enzymes and protein levels. The present findings highlight the importance of dietary MO-NPs in aquaculture as a tool to enhance fish growth performance and mitigate ZnO-NPs-induced toxicity for both fish and fish consumers. Further studies are much needed to investigate the impact of dietary MO-NPs on gene expression and assess their impacts on other fish species.
Author Contributions
Conceptualization; H.S.H., R.M.A., A.H.E., H.H.M., H.E., A.M.A., H.A.M., M.A.E.-B., M.A., E.M.M.Y. and A.K.I.; methodology, H.S.H., R.M.A., A.H.E., H.H.M., H.E., A.M.A., H.A.M., M.A.E.-B., M.A., E.M.M.Y. and A.K.I.; formal analysis, H.S.H., R.M.A., A.H.E., H.H.M., H.E., A.M.A., H.A.M., M.A.E.-B., M.A., E.M.M.Y. and A.K.I.; investigation, H.S.H., R.M.A., A.H.E., H.H.M., H.E., A.M.A., H.A.M., M.A.E.-B., M.A., E.M.M.Y. and A.K.I.; writing—original draft preparation, H.S.H., R.M.A., H.H.M. and H.E.; writing—review and editing, H.H.M. and H.E. All authors have read and agreed to the published version of the manuscript.
Funding
Taif University Researchers Supporting Project number (TURSP-2020/57), Taif University, P.O. Box 11099, Taif 21944, Saudi Arabia.
Institutional Review Board Statement
The animal study was reviewed and approved by University of Sadat City (approval code VUSC-020-1-22, approved on 7 October 2022).
Data Availability Statement
All data are available in this manuscript.
Acknowledgments
All the authors are grateful to Taif University Researchers Supporting Project number (TURSP-2020/57), Taif University, P.O. Box 11099, Taif 21944, Saudi Arabia for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nasr-Eldahan, S.; Nabil-Adam, A.; Shreadah, M.A.; Maher, A.M.; Ali, T.E. A review article on nanotechnology in quaculture sustainability as a novel tool in fish disease control. Aquac. Int. 2021, 29, 1459–1480. [Google Scholar] [CrossRef] [PubMed]
- El-Bouhy, Z.M.; Reda, R.M.; Mahboub, H.H.; Gomaa, F.N. Bioremediation effect of pomegranate peel on subchronic mercury immunotoxicity on African catfish (Clarias gariepinus). Environ. Sci. Pollut. Res. 2021, 28, 2219–2235. [Google Scholar] [CrossRef] [PubMed]
- El-Bouhy, Z.M.; Reda, R.M.; Mahboub, H.H.; Gomaa, F.N. Chelation of mercury intoxication and testing different protective aspects of Lactococcus lactis probiotic in African catfish. Aquac. Res. 2021, 52, 3815–3828. [Google Scholar] [CrossRef]
- Mahboub, H.H.; Khedr, M.H.E.; Elshopakey, G.E.; Shakweer, M.S.; Mohamed, D.I.; Ismail, T.A.; Ismail, S.H.; Abdel Rahman, A.N. Impact of silver nanoparticles exposure on neuro-behavior, hematology, and oxidative stress biomarkers of African catfish (Clarias gariepinus). Aquaculture 2021, 544, 737082. [Google Scholar] [CrossRef]
- Al Jabri, H.; Saleem, M.H.; Rizwan, M.; Hussain, I.; Usman, K.; Alsafran, M. Zinc Oxide Nanoparticles and Their Biosynthesis: Overview. Life 2022, 12, 594. [Google Scholar] [CrossRef]
- Mahboub, H.H.; Shahin, K.; Zaglool, A.W.; Roushdy, E.M.; Ahmed, S.S.A. Efficacy of nano zinc oxide dietary supplements on growth performance, immunomodulation and disease resistance of African Catfish, Clarias gariepinus. Dis. Aquat. Org. 2020, 142, 147–160. [Google Scholar] [CrossRef]
- Mahboub, H.H.; Beheiry, R.R.; Shahin, S.E.; Behairy, A.; Khedr, M.H.E.; Ibrahim, S.M.; Elshopakey, G.E.; Daoush, W.M.; Altohamy, D.E.; Ismail, T.A.; et al. Adsorptivity of mercury on magnetite nano-particles and their influences on growth, economical, hemato-biochemical, histological parameters and bioaccumulation in Nile tilapia (Oreochromis niloticus). Aquat. Toxicol. 2021, 235, 105828. [Google Scholar] [CrossRef] [PubMed]
- Abdel Rahman, A.N.; Elshopakey, G.E.; Behairy, A.; Altohamy, D.E.; Ahmed, A.I.; Farroh, K.Y.; Alkafafy, M.; Shahin, S.A.; Ibrahim, R.E. Chitosan-Ocimum basilicum nanocomposite as a dietary additive in Oreochromis niloticus: Effects on immune-antioxidant response, head kidney gene expression, intestinal architecture, and growth. Fish Shellfish Immunol. 2022, 128, 425–435. [Google Scholar] [CrossRef]
- Elabd, H.; Youssuf, H.; Mahboub, H.H.; Salem, S.M.R.; Husseiny, W.A.; Khalid, A.; El-Desouky, H.S.; Faggio, C. Growth, hemato-biochemical, immune-antioxidant response, and gene expression in Nile tilapia (Oreochromis niloticus) received nano iron oxide-incorporated diets. Fish Shellfish Immunol. 2022, 128, 574–581. [Google Scholar] [CrossRef]
- Abdel Rahman, A.N.; Shakweer, M.S.; Algharib, S.A.; Abdelaty, A.I.; Kamel, S.; Ismail, T.A.; Daoush, W.M.; Ismail, S.H.; Mahboub, H.H. Silica nanoparticles acute toxicity alters ethology, neuro-stress indices, and physiological status of African catfish (Clarias gariepinus). Aquac. Rep. 2022, 23, 101034. [Google Scholar] [CrossRef]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanoparticle Res. 2012, 14, 1109. [Google Scholar] [CrossRef]
- Farre, J.C.; Krick, R.; Subramani, S.; Thumm, M. Turnover of organelles by autophagy in yeast. Curr. Opin. Cell Biol. 2009, 21, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Chen, L. Oxidative stress responses in different organs of carp (Cyprinus carpio) with exposure to ZnO nanoparticles. Ecotoxicol. Environ. Saf. 2012, 80, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Chen, L.; Hao, J.; Zhong, N. Bioaccumulation and sub-acute toxicity of zinc oxide nanoparticles in juvenile carp (Cyprinus carpio): A comparative study with its bulk counterparts. Ecotoxicol. Environ. Saf. 2013, 91, 52–60. [Google Scholar] [CrossRef]
- Rashidian, G.; Lazado, C.C.; Mahboub, H.H.; Mohammadi-Aloucheh, R.; Proki´c, M.D.; Nada, H.S.; Faggio, C. Chemically and green synthesized ZnO nanoparticles alter key immunological molecules in common carp (Cyprinus carpio) Skin Mucus. Int. J. Mol. Sci. 2021, 22, 3270. [Google Scholar] [CrossRef]
- Rashidian, G.; Mahboub, H.H.; Hoseinifar, S.H.; Ghafarifarsani, H.; Zare, M.; Punyatong, M.; Doan, H.V. Allium hirtifolium protects Cyprinus carpio against the detrimental responses mediated by foodborne zinc oxide nanoparticle. Aquaculture 2022, 555, 738252. [Google Scholar] [CrossRef]
- Xiong, D.; Fang, T.; Yu, L.; Sima, X.; Zhu, W. Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: Acute toxicity, oxidative stress and oxidative damage. Sci. Total Environ. 2011, 409, 1444–1452. [Google Scholar] [CrossRef]
- Kaya, H.; Aydın, F.; Gürkan, M.; Yılmaz, S.; Ates, M.; Demir, V.; Arslan, Z. Effects of zinc oxide nanoparticles on bioaccumulation and oxidative stress in different organs of tilapia (Oreochromis niloticus). Environ. Toxicol. Pharmacol. 2015, 40, 936–947. [Google Scholar] [CrossRef]
- Shahzad, K.; Naeem Khan, M.; Jabeen, F.; Kosour, N.; Shakoor Chaudhry, A.; Sohail, M.; Ahmad, N. Toxicity of zinc oxide nanoparticles (ZnO-NPs) in tilapia (Oreochromis mossambicus): Tissue accumulation, oxidative stress, histopathology and genotoxicity. Int. J. Environ. Sci. Technol. 2019, 16, 1973–1984. [Google Scholar] [CrossRef]
- Dezfoulnejad, M.C.; Molayemraftar, T. Use of dietary rosemary (Rosmarinus officinalis L.) extract as a growth promotor and immunostimulant in common carp. Aquac. Res. 2022, 53, 1553–1562. [Google Scholar] [CrossRef]
- Kurian, A.; Elumalai, P. Study on the impacts of chemical and green synthesized (Leucas aspera and oxy-cyclodextrin complex) dietary zinc oxide nanoparticles in Nile tilapia (Oreochromis niloticus). Environ. Sci. Pollut. Res. 2021, 28, 20344–20361. [Google Scholar] [CrossRef]
- Jeyavani, J.; Sibiya, A.; Sivakamavalli, J.; Divya, M.; Preetham, E.; Vaseeharan, B.; Faggio, C. Phytotherapy and combined nanoformulations as a promising disease management in aquaculture: A review. Aquac. Int. 2022, 30, 1071–1086. [Google Scholar] [CrossRef]
- Moyo, B.; Masika, P.J.; Hugo, A.; Muchenje, V. Nutritional characterization of Moringa (Moringa oleiferalam.) leaves. Afr. J. Biotechnol. 2011, 10, 12925–12933. [Google Scholar]
- Fakurazi, S.; Sharifudin, S.A.; Arulselvan, P. Moringa oleifera hydroethanolic extracts effectively alleviate acetaminopheninduced hepatotoxicity in experimental rats through their antioxidant nature. Molecules 2012, 17, 8334–8350. [Google Scholar] [CrossRef]
- Amaglo, N.K.; Bennett, R.N.; Lo Curto, R.B.; Rosa, E.A.S.; Lo Turco, V.; Giuffrida, A.; Curto, A.L.; Crea, F.; Timpo, G.M. Profiling selected phytochemicals and nutrients in different tissues of the multipurpose tree Moringa oleifera L., grown in Ghana. Food Chem. 2010, 122, 1047–1054. [Google Scholar] [CrossRef]
- Hamed, H.S.; El-Sayed, Y.S. Antioxidant activities of Moringa oleifera leaf extract against pendimethalin-induced oxidative stress and genotoxicity in Nile tilapia, Oreochromis niloticus (L.). Fish Physiol. Biochem. 2019, 45, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, L.; Doriya, K.; Kumar, D.S. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci. Hum. Wellness 2016, 5, 49–56. [Google Scholar] [CrossRef]
- Oduro, I.; Ellis, W.O.; Owusu, D. Nutritional potential of two leafy vegetables: Moringa oleifera and Ipomoea batatas leaves. Sci. Res. Essays 2008, 3, 57–60. [Google Scholar]
- Kamalakkannan, N.; Prince, P.S.M. Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats. Basic Clin. Pharmacol. Toxicol. 2006, 98, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Al-Malki, A.L.; El Rabey, H.A. The antidiabetic effect of low doses of Moringa oleifera Lam. seeds on streptozotocin induced diabetes and diabetic nephropathy in male rats. Biomed. Res. Int. 2015, 2015, 381040. [Google Scholar] [CrossRef]
- Baker, K.; Marcus, C.B.; Huffman, K.; Kruk, H.; Malfroy, B.; Doctrow, S.R. Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: A key role for reactive oxygen species in ischemic brain injury. J. Pharmacol. Exp. Ther. 1998, 284, 215–221. [Google Scholar] [PubMed]
- Kirisattayakul, W.; Wattanathorn, J.; Tong-Un, T.; Muchimapura, S.; Wannanon, P.; Jittiwat, J. Cerebroprotective effect of Moringa oleifera against focal ischemic stroke induced by middle cerebral artery occlusion. Oxid. Med. Cell. Longev. 2013, 2013, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. 2007, 21, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Coppin, J.P.; Xu, Y.; Chen, H.; Pan, M.H.; Ho, C.T.; Juliani, R.; Simon, J.E.; Wu, Q. Determination of flavonoids by LC/MS and anti-inflammatory activity in Moringa oleifera. J. Funct. Foods 2013, 5, 1892–1899. [Google Scholar] [CrossRef]
- Sharifudin, S.A.; Fakurazi, S.; Hidayat, M.T.; Hairuszah, I.; Moklas, M.A.; Arulselvan, P. Therapeutic potential of Moringa oleifera extracts against acetaminophen-induced hepatotoxicity in rats. Pharm. Biol. 2013, 51, 279–288. [Google Scholar] [CrossRef]
- Ibrahim, R.E.; El-Houseiny, W.; Behairy, A.; Mansour, M.F.; Abd-Elhakim, Y.M. Ameliorative effects of Moringa oleifera seeds and leaves on chlorpyrifos-induced growth retardation, immune suppression, oxidative stress, and DNA damage in Oreochromis niloticus. Aquaculture 2019, 505, 225–234. [Google Scholar] [CrossRef]
- Mahboub, H.H.; Rashidian, G.; Hoseinifar, S.H.; Kamel, S.; Zare, M.; Ghafarifarsani, H.; Algharib, S.A.; Moonmanee, T.; Doan, H.V. Protective effects of Allium hirtifolium extract against foodborne toxicity of Zinc oxide nanoparticles in common carp (Cyprinus carpio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 257, 109345. [Google Scholar] [CrossRef]
- Ismail, S.H.; Hamdy, A.; Ismail, T.A.; Mahboub, H.H.; Mahmoud, W.H.; Daoush, W.M. Synthesis and Characterization of Antibacterial Carbopol/ZnO Hybrid Nanoparticles Gel. Crystals 2021, 11, 1092. [Google Scholar] [CrossRef]
- Goda, M.N.; Shaheen, A.A.M.; Hamed, H.S. Antagonistic effects of dietary parsley and parsley nanoparticles against zinc oxide nanoparticles induced behavioral, physiological, histopathological alterations and oxidative stress in Nile Tilapia, Oreochromis niloticus. Fishes, 2022; in press. [Google Scholar]
- Tietz, N.W.; Burtis, C.A.; Duncan, P.; Ervin, K.; Petitclerc, C.J.; Rinker, A.D.; Shuey, D.; Zygowicz, E.R. A reference method for measurement of alkaline phosphatase activity in human serum. Clin. Chem. 1983, 29, 751–761. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Vassault, A. Lactate dehydrogenase: UV-method with pyruvate and NADH. In Methods of Enzymatic Analysis, 3rd ed.; Bergmeyer, H.U., Ed.; Plenum: New York, NY, USA, 1983; pp. 118–125. [Google Scholar]
- Larsen, K. Creatinine assay by a reaction-kinetic principle. Clin. Chim. Acta 1972, 41, 209–217. [Google Scholar] [CrossRef]
- Henry, R.J. Colorimetric Determination of Total Protein. In Clinical Chemistry; Harper & Row Publishers: New York, NY, USA, 1964; Volume 181, pp. 166–173. [Google Scholar]
- Trinder, P. Determination of blood glucose using 4-amino phenazone as oxygen acceptor. J. Clin. Pathol. 1969, 22, 246. [Google Scholar] [CrossRef] [PubMed]
- Foster, L.; Dunn, R. Single antibody technique for radioimmunoassay of cortisol in un extracted serum or plasma. Clin. Chem. 1974, 20, 365. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Doumas, B.T.; Watson, W.A.; Biggs, H.G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta 1971, 31, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Uchiyama, M. Determination of malonaldehydeprecursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar]
- Nishikimi, M.; Appaji, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reducedphenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods Enzymology; Academic Press: Cambridge, MA, USA, 1984; pp. 121–126. [Google Scholar]
- Beutler, E.; Duron, O.; Kelly, B.M. Improve method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Secombes, C.J. Isolation of salmonid macrophages and analysis of their killing activity. Tech. Fish Immunol. 1990, 1, 137–154. [Google Scholar]
- Abdel-Tawwab, M.; Adeshina, I.; Jenyo-Oni, A.; Ajani, E.K.; Emikpe, B.O. Growth, physiological, antioxidants, and immune response of African catfish, Clarias gariepinus (B.) to dietary clove basil, Ocimum gratissimum leaf extract and its susceptibility to Listeria monocytogenes infection. Fish Shellfish Immunol. 2018, 78, 346–354. [Google Scholar] [CrossRef]
- Ellis, A.E. Lysozyme assays. In Techniques in Fish Immunology; Stolen, J.S., Ed.; SOS Publications: Fair Haven, CT, USA, 1990; pp. 101–103. [Google Scholar]
- Siwicki, A.; Anderson, D. Nonspecific defense mechanisms assay in fish: II. Potential killing activity of neutrophils and macrophages, lysozyme activity in serum and organs and total immunoglobulin level in serum. In Fish Diseases Diagnosis and Preventions Methods; Siwicki, A.K., Anderson, D.P., Waluga, J., Eds.; IRS: Olsztyn, Poland, 1993; pp. 105–112. [Google Scholar]
- Dytham, C. Choosing and Using Statistics: A Biologist ’s Guide; Blackwell Science Ltd.: London, UK, 2011. [Google Scholar]
- Elabd, H.; Faggio, C.; Mahboub, H.H.; Emam, M.A.; Kamel, S.; El Kammar, R.; Abdelnaeim, N.S.; Shaheen, A.; Tresnakova, N.; Matter, A. Mucuna pruriens seeds extract boosts growth, immunity, testicular histology, and expression of immune-related genes of mono-sex Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2022, 127, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Hamed, H.S.; Abdel Tawwab, M. Dietary pomegranate (Punica granatum) peel mitigated the adverse effects of silver nanoparticles on the performance, haemato-biochemical, antioxidant, and immune responses of Nile tilapia fingerlings. Aquaculture 2021, 540, 736742. [Google Scholar] [CrossRef]
- Hamed, H.S.; Ali, R.M.; Shaheen, A.A.; Hussein, N.M. Chitosan nanoparticles alleviated endocrine disruption, oxidative damage, and genotoxicity of Bisphenol-A- intoxicated female African catfish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 248, 109104. [Google Scholar] [CrossRef]
- Sayed, A.H.; Hamed, H.S. Induction of apoptosis and DNA damage by 4-nonylphenol in African catfish (Clarias gariepinus) and the antioxidant role of Cydonia oblonga. Ecotoxicol. Environ. Saf. 2017, 139, 97–101. [Google Scholar] [CrossRef]
- Andrawes, N.G.; Abo Nour, A.A.; Shaheen, A.A.; Hamed, H.S. Effect of Nigella sativa enriched diet on biochemical variables and antioxidant damage caused by silver nanoparticles toxicity in African catfish, Clarias gariepinus. Egypt J. Aquat. Biol. Fish 2021, 25, 63–76. [Google Scholar] [CrossRef]
- Abdel Rahman, A.N.; Mansour, D.A.; Abd El-Rahman, G.I.; Elseddawy, N.M.; Zaglool, A.W.; Khamis, T.; Mahmoud, S.F.; Mahboub, H.H. Imidacloprid toxicity in Clarias gariepinus: Protective role of dietary Hyphaene thebaica against biochemical and histopathological disruption, oxidative stress, immune genes expressions, and Aeromonas sobria infection. Aquaculture 2022, 555, 738170. [Google Scholar] [CrossRef]
- Abdel Rahman, A.N.; Mahmoud, S.M.; Khamis, T.; Rasheed, N.; Mohamed, D.I.; Ghanem, R.; Mansour, D.M.; Ismail, T.A.; Mahboub, H.H. Palliative effect of dietary common sage leaves against toxic impacts of nonylphenol in Mirror carp. (Cyprinus carpio var specularis): Growth, gene expression, immune-antioxidant status, and histopathological alterations. Aquac. Rep. 2022, 25, 101200. [Google Scholar] [CrossRef]
- Ahmed, S.A.A.; Nada, H.S.; Ibrahim, S.M.; Fahmy, E.M.; Khedr, M.H.E.; Moustafa, S.M.; Mahboub, H.H. Comparative antitoxic potency of honey and natamycin- supplemented diets against aflatoxicosis and their influences on growth, serum biochemistry, immunohistochemistry, and residual deposition in Nile tilapia (Oreochromis niloticus). Aquaculture 2022, 551, 737934. [Google Scholar] [CrossRef]
- Hamed, H.S.; Ismal, S.M.; Abdel Tawwab, M. Modulatory effects of cinnamon (Cinnamomum zeylanicum) against waterborne lead toxicity in Niletilapia fingerlings: Growth performance, haemato-biochemical, innate immunity and hepatic antioxidant capacity. Aquac. Rep. 2022, 25, 101190. [Google Scholar] [CrossRef]
- Kizhakkumpat, A.; Syed, A.; Elgorban, A.M.; Bahkali, A.H.; Khan, S.S. The toxicity analysis of PVP, PVA and PEG surface functionalized ZnO nanoparticles on embryonicas well as adult Danio rerio. Environ. Monit. Assess. 2021, 193, 824. [Google Scholar] [CrossRef]
- Sarkar, M.; Bhowmick, S.; Hussain, J.; Hasan, M.; Hossain, S. Hot water extract of Moringa oleifera leaves protects erythrocytes from hemolysis and major organs from oxidative stress in vitro. J. Basic Appl. Res. 2017, 3, 120–126. [Google Scholar]
- Guyton, A.; Hall, J. Blood cells, immunity, and blood clotting: Blood groups. In Text Book of Medical Physiology, 11th ed.; WB Saunders Company: Philadelphia, PA, USA, 2005; pp. 458–459. [Google Scholar]
- Glencross, B.D.; Booth, M.; Allen, G.I. A feed is only as good as its ingredients-a review of ingredient evaluation strategies for aquaculture feeds. Aquac. Nutr. 2007, 13, 17–34. [Google Scholar] [CrossRef]
- Camacho-Murillo, M.; Obando-Víquez, M.; Lilia, S.; Vega-Baudrit, J.R. Toxicity of nanoparticles: A development opportunity in environment and health. J. Clin. Exp. Tox. 2022, 6, 1–6. [Google Scholar]
- Taheri, S.; Banaee, M.; Haghi, B.N.; Mohiseni, M. Effects of dietary supplementation of zinc oxide nanoparticles on some biochemical biomarkers in common carp (Cyprinus carpio). Int. J. Aquat. Biol. 2017, 5, 286–294. [Google Scholar]
- Selvakumar, D.; Natarajan, P. Hepato-protective activity of Moringa oleifera Lam leaves in carbon tetrachloride induced hepato-toxicity in albino rats. Pharmacogn. Mag. 2008, 4, 97. [Google Scholar]
- Sensi, S.L.; Yin, H.Z.; Carriedo, S.G.; Rao, S.S.; Weiss, J.H. Preferential Zn2+ influx through Ca2+-permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc. Natl. Acad. Sci. USA 1999, 96, 2414–2419. [Google Scholar] [CrossRef]
- Heng, B.C.; Zhao, X.; Xiong, S.; Ng, K.W.; Boey, F.Y.C.; Loo, J.S.C. Toxicity of zinc oxide (ZnO) nanoparticles on human bronchial epithelial cells (BEAS-2B) is accentuated by oxidative stress. Food Chem. Toxicol. 2010, 48, 1762–1766. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Zhang, X.; Chang, Y.; Chen, Y. The impact of ZnO nanoparticle aggregates on the embryonic development of zebrafish (Danio rerio). Nanotechnology 2009, 20, 195103. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Jabeen, F.; Asghar, M.S.; Qureshi, N.A.; Shakeel, M.; Noureen, A.; Shabbir, S. Role of nao-ceria in the amelioration of oxidative stress: Current and future applications in medicine. Int. J. Biosci. 2015, 6, 89–109. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nano level. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Buerki-Thurnherr, T.; Xiao, L.; Diener, L.; Arslan, O.; Hirsch, C.; Maeder-althaus, X. In vitro mechanistic study towards a better understanding of ZnO nanoparticle toxicity. Nanotoxicology 2013, 7, 402–416. [Google Scholar] [CrossRef] [PubMed]
- Vimercati, L.; Cavone, D.; Caputi, A.; De Maria, L.; Tria, M.; Prato, E.; Ferri, G.M. Nanoparticles: An experimental study of zinc nanoparticles toxicity on marine crustaceans. General overview on the health implications in humans. Front. Public Health 2020, 8, 192. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Machado, D.I.; López-Cervantes, J.; Ríos Vázquez, N.J. High-performance liquid chromatography method to measureα-andγ-tocopherol in leaves, flowers and freshbeans from Moringa oleifera. J. Chromatogr. A 2006, 1105, 111–114. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. Antioxidant properties of various solvent extracts of totalphenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef]
- De Pablo, M.A.; De Cienfuegos, G.Á. Modulatory effects of dietary lipids on im-mune system functions. Immunol. Cell Biol. 2000, 78, 31–39. [Google Scholar]
- Mora, J.R.; Iwata, M.; Von Andrian, U.H. Vitamin effects on the immune system:vitamins a and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).