Transferrin Mediated NCC Killing Activity through NCCRP-1 in Nile Tilapia (Oreochromis niloticus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Rearing and Sample Collection
2.2. RNA Extraction and cDNA Synthesis
2.3. Construction of pGBKT7-On-NCCRP-1 Plasmid
2.4. Construction of the Liver and Head Kidney cDNA Y2H Library
2.5. Screening the cDNA Library by Yeast Mating
2.6. Cloning and Bioinformatics Analysis of On-TF
2.7. Verification of On-TF and On-NCCRP-1 Interaction
2.8. Construction, Expression, and Purification of the rOn-TF Plasmid
2.9. Activation and Regulation of rOn-TF on NCC Activity
2.10. Assay for the Killing Effect of NCCs
2.11. Statistical Analysis
3. Results
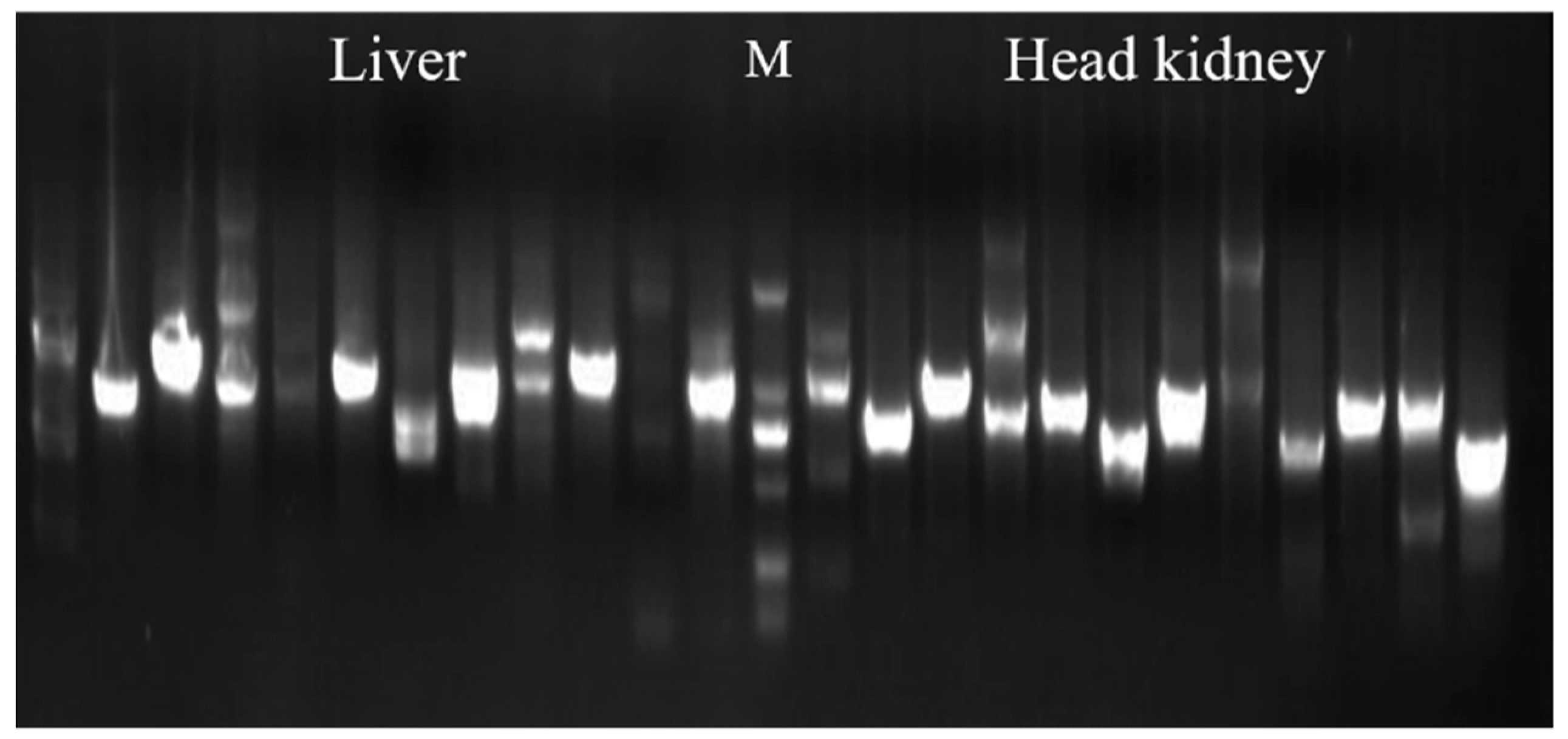
3.1. Evaluation and Screening of Y2H Library
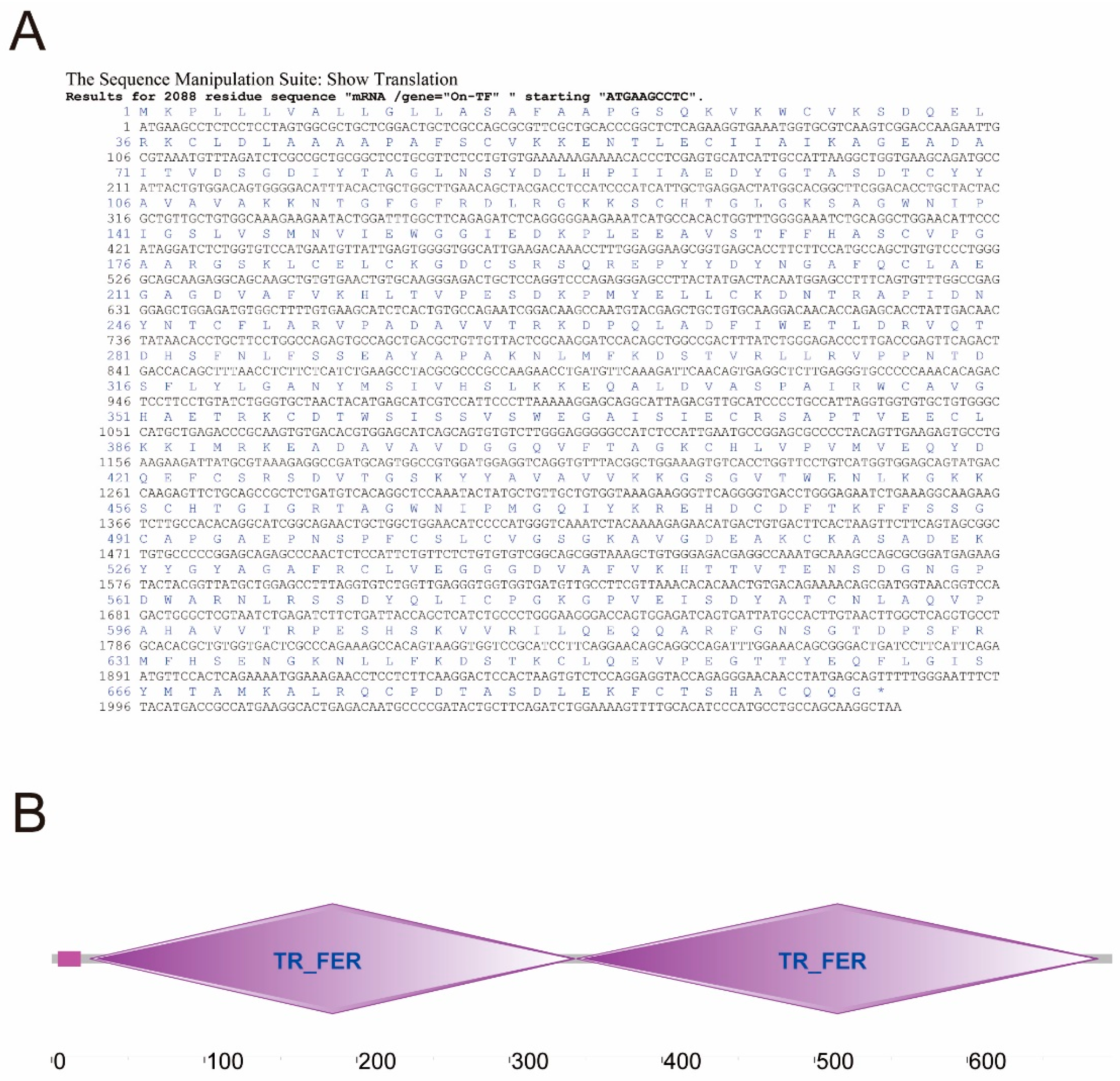
3.2. Cloning and Sequence Analysis of On-TF Gene
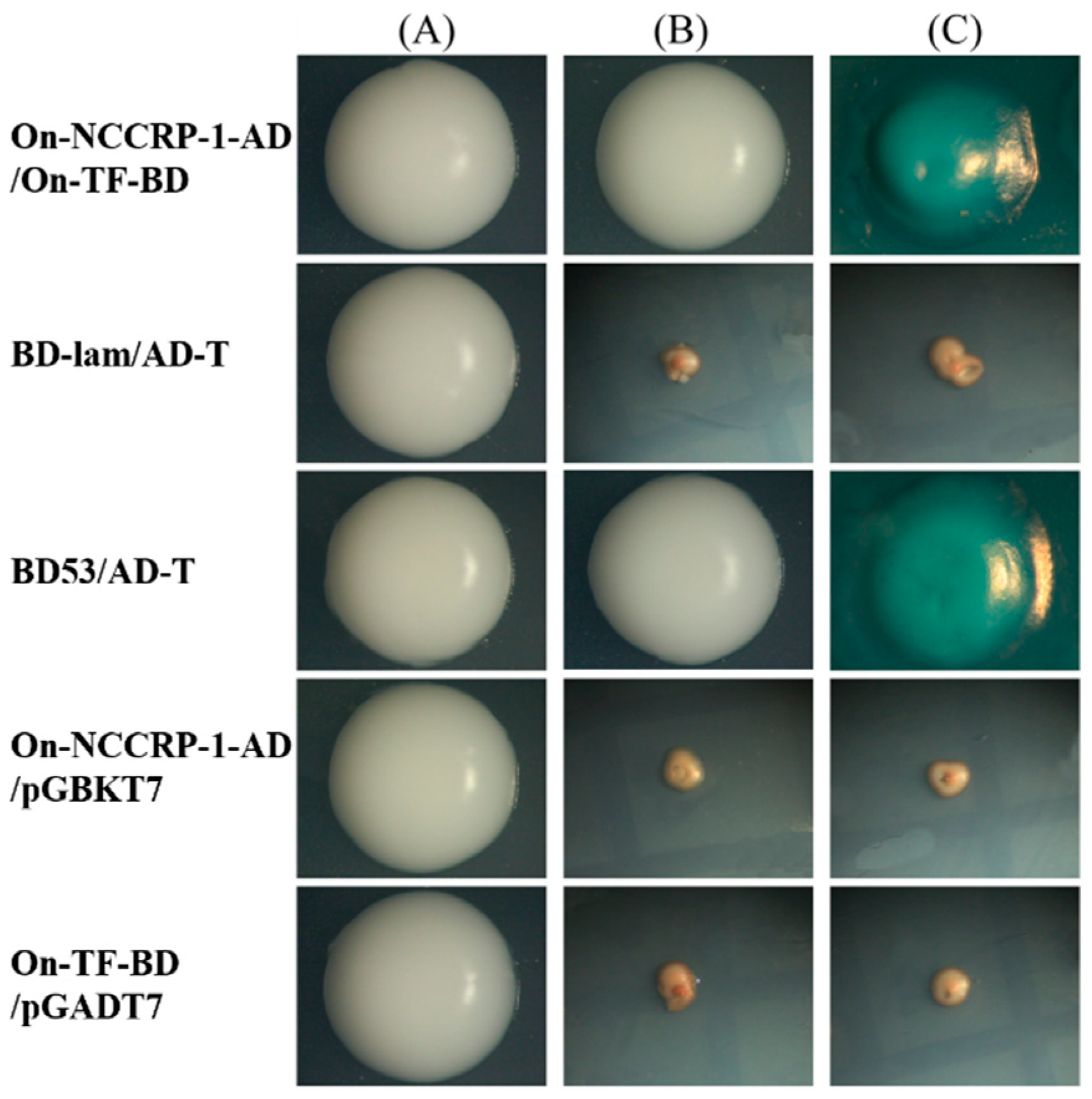
3.3. Verification of On-NCCRP-1 and On-TF Interaction
3.4. Expression, Purification, and Western Blot Analysis of rOn-TF
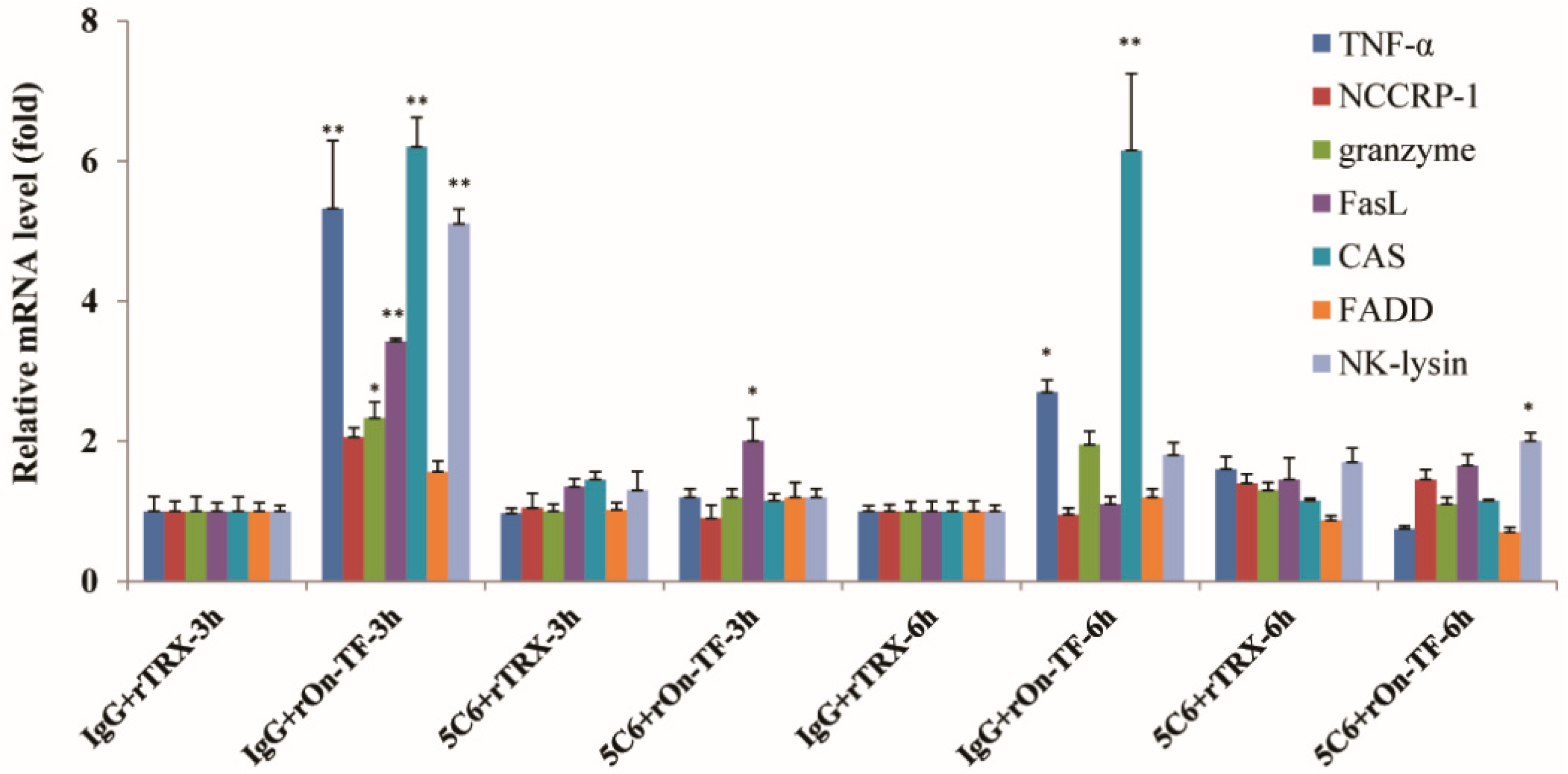
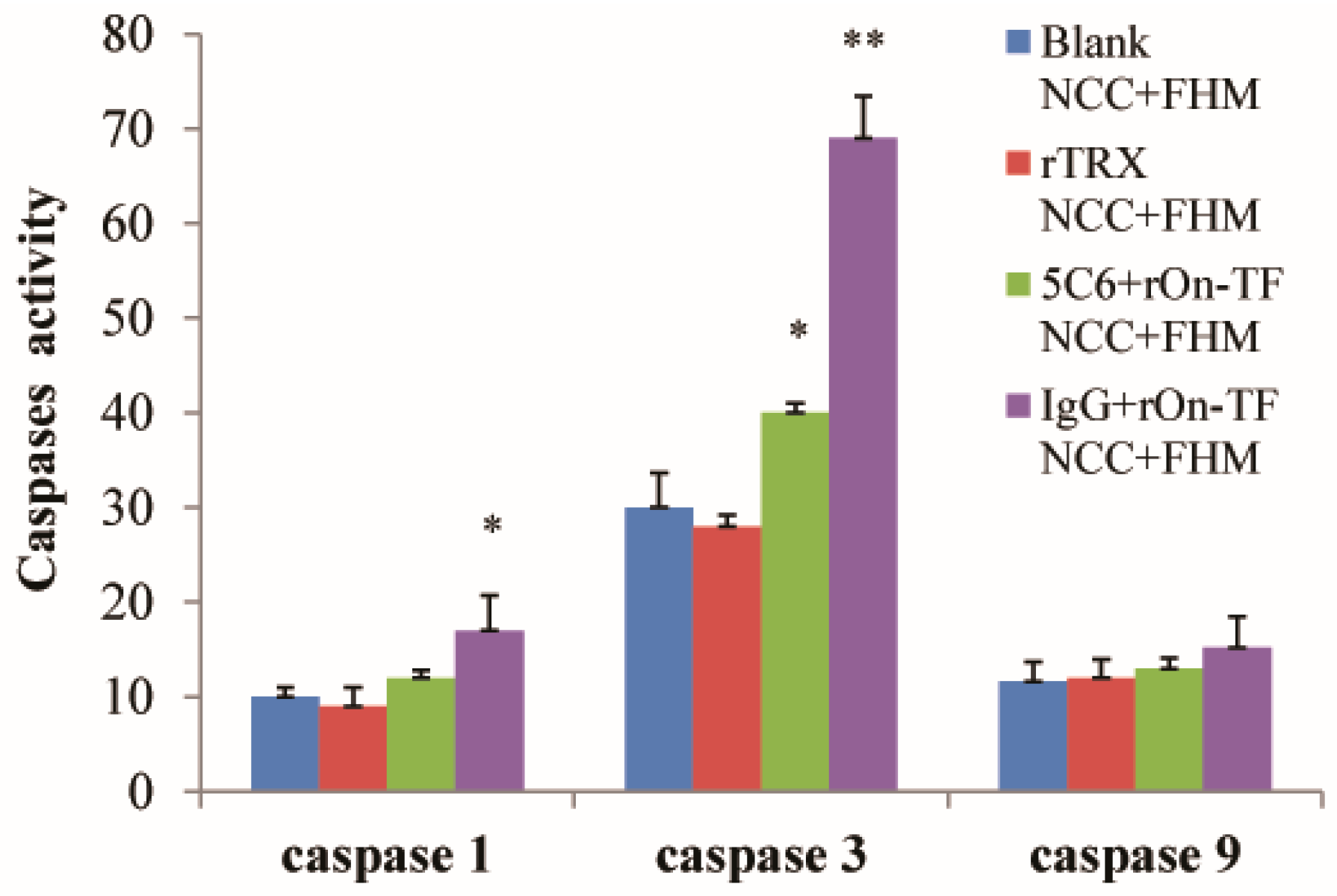
3.5. Effect of rOn-TF Protein on the Expression of NCC Effector Molecules
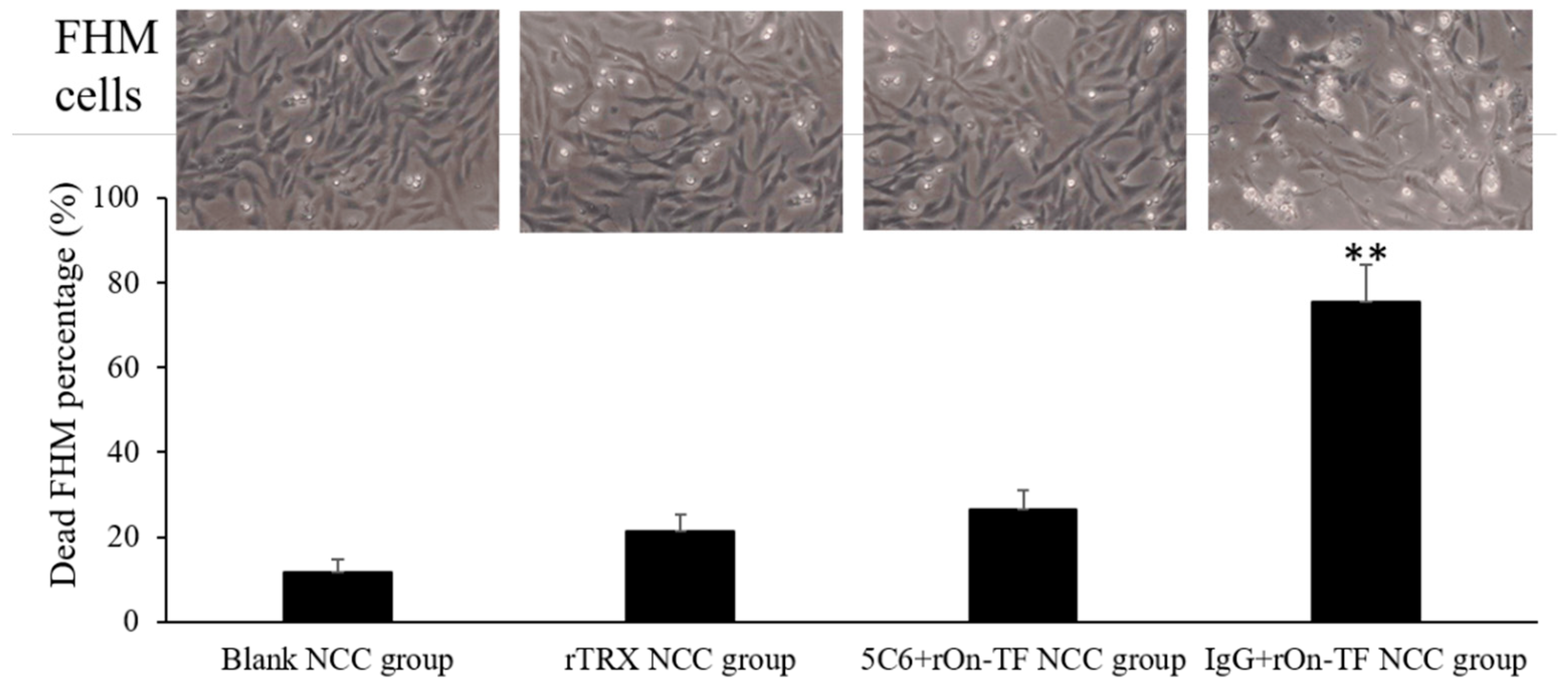
3.6. Effect of rOn-TF Protein on the Cytotoxicity of NCC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Razeghian, E.; Kameh, M.C.; Shafiee, S.; Khalafi, F.; Jafari, F.; Asghari, M.; Kazemi, K.; Ilkhani, S.; Shariatzadeh, S.; Haj-Mirzaian, A. The role of the natural killer (NK) cell modulation in breast cancer incidence and progress. Mol. Biol. Rep. 2022, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Praveen, K.; Leary, J.H., 3rd; Evans, D.L.; Jaso-Friedmann, L. Nonspecific cytotoxic cells of teleosts are armed with multiple granzymes and other components of the granule exocytosis pathway. Mol. Immunol. 2006, 43, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Jaso-Friedmann, L.; Leary, J.H.; Evans, D.L. Nonspecific cytotoxic cells in fish: Antigenic cross-reactivity of a function associated molecule with the intermediate filament vimentin. Cell. Immunol. 1993, 148, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.L.; Carlson, R.L.; Graves, S.S.; Hogan, K.T. Nonspecific cytotoxic cells in fish (Ictalurus punctatus). IV. Target cell binding and recycling capacity. Dev. Comp. Immunol. 1984, 8, 823–833. [Google Scholar] [CrossRef]
- Evans, D.L.; Hogan, K.T.; Graves, S.S.; Carlson, R.L.; Floyd, E.; Dawe, D.L. Nonspecific cytotoxic cells in fish (Ictalurus punctatus). III. Biophysical and biochemical properties affecting cytolysis. Dev. Comp. Immunol. 1984, 8, 599–610. [Google Scholar] [CrossRef]
- Cannon, J.P.; Haire, R.N.; Magis, A.T.; Eason, D.D.; Winfrey, K.N.; Hernandez Prada, J.A.; Bailey, K.M.; Jakoncic, J.; Litman, G.W.; Ostrov, D.A. A bony fish immunological receptor of the NITR multigene family mediates allogeneic recognition. Immunity 2008, 29, 228–237. [Google Scholar] [CrossRef]
- Yoder, J.A. Investigating the morphology, function and genetics of cytotoxic cells in bony fish. Comp. Biochem. Phys. C 2004, 138, 271–280. [Google Scholar] [CrossRef]
- Evans, D.L.; Leary, J.H.; Jaso-Friedmann, L. Nonspecific cytotoxic cells and innate immunity: Regulation by programmed cell death. Dev. Comp. Immunol. 2001, 25, 791–805. [Google Scholar] [CrossRef]
- Dautremepuits, C.; Fortier, M.; Croisetiere, S.; Belhumeur, P.; Fournier, M. Modulation of juvenile brook trout (Salvelinus fontinalis) cellular immune system after Aeromonas salmonicida challenge. Vet. Immunol. Immunopathol. 2006, 110, 27–36. [Google Scholar] [CrossRef]
- Huang, X.Z.; Li, Y.W.; Mai, Y.Z.; Luo, X.C.; Dan, X.M.; Li, A.X. Molecular cloning of NCCRP-1 gene from orange-spotted grouper (Epinephelus coioides) and characterization of NCCRP-1(+) cells post Cryptocaryon irritans infection. Dev. Comp. Immunol. 2014, 46, 267–278. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.; Cai, J.; Tang, J.; Cai, S.; Lu, Y.; Wang, B.; Jian, J. Biological characterisation, expression and functional analysis of non-specific cytotoxic cell receptor protein 1 in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2020, 104, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Jaso-Friedmann, L.; Leary, J.H.; Weisman, Z.; Evans, D.L. Activation of nonspecific cytotoxic cells with a multiple antigenic peptide: Specificity and requirements for receptor crosslinkage. Cell. Immunol. 1996, 170, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Jaso-Friedmann, L.; Leary, J.H.; Evans, D.L. Role of nonspecific cytotoxic cells in the induction of programmed cell death of pathogenic protozoans: Participation of the Fas ligand-Fas receptor system. Exp. Parasitol. 2000, 96, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Chen, L.; Chen, L.; Wang, B.; Wang, Y.; Zhan, X. Chimeric antigen receptor-natural killer cells: Novel insight into immunotherapy for solid tumors (Review). Exp. Ther. Med. 2021, 21, 340. [Google Scholar] [CrossRef]
- Kallio, H.; Tolvanen, M.; Jänis, J.; Pan, P.W.; Laurila, E.; Kallioniemi, A.; Kilpinen, S.; Tuominen, V.J.; Isola, J.; Valjakka, J.; et al. Characterization of non-specific cytotoxic cell receptor protein 1: A new member of the lectin-type subfamily of F-box proteins. PLoS ONE 2011, 6, e27152. [Google Scholar] [CrossRef]
- Niu, J.; Huang, Y.; Liu, X.; Wu, F.; Tang, J.; Wang, B.; Lu, Y.; Cai, J.; Jian, J. Fish galectin8-Like exerts positive regulation on immune response against bacterial infection. Front. Immunol. 2020, 11, 1140. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, Q.; Niu, J.; Tang, J.; Wang, B.; Abarike, E.D.; Lu, Y.; Cai, J.; Jian, J. NK-lysin from Oreochromis niloticus improves antimicrobial defence against bacterial pathogens. Fish Shellfish Immunol. 2018, 72, 259–265. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Z.; Zheng, Q.; Tang, J.; Cai, J.; Lu, Y.; Jian, J. Conservation of structural and interactional features of CD28 and CD80/86 molecules from Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2018, 72, 95–103. [Google Scholar] [CrossRef]
- Zhang, Z.; Niu, J.; Li, Q.; Huang, Y.; Jiang, B.; Wu, Y.; Huang, Y.; Jian, J. HMG20A from Nile tilapia (Oreochromis niloticus) involved in the immune response to bacterial infection. Fish Shellfish Immunol. 2021, 119, 499–507. [Google Scholar] [CrossRef]
- Wang, S.; Wu, R.; Lu, J.; Jiang, Y.; Huang, T.; Cai, Y.D. Protein-protein interaction networks as miners of biological discovery. Proteomics 2022, 22, e2100190. [Google Scholar] [CrossRef]
- Paiano, A.; Margiotta, A.; De Luca, M.; Bucc, I.C. Yeast two-Hybrid assay to identify interacting proteins. Curr. Protoc. Protein Sci. 2019, 95, e70. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Mu, L.; Wu, H.; Han, K.; Guo, Z.; Ye, J. Expression and functional analysis of Nile tilapia transferrin receptors (TfRs) in host resistance to pathogenic bacteria and iron ion metabolism. Fish Shellfish Immunol. 2020, 100, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.V.; Wilson, J.M.; Rodrigues, P.N. Transferrin and ferritin response to bacterial infection: The role of the liver and brain in fish. Dev. Comp. Immunol. 2009, 33, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Trites, M.J.; Barreda, D.R. Contributions of transferrin to acute inflammation in the goldfish, C. auratus. Dev. Comp. Immunol. 2017, 67, 300–309. [Google Scholar] [CrossRef]
- Gao, J.; Ding, S.; Huang, X.; Shi, X. Cloning and expression characterization of the serum transferrin gene in the Chinese black sleeper (Bostrichthys sinensis). Gene 2013, 515, 89–98. [Google Scholar] [CrossRef]
- Dietrich, M.A.; Zmijewski, D.; Karol, H.; Hejmej, A.; Bilińska, B.; Jurecka, P.; Irnazarow, I.; Słowińska, M.; Hliwa, P.; Ciereszko, A. Isolation and characterization of transferrin from common carp (Cyprinus carpio L.) seminal plasma. Fish Shellfish Immunol. 2010, 29, 66–74. [Google Scholar] [CrossRef]
- Rao, V.S.; Srinivas, K.; Sujini, G.N.; Kumar, G.N. Protein-protein interaction detection: Methods and analysis. Int. J. Proteom. 2014, 2014, 147648. [Google Scholar] [CrossRef]
- Praveen, K.; Evans, D.L.; Jaso-Friedmann, L. Constitutive expression of tumor necrosis factor-alpha in cytotoxic cells of teleosts and its role in regulation of cell-mediated cytotoxicity. Mol. Immunol. 2006, 43, 279–291. [Google Scholar] [CrossRef]
- Li, K.; Qiu, H.; Yan, J.; Shen, X.; Wei, X.; Duan, M.; Yang, J. The involvement of TNF-α and TNF-β as proinflammatory cytokines in lymphocyte-mediated adaptive immunity of Nile tilapia by initiating apoptosis. Dev. Comp. Immunol. 2021, 115, 103884. [Google Scholar] [CrossRef]
- Walch, M.; Dotiwala, F.; Mulik, S.; Thiery, J.; Kirchhausen, T.; Clayberger, C.; Krensky, A.M.; Martinvalet, D.; Lieberman, J. Cytotoxic cells kill intracellular bacteria through granulysin-mediated delivery of granzymes. Cell 2014, 157, 1309–1323. [Google Scholar] [CrossRef] [Green Version]
- Bots, M.; Medema, J.P. Granzymes at a glance. J. Cell Sci. 2006, 119, 5011–5014. [Google Scholar] [CrossRef] [PubMed]
- Praveen, K.; Leary, J.H.; Evans, D.L.; Jaso-Friedmann, L. Molecular cloning of cellular apoptosis susceptibility (CAS) gene in Oreochromis niloticus and its proposed role in regulation of non-specific cytotoxic cell (NCC) functions. Fish Shellfish Immunol. 2006, 20, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zheng, Q.; Wang, Z.; Tang, J.; Lu, Y.; Qin, Q.; Cai, J.; Jian, J. Fish natural killer enhancing factor-A (NKEF-A) enhance cytotoxicity of nonspecific cytotoxic cells against bacterial infection. Mol. Immunol. 2021, 133, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.; Liu, Y.P.; Tong, X.X.; Zhang, T.N.; Yang, N. Molecular mechanisms and functions of pyroptosis in sepsis and sepsis-associated organ dysfunction. Front. Cell. Infect. Microbiol. 2022, 12, 962139. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Chen, Z.; Xie, R.; Wang, P.; Zhang, Z.; Cai, J.; Wang, B.; Jian, J. Transferrin Mediated NCC Killing Activity through NCCRP-1 in Nile Tilapia (Oreochromis niloticus). Fishes 2022, 7, 253. https://doi.org/10.3390/fishes7050253
Huang Y, Chen Z, Xie R, Wang P, Zhang Z, Cai J, Wang B, Jian J. Transferrin Mediated NCC Killing Activity through NCCRP-1 in Nile Tilapia (Oreochromis niloticus). Fishes. 2022; 7(5):253. https://doi.org/10.3390/fishes7050253
Chicago/Turabian StyleHuang, Yu, Zhengsi Chen, Ruitao Xie, Pei Wang, Zhiqiang Zhang, Jia Cai, Bei Wang, and Jichang Jian. 2022. "Transferrin Mediated NCC Killing Activity through NCCRP-1 in Nile Tilapia (Oreochromis niloticus)" Fishes 7, no. 5: 253. https://doi.org/10.3390/fishes7050253
APA StyleHuang, Y., Chen, Z., Xie, R., Wang, P., Zhang, Z., Cai, J., Wang, B., & Jian, J. (2022). Transferrin Mediated NCC Killing Activity through NCCRP-1 in Nile Tilapia (Oreochromis niloticus). Fishes, 7(5), 253. https://doi.org/10.3390/fishes7050253






