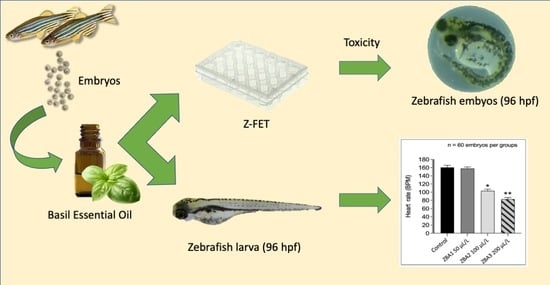
Evaluation of Anaesthetic Effect of Commercial Basil Ocimum basilicum on Zebrafish (Danio rerio) Embryos
Abstract
1. Introduction
2. Materials and Methods
2.1. Ocimum basilicum Composition
2.2. Fish Management
2.3. ZFET Trial
2.4. Anesthetic Trial
2.5. Heart Rate Determination
2.6. Statistical Analysis
3. Results
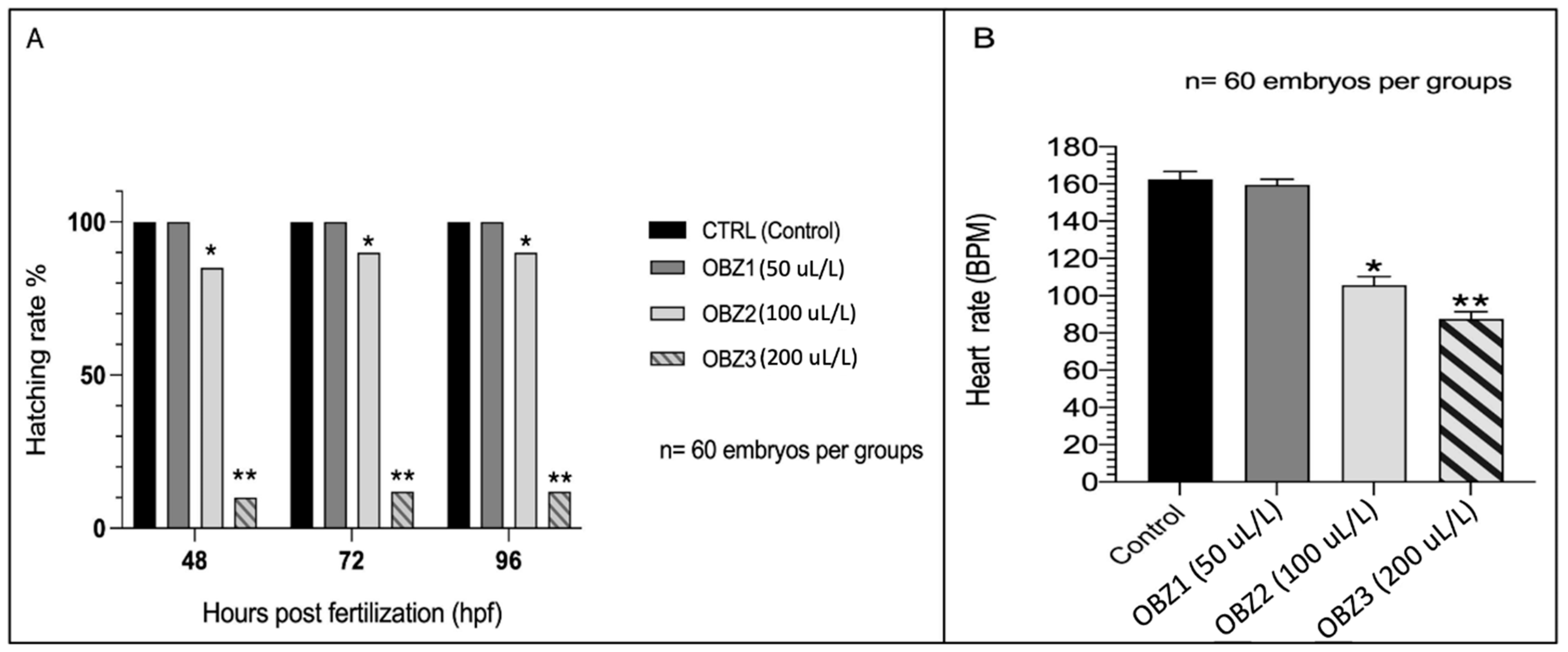
3.1. ZFET Trial
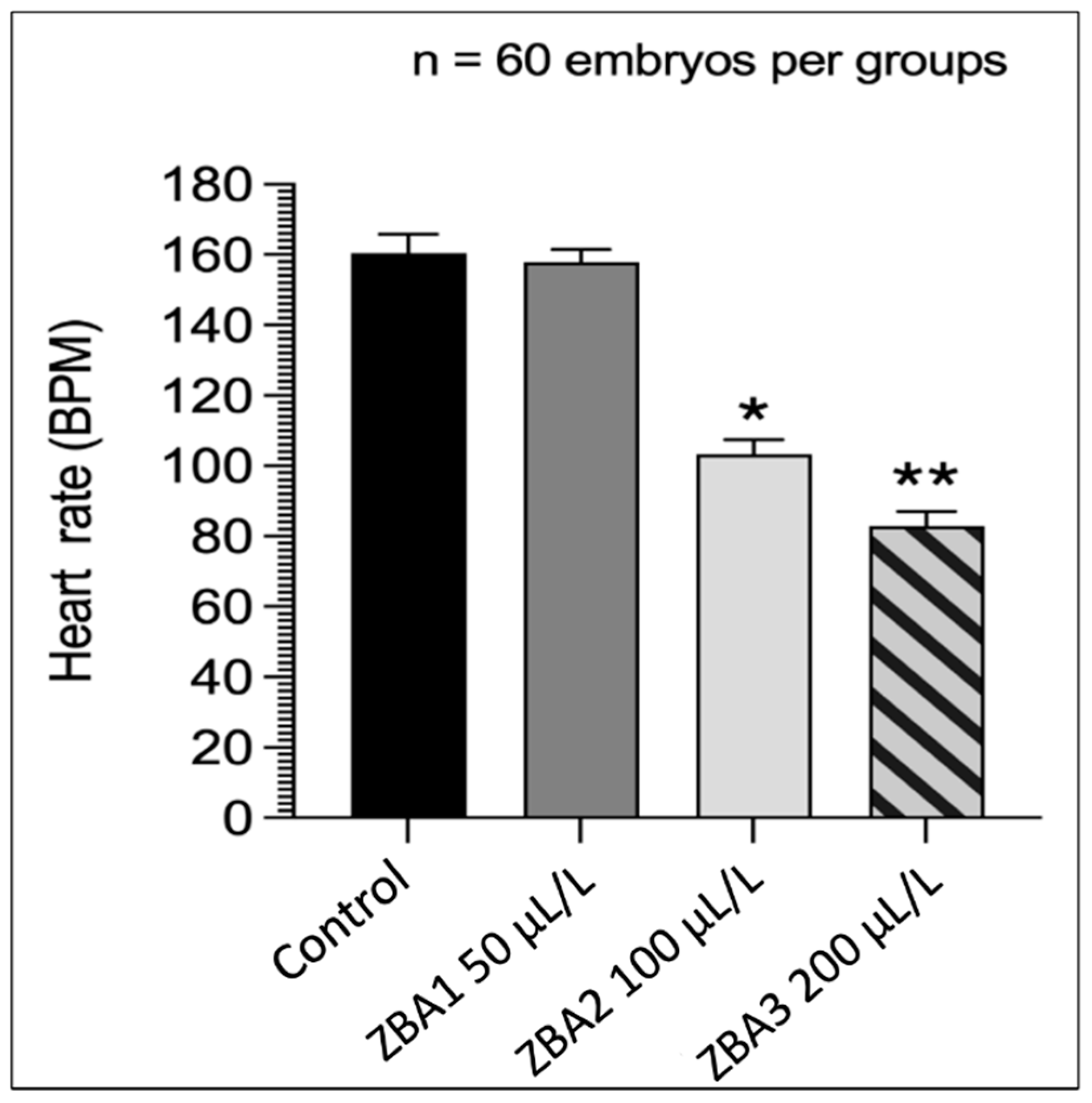
3.2. Anesthetic Trial
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.J.; Umano, K.; Shibamoto, T.; Lee, K.G. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus Vulgaris L.) and their antioxidant properties. Food Chem. 2005, 91, 131–137. [Google Scholar] [CrossRef]
- Dumbravă, D.G.; Moldovan, C.; Raba, D.N.; Popa, M.V. Vitamin C, chlorophylls, carotenoids and xanthophylls content in some basil (Ocimum basilicum L.) and rosemary (Rosmarinus officinalis L.) leaves extracts. J. Agroaliment. Process. Technol. 2012, 18, 253–258. [Google Scholar]
- Delille, L. Les Plantes Médicinales D’Algérie; BERTI: Algiers, Algeria, 2007; pp. 47–48. [Google Scholar]
- Adigüzel, A.; Medine, G.; Meryem, B.; Hatice, U.T.C.; Fikrettin, A.; Üsa, K. Antimicrobial effects of Ocimum basilicum (Labiatae) extract. Turk. J. Biol. 2005, 29, 155–160. [Google Scholar]
- Gaio, I.; Saggiorato, A.G.; Treichel, H.; Cichoski, A.J.; Astolfi, V.; Cardoso, R.I.; Toniazzo, G.; Valduga, E.; Paroul, N.; Cansian, R.L. Antibacterial activity of basil essential oil (Ocimum basilicum L.) in Italian-type sausage. J. Verbrauch. Lebensm. 2015, 10, 323–329. [Google Scholar] [CrossRef]
- Silva, L.L.; Garlet, Q.I.; Koakoski, G.; Oliveira, T.A.; Barcellos, L.J.G.; Baldisserotto, B.; Heinzmann, B.M. Effects of anesthesia with the essential oil of Ocimum gratissimum L. in parameters of fish stress. Rev. Bras. Plantas Med. 2015, 17, 215–223. [Google Scholar] [CrossRef]
- Arranz, E.; Jaime, L.; López de las Hazas, M.C.; Reglero, G.; Santoyo, S. Supercritical fluid extraction as an alternative process to obtain essential oils with anti-inflammatory properties from marjoram and sweet basil. Ind. Crop. Prod. 2015, 67, 121–129. [Google Scholar] [CrossRef]
- Nahak, G.; Sahu, R.K. Immunostimulatory effect of Ocimum sanctum linn. leaf extract in Clarias batrachus liNN. A. J. Pharm. Cli. Res. 2014, 7, 157–163. [Google Scholar]
- Amirkhani, N.; Firouzbakhsh, F. Protective effects of basil (Ocimum basilicum) ethanolic extract supplementation diets against experimental Aeromonas hydrophila infection in common carp (Cyprinus carpio). Aquac. Res. 2015, 46, 716–724. [Google Scholar] [CrossRef]
- Danesi, F.; Elementi, S.; Neri, R.; Maranesi, M.; D’Antuono, L.F.; Bordoni, A. Effect of cultivar on the protection of cardiomyocytes from oxidative stress by essential oils and aqueous extracts of basil (Ocimum basilicum L.). J. Agric. Food Chem. 2008, 12, 9911–9917. [Google Scholar] [CrossRef]
- Mahapatra, S.K.; Roy, S. Phytopharmacological approach of free radical scavenging and anti-oxidative potential of eugenol and Ocimum gratissimum Linn. Asian Pac. J. Trop. Med. 2014, 7, S391–S397. [Google Scholar] [CrossRef]
- Eftekhar, N.; Moghimi, A.; Boskabady, M.H. The Effects of Ocimum basilicum Extract and Its Constituent, Rosmarinic Acid on Total and Differential Blood WBC, Serum Levels of NO, MDA, Thiol, SOD, and CAT in Ovalbumin Sensitized Rats. Iran. J. Pharm. Res. IJPR 2018, 17, 1371–1385. [Google Scholar]
- Kris-Etherton, P.M.; Lefevre, M.; Beecher, G.R.; Gross, M.D.; Keen, C.L.; Etherton, T.D. Bioactive compounds in nutrition and health-research methodologies for establishing biological function: The antioxidant and anti-inflammatory effects of flavonoids on atherosclerosis. Annu. Rev. Nutr. 2004, 24, 511–538. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, M.; Sivakumar, R.; Rajeswary, M.; Yogalakshmi, K. Chemical composition and larvicidal activity of essential oil from Ocimum basilicum (L.) against Culex tritaeniorhynchus, Aedes albopictus and Anopheles subpictus (Diptera: Culicidae). Exp. Parasitol. 2013, 134, 7–11. [Google Scholar] [CrossRef]
- de Almeida, I.; Alviano, D.S.; Vieira, D.P.; Alves, P.B.; Blank, A.F.; Lopes, A.H.; Alviano, C.S.; Rosa, M. Antigiardial activity of Ocimum basilicum essential oil. Parasitol. Res. 2007, 101, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Boijink, C.L.; Queiroz, C.A.; Chagas, E.C.; Chaves, F.C.M.; Inoue, L.A.K.A. Anesthetic and anthelminthic effects of clove basil (Ocimum gratissimum) essential oil for tambaqui (Colossoma macropomum). Aquaculture 2016, 457, 24–28. [Google Scholar] [CrossRef]
- Sutili, F.J.; Murari, A.L.; Silva, L.L.; Gressler, L.T.; Heinzmann, B.M.; de Vargas, A.C.; Schmidt, D.; Baldisserotto, B. The use of Ocimum americanum essential oil against the pathogens Aeromonas hydrophila and Gyrodactylus sp. in silver catfish (Rhamdia quelen). Lett. Appl. Microbiol. 2016, 63, 82–88. [Google Scholar] [CrossRef]
- Di Paola, D.; Capparucci, F.; Abbate, J.M.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Genovese, T.; Impellizzeri, D.; et al. Environmental Risk Assessment of Oxaliplatin Exposure on Early Life Stages of Zebrafish (Danio rerio). Toxics 2022, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- OECD. Guideline for the Testing of Chemicals; Fish Embryo Toxicity (FET): Paris, Frence, 2013. [Google Scholar]
- Sampurna, B.P.; Audira, G.; Juniardi, S.; Lai, Y.-H.; Hsiao, C.-D. A Simple ImageJ-Based Method to Measure Cardiac Rhythm in Zebrafish Embryos. Inventions 2018, 3, 21. [Google Scholar] [CrossRef]
- Schwerte, T.; Überbacher, D.; Pelster, B. Non-Invasive Imaging of Blood Cell Concentration and Blood Distribution in Zebrafish Danio rerio Incubated in Hypoxic Conditions in Vivo. J. Exp. Biol. 2003, 206, 1299–1307. [Google Scholar] [CrossRef]
- Pylatiuk, C.; Sanchez, D.; Mikut, R.; Alshut, R.; Reischl, M.; Hirth, S.; Rottbauer, W.; Just, S. Automatic zebrafish heartbeat detection and analysis for zebrafish embryos. Zebrafish 2014, 11, 379–383. [Google Scholar] [CrossRef] [PubMed]
- McFarland, W.N.; Klontz, G.W. Anesthesia in fishes. Fed. Proc. 1969, 28, 1535–1540. [Google Scholar]
- Ross, B. Anaesthetic and Sedative Techniques for Aquatic Animals, 3rd ed.; Blackwell: Oxford, UK; Ames, IA, USA, 2008; ISBN 9781405149389. [Google Scholar]
- Adewale, A.Y.; Adeshina, I.; Yusuf, O.Y. Anaesthetic effect of Ocimum gratissimum extract on Oreochromis niloticus juveniles. Eur. Exp. Biol. 2017, 7, 2–7. [Google Scholar] [CrossRef]
- Ventura, A.; Filho, R.; Teodoro, G.; Laice, L.; Barbosa, P.; Stringhetta, G.; Povh, J. Essential oil of Ocimum basilicum and Eugenol as Sedatives for Nile Tilapia. J. Agric. Stud. 2020, 8, 657–665. [Google Scholar] [CrossRef]
- Öğretmen, F.; Gölbaşi, S.; Kutluyer Kocabaş, F. Efficacy of Clove Oil, Benzocaine, Eugenol, 2-Phenoxyethanol as Anaesthetics on Shabbout Fish (Barbus Grypus Heckel, 1843). Iran. J. Fish. Sci. 2016, 15, 470–478. [Google Scholar]
- Hill, J.V.; Forster, M.E. Cardiovascular responses of Chinook salmon (Oncorhynchus tshawytscha) during rapid anaesthetic induction and recovery. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 137, 167–177. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils. A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, N.N.; Erasmus, V.N.; Namwoonde, A. Effects of different fish sizes, temperatures, and concentration levels of sodium bicarbonate on anaesthesia in Mozambique tilapia (Oreochromis mossambicus). Aquaculture 2020, 529, 735716. [Google Scholar] [CrossRef]
- Priborsky, J.; Velisek, J. A Review of Three Commonly Used Fish Anesthetics. Rev. Fish. Sci. Aquac. 2018, 26, 417–442. [Google Scholar] [CrossRef]
- Matthews, M.; Varga, Z.M. Anesthesia and Euthanasia in Zebrafish. ILAR J. 2012, 53, 192–204. [Google Scholar] [CrossRef]
- Craig, M.P.; Gilday, S.D.; Hove, J.R. Dose-Dependent Effects of Chemical Immobilization on the Heart Rate of Embryonic Zebrafish. Lab. Anim. 2006, 35, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Félix, L.M.; Luzio, A.; Themudo, M.; Antunes, L.; Matos, M.; Coimbra, A.M.; Valentim, A.M. MS-222 Short Exposure Induces Developmental and Behavioural Alterations in Zebrafish Embryos. Reprod. Toxicol. 2018, 81, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Strykowski, J.L.; Schech, J.M. Effectiveness of recommended euthanasia methods in larval zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 81–84. [Google Scholar] [PubMed]
- Ehrlich, O.; Karamalakis, A.; Krylov, A.J.; Dudczig, S.; Hassell, K.L.; Jusuf, P.R. Clove Oil and AQUI-S Efficacy for Zebrafish Embryo, Larva, and Adult Anesthesia. Zebrafish 2019, 16, 451–459. [Google Scholar] [CrossRef]
- de Freitas Souza, C.; Baldissera, M.D.; Bianchini, A.E.; da Silva, E.G.; Mourão, R.; da Silva, L.; Schmidt, D.; Heinzmann, B.M.; Baldisserotto, B. Citral and linalool chemotypes of Lippia alba essential oil as anesthetics for fish: A detailed physiological analysis of side effects during anesthetic recovery in silver catfish (Rhamdia quelen). Fish Physiol. Biochem. 2018, 44, 21–34. [Google Scholar] [CrossRef]
- Li, Y.D.; She, Q.X.; Han, Z.B.; Sun, N.; Liu, X.; Li, X.D. Anaesthetic Effects of Eugenol on Grass Shrimp (Palaemonetes sinensis) of Different Sizes at Different Concentrations and Temperatures. Sci. Rep. 2018, 8, 11007. [Google Scholar] [CrossRef]
- Heldwein, C.G.; Silva, L.L.; Reckzieguel, P.; Barros, F.M.; Bürger, M.E.; Baldisserotto, B.; Mallmann, C.A.; Schmidt, D.; Caron, B.O.; Heinzmann, B.M. Participation of the GABAergic system in the anesthetic effect of Lippia alba (Mill.) N.E. Brown essential oil. Braz. J. Med. Biol. Res. 2012, 45, 436–443. [Google Scholar] [CrossRef]
- Sadamitsu, K.; Shigemitsu, L.; Suzuki, M.; Ito, D.; Kashima, M.; Hirata, H. Characterization of zebrafish GABAA receptor subunits. Sci. Rep. 2021, 11, 6242. [Google Scholar] [CrossRef]
- Bentzen, B.H.; Grunnet, M. Central and Peripheral GABA(A) Receptor Regulation of the Heart Rate Depends on the Conscious State of the Animal. Adv. Pharmacol. Sci. 2011, 2011, 578273. [Google Scholar] [CrossRef]
- Askari, V.R.; Baradaran Rahimi, V.; Ghorbani, A.; Rakhshandeh, H. Hypnotic Effect of Ocimum basilicum on Pentobarbital-Induced Sleep in Mice. Iran. Red Crescent Med. J. 2016, 18, 24261. [Google Scholar] [CrossRef]
- Bianchini, A.E.; Garlet, Q.I.; da Cunha, J.A.; Bandeira, G.J.; Brusque, I.C.M.; Salbego, J.; Heinzmann, B.M.; Baldisserotto, B. Monoterpenoids (thymol, carvacrol and S-(+)-linalool) with anesthetic activity in silver catfish (Rhamdia quelen): Evaluation of acetylcholinesterase and GABAergic activity. Braz. J. Med. Biol. Res. 2017, 50, 6346. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Moon, J.Y.; Jung, S.J.; Kang, J.G.; Choi, S.P.; Jang, J.H. Eugenol inhibits the GABAA current in trigeminal ganglion neurons. PLoS ONE 2015, 10, 0117316. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, S.; Interaminense, L.F.; Magalhães, P.J.; Leal-Cardoso, J.H.; Duarte, G.P. Cardiovascular effects of eugenol, a phenolic compound present in many plant essential oils, in normotensive rats. J. Cardiovasc. Pharmacol. 2004, 43, 250–257. [Google Scholar] [CrossRef]
- Majewski, M.; Kasica, N.; Jakubowski, P.; Podlasz, P. Influence of fresh garlic (Allium sativum L.) juice on zebrafish (Danio rerio) embryos: Developmental effects. J. Elem. 2017, 22, 475–486. [Google Scholar] [CrossRef]
- Silva, L.L.; Parodi, T.V.; Reckziegel, P.; de Oliveira Garcia, V.; Escobar Bürger, M.; Baldisserotto, B.; Malmann, C.A.; Soares Pereira, A.M.; Heinzmann, B.M. Essential oil of Ocimum gratissimum L.: Anesthetic effects, mechanism of action and tolerance in silver catfish. Rhamdia Quelen Aquac. 2012, 350–353, 91–97. [Google Scholar] [CrossRef]
- Netto, J.D.L.; Oliveira, R.S.; Copatti, C.E. Efficiency of essential oils of Ocimum basilicum and Cymbopogum flexuosus in the sedation and anaesthesia of Nile tilapia juveniles. An. Acad. Bras. Ciências 2017, 89, 2971–2974. [Google Scholar] [CrossRef]
- von Hellfeld, R.; Brotzmann, K.; Baumann, L.; Strecker, R.; Braunbeck, T. Adverse effects in the fish embryo acute toxicity (FET) test: A catalogue of unspecific morphological changes versus more specific effects in zebrafish (Danio rerio) embryos. Environ. Sci. Eur. 2020, 32, 122. [Google Scholar] [CrossRef]
- Ni, J.; Wang, H.; Wei, X.; Shen, K.; Sha, Y.; Dong, Y.; Shu, Y.; Wan, X.; Cheng, J.; Wang, F.; et al. Isoniazid causes heart looping disorder in zebrafish embryos by the induction of oxidative stress. BMC Pharmacol. Toxicol. 2020, 21, 22. [Google Scholar] [CrossRef]
- Elfawy, H.A.; Anupriya, S.; Mohanty, S.; Patel, P.; Ghosal, S.; Panda, P.K.; Das, B.; Verma, S.K.; Patnaik, S. Molecular toxicity of Benzo(a)pyrene mediated by elicited oxidative stress infer skeletal deformities and apoptosis in embryonic zebrafish. Sci. Total Environ. 2021, 789, 147989. [Google Scholar] [CrossRef]
- Inohaya, K.; Yasumasu, S.; Araki, K.; Naruse, K.; Yamazaki, K.; Yasumasu, I.; Iuchi, I.; Yamagami, K. Species-dependent migration of fish hatching gland cells that commonly express astacin-like proteases in common. Dev. Growth Differ. 1997, 39, 191–197. [Google Scholar] [CrossRef]
- Zahl, I.H.; Kiessling, A.; Samuelsen, O.B.; Hansen, M.K. Anaesthesia of Atlantic Cod (Gadus morhua)—Effect of Pre-Anaesthetic Sedation, and Importance of Body Weight, Temperature and Stress. Aquaculture 2009, 295, 52–59. [Google Scholar] [CrossRef]
- Skår, M.W.; Haugland, G.T.; Powell, M.D.; Wergeland, H.I.; Samuelsen, O.B. Development of Anaesthetic Protocols for Lumpfish (Cyclopterus Lumpus L.): Effect of Anaesthetic Concentrations, Sea Water Temperature and Body Weight. PLoS ONE 2017, 12, e0179344. [Google Scholar] [CrossRef] [PubMed]
- Dawit Moges, F.; Patel, P.; Parashar, S.K.S.; Das, B. Mechanistic Insights into Diverse Nano-Based Strategies for Aquaculture Enhancement: A Holistic Review. Aquaculture 2020, 519, 734770. [Google Scholar] [CrossRef]
- Mitjana, O.; Bonastre, C.; Tejedor, M.T.; Garza, L.; Esteban, J.; Falceto, M.V. Simultaneous Effect of Sex and Dose on Efficacy of Clove Oil, Tricaine Methanesulfonate, 2-Phenoxyethanol and Propofol as Anaesthetics in Guppies, Poecilia Reticulata (Peters). Aquac. Res. 2018, 49, 2140–2146. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capparucci, F.; De Benedetto, G.; Natale, S.; Pecoraro, R.; Iaria, C.; Marino, F. Evaluation of Anaesthetic Effect of Commercial Basil Ocimum basilicum on Zebrafish (Danio rerio) Embryos. Fishes 2022, 7, 318. https://doi.org/10.3390/fishes7060318
Capparucci F, De Benedetto G, Natale S, Pecoraro R, Iaria C, Marino F. Evaluation of Anaesthetic Effect of Commercial Basil Ocimum basilicum on Zebrafish (Danio rerio) Embryos. Fishes. 2022; 7(6):318. https://doi.org/10.3390/fishes7060318
Chicago/Turabian StyleCapparucci, Fabiano, Giovanni De Benedetto, Sabrina Natale, Roberta Pecoraro, Carmelo Iaria, and Fabio Marino. 2022. "Evaluation of Anaesthetic Effect of Commercial Basil Ocimum basilicum on Zebrafish (Danio rerio) Embryos" Fishes 7, no. 6: 318. https://doi.org/10.3390/fishes7060318
APA StyleCapparucci, F., De Benedetto, G., Natale, S., Pecoraro, R., Iaria, C., & Marino, F. (2022). Evaluation of Anaesthetic Effect of Commercial Basil Ocimum basilicum on Zebrafish (Danio rerio) Embryos. Fishes, 7(6), 318. https://doi.org/10.3390/fishes7060318