Effects of Diets with Varying Astaxanthin from Yarrowia lipolytica Levels on the Growth, Feed Utilization, Metabolic Enzymes Activities, Antioxidative Status and Serum Biochemical Parameters of Litopenaeus vannamei
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Experimental Shrimp and Feeding Trial
2.3. Methods of Sample Collection and Analysis
2.4. Statistical Analysis
3. Results
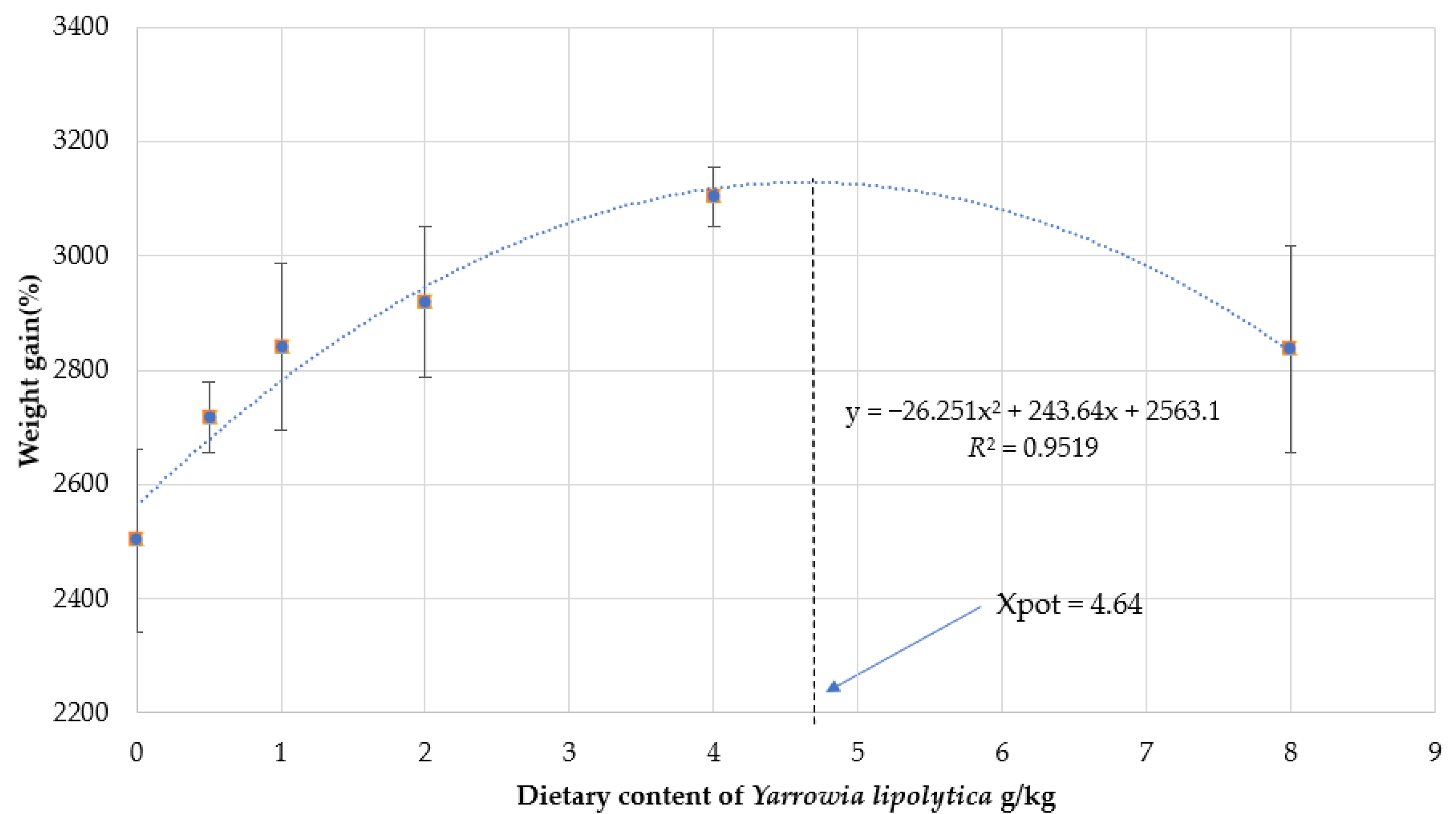
3.1. Growth Performance and Morphological Index
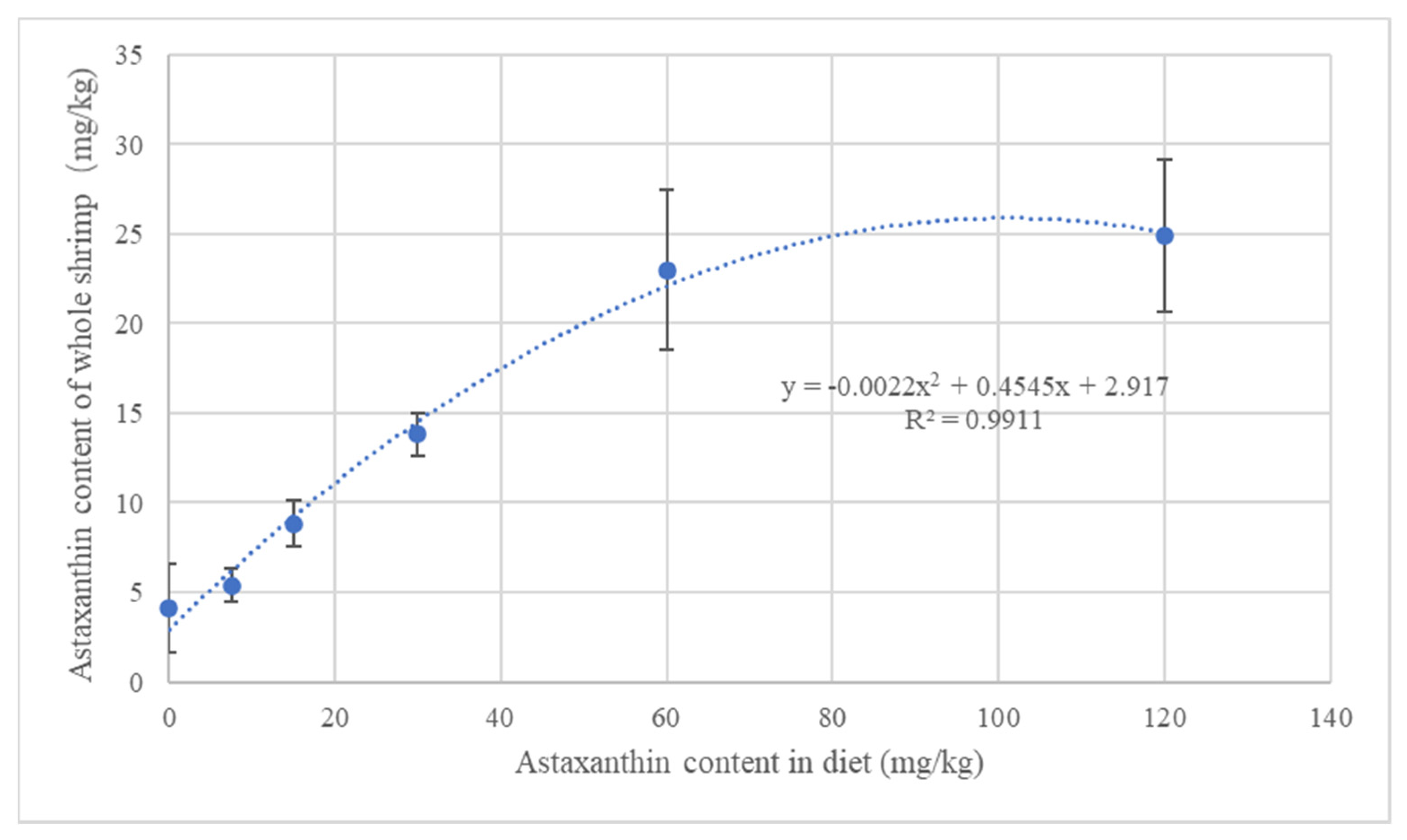
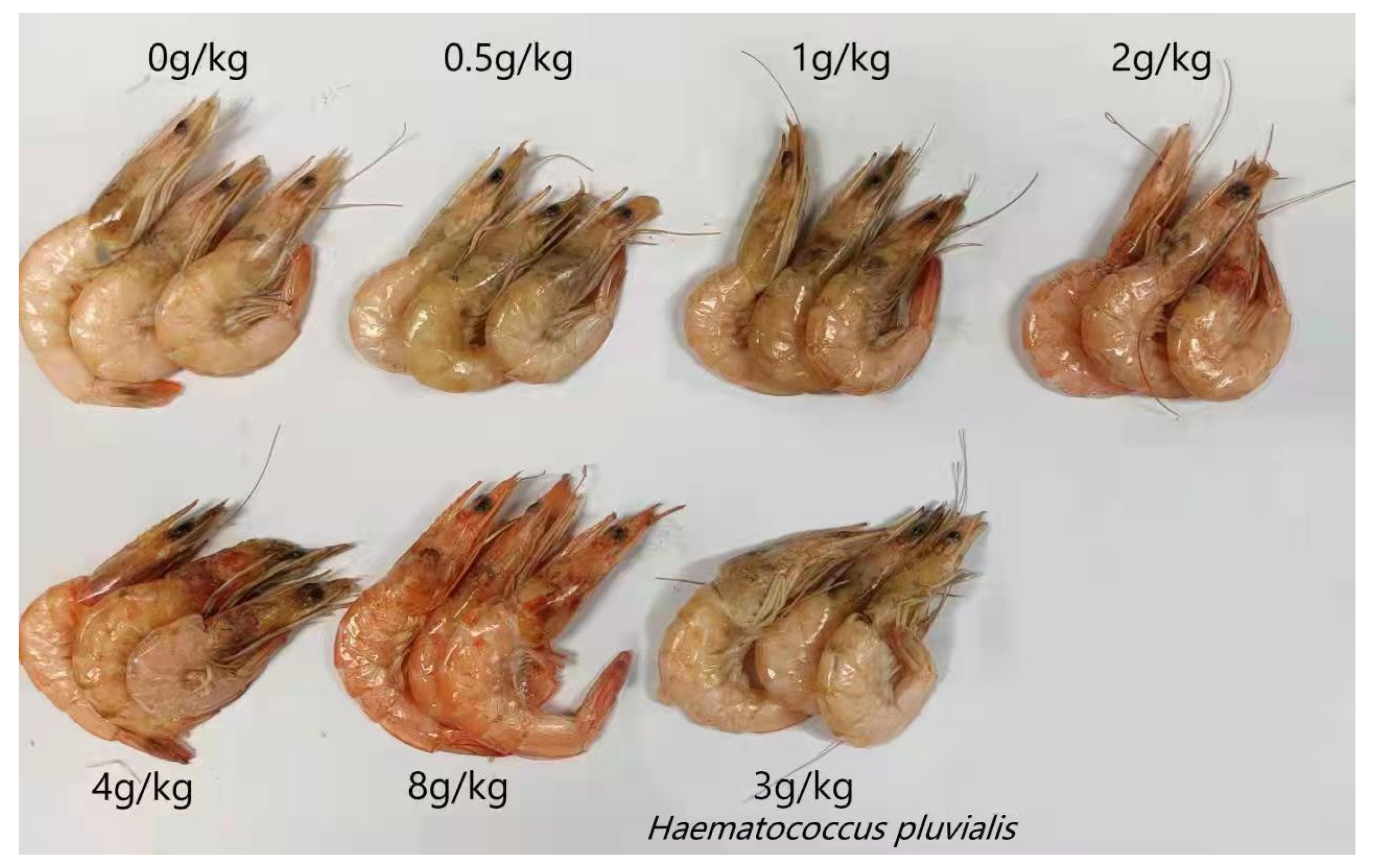
3.2. Effect on Body Color of Shrimp
3.3. Proximate Composition of the Whole Body and Muscles
3.4. Serum Biochemical and Antioxidative Indexes
3.5. Immune and Antioxidant Indexes of Hepatopancreas
3.6. Gastrointestinal Digestive Enzyme Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ekezie, F.-G.C.; Sun, D.-W.; Cheng, J.-H. Altering the IgE binding capacity of king prawn (Litopenaeus vannamei) tropomyosin through conformational changes induced by cold argon-plasma jet. Food Chem. 2019, 300, 125143. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.-J.; Tian, L.-X.; Yang, H.-J.; Liang, G.-Y.; Yue, Y.-R.; Xu, D.-H. Effects of dietary astaxanthin on growth, antioxidant capacity and gene expression in Pacific white shrimp Litopenaeus vannamei. Aquac. Nutr. 2013, 19, 917–927. [Google Scholar] [CrossRef]
- Flegel, K.M. Physicians, finder’s fees and free, informed consent. Can. Med. Assoc. J. 1997, 157, 1373–1374. [Google Scholar]
- Sirirustananun, N.; Chen, J.-C.; Lin, Y.-C.; Yeh, S.-T.; Liou, C.-H.; Chen, L.-L.; Sim, S.S.; Chiew, S.L. Dietary administration of a Gracilaria tenuistipitata extract enhances the immune response and resistance against Vibrio alginolyticus and white spot syndrome virus in the white shrimp Litopenaeus vannamei. Fish Shellfish. Immunol. 2011, 31, 848–855. [Google Scholar] [CrossRef]
- Choi, S.; Koo, S. Efficient Syntheses of the Keto-carotenoids Canthaxanthin, Astaxanthin, and Astacene. J. Org. Chem. 2005, 70, 3328–3331. [Google Scholar] [CrossRef] [PubMed]
- Margalith, P.Z. Production of ketocarotenoids by microalgae. Appl. Microbiol. Biotechnol. 1999, 51, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Mularczyk, M.; Michalak, I.; Marycz, K. Astaxanthin and other Nutrients from Haematococcus pluvialis—Multifunctional Applications. Mar. Drugs 2020, 18, 459. [Google Scholar] [CrossRef]
- Kildegaard, K.R.; Adiego-Pérez, B.; Belda, D.D.; Khangura, J.K.; Holkenbrink, C.; Borodina, I. Engineering of Yarrowia lipolytica for production of astaxanthin. Synth. Syst. Biotechnol. 2017, 2, 287–294. [Google Scholar] [CrossRef]
- Zhu, H.-Z.; Jiang, S.; Wu, J.-J.; Zhou, X.-R.; Liu, P.-Y.; Huang, F.-H.; Wan, X. Production of High Levels of 3S, 3′S-Astaxanthin in Yarrowia lipolytica via Iterative Metabolic Engineering. J. Agric. Food Chem. 2022, 70, 2673–2683. [Google Scholar] [CrossRef]
- Barth, G.; Gaillardin, C. Physiology and genetics of the dimorphic fungus Yarrowia lipolytica. FEMS Microbiol. Rev. 1997, 19, 219–237. [Google Scholar] [CrossRef]
- Berge, G.; Hatlen, B.; Odom, J.M.; Ruyter, B. Physical treatment of high EPA Yarrowia lipolytica biomass increases the availability of n-3 highly unsaturated fatty acids when fed to Atlantic salmon. Aquac. Nutr. 2013, 19, 110–121. [Google Scholar] [CrossRef]
- Xu, B.; Liu, Y.; Chen, K.; Wang, L.; Sagada, G.; Tegomo, A.F.; Yang, Y.; Sun, Y.; Zheng, L.; Ullah, S.; et al. Evaluation of Methanotroph (Methylococcus capsulatus, Bath) Bacteria Meal (FeedKind®) as an Alternative Protein Source for Juvenile Black Sea Bream, Acanthopagrus schlegelii. Front. Mar. Sci. 2021, 8, 778301. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Y.; Xu, B.; Sagada, G.; Chen, K.; Xiao, J.; Zhang, J.; Shao, Q. Effects of berberine supplementation in high starch diet on growth performance, antioxidative status, immune parameters and ammonia stress response of fingerling black sea bream (Acanthopagrus schlegelii). Aquaculture 2020, 527, 735473. [Google Scholar] [CrossRef]
- Chien, Y.-H.; Jeng, S.-C. Pigmentation of kuruma prawn, Penaeus japonicus Bate, by various pigment sources and levels and feeding regimes. Aquaculture 1992, 102, 333–346. [Google Scholar] [CrossRef]
- Wade, N.M.; Gabaudan, J.; Glencross, B.D. A review of carotenoid utilisation and function in crustacean aquaculture. Rev. Aquac. 2017, 9, 141–156. [Google Scholar] [CrossRef]
- Flores, M.; Díaz, F.; Medina, R.; Re, A.D.; Licea, A. Physiological, metabolic and haematological responses in white shrimp Litopenaeus vannamei (Boone) juveniles fed diets supplemented with astaxanthin acclimated to low-salinity water. Aquac. Res. 2007, 38, 740–747. [Google Scholar] [CrossRef]
- Niu, J.; Tian, L.-X.; Liu, Y.-J.; Yang, H.-J.; Ye, C.-X.; Gao, W.; Mai, K.-S. Effect of Dietary Astaxanthin on Growth, Survival, and Stress Tolerance of Postlarval Shrimp, Litopenaeus vannamei. J. World Aquac. Soc. 2009, 40, 795–802. [Google Scholar] [CrossRef]
- Kumar, V. Effect of dietary astaxanthin on growth and immune response of Giant freshwater prawn Macrobrachium rosenbergii (de man). Asian Fish. Sci. 2009, 22, 61–69. [Google Scholar] [CrossRef]
- Daly, B.; Swingle, J.S.; Eckert, G.L. Dietary astaxanthin supplementation for hatchery-cultured red king crab, Paralithodes camtschaticus, juveniles. Aquac. Nutr. 2012, 19, 312–320. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, S. Effect of dietary astaxanthin on the growth performance and nonspecific immunity of red swamp crayfish Procambarus clarkii. Aquaculture 2019, 512, 734341. [Google Scholar] [CrossRef]
- Wang, W.; Ishikawa, M.; Koshio, S.; Yokoyama, S.; Dawood, M.A.; Zhang, Y. Effects of dietary astaxanthin supplementation on survival, growth and stress resistance in larval and post-larval kuruma shrimp, Marsupenaeus japonicus. Aquac. Res. 2018, 49, 2225–2232. [Google Scholar] [CrossRef]
- Fang, H.; He, X.; Zeng, H.; Liu, Y.; Tian, L.; Niu, J. Replacement of Astaxanthin With Lutein in Diets of Juvenile Litopenaeus vannamei: Effects on Growth Performance, Antioxidant Capacity, and Immune Response. Front. Mar. Sci. 2021, 8, 1834. [Google Scholar] [CrossRef]
- Baron, M.; Davies, S.; Alexander, L.; Snellgrove, D.; Sloman, K.A. The effect of dietary pigments on the coloration and behaviour of flame-red dwarf gourami, Colisa lalia. Anim. Behav. 2008, 75, 1041–1051. [Google Scholar] [CrossRef]
- Ettefaghdoost, M.; Haghighi, H. Impact of different dietary lutein levels on growth performance, biochemical and immuno-physiological parameters of oriental river prawn (Macrobrachium nipponense). Fish Shellfish. Immunol. 2021, 115, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Guo, N.; Sun, J.; Xue, C. Comprehensive utilization of shrimp waste based on biotechnological methods: A review. J. Clean. Prod. 2017, 143, 814–823. [Google Scholar] [CrossRef]
- Xie, S.; Yin, P.; Tian, L.; Yu, Y.; Liu, Y.; Niu, J. Dietary Supplementation of Astaxanthin Improved the Growth Performance, Antioxidant Ability and Immune Response of Juvenile Largemouth Bass (Micropterus salmoides) Fed High-Fat Diet. Mar. Drugs 2020, 18, 642. [Google Scholar] [CrossRef]
- Kang, C.D.; Han, S.J.; Choi, S.P.; Sim, S.J. Fed-batch culture of astaxanthin-rich Haematococcus pluvialis by exponential nutrient feeding and stepwise light supplementation. Bioprocess Biosyst. Eng. 2010, 33, 133–139. [Google Scholar] [CrossRef]
- Shah, M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Wang, W.; Liu, M.; Fawzy, S.; Xue, Y.; Wu, M.; Huang, X.; Yi, G.; Lin, Q. Effects of Dietary Phaffia rhodozyma Astaxanthin on Growth Performance, Carotenoid Analysis, Biochemical and Immune-Physiological Parameters, Intestinal Microbiota, and Disease Resistance in Penaeus monodon. Front. Microbiol. 2021, 12, 762689. [Google Scholar] [CrossRef]
- Babin, A.; Moreau, J.; Moret, Y. Storage of Carotenoids in Crustaceans as an Adaptation to Modulate Immunopathology and Optimize Immunological and Life-History Strategies. BioEssays 2019, 41, e1800254. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Zhang, L.; Wang, J.; Ma, J.; Meng, X.; Duan, P.; Sun, L.; Sun, Y. Effect of dietary lipid level on the growth performance, feed utilization, body composition and blood chemistry of juvenile starry flounder (Platichthys stellatus). Aquac. Res. 2009, 41, 1470–1478. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, F.; Wang, L.-L.; Shao, Q.; Xu, Z.; Xu, J. Dietary Protein Requirement of Juvenile Black Sea Bream, Sparus macrocephalus. J. World Aquac. Soc. 2010, 41, 151–164. [Google Scholar] [CrossRef]
- Chuchird, N.; Rorkwiree, P.; Rairat, T. Effect of dietary formic acid and astaxanthin on the survival and growth of Pacific white shrimp (Litopenaeus vannamei) and their resistance to Vibrio parahaemolyticus. SpringerPlus 2015, 4, 440. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Yuan, Y.; Lu, Y.; Ma, H.; Sun, P.; Li, Y.; Qiu, H.; Ding, L.; Zhou, Q. Regulation of growth, tissue fatty acid composition, biochemical parameters and lipid related genes expression by different dietary lipid sources in juvenile black seabream, Acanthopagrus schlegelii. Aquaculture 2017, 479, 25–37. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, W.; Gladstone, S.; Ng, W.-K.; Zhang, J.; Shao, Q. Effects of isoenergetic diets with varying protein and lipid levels on the growth, feed utilization, metabolic enzymes activities, antioxidative status and serum biochemical parameters of black sea bream (Acanthopagrus schlegelii). Aquaculture 2019, 513, 734397. [Google Scholar] [CrossRef]
- Bi, J.; Cui, R.; Li, Z.; Liu, C.; Zhang, J. Astaxanthin alleviated acute lung injury by inhibiting oxidative/nitrative stress and the inflammatory response in mice. Biomed. Pharmacother. 2017, 95, 974–982. [Google Scholar] [CrossRef]
- Bhuvaneswari, S.; Yogalakshmi, B.; Sreeja, S.; Anuradha, C.V. Astaxanthin reduces hepatic endoplasmic reticulum stress and nuclear factor-kappa B-mediated inflammation in high fructose and high fat diet-fed mice. Cell Stress Chaperones 2014, 19, 183–191. [Google Scholar] [CrossRef]

| Ingredients (%) | A0 | A1 | A2 | A3 | A4 | A5 | A6 |
|---|---|---|---|---|---|---|---|
| Fishmeal | 22 | 22 | 22 | 22 | 22 | 22 | 22 |
| Peeled soybean meal | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Fermented soybean meal | 13 | 13 | 13 | 13 | 13 | 13 | 13 |
| Soy protein concentrate | 10.5 | 10.5 | 10.5 | 10.5 | 10.5 | 10.5 | 10.5 |
| Squid liver powder | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Chicken meal | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| High gluten flour | 22 | 22 | 22 | 22 | 22 | 22 | 22 |
| Fish oil | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Soy phospholipids | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| L-lysine | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| DL-methionine | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Taurine | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 |
| Sodium carboxymethyl cellulose | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.5 | 0.50 |
| Carrageenan | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.2 | 0.20 |
| Calcium dihydrogen phosphate | 2.1 | 2.1 | 2.1 | 2.1 | 2.1 | 2.1 | 2.1 |
| Vc phosphate | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Vitamin mixture | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Mineral premix | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Zeolite powder | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Antioxidant | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Antifungal agent | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Yarrowia lipolytica (astaxanthin 1.5%) | 0 | 0.05 | 0.1 | 0.2 | 0.4 | 0.8 | 0 |
| Haematococcus pluvialis (astaxanthin 2.0%) | 0 | 0 | 0 | 0 | 0 | 0 | 0.4 |
| Beer yeast | 0.96 | 0.93 | 0.9 | 0.84 | 0.72 | 0.48 | 0.72 |
| Alpha cellulose | 2.07 | 2.05 | 2.03 | 1.99 | 1.91 | 1.75 | 1.91 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Astaxanthin (mg kg−1) | 0 | 7.5 | 15 | 30 | 60 | 120 | 60 |
| Proximate analysis (%) | |||||||
| Crude protein | 39.55 | 39.04 | 39.09 | 39.54 | 38.97 | 39.26 | 39.65 |
| Crude lipid | 7.31 | 7.09 | 7.85 | 7.60 | 7.51 | 7.44 | 7.54 |
| Gross energy (kJ g−1) | 16.93 | 16.72 | 17.03 | 17.04 | 16.87 | 16.91 | 17.04 |
| Gross phosphorus | 0.82 | 0.78 | 0.86 | 0.81 | 0.85 | 0.82 | 0.80 |
| Index 1 | A0 | A1 | A2 | A3 | A4 | A5 | A6 |
|---|---|---|---|---|---|---|---|
| SR 2 | 96.33 ± 4.80 | 99.60 ± 0.89 | 95.33 ± 5.75 | 95.67 ± 4.63 | 96.80 ± 3.03 | 96.67 ± 4.68 | 97.00 ± 2.76 |
| WGR 3 | 2502.43 ± 160.01 c | 2717.21 ± 62.32 b | 2839.99 ± 146.13 b | 2918.19 ± 131.99 ab | 3102.94 ± 51.20 a | 2836.46 ± 181.21 b | 2876.05 ± 143.69 b |
| FBL 4 | 9.08 ± 0.67 | 9.25 ± 0.56 | 9.22 ± 0.54 | 9.34 ± 0.52 | 9.50 ± 0.53 | 9.61 ± 0.52 | 9.48 ± 0.55 |
| CF 5 | 0.81 ± 0.02 b | 0.80 ± 0.02 b | 0.82 ± 0.1 b | 0.81 ± 0.01 b | 0.84 ± 0.01 a | 0.82 ± 0.01 b | 0.78 ± 0.01 c |
| HIS 6 | 4.99 ± 0.44 ab | 5.50 ± 0.60 a | 4.80 ± 0.35 bc | 4.75 ± 0.39 bc | 4.35 ± 0.68 cd | 4.04 ± 0.41 d | 4.84 ± 0.63 bc |
| FR 7 | 4.46 ± 0.60 | 4.47 ± 0.83 | 4.49 ± 0.11 | 4.72 ± 0.36 | 4.26 ± 0.28 | 4.36 ± 0.29 | 4.32 ± 0.29 |
| FCR 8 | 1.23 ± 0.16 | 1.23 ± 0.23 | 1.23 ± 0.03 | 1.30 ± 0.10 | 1.17 ± 0.08 | 1.20 ± 0.08 | 1.19 ± 0.08 |
| PER 9 | 2.05 ± 0.29 | 2.08 ± 0.42 | 2.01 ± 0.05 | 1.92 ± 0.14 | 2.13 ± 0.14 | 2.08 ± 0.14 | 2.10 ± 0.15 |
| PPV 10 | 36.33 ± 5.02 | 37.75 ± 7.57 | 36.56 ± 0.95 | 35.65 ± 2.68 | 39.12 ± 2.60 | 38.97 ± 2.69 | 39.10 ± 2.67 |
| Index 1 | A0 | A1 | A2 | A3 | A4 | A5 | A6 |
|---|---|---|---|---|---|---|---|
| Whole Shrimp | |||||||
| Moisture | 75.78 ± 0.10 a | 75.14 ± 0.14 bc | 75.37 ± 0.18 b | 74.95 ± 0.13 c | 75.09 ± 0.18 bc | 74.78 ± 0.11 c | 74.69 ± 0.12 c |
| Crude protein | 17.05 ± 0.21 | 17.53 ± 0.14 | 17.56 ± 0.23 | 17.97 ± 0.08 | 17.78 ± 0.16 | 18.10 ± 0.06 | 17.58 ± 0.12 |
| Crude lipid | 0.96 ± 0.02 | 1.01 ± 0.05 | 1.01 ± 0.05 | 0.95 ± 0.06 | 0.94 ± 0.04 | 1.01 ± 0.03 | 0.99 ± 0.03 |
| Ash | 3.42 ± 0.05 | 3.36 ± 0.05 | 3.40 ± 0.04 | 3.30 ± 0.09 | 3.90 ± 0.08 | 3.42 ± 0.06 | 3.32 ± 0.05 |
| Muscle | |||||||
| Moisture | 76.08 ± 0.07 | 76.07 ± 0.13 | 75.54 ± 0.15 | 75.80 ± 0.15 | 76.53 ± 0.19 | 75.91 ± 0.15 | 75.81 ± 0.16 |
| Crude protein | 22.13 ± 0.14 | 22.19 ± 0.02 | 22.20 ± 0.16 | 22.25 ± 0.07 | 22.31 ± 0.20 | 22.50 ± 0.14 | 22.40 ± 0.14 |
| Crude lipid | 0.38 ± 0.01 | 0.40 ± 0.02 | 0.41 ± 0.03 | 0.39 ± 0.01 | 0.40 ± 0.02 | 0.41 ± 0.02 | 0.40 ± 0.02 |
| Ash | 1.56 ± 0.07 | 1.52 ± 0.04 | 1.51 ± 0.10 | 1.64 ± 0.10 | 1.54 ± 0.08 | 1.72 ± 0.03 | 1.65 ± 0.03 |
| Index 1 | A0 | A1 | A2 | A3 | A4 | A5 | A6 |
|---|---|---|---|---|---|---|---|
| TP 2 | 20.80 ± 1.49 c | 27.22 ± 4.33 b | 27.99 ± 2.82 b | 25.38 ± 4.44 b | 28.46 ± 6.85 b | 34.74 ± 2.13 a | 35.05 ± 1.81 a |
| ALB 3 | 10.12 ± 0.84 c | 12.39 ± 1.36 b | 12.34 ± 0.71 b | 11.94 ± 1.66 b | 12.80 ± 2.40 b | 14.53 ± 0.49 a | 15.44 ± 0.99 a |
| SOD 4 | 19.80 ± 4.87 | 21.69 ± 3.94 | 19.84 ± 1.72 | 21.10 ± 4.78 | 19.92 ± 6.44 | 19.54 ± 3.22 | 19.62 ± 5.87 |
| MDA 5 | 12.48 ± 2.25 a | 10.69 ± 1.04 b | 9.58 ± 2.36 b | 9.18 ± 3.53 c | 9.11 ± 2.57 c | 7.97 ± 1.14 d | 9.10 ± 2.01 c |
| GSH-Px 6 | 127.51 ± 17.72 c | 140.07 ± 38.40 b | 153.80 ± 41.35 b | 153.09 ± 41.48 b | 153.85 ± 56.20 b | 181.29 ± 42.21 a | 156.93 ± 19.64 b |
| CAT 7 | 0.68 ± 0.19 | 0.72 ± 0.13 | 0.72 ± 0.17 | 0.78 ± 0.19 | 0.73 ± 0.29 | 0.65 ± 0.13 | 0.63 ± 0.19 |
| T-AOC 8 | 0.19 ± 0.04 c | 0.24 ± 0.04 b | 0.25 ± 0.03 b | 0.29 ± 0.13 b | 0.34 ± 0.15 a | 0.31 ± 0.05 b | 0.30 ± 0.06 b |
| LZM 9 | 254.39 ± 32.22 | 238.60 ± 81.99 | 263.16 ± 23.06 | 201.75 ± 28.57 | 208.77 ± 21.49 | 233.33 ± 47.27 | 228.07 ± 45.96 |
| AKP 10 | 0.34 ± 0.16 | 0.28 ± 0.08 | 0.35 ± 0.16 | 0.25 ± 0.05 | 0.27 ± 0.08 | 0.29 ± 0.04 | 0.29 ± 0.06 |
| PPO 11 | 9.06 ± 1.08 | 9.78 ± 2.66 | 9.17 ± 3.72 | 9.56 ± 1.61 | 10.50 ± 5.18 | 12.50 ± 3.90 | 11.83 ± 3.05 |
| AST 12 | 1.81 ± 0.70 | 1.96 ± 0.42 | 1.86 ± 0.35 | 1.83 ± 0.91 | 2.12 ± 1.49 | 1.93 ± 0.69 | 1.84 ± 0.59 |
| TG 13 | 0.86 ± 0.19 | 0.76 ± 0.21 | 0.79 ± 0.15 | 0.61 ± 0.17 | 0.62 ± 0.16 | 0.70 ± 0.16 | 0.75 ± 0.13 |
| TC 14 | 1.38 ± 0.22 | 1.47 ± 0.38 | 1.44 ± 0.32 | 1.22 ± 0.46 | 1.32 ± 0.37 | 1.42 ± 0.31 | 1.26 ± 0.28 |
| Index 1 | A0 | A1 | A2 | A3 | A4 | A5 | A6 |
|---|---|---|---|---|---|---|---|
| MDA 2 | 3.82 ± 1.21 | 3.55 ± 0.77 | 3.69 ± 1.53 | 3.42 ± 0.29 | 3.89 ± 0.90 | 4.44 ± 1.04 | 4.56 ± 0.92 |
| CAT 3 | 0.15 ± 0.02 | 0.24 ± 0.08 | 0.23 ± 0.10 | 0.18 ± 0.06 | 0.23 ± 0.09 | 0.27 ± 0.07 | 0.18 ± 0.05 |
| SOD 4 | 9.04 ± 1.21 | 9.51 ± 1.70 | 10.96 ± 1.05 | 7.86 ± 1.18 | 8.84 ± 1.76 | 8.58 ± 2.07 | 10.25 ± 2.41 |
| GSH-Px 5 | 63.22 ± 9.54 b | 62.98 ± 6.69 b | 65.31 ± 12.71 b | 56.20 ± 8.35 c | 71.43 ± 16.60 b | 59.03 ± 4.47 b | 85.60 ± 15.66 a |
| i-NOS 6 | 1.75 ± 0.34 c | 2.70 ± 0.40 b | 2.37 ± 0.57 bc | 2.76 ± 0.67 b | 2.94 ± 0.28 b | 3.84 ± 0.38 a | 3.05 ± 0.84 b |
| NK-κB 7 | 0.42 ± 0.05 | 0.40 ± 0.02 | 0.41 ± 0.02 | 0.38 ± 0.10 | 0.41 ± 0.09 | 0.44 ± 0.03 | 0.49 ± 0.06 |
| COX-2 8 | 2.67 ± 0.23 | 2.37 ± 0.15 | 2.31 ± 0.27 | 2.28 ± 0.45 | 2.44 ± 0.70 | 2.33 ± 0.08 | 2.75 ± 0.39 |
| Caspase-3 activation 9 | 0.61 ± 0.11 | 0.64 ± 0.14 | 0.73 ± 0.12 | 0.54 ± 0.21 | 0.55 ± 0.25 | 0.51 ± 0.30 | 1.02 ± 0.66 |
| Caspase-9 activation 10 | 0.70 ± 0.16 a | 0.68 ± 0.18 a | 0.61 ± 0.16 a | 0.40 ± 0.17 c | 0.31 ± 0.17 c | 0.44 ± 0.09 b | 0.50 ± 0.06 b |
| Index 1 | A0 | A1 | A2 | A3 | A4 | A5 | A6 |
|---|---|---|---|---|---|---|---|
| Trypsin (U mgprot−1) | 12.77 ± 1.82 | 11.19 ± 1.11 | 11.76 ± 1.10 | 12.09 ± 1.07 | 11.10 ± 1.99 | 12.64 ± 1.65 | 12.38 ± 1.40 |
| Lipase (U gprot−1) | 5.88 ± 0.94 | 5.84 ± 0.91 | 5.05 ± 1.46 | 6.30 ± 0.84 | 5.80 ± 1.50 | 6.59 ± 1.05 | 5.85 ± 2.07 |
| Amylase (U mgprot−1) | 7.01 ± 1.91 | 7.54 ± 1.38 | 7.60 ± 1.06 | 6.20 ± 1.05 | 6.92 ± 1.99 | 8.60 ± 1.19 | 8.28 ± 1.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zheng, L.; Xu, B.; Sagada, G.; Zhang, J.; Shao, Q. Effects of Diets with Varying Astaxanthin from Yarrowia lipolytica Levels on the Growth, Feed Utilization, Metabolic Enzymes Activities, Antioxidative Status and Serum Biochemical Parameters of Litopenaeus vannamei. Fishes 2022, 7, 352. https://doi.org/10.3390/fishes7060352
Liu Y, Zheng L, Xu B, Sagada G, Zhang J, Shao Q. Effects of Diets with Varying Astaxanthin from Yarrowia lipolytica Levels on the Growth, Feed Utilization, Metabolic Enzymes Activities, Antioxidative Status and Serum Biochemical Parameters of Litopenaeus vannamei. Fishes. 2022; 7(6):352. https://doi.org/10.3390/fishes7060352
Chicago/Turabian StyleLiu, Yuechong, Lu Zheng, Bingying Xu, Gladstone Sagada, Jinzhi Zhang, and Qingjun Shao. 2022. "Effects of Diets with Varying Astaxanthin from Yarrowia lipolytica Levels on the Growth, Feed Utilization, Metabolic Enzymes Activities, Antioxidative Status and Serum Biochemical Parameters of Litopenaeus vannamei" Fishes 7, no. 6: 352. https://doi.org/10.3390/fishes7060352
APA StyleLiu, Y., Zheng, L., Xu, B., Sagada, G., Zhang, J., & Shao, Q. (2022). Effects of Diets with Varying Astaxanthin from Yarrowia lipolytica Levels on the Growth, Feed Utilization, Metabolic Enzymes Activities, Antioxidative Status and Serum Biochemical Parameters of Litopenaeus vannamei. Fishes, 7(6), 352. https://doi.org/10.3390/fishes7060352





