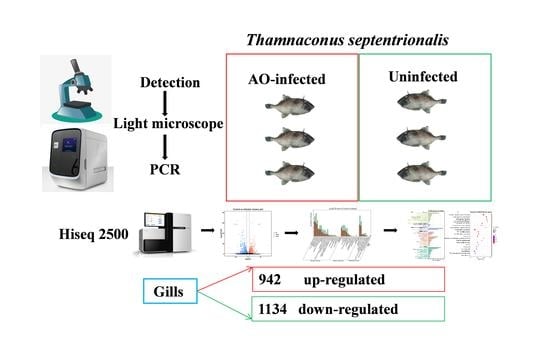
Transcriptome Analysis of Greenfin Horse-Faced Filefish (Thamnaconus septentrionalis) Gills in Response to Amyloodinium ocellatum (AO) Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. AO Infection and Sample Collection
2.2. DNA Extraction and PCR Detection
2.3. RNA Extraction and Library Construction
2.4. Filtering Reads and Alignment with Ribosome RNA (rRNA)
2.5. Alignment with Reference Genome
2.6. Quantification of Gene Expression Abundance and Differentially Expressed Genes (DEGs)
2.7. Relationship Analysis of Samples
2.8. GO Enrichment and Pathway Enrichment Analysis
3. Results
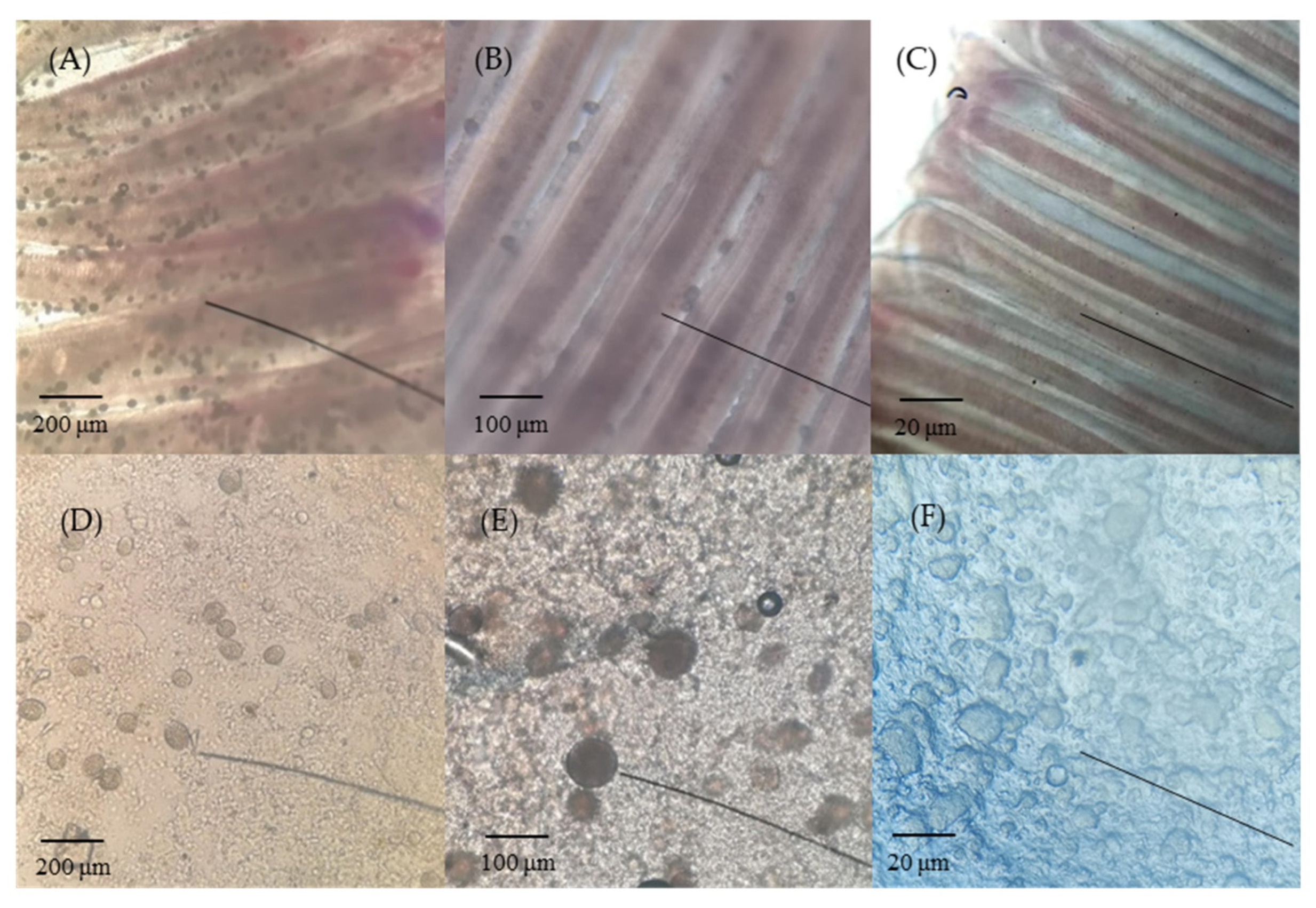
3.1. Identification and Disease Signs of AO Infection in Greenfin Horse-Faced Filefish
3.2. Transcriptome Assembly and Sequence Description
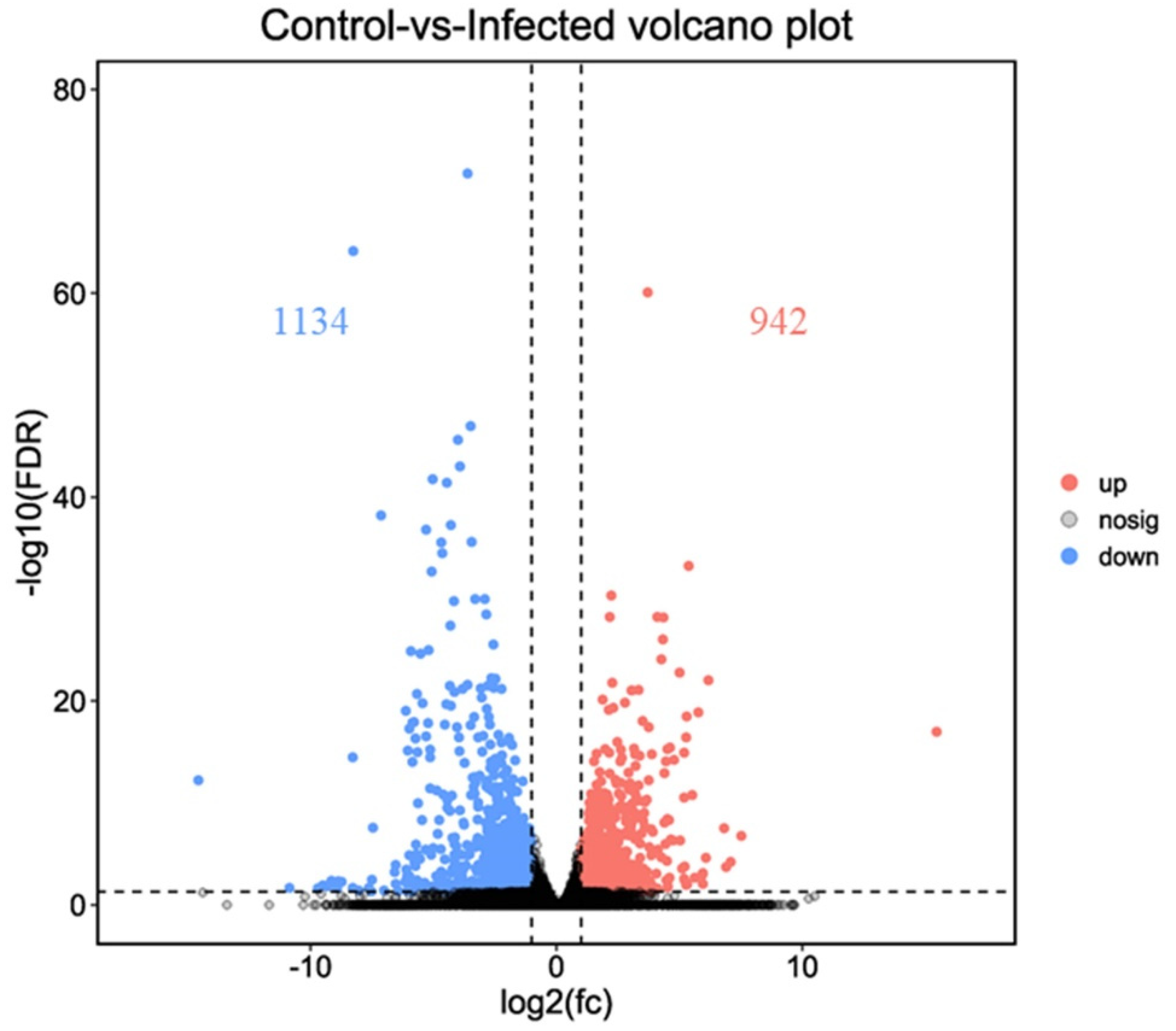
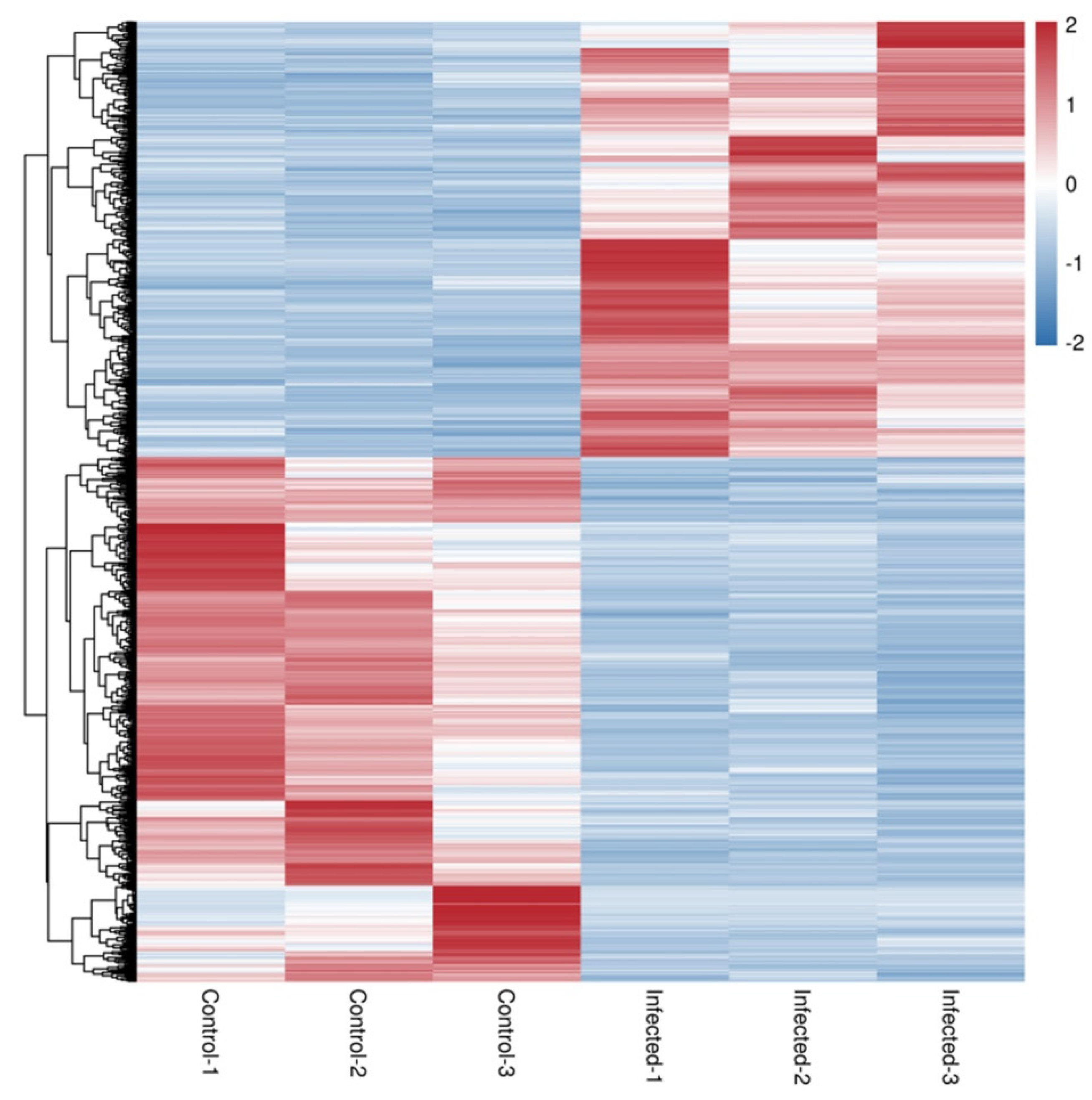
3.3. Differentially Expressed Genes after AO Infection
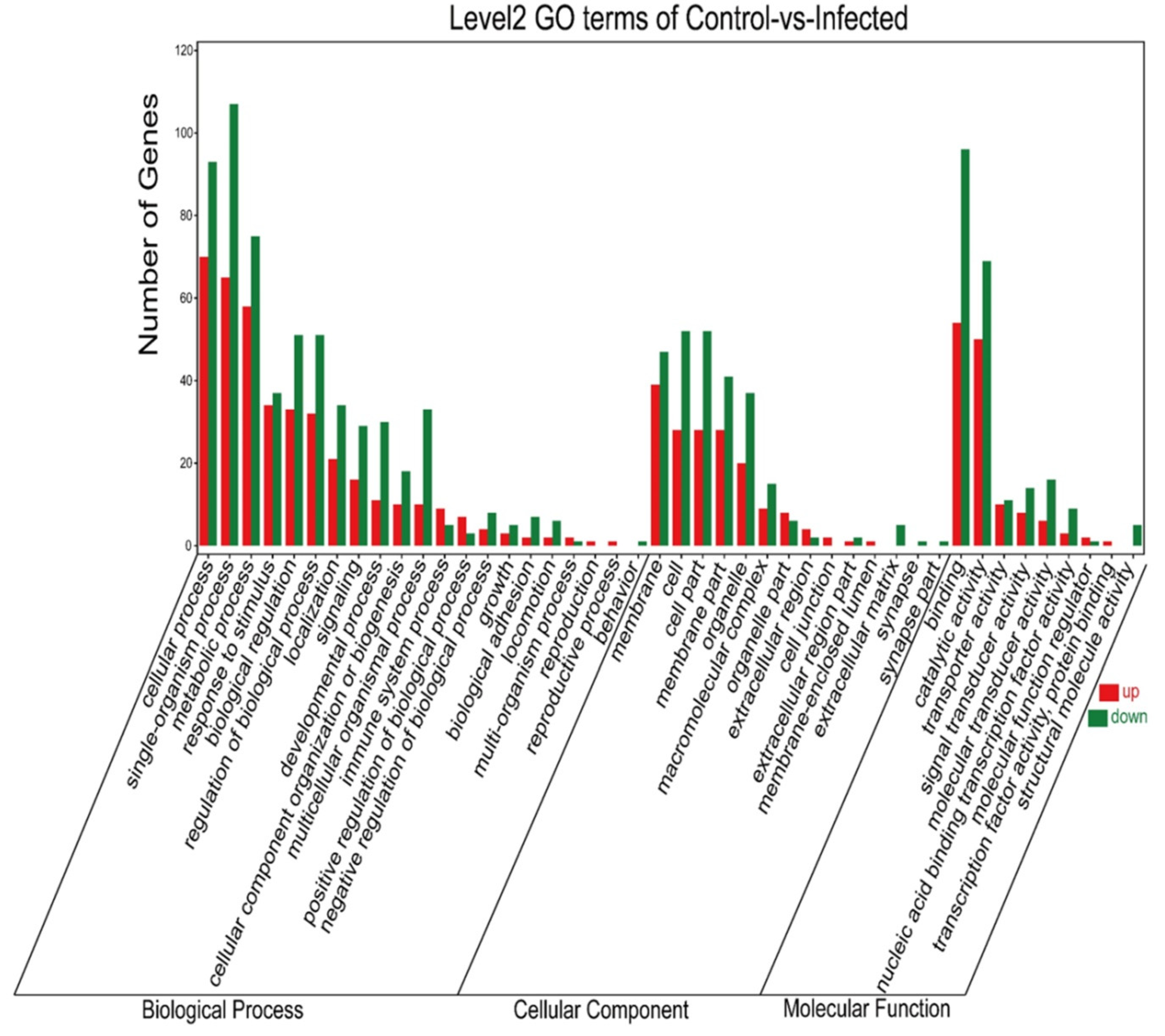
3.4. GO Annotated after AO Infection
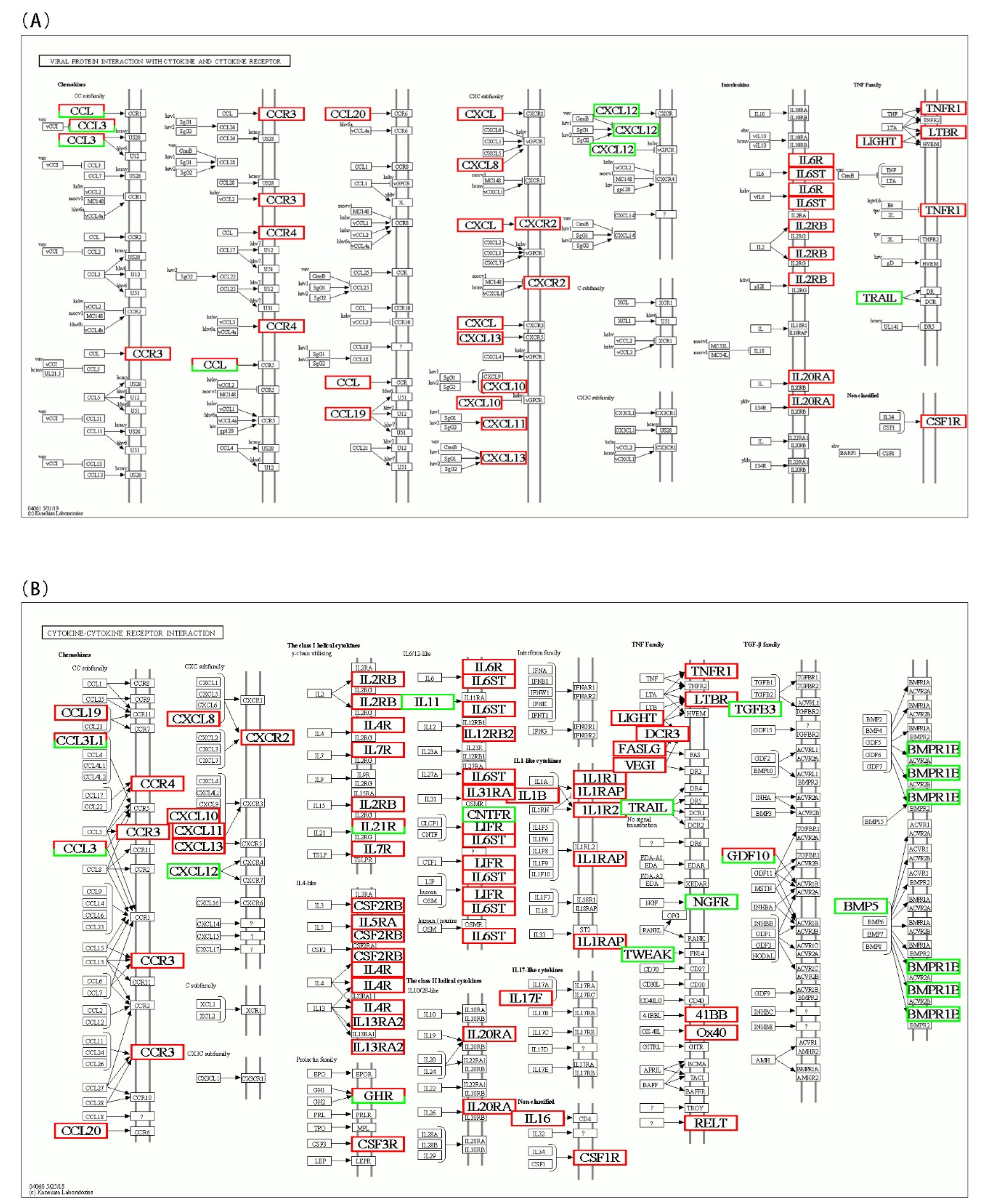
3.5. KEGG Pathway Enrichment of DEGs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Noga, E.J. Amyloodinium ocellatum. In Fish Parasites Pathobiology and Protection; Woo, P.T.K., Buchman, K., Eds.; CAB International: London, UK, 2012; p. 383. [Google Scholar]
- Byadgi, O.; Marroni, F.; Dirks, R.; Massimo, M.; Volpatti, D.; Galeotti, M.; Beraldo, P. Transcriptome Analysis of Amyloodinium ocellatum Tomonts Revealed Basic Information on the Major Potential Virulence Factors. Genes 2020, 11, 1252. [Google Scholar] [CrossRef]
- Alvarezpellitero, P.; Eiras, J.; Segner, H.; Wahli, T.; Kapoor, B.G. Diseases caused by flagellates. Fish Dis. 2008, 2, 435–582. [Google Scholar]
- Byadgi, O.; Beraldo, P.; Volpatti, D.; Massimo, M.; Bulfon, C.; Galeotti, M. Expression of infection-related immune response in European sea bass (Dicentrarchus labrax) during a natural outbreak from a unique dinoflagellate Amyloodinium ocellatum. Fish Shellfish Immunol. 2019, 84, 62–72. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Li, Y.; Zhang, M.; Jacques, K.J.; Gu, W.; Sun, Y.; Sun, J.; Yang, Y.; Xu, S.; et al. Immune response of silver pomfret (Pampus argenteus) to Amyloodinium ocellatum infection. J. Fish Dis. 2021, 44, 2111–2123. [Google Scholar] [CrossRef]
- Levy, M.; Poore, M.; Colorni, A.; Noga, E.; Vandersea, M.; Litaker, R.W. A highly specific PCR assay for detecting the fish ectoparasite Amyloodinium ocellatum. Dis. Aquat. Org. 2007, 73, 219–226. [Google Scholar] [CrossRef]
- Paperna, I. Reproduction cycle and tolerance to temperature and salinity of Amyloodinium ocellatum (Brown, 1931) (Dinoflagellida). Ann. Parasitol. Hum. Comp. 1984, 59, 7–30. [Google Scholar] [CrossRef]
- Noga, E.J. Propagation in cell culture of the dinoflagellate amyloodinium, an ectoparasite of marine fishes. Science 1987, 236, 1302–1304. [Google Scholar] [CrossRef]
- Picón-Camacho, S.M.; Thompson, W.P.; Blaylock, R.B.; Lotz, J.M. Development of a rapid assay to detect the dinoflagellate Amyloodinium ocellatum using loop-mediated isothermal amplification (LAMP). Veter- Parasitol. 2013, 196, 265–271. [Google Scholar] [CrossRef]
- Oestmann, D.J.; Lewis, D.H.; Zettler, B.A. Communications: Clearance of Amyloodinium ocellatum Dinospores by Artemia salina. J. Aquat. Anim. Health 1995, 7, 257–261. [Google Scholar] [CrossRef]
- Fajer-Ávila, E.J.; la Parra, I.A.-D.; Aguilar-Zarate, G.; Contreras-Arce, R.; Zaldívar-Ramírez, J.; Betancourt-Lozano, M. Toxicity of formalin to bullseye puffer fish (Sphoeroides annulatus Jenyns, 1843) and its effectiveness to control ectoparasites. Aquaculture 2003, 223, 41–50. [Google Scholar] [CrossRef]
- Owatari, M.S.; Sterzelecki, F.C.; da Silva, C.S.; Magnotti, C.; Mouriño, J.L.P.; Cerqueira, V.R. Amyloodiniosis in farmed Sardinella brasiliensis (Steindachner, 1879): Case report of successful therapeutic control with copper sulfate. Parasitol. Int. 2020, 76, 102091. [Google Scholar] [CrossRef]
- Tedesco, P.; Beraldo, P.; Massimo, M.; Fioravanti, M.L.; Volpatti, D.; Dirks, R.; Galuppi, R. Comparative Therapeutic Effects of Natural Compounds Against Saprolegnia spp. (Oomycota) and Amyloodinium ocellatum (Dinophyceae). Front. Veter Sci. 2020, 7, 83. [Google Scholar] [CrossRef]
- Sudhagar, A.; Kumar, G.; El-Matbouli, M. Transcriptome Analysis Based on RNA-Seq in Understanding Pathogenic Mechanisms of Diseases and the Immune System of Fish: A Comprehensive Review. Int. J. Mol. Sci. 2018, 19, 245. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, T.; Chen, B.; Bai, H.; Bai, Y.; Zhao, J.; Pu, F.; Wu, Y.; Chen, L.; Shi, Y.; et al. Identification and Expression Analysis of Long Non-coding RNA in Large Yellow Croaker (Larimichthys crocea) in Response to Cryptocaryon irritans Infection. Front. Genet. 2020, 11, 590475. [Google Scholar] [CrossRef]
- Xie, X.; Jiang, Y.; Miao, R.; Huang, J.; Zhou, L.; Kong, J.; Yin, F. The gill transcriptome reveals unique antimicrobial features that protect Nibea albiflora from Cryptocaryon irritans infection. J. Fish Dis. 2021, 44, 1215–1227. [Google Scholar] [CrossRef]
- Byadgi, O.; Massimo, M.; Dirks, R.P.; Pallavicini, A.; Bron, J.E.; Ireland, J.H.; Volpatti, D.; Galeotti, M.; Beraldo, P. Innate immune-gene expression during experimental amyloodiniosis in European seabass (Dicentrarchus labrax). Veter- Immunol. Immunopathol. 2021, 234, 110217. [Google Scholar] [CrossRef]
- Kang, K.H.; Kang, H.K.; Zong, T.C.; Kim, J.M.; Zhi, F.Z. Cryopreservation of filefish (Thamnaconus septentrionalis gunther, 1877) sperm. Aquac. Res. 2015, 35, 1429–1433. [Google Scholar] [CrossRef]
- Bian, L.; Li, F.; Ge, J.; Wang, P.; Chang, Q.; Zhang, S.; Li, J.; Liu, C.; Liu, K.; Liu, X.; et al. Chromosome-level genome assembly of the greenfin horse-faced filefish (Thamnaconus septentrionalis) using Oxford Nanopore PromethION sequencing and Hi-C technology. Mol. Ecol. Resour. 2020, 20, 1069–1079. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Bauer, S.; Grossmann, S.; Vingron, M.; Robinson, P.N. Ontologizer 2.0--A multifunctional tool for GO term enrichment analysis and data exploration. Bioinformatics 2008, 24, 1650–1651. [Google Scholar] [CrossRef]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 29–34. [Google Scholar] [CrossRef]
- Fringuelli, E.; Gordon, A.W.; Rodger, H.; Welsh, M.D.; A Graham, D. Detection of Neoparamoeba perurans by duplex quantitative Taqman real-time PCR in formalin-fixed, paraffin-embedded Atlantic salmonid gill tissues. J. Fish Dis. 2012, 35, 711–724. [Google Scholar] [CrossRef]
- Cervera, L.; González-Fernández, C.; Arizcun, M.; Cuesta, A.; Chaves-Pozo, E. Severe Natural Outbreak of Cryptocaryon irritans in Gilthead Seabream Produces Leukocyte Mobilization and Innate Immunity at the Gill Tissue. Int. J. Mol. Sci. 2022, 23, 937. [Google Scholar] [CrossRef]
- Massimo, M.; Volpatti, D.; Galeotti, M.; Bron, J.E.; Beraldo, P. News Insights into the Host-Parasite Interactions of Amyloodiniosis in European Sea Bass: A Multi-Modal Approach. Pathogens 2022, 11, 62. [Google Scholar] [CrossRef]
- Xu, G.; Huang, T.; Gu, W.; Zhang, Y.; Yao, Z.; Zhao, C.; Wang, B. Characterization, expression, and functional analysis of the hepcidin gene from Brachymystax lenok. Dev. Comp. Immunol. 2018, 89, 131–140. [Google Scholar] [CrossRef]
- Neves, J.V.; Caldas, C.; Vieira, I.; Ramos, M.F.; Rodrigues, P.N. Multiple hepcidins in a teleost fish, Dicentrarchus labrax: Different hepcidins for different roles. J. Immunol. 2015, 195, 2696–2709. [Google Scholar] [CrossRef]
- Drakesmith, H.; Prentice, A.M.; Parducci, L.; Edwards, M.E.; Bennett, K.D.; Alm, T.; Elverland, E.; Tollefsrud, M.M.; Jørgensen, T.; Houmark-Nielsen, M.; et al. Hepcidin and the Iron-Infection Axis. Science 2012, 338, 768–772. [Google Scholar] [CrossRef]
- Zhang, Y.; Chua, S., Jr. Leptin function and regulation. Compr. Physiol. 2017, 8, 351–369. [Google Scholar]
- Yu, R.M.K.; Chu, D.L.H.; Tan, T.-F.; Li, V.W.T.; Chan, A.K.Y.; Giesy, J.P.; Cheng, S.H.; Wu, R.S.S.; Kong, R.Y.C. Leptin-Mediated Modulation of Steroidogenic Gene Expression in Hypoxic Zebrafish Embryos: Implications for the Disruption of Sex Steroids. Environ. Sci. Technol. 2012, 46, 9112–9119. [Google Scholar] [CrossRef]
- Zhang, D.; Mohammed, H.; Ye, Z.; Rhodes, M.A.; Thongda, W.; Zhao, H.; Jescovitch, L.N.; Fuller, S.A.; Davis, D.A.; Peatman, E. Transcriptomic profiles of Florida pompano (Trachinotus carolinus) gill following infection by the ectoparasite Amyloodinium ocellatum. Fish Shellfish Immunol. 2022, 125, 171–179. [Google Scholar] [CrossRef]
- Han, H.-H.; Sedgwick, A.C.; Shang, Y.; Li, N.; Liu, T.; Li, B.-H.; Yu, K.; Zang, Y.; Brewster, J.T.; Odyniec, M.L.; et al. Protein encapsulation: A new approach for improving the capability of small-molecule fluorogenic probes. Chem. Sci. 2019, 11, 1107–1113. [Google Scholar] [CrossRef]
- Vilar, J.M.; Saiz, L. Control of gene expression by modulated self-assembly. Nucleic Acids Res. 2011, 39, 6854–6863. [Google Scholar] [CrossRef]
- Lie, K.K.; Kvalheim, K.; Rasinger, J.D.; Harboe, T.; Nordgreen, A.; Moren, M. Vitamin A and arachidonic acid altered the skeletal mineralization in Atlantic cod ( Gadus morhua ) larvae without any interactions on the transcriptional level. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 191, 80–88. [Google Scholar] [CrossRef]
- Wang, T.; Secombes, C.J. The cytokine networks of adaptive immunity in fish. Fish Shellfish. Immunol. 2013, 35, 1703–1718. [Google Scholar] [CrossRef]


| Sample | Filtered Raw Data | Clean Data (%) | Total Mapped (%) | AF Q20 (%) |
|---|---|---|---|---|
| Control-1 | 42,876,748 | 99.64% | 89.21% | 97.68% |
| Control-2 | 45,551,512 | 99.60% | 88.41% | 97.63% |
| Control-3 | 39,302,198 | 99.64% | 87.77% | 97.62% |
| Infected-1 | 55,153,762 | 99.65% | 86.18% | 97.61% |
| Infected-2 | 46,143,972 | 99.64% | 86.57% | 97.68% |
| Infected-3 | 40,601,192 | 99.66% | 87.20% | 97.68% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.-G.; Wang, Y.; Xu, W.-B.; Qin, B.; Ying, N.; Song, X.-F.; Yue, Y.-F.; Wang, X.-S.; Zhang, B.-B.; Wu, Y.-Q. Transcriptome Analysis of Greenfin Horse-Faced Filefish (Thamnaconus septentrionalis) Gills in Response to Amyloodinium ocellatum (AO) Infection. Fishes 2022, 7, 252. https://doi.org/10.3390/fishes7050252
Yang L-G, Wang Y, Xu W-B, Qin B, Ying N, Song X-F, Yue Y-F, Wang X-S, Zhang B-B, Wu Y-Q. Transcriptome Analysis of Greenfin Horse-Faced Filefish (Thamnaconus septentrionalis) Gills in Response to Amyloodinium ocellatum (AO) Infection. Fishes. 2022; 7(5):252. https://doi.org/10.3390/fishes7050252
Chicago/Turabian StyleYang, Li-Guo, Yue Wang, Wen-Bin Xu, Bo Qin, Na Ying, Xue-Feng Song, Yan-Feng Yue, Xiao-Shan Wang, Bian-Bian Zhang, and Yan-Qing Wu. 2022. "Transcriptome Analysis of Greenfin Horse-Faced Filefish (Thamnaconus septentrionalis) Gills in Response to Amyloodinium ocellatum (AO) Infection" Fishes 7, no. 5: 252. https://doi.org/10.3390/fishes7050252
APA StyleYang, L.-G., Wang, Y., Xu, W.-B., Qin, B., Ying, N., Song, X.-F., Yue, Y.-F., Wang, X.-S., Zhang, B.-B., & Wu, Y.-Q. (2022). Transcriptome Analysis of Greenfin Horse-Faced Filefish (Thamnaconus septentrionalis) Gills in Response to Amyloodinium ocellatum (AO) Infection. Fishes, 7(5), 252. https://doi.org/10.3390/fishes7050252