Astragalus membranaceus Extract (AME) Enhances Growth, Digestive Enzymes, Antioxidant Capacity, and Immunity of Pangasianodon hypophthalmus Juveniles
Abstract
1. Introduction
2. Materials and Methods
2.1. Herbal Extract and Analysis of Its Bioactive Constituents
2.2. Fish and Adaptation Conditions
2.3. Formulation of AME-Based Diets
2.4. Fish Rearing and Experimental Design
2.5. Water Quality Measurements
2.6. Determination of Growth, Feed Utilization, and Survival Rates
2.7. Proximate Composition of the Whole-Body and Amino Acid Retention
2.8. Sampling Procedures
Serum Collection and Preparation of Tissue Homogenates
2.9. Serum Biochemical Assays
2.10. Serum Immunity Parameters
2.11. Hepatic Antioxidant Biomarkers
2.12. Statistical Analysis
3. Results
3.1. The Phyto-Components, Flavonoids, and Phenolics Present in AME Supplement
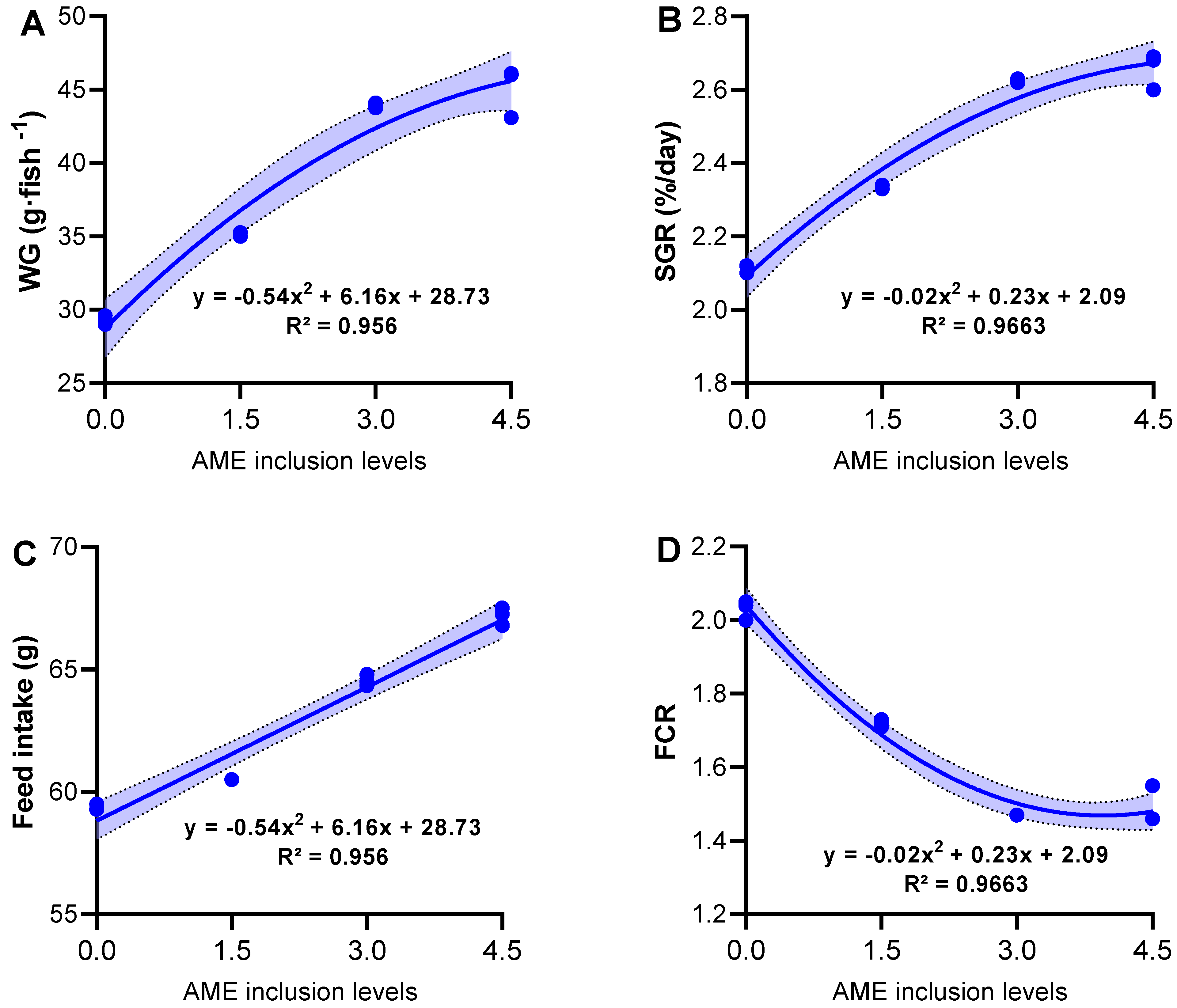
3.2. Growth Performance, Feed Utilization, and Survival Rates
3.3. Whole-Body Proximate Analysis and Amino acid Composition
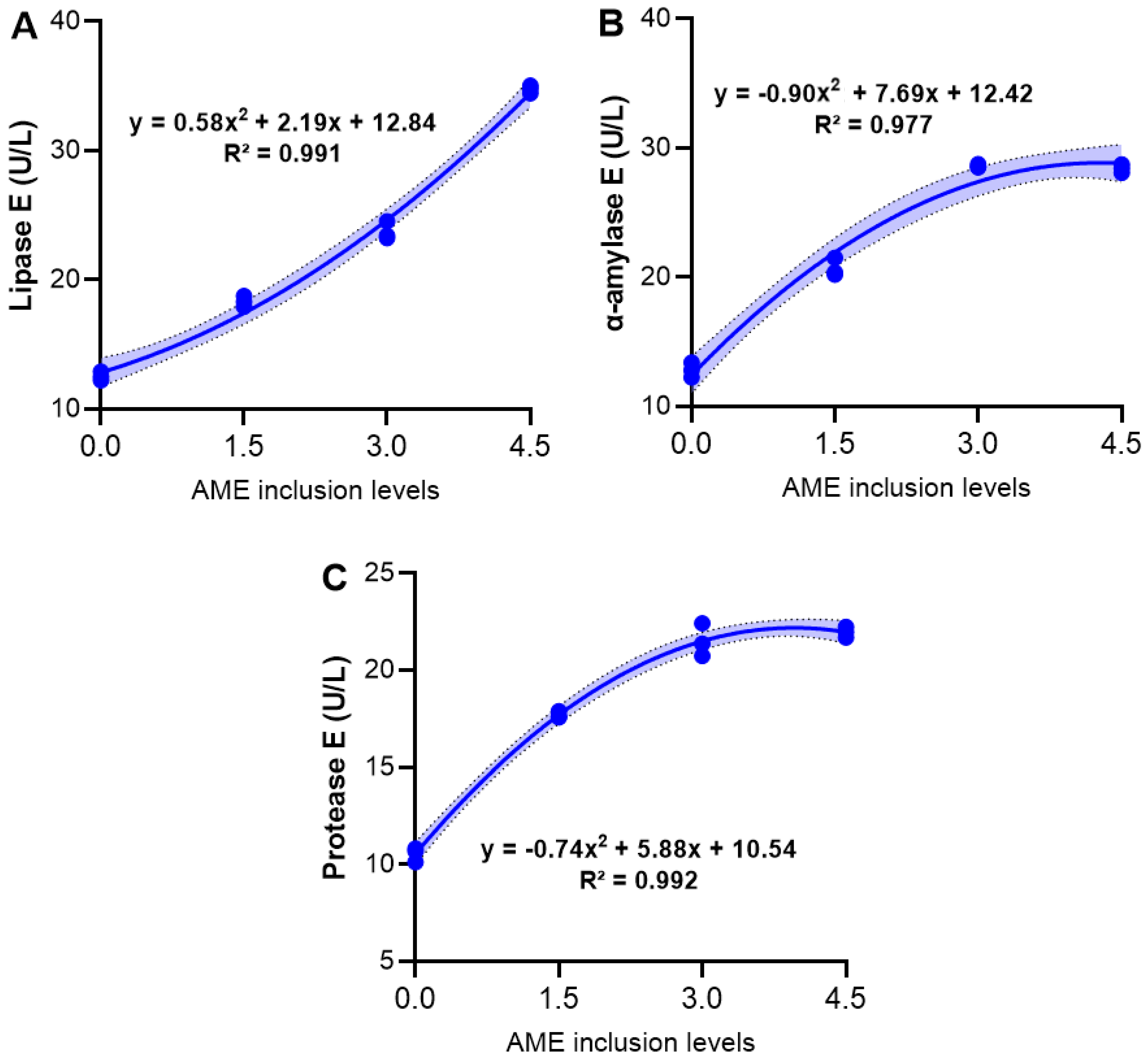
3.4. Digestive Enzymes
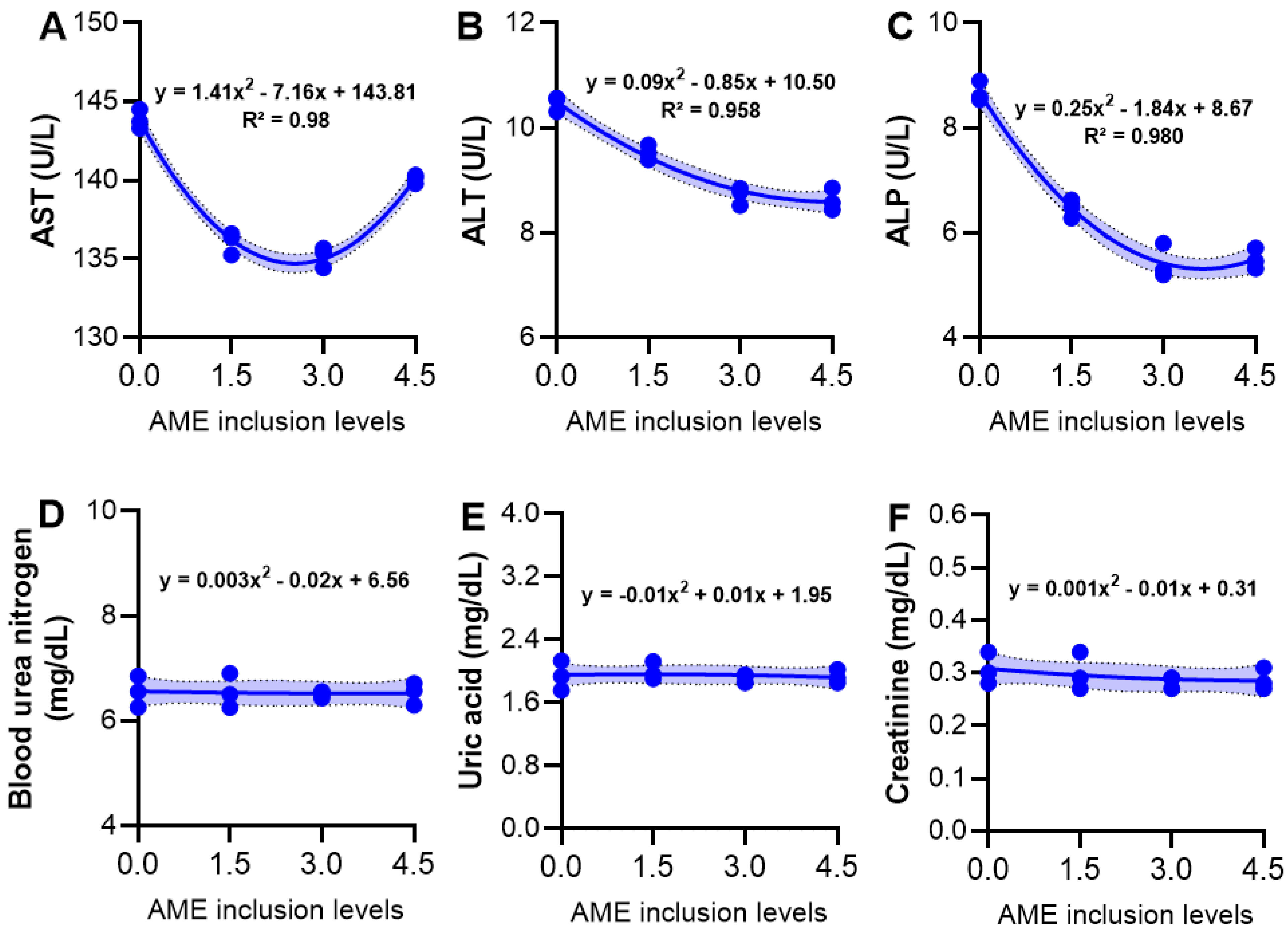
3.5. Serum Biochemical Variables
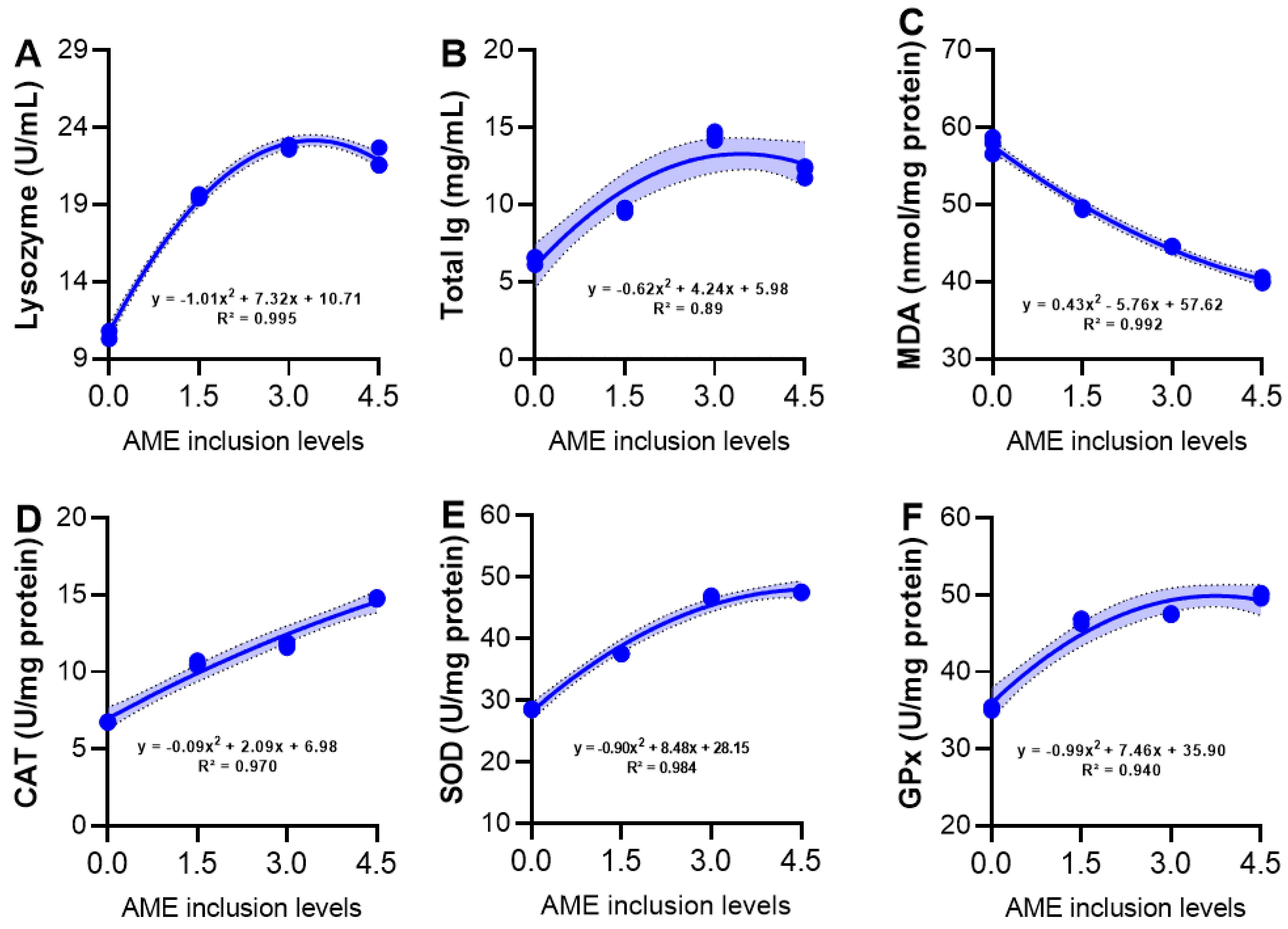
3.6. Serum Immunity and Hepatic Antioxidant Activity
4. Discussion
5. Conclusions and Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, H.; Haque, M.M.; Belton, B. Striped catfish (Pangasianodon hypophthalmus, Sauvage, 1878) aquaculture in Bangladesh: An overview. Aquac. Res. 2013, 44, 950–965. [Google Scholar] [CrossRef]
- Phan, L.T.; Bui, T.M.; Nguyen, T.T.T.; Gooley, G.J.; Ingram, B.A.; Nguyen, H.V.; Nguyen, P.T.; De Silva, S.S. Current status of farming practices of striped catfish, Pangasianodon hypophthalmus in the Mekong Delta, Vietnam. Aquaculture 2009, 296, 227–236. [Google Scholar] [CrossRef]
- De Silva, S.S.; Phuong, N.T. Striped catfish farming in the Mekong Delta, Vietnam: A tumultuous path to a global success. Rev. Aquac. 2011, 3, 45–73. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Chaklader, M.R.; Shukry, M.; Ahmed, H.A.; Khallaf, M.A. A multispecies probiotic modulates growth, digestive enzymes, immunity, hepatic antioxidant activity, and disease resistance of Pangasianodon hypophthalmus fingerlings. Aquaculture 2023, 563, 738948. [Google Scholar] [CrossRef]
- Dong, H.T.; Nguyen, V.V.; Phiwsaiya, K.; Gangnonngiw, W.; Withyachumnarnkul, B.; Rodkhum, C.; Senapin, S. Concurrent infections of Flavobacterium columnare and Edwardsiella ictaluri in striped catfish, Pangasianodon hypophthalmus in Thailand. Aquaculture 2015, 448, 142–150. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Abdel-Daim, M.M.; Shukry, M.; Nowosad, J.; Kucharczyk, D. Benefits and applications of Moringa oleifera as a plant protein source in Aquafeed: A review. Aquaculture 2022, 547, 737369. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Hendam, B.M.; Nofal, M.I.; El-Son, M.A.M. Ginkgo biloba leaf extract improves growth, intestinal histomorphometry, immunity, antioxidant status and modulates transcription of cytokine genes in hapa-reared Oreochromis niloticus. Fish Shellfish Immunol. 2021, 117, 339–349. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Shukry, M.; Noreldin, A.E.; Ahmed, H.A.; El-Bahrawy, A.; Ghetas, H.A.; Khalifa, E. Milk thistle (Silybum marianum) extract improves growth, immunity, serum biochemical indices, antioxidant state, hepatic histoarchitecture, and intestinal histomorphometry of striped catfish, Pangasianodon hypophthalmus. Aquaculture 2023, 562, 738761. [Google Scholar] [CrossRef]
- Nhu, T.Q.; Bich Hang, B.T.; Bach, L.T.; Buu Hue, B.T.; Quetin-Leclercq, J.; Scippo, M.-L.; Phuong, N.T.; Kestemont, P. Plant extract-based diets differently modulate immune responses and resistance to bacterial infection in striped catfish (Pangasianodon hypophthalmus). Fish Shellfish Immunol. 2019, 92, 913–924. [Google Scholar] [CrossRef]
- Nhu, T.Q.; Bich Hang, B.T.; Cornet, V.; Oger, M.; Bach, L.T.; Anh Dao, N.L.; Thanh Huong, D.T.; Quetin-Leclercq, J.; Scippo, M.-L.; Phuong, N.T.; et al. Single or Combined Dietary Supply of Psidium guajava and Phyllanthus amarus Extracts Differentially Modulate Immune Responses and Liver Proteome in Striped Catfish (Pangasianodon hyphophthalmus). Front. Immunol. 2020, 11, 797. [Google Scholar] [CrossRef]
- Maiti, S.; Saha, S.; Jana, P.; Chowdhury, A.; Khatua, S.; Ghosh, T.K. Effect of dietary Andrographis paniculata leaf extract on growth, immunity, and disease resistance against Aeromonas hydrophila in Pangasianodon hypopthalmus. J. Appl. Aquac. 2021, 1–25. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Z.; Huang, L.; Zheng, S.; Wang, D.; Chen, S.; Zhang, H.; Yang, S. Review of the Botanical Characteristics, Phytochemistry, and Pharmacology of Astragalus membranaceus (Huangqi). Phytother. Res. 2014, 28, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Zhao, K.; Huang, Q.; Shang, P. Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int. J. Biol. Macromol. 2014, 64, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ren, W.; Zhang, L.; Zhang, Y.; Liu, D.; Liu, Y. A review of the pharmacological action of Astragalus polysaccharide. Front. Pharmacol. 2020, 11, 349. [Google Scholar] [CrossRef]
- Ardó, L.; Yin, G.; Xu, P.; Váradi, L.; Szigeti, G.; Jeney, Z.; Jeney, G. Chinese herbs (Astragalus membranaceus and Lonicera japonica) and boron enhance the non-specific immune response of Nile tilapia (Oreochromis niloticus) and resistance against Aeromonas hydrophila. Aquaculture 2008, 275, 26–33. [Google Scholar] [CrossRef]
- Elabd, H.; Wang, H.-P.; Shaheen, A.; Yao, H.; Abbass, A. Feeding Glycyrrhiza glabra (liquorice) and Astragalus membranaceus (AM) alters innate immune and physiological responses in yellow perch (Perca flavescens). Fish Shellfish Immunol. 2016, 54, 374–384. [Google Scholar] [CrossRef]
- Elabd, H.; Wang, H.-P.; Shaheen, A.; Yao, H.; Abbass, A. Astragalus membranaceus (AM) enhances growth performance and antioxidant stress profiles in bluegill sunfish (Lepomis macrochirus). Fish Physiol. Biochem. 2016, 42, 955–966. [Google Scholar] [CrossRef]
- Angela, C.; Wang, W.; Lyu, H.; Zhou, Y.; Huang, X. The effect of dietary supplementation of Astragalus membranaceus and Bupleurum chinense on the growth performance, immune-related enzyme activities and genes expression in white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2020, 107, 379–384. [Google Scholar] [CrossRef]
- Sun, Z.; Wei, Z.; Liu, Q.; Mai, H.; Liu, Y.; Liu, B.; Tan, X.; Ye, C. Effects of dietary Astragalus membranaceus (Fisch.) Bge. root extract on growth performance, plasma biochemical parameters, fish composition, liver and intestinal morphology, and genes expression in head kidney of hybrid grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus♀). Aquac. Rep. 2022, 22, 100934. [Google Scholar] [CrossRef]
- Liu, Y.; Miao, Y.; Xu, N.; Ding, T.; Cui, K.; Chen, Q.; Zhang, J.; Fang, W.; Mai, K.; Ai, Q. Effects of dietary Astragalus polysaccharides (APS) on survival, growth performance, activities of digestive enzyme, antioxidant responses and intestinal development of large yellow croaker (Larimichthys crocea) larvae. Aquaculture 2020, 517, 734752. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Zhou, H.; Mai, K.; He, G. Dietary Astragalus polysaccharides ameliorates the growth performance, antioxidant capacity and immune responses in turbot (Scophthalmus maximus L.). Fish Shellfish Immunol. 2020, 99, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Wu, S. Dietary Astragalus membranaceus polysaccharide ameliorates the growth performance and innate immunity of juvenile crucian carp (Carassius auratus). Int. J. Biol. Macromol. 2020, 149, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ran, C.; Wei, K.; Xie, Y.; Xie, M.; Zhou, W.; Yang, Y.; Zhang, Z.; Lv, H.; Ma, X.; et al. The effect of Astragalus polysaccharide on growth, gut and liver health, and anti-viral immunity of zebrafish. Aquaculture 2021, 540, 736677. [Google Scholar] [CrossRef]
- Shi, F.; Lu, Z.; Yang, M.; Li, F.; Zhan, F.; Zhao, L.; Li, Y.; Li, Q.; Li, J.; Li, J.; et al. Astragalus polysaccharides mediate the immune response and intestinal microbiota in grass carp (Ctenopharyngodon idellus). Aquaculture 2021, 534, 736205. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Devi, G.; Doan, H.V.; Tapingkae, W.; Balasundaram, C.; Arockiaraj, J.; Ringø, E. Changes in immune genes expression, immune response, digestive enzymes -antioxidant status, and growth of catla (Catla catla) fed with Astragalus polysaccharides against edwardsiellosis disease. Fish Shellfish Immunol. 2022, 121, 418–436. [Google Scholar] [CrossRef]
- Pu, Y.; Wu, S. The growth performance, body composition and nonspecific immunity of white shrimps (Litopenaeus vannamei) affected by dietary Astragalus membranaceus polysaccharide. Int. J. Biol. Macromol. 2022, 209, 162–165. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Khalil, S.R.; El-Hady, E.W.; Elhady, W.M.; Ismail, T.A.; Marini, C.; Di Cerbo, A.; Abdel-Latif, H.M.R. Immunosuppressive Effects of Thallium Toxicity in Nile Tilapia Fingerlings: Elucidating the Rescue Role of Astragalus membranaceus Polysaccharides. Front. Vet. Sci. 2022, 9, 843031. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Khalil, S.R.; Moustafa, A.A.; Mahmoud, H.K.; Abdel-Latif, H.M.R. Astragalus membranaceus polysaccharides modulate growth, hemato-biochemical indices, hepatic antioxidants, and expression of HSP70 and apoptosis-related genes in Oreochromis niloticus exposed to sub-lethal thallium toxicity. Fish Shellfish Immunol. 2021, 118, 251–260. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Dawood, M.A.O.; Menanteau-Ledouble, S.; El-Matbouli, M. The nature and consequences of co-infections in tilapia: A review. J. Fish Dis. 2020, 43, 651–664. [Google Scholar] [CrossRef]
- Gomathi, D.; Kalaiselvi, M.; Ravikumar, G.; Devaki, K.; Uma, C. GC-MS analysis of bioactive compounds from the whole plant ethanolic extract of Evolvulus alsinoides (L.) L. J. Food Sci. Technol. 2015, 52, 1212–1217. [Google Scholar] [CrossRef]
- Mattila, P.; Astola, J.; Kumpulainen, J. Determination of Flavonoids in Plant Material by HPLC with Diode-Array and Electro-Array Detections. J. Agric. Food Chem. 2000, 48, 5834–5841. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, N.; Tunçel, M.; Tunçel, N.B. Determination of Phenolic Acids by a Modified HPLC: Its Application to Various Plant Materials. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 587–596. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Fish and Shrimp; National Academy Press: Washington, DC, USA, 2011; 392p. [Google Scholar]
- AOAC. Official Methods of Analysis, 13th ed.; Association of Analytical Chemists: Washington, DC, USA, 2012; 1018p. [Google Scholar]
- Belfield, A.; Goldberg, D. Colorimetric determination of alkaline phosphatase activity. Enzyme 1971, 12, 561–568. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Bernfeld, P. Amylases, α and β. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1955; Volume 1, pp. 149–158. [Google Scholar]
- Khantaphant, S.; Benjakul, S. Comparative study on the proteases from fish pyloric caeca and the use for production of gelatin hydrolysate with antioxidative activity. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 151, 410–419. [Google Scholar] [CrossRef]
- Shihabi, Z.K.; Bishop, C. Simplified Turbidimetric Assay for Lipase Activity. Clin. Chem. 1971, 17, 1150–1153. [Google Scholar] [CrossRef]
- Barham, D.; Trinder, P. Enzymatic determination of uric acid. Analyst 1972, 97, 142–145. [Google Scholar] [CrossRef]
- Coulombe, J.J.; Favreau, L. A new simple semimicro method for colorimetric determination of urea. Clin. Chem. 1963, 9, 102–108. [Google Scholar] [CrossRef]
- Heinegård, D.; Tiderström, G. Determination of serum creatinine by a direct colorimetric method. Clin. Chim. Acta 1973, 43, 305–310. [Google Scholar] [CrossRef]
- Ellis, A. Lysozyme assays. Tech. Fish Immunol. 1990, 1, 101–103. [Google Scholar]
- Cuesta, A.; Meseguer, J.; Esteban, M.A. Total serum immunoglobulin M levels are affected by immunomodulators in seabream (Sparus aurata L.). Vet. Immunol. Immunopathol. 2004, 101, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Siwicki, A.; Anderson, D. An easy spectrophotometric assay for determining total protein and immunoglobulin levels in fish sera: Correlation to fish health. Tech. Fish Immunol. 1993, 3, 23–30. [Google Scholar]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- McCord, J.M.; Fridovich, I. Superoxide Dismutase: An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Wu, F.; Yang, C.; Wen, H.; Zhang, C.; Jiang, M.; Liu, W.; Tian, J.; Yu, L.; Lu, X. Improving low-temperature stress tolerance of tilapia, Oreochromis niloticus: A functional analysis of Astragalus membranaceus. J. World Aquac. Soc. 2019, 50, 749–762. [Google Scholar] [CrossRef]
- Xiang, X.; Chen, J.; Zhou, X.; Wang, W. Effect of astragalus polysaccharides on growth, body composition and immune index in Schizothorax prenanti. Acta Hydrobiol. Sin. 2011, 35, 291–299. [Google Scholar] [CrossRef]
- Zahran, E.; Risha, E.; AbdelHamid, F.; Mahgoub, H.A.; Ibrahim, T. Effects of dietary Astragalus polysaccharides (APS) on growth performance, immunological parameters, digestive enzymes, and intestinal morphology of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2014, 38, 149–157. [Google Scholar] [CrossRef]
- Lin, S.-M.; Jiang, Y.; Chen, Y.-J.; Luo, L.; Doolgindachbaporn, S.; Yuangsoi, B. Effects of Astragalus polysaccharides (APS) and chitooligosaccharides (COS) on growth, immune response and disease resistance of juvenile largemouth bass, Micropterus salmoides. Fish Shellfish Immunol. 2017, 70, 40–47. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Yousefi, M.; Karimi, M.; Fadaei Raieni, R.; Dadar, M.; Yilmaz, S.; Dawood, M.A.O.; Abdel-Latif, H.M.R. Benefits of Dietary Polyphenols and Polyphenol-Rich Additives to Aquatic Animal Health: An Overview. Rev. Fish. Sci. Aquac. 2021, 29, 478–511. [Google Scholar] [CrossRef]
- Citarasu, T. Herbal biomedicines: A new opportunity for aquaculture industry. Aquac. Int. 2010, 18, 403–414. [Google Scholar] [CrossRef]
- Bratkov, V.M.; Shkondrov, A.M.; Zdraveva, P.K.; Krasteva, I.N. Flavonoids from the Genus Astragalus: Phytochemistry and Biological Activity. Pharm. Rev. 2016, 10, 11–32. [Google Scholar] [CrossRef]
- Matkowski, A.; Woźniak, D.; Lamer-Zarawska, E.; Oszmiański, J.; Leszczyńska, A. Flavonoids and Phenol Carboxylic Acids in the Oriental Medicinal Plant Astragalus membranaceus Acclimated in Poland. Z. Für Nat. C 2003, 58, 602–604. [Google Scholar] [CrossRef]
- Güroy, B.; Mantoğlu, S.; Kayalı, S.; Şahin, İ. Effect of dietary Yucca schidigera extract on growth, total ammonia–nitrogen excretion and haematological parameters of juvenile striped catfish Pangasianodon hypophthalmus. Aquac. Res. 2014, 45, 647–654. [Google Scholar] [CrossRef]
- Güroy, B.; Mantoğlu, S.; Merrifield, D.L.; Guroy, D. Effects of dietary Nutrafito Plus on growth, haemotological parameters and total ammonia-nitrogen excretion of juvenile striped catfish Pangasianodon hypophthalmus. Aquac. Res. 2016, 47, 1770–1777. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Dadar, M.; Ringø, E. Modulation of nutrient digestibility and digestive enzyme activities in aquatic animals: The functional feed additives scenario. Aquac. Res. 2017, 48, 3987–4000. [Google Scholar] [CrossRef]
- Shi, H.-T.; Zhao, S.-Z.; Wang, K.-L.; Fan, M.-X.; Han, Y.-Q.; Wang, H.-L. Effects of dietary Astragalus membranaceus supplementation on growth performance, and intestinal morphology, microbiota and metabolism in common carp (Cyprinus carpio). Aquac. Rep. 2022, 22, 100955. [Google Scholar] [CrossRef]
- Rudy, A.N.; Meylianawati, O.F.A.; Yanti, P.S.; Esti, H.H. The effects of dietary Eleutherine bulbosa on the growth, leukocyte profile, and digestive enzyme activity of the striped catfish Pangasianodon hypophthalmus. Nusant. Biosci. 2018, 10, 46–51. [Google Scholar]
- Yu, W.; Yang, Y.; Zhou, Q.; Huang, X.; Huang, Z.; Li, T.; Wu, Q.; Zhou, C.; Ma, Z.; Lin, H. Effects of dietary Astragalus polysaccharides on growth, health and resistance to Vibrio harveyi of Lates calcarifer. Int. J. Biol. Macromol. 2022, 207, 850–858. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Abdel-Tawwab, M.; Khafaga, A.F.; Dawood, M.A.O. Dietary oregano essential oil improved the growth performance via enhancing the intestinal morphometry and hepato-renal functions of common carp (Cyprinus carpio L.) fingerlings. Aquaculture 2020, 526, 735432. [Google Scholar] [CrossRef]
- Bruslé, J.; Anadon, G.G. The structure and function of fish liver. Fish Morphol. 1996, 76, 545–551. [Google Scholar]
- Jia, R.; Cao, L.; Xu, P.; Jeney, G.; Yin, G. In vitro and in vivo hepatoprotective and antioxidant effects of Astragalus polysaccharides against carbon tetrachloride-induced hepatocyte damage in common carp (Cyprinus carpio). Fish Physiol. Biochem. 2012, 38, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Davila, J.C.; Lenherr, A.; Acosta, D. Protective effect of flavonoids on drug-induced hepatotoxicity in vitro. Toxicology 1989, 57, 267–286. [Google Scholar] [CrossRef]
- Zhen-Lun, Z.; Qi-Zhen, W.; Chang-Xiao, L. Hepatoprotective effects of Astraglus root. J. Ethnopharmacol. 1990, 30, 145–149. [Google Scholar] [CrossRef]
- Hamid, M.; Liu, D.; Abdulrahim, Y.; Liu, Y.; Qian, G.; Khan, A.; Gan, F.; Huang, K. Amelioration of CCl4-induced liver injury in rats by selenizing Astragalus polysaccharides: Role of proinflammatory cytokines, oxidative stress and hepatic stellate cells. Res. Vet. Sci. 2017, 114, 202–211. [Google Scholar] [CrossRef]
- Shahzad, M.; Shabbir, A.; Wojcikowski, K.; Wohlmuth, H.; C Gobe, G. The antioxidant effects of Radix Astragali (Astragalus membranaceus and related species) in protecting tissues from injury and disease. Curr. Drug Targets 2016, 17, 1331–1340. [Google Scholar] [CrossRef]
- Nelson, K.; Jones, J.; Jacobson, S.; Reimschuessel, R. Elevated Blood Urea Nitrogen (BUN) Levels in Goldfish as an Indicator of Gill Dysfunction. J. Aquat. Anim. Health 1999, 11, 52–60. [Google Scholar] [CrossRef]
- Zhang, H.W.; Lin, Z.X.; Xu, C.; Leung, C.; Chan, L.S. Astragalus (a traditional Chinese medicine) for treating chronic kidney disease. Cochrane Database Syst. Rev. 2014, CD008369. [Google Scholar] [CrossRef]
- Li, M.; Wang, W.; Xue, J.; Gu, Y.; Lin, S. Meta-analysis of the clinical value of Astragalus membranaceus in diabetic nephropathy. J. Ethnopharmacol. 2011, 133, 412–419. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, X.; Li, C.; Fu, P. Systematic review of the renal protective effect of Astragalus membranaceus (root) on diabetic nephropathy in animal models. J. Ethnopharmacol. 2009, 126, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.B.; Ingram, G.A. Noncellular nonspecific defence mechanisms of fish. Annu. Rev. Fish Dis. 1992, 2, 249–279. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, M.; Cheng, A.; Hao, E.; Huang, X.; Chen, X. Immunomodulatory and antioxidant effects of Astragalus polysaccharide liposome in large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2020, 100, 126–136. [Google Scholar] [CrossRef]
- Wang, X.; Ding, L.; Yan, M.; Chai, X.; Lu, R.; Wang, Q.; Li, F. Polysaccharides, saponins, and water decoction of Astragalus membranaceus significantly enhance the non-specific immune response of spotted maigre (Nibea albiflora). Isr. J. Aquac.—Bamidgeh 2012, 64, 1–6. [Google Scholar] [CrossRef]
- Chakraborty, S.B.; Hancz, C. Application of phytochemicals as immunostimulant, antipathogenic and antistress agents in finfish culture. Rev. Aquac. 2011, 3, 103–119. [Google Scholar] [CrossRef]
- Qi, Y.; Gao, F.; Hou, L.; Wan, C. Anti-inflammatory and immunostimulatory activities of astragalosides. Am. J. Chin. Med. 2017, 45, 1157–1167. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saikia, S.K. Oxidative Stress in Fish: A Review. J. Sci. Res. 2020, 12, 145–160. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Yousefi, S.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Maradonna, F.; Carnevali, O. Oxidative Stress and Antioxidant Defense in Fish: The Implications of Probiotic, Prebiotic, and Synbiotics. Rev. Fish. Sci. Aquac. 2021, 29, 198–217. [Google Scholar] [CrossRef]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant Defenses in Fish: Biotic and Abiotic Factors. Rev. Fish Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Li, R.; Chen, W.-C.; Wang, W.-P.; Tian, W.-Y.; Zhang, X.-G. Antioxidant activity of Astragalus polysaccharides and antitumour activity of the polysaccharides and siRNA. Carbohydr. Polym. 2010, 82, 240–244. [Google Scholar] [CrossRef]
- Bors, W.; Michel, C.; Stettmaier, K. Antioxidant effects of flavonoids. BioFactors 1997, 6, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Huyut, Z.; Beydemir, Ş.; Gülçin, İ. Antioxidant and Antiradical Properties of Selected Flavonoids and Phenolic Compounds. Biochem. Res. Int. 2017, 2017, 7616791. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
| Ration Elements | % DM Basis |
|---|---|
| Fish meal (FM; 72% CP) 1 | 10.0 |
| Soybean meal (SBM; 46% CP) 2 | 34.0 |
| Corn gluten meal (CGM; 60% CP) | 3.5 |
| Rice bran | 14.0 |
| Yellow corn meal | 15.0 |
| Wheat bran | 9.0 |
| Wheat flour | 13.0 |
| Sunflower oil | 0.70 |
| Vitamin and Mineral premix 3 | 0.30 |
| Di-calcium phosphate | 0.50 |
| Total | 100 |
| Chemical composition (% on DM basis) | |
| Dry matter (DM) | 90.19 |
| Crude protein (CP) | 30.82 |
| Ether extract (EE) | 6.93 |
| Ash | 6.59 |
| Crude fiber (CF) | 8.81 |
| Nitrogen-free extract (NFE) 4 | 46.85 |
| Gross energy (GE; KJ/g diet DM) 5 | 18.06 |
| Protein to energy ratio (P/E ratio) 6 | 17.06 |
| Phytochemicals | RT (min) | Concentration (µg/mL) |
|---|---|---|
| Flavonoids | ||
| Rutin | 4.6 | 5.23 |
| Catechin | 12.01 | 4.14 |
| Quercetin | 6.9 | 9.21 |
| Kaempferol | 8.1 | 4.05 |
| Luteolin | 9.0 | 6.09 |
| Chrysoeriol | 15.0 | 19.08 |
| Naringin | 5.2 | 4.12 |
| Apigenin | 10.0 | 12.45 |
| Phenolics | ||
| Syringic acid | 3.0 | 8.69 |
| Caffeic acid | 4.7 | 3.22 |
| Ferulic acid | 6.8 | 5.66 |
| Protocatechuic acid | 7.8 | 3.31 |
| Gallic acid | 9.0 | 3.77 |
| Ellagic acid | 11.0 | 6.33 |
| p-Coumaric acid | 4.0 | 6.14 |
| Resveratrol | 13.8 | 7.01 |
| Vanillic acid | 14.6 | 6.79 |
| Gentisic acid | 2.0 | 6.55 |
| Parameters | AST0.0 | AST1.5 | AST3.0 | AST4.5 |
|---|---|---|---|---|
| Moisture (%) | 73.20 ± 0.20 | 73.55 ± 0.71 | 74.47 ± 0.75 | 73.69 ± 0.64 |
| Crude protein (%) | 14.24 ± 0.21 | 14.68 ± 0.21 | 14.58 ± 0.29 | 14.63 ± 0.52 |
| Ether extract (%) | 4.60 ± 0.17 | 4.62 ± 0.24 | 4.66 ± 0.20 | 4.54 ± 0.21 |
| Ash (%) | 3.10 ± 0.03 | 3.17 ± 0.03 | 3.15 ± 0.02 | 3.17 ± 0.03 |
| Amino Acid Content | Experimental Groups | |||
|---|---|---|---|---|
| AME0.0 | AME1.5 | AME3.0 | AME4.5 | |
| Essential amino acids (% of total amino acids) | ||||
| Threonine | 2.86 ± 0.82 | 2.07 ± 0.01 | 2.11 ± 0.06 | 2.16 ± 0.03 |
| Valine | 3.11 ± 0.69 | 2.56 ± 0.02 | 2.54 ± 0.07 | 2.65 ± 0.08 |
| Methionine | 1.00 ± 0.06 | 1.10 ± 0.01 | 1.12 ± 0.05 | 1.16 ± 0.08 |
| Phenylalanine | 1.67 ± 0.14 | 1.84 ± 0.03 | 1.93 ± 0.10 | 2.00 ± 0.19 |
| Lysine | 3.25 ± 0.11 | 3.33 ± 0.03 | 3.42 ± 0.12 | 3.51 ± 0.13 |
| Leucine | 3.18 ± 0.07 | 3.27 ± 0.02 | 3.35 ± 0.11 | 3.46 ± 0.11 |
| Histidine | 0.99 ± 0.04 | 1.14 ± 0.04 | 1.11 ± 0.04 | 1.12 ± 0.01 |
| Arginine | 3.41 ± 0.02 | 3.44 ± 0.01 | 3.53 ± 0.07 | 3.52 ± 0.01 |
| Tryptophan | 0.31 ± 0.01 | 0.34 ± 0.003 | 0.36 ± 0.04 | 0.41 ± 0.04 |
| Non-essential amino acids (% of total amino acids) | ||||
| Aspartic Acid | 4.55 ± 0.04 | 4.68 ± 0.03 | 4.70 ± 0.10 | 4.77 ± 0.04 |
| Alanine | 3.63 ± 0.61 | 4.25 ± 0.02 | 4.23 ± 0.02 | 4.22 ± 0.11 |
| Isoleucine | 1.63 ± 0.09 | 1.72 ± 0.02 | 1.81 ± 0.08 | 1.87 ± 0.15 |
| Serine | 2.20 ± 0.10 | 2.08 ± 0.04 | 2.15 ± 0.03 | 2.16 ± 0.07 |
| Glutamate | 6.67 ± 0.10 | 6.50 ± 0.10 | 6.62 ± 0.10 | 6.76 ± 0.17 |
| Glycine | 5.76 ± 0.15 | 5.58 ± 0.02 | 5.71 ± 0.04 | 5.52 ± 0.14 |
| Tyrosine | 1.32 ± 0.06 | 1.23 ± 0.03 | 1.31 ± 0.04 | 1.29 ± 0.03 |
| Proline | 2.81 ± 0.11 | 2.70 ± 0.01 | 2.79 ± 0.03 | 2.75 ± 0.07 |
| Cysteine | 0.40 ± 0.01 | 0.40 ± 0.01 | 0.42 ± 0.01 | 0.45 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Latif, H.M.R.; Ahmed, H.A.; Shukry, M.; Chaklader, M.R.; Saleh, R.M.; Khallaf, M.A. Astragalus membranaceus Extract (AME) Enhances Growth, Digestive Enzymes, Antioxidant Capacity, and Immunity of Pangasianodon hypophthalmus Juveniles. Fishes 2022, 7, 319. https://doi.org/10.3390/fishes7060319
Abdel-Latif HMR, Ahmed HA, Shukry M, Chaklader MR, Saleh RM, Khallaf MA. Astragalus membranaceus Extract (AME) Enhances Growth, Digestive Enzymes, Antioxidant Capacity, and Immunity of Pangasianodon hypophthalmus Juveniles. Fishes. 2022; 7(6):319. https://doi.org/10.3390/fishes7060319
Chicago/Turabian StyleAbdel-Latif, Hany M. R., Hamada A. Ahmed, Mustafa Shukry, Md Reaz Chaklader, Rasha M. Saleh, and Mohamed A. Khallaf. 2022. "Astragalus membranaceus Extract (AME) Enhances Growth, Digestive Enzymes, Antioxidant Capacity, and Immunity of Pangasianodon hypophthalmus Juveniles" Fishes 7, no. 6: 319. https://doi.org/10.3390/fishes7060319
APA StyleAbdel-Latif, H. M. R., Ahmed, H. A., Shukry, M., Chaklader, M. R., Saleh, R. M., & Khallaf, M. A. (2022). Astragalus membranaceus Extract (AME) Enhances Growth, Digestive Enzymes, Antioxidant Capacity, and Immunity of Pangasianodon hypophthalmus Juveniles. Fishes, 7(6), 319. https://doi.org/10.3390/fishes7060319









