Widespread Hybridization between Invasive Bleak (Alburnus alburnus) and Iberian Chub (Squalius spp.): A Neglected Conservation Threat
Abstract
1. Introduction
2. Materials and Methods
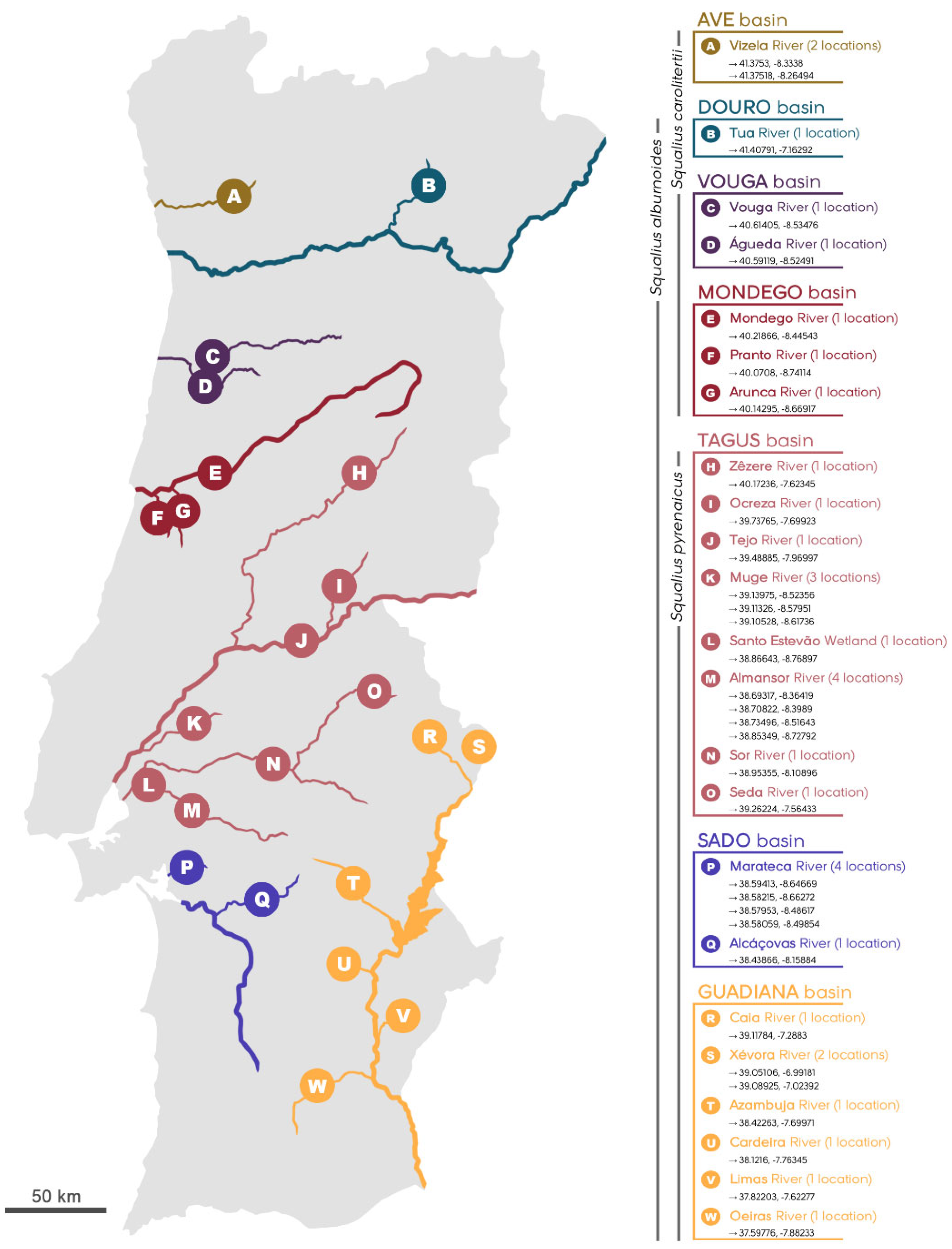
2.1. Occurrence Data and Mapping
2.2. Molecular Analysis of Hybrids between A. alburnus and S. carolitertii
2.3. Ethical Statement
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lodge, D.M. Biological Invasions: Lessons for Ecology. Trends Ecol. Evol. 1993, 8, 133–137. [Google Scholar] [CrossRef]
- Olden, J.D.; Poff, N.L.; Douglas, M.R.; Douglas, M.E.; Fausch, K.D. Ecological and Evolutionary Consequences of Biotic Homogenization. Trends Ecol. Evol. 2004, 19, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Vellend, M.; Harmon, L.J.; Lockwood, J.L.; Mayfield, M.M.; Hughes, A.R.; Wares, J.P.; Sax, D.F. Effects of Exotic Species on Evolutionary Diversification. Trends Ecol. Evol. 2007, 22, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Bellard, C.; Genovesi, P.; Jeschke, J. Global Patterns in Threats to Vertebrates by Biological Invasions. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152454. [Google Scholar] [CrossRef]
- Rhymer, J.M.; Simberloff, D. Extinction by Hybridization and Introgression. Annu. Rev. Ecol. Syst. 1996, 27, 83–109. [Google Scholar] [CrossRef]
- Allendorf, F.W.; Leary, R.F.; Spruell, P.; Wenburg, J.K. The Problems with Hybrids: Setting Conservation Guidelines. Trends Ecol. Evol. 2001, 16, 613–622. [Google Scholar] [CrossRef]
- Ribeiro, F.; Leunda, P. Non-Native Fish Impacts on Mediterranean Freshwater Ecosystems: Current Knowledge and Research Needs. Fish. Manag. Ecol. 2012, 19, 142–156. [Google Scholar] [CrossRef]
- Largiadèr, C.R. Hybridization and Introgression between Native and Alien Species. In Biological Invasions; Springer: Berlin/Heidelberg, Germany, 2008; pp. 275–292. [Google Scholar]
- Dowling, T.E.; Secor, C.L. The Role of Hybridization and Introgression in the Diversification of Animals. Annu. Rev. Ecol. Syst. 1997, 593–619. [Google Scholar] [CrossRef]
- Perry, W.L.; Feder, J.L.; Lodge, D.M. Implications of Hybridization between Introduced and Resident Orconectes Crayfishes. Conserv. Biol. 2001, 15, 1656–1666. [Google Scholar] [CrossRef]
- Quilodrßn, C.S.; Montoya-Burgos, J.I.; Currat, M. Modelling Interspecific Hybridization with Genome Exclusion to Identify Conservation Actions: The Case of Native and Invasive Pelophylax Waterfrogs. Evol. Appl. 2015, 8, 199–210. [Google Scholar] [CrossRef]
- Huxel, G.R. Rapid Displacement of Native Species by Invasive Species: Effects of Hybridization. Biol. Conserv. 1999, 89, 143–152. [Google Scholar] [CrossRef]
- Mack, R.N.; Simberloff, D.; Mark Lonsdale, W.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic Invasions: Causes, Epidemiology, Global Consequences, and Control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Fitzpatrick, B.M.; Johnson, J.R.; Kump, D.K.; Smith, J.J.; Voss, S.R.; Shaffer, H.B. Rapid Spread of Invasive Genes into a Threatened Native Species. Proc. Natl. Acad. Sci. USA 2010, 107, 3606–3610. [Google Scholar] [CrossRef] [PubMed]
- Currat, M.; Ruedi, M.; Petit, R.J.; Excoffier, L. The Hidden Side of Invasions: Massive Introgression by Local Genes. Evol. Int. J. Org. Evol. 2008, 62, 1908–1920. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.J.; Ayres, D.R. What Can Mathematical Modeling Tell Us about Hybrid Invasions? Biol. Invasions 2009, 11, 1217–1224. [Google Scholar] [CrossRef]
- Leprieur, F.; Beauchard, O.; Blanchet, S.; Oberdorff, T.; Brosse, S. Fish Invasions in the World’s River Systems: When Natural Processes Are Blurred by Human Activities. PLoS Biol. 2008, 6, e28. [Google Scholar]
- Verspoor, E.; Hammart, J. Introgressive Hybridization in Fishes: The Biochemical Evidence. J. Fish Biol. 1991, 39, 309–334. [Google Scholar] [CrossRef]
- Scribner, K.T.; Page, K.S.; Bartron, M.L. Hybridization in Freshwater Fishes: A Review of Case Studies and Cytonuclear Methods of Biological Inference. Rev. Fish Biol. Fish. 2000, 10, 293–323. [Google Scholar] [CrossRef]
- Docker, M.F.; Dale, A.; Heath, D.D. Erosion of Interspecific Reproductive Barriers Resulting from Hatchery Supplementation of Rainbow Trout Sympatric with Cutthroat Trout. Mol. Ecol. 2003, 12, 3515–3521. [Google Scholar] [CrossRef]
- Hernandez Chavez, C.; Turgeon, J. Asexual and Sexual Hybrids between Fundulus Diaphanus and F. Heteroclitus in the Canadian Atlantic Region. Mol. Ecol. 2007, 16, 1467–1480. [Google Scholar] [CrossRef]
- Latorre, D.; Masó, G.; Hinckley, A.; Verdiell-Cubedo, D.; Tarkan, A.S.; Vila-Gispert, A.; Copp, G.H.; Cucherousset, J.; Da Silva, E.; Fernández-Delgado, C.; et al. Inter-Population Variability in Growth and Reproduction of Invasive Bleak Alburnus Alburnus (Linnaeus, 1758) across the Iberian Peninsula. Mar. Freshw. Res. 2018, 69, 1326–1332. [Google Scholar] [CrossRef]
- Collares-Pereira, M.; Alves, M.; Ribeiro, F.; Domingos, I.; Almeida, P.; Costa, L.; Gante, H.; Filipe, A.; Aboim, M.; Rodrigues, P.; et al. Guia Dos Peixes de Água Doce e Migradores de Portugal Continental; Edições Afrontamento: Porto, Portugal, 2021; p. 292. [Google Scholar]
- Martelo, J.; da Costa, L.; Ribeiro, D.; Gago, J.; Magalhães, M.F.; Gante, H.F.; Alves, M.J.; Cheoo, G.; Gkenas, C.; Banha, F.; et al. Evaluating the Range Expansion of Recreational Non-Native Fishes in Portuguese Freshwaters Using Scientific and Citizen Science Data. BioInvasions Rec. 2021, 10, 378–389. [Google Scholar] [CrossRef]
- Paterna, F.J.O.; Trigo, F.A.; Pérez, A.S.; Marín, J.M.Z.; Ruiz-Navarro, A.; Ferrero, A.T. Peces Exóticos En La Cuenca Del Río Segura: Impactos Potenciales y Prioridad En La Gestión. In Proceedings of the Biodiversidad y procesos ecológicos en el Sureste Ibérico; Universidad de Murcia, Servicio de Publicaciones: Murcia, Spain, 2017; pp. 250–260. [Google Scholar]
- Wheeler, A. Hybrids of Bleak, Alburnus Alburnus, and Chub, Leuciscus Cephalus in English Rivers. J. Fish Biol. 1978, 13, 467–473. [Google Scholar] [CrossRef]
- Crivelli, A.; Dupont, F. Biometrical and Biological Features of Alburnus Alburnus × Rutilus Rubilio Natural Hybrids from Lake Mikri Prespa, Northern Greece. J. Fish Biol. 1987, 31, 721–733. [Google Scholar] [CrossRef]
- Berrebi, P.; Dupont, F.; Cattaneo-Berrebi, G.; Crivelli, A. An Isozyme Study of the Natural Cyprinid Hybrid Alburnus Alburnus × Rutilus Rubilio in Greece. J. Fish Biol. 1989, 34, 307–313. [Google Scholar] [CrossRef]
- Šorić, V.M. A Natural Hybrid of Leuciscus Cephalus and Alburnus Alburnus (Pisces, Cyprinidae) from the Ibar River, Western Serbia. Arch. Biol. Sci. 2004, 56, 23–32. [Google Scholar] [CrossRef]
- Witkowski, A.; Kotusz, J.; Wawer, K.; Stefaniak, J.; Popiołek, M.; Błachuta, J. A Natural Hybrid of Leuciscus Leuciscus (L.) and Alburnus Alburnus (L.) (Osteichthyes: Cyprinidae) from the Bystrzyca River (Poland). Ann. Zool. 2015, 65, 287–293. [Google Scholar] [CrossRef]
- Tsyba, A.; Ghazali, M.; Kokodiy, S.; Mezhzherin, S. Regular Intergeneric Hybridization of Leuciscine Cyprinids (Cyprinidae, Leuciscinae) in the Dnipro Affluants. Zoodiversity 2021, 55, 295–306. [Google Scholar] [CrossRef]
- Almodóvar, A.; Nicola, G.; Leal, S.; Torralva, M.; Elvira, B. Natural Hybridization with Invasive Bleak Alburnus Alburnus Threatens the Survival of Iberian Endemic Calandino Squalius Alburnoides Complex and Southern Iberian Chub Squalius Pyrenaicus. Biol. Invasions 2012, 14, 2237–2242. [Google Scholar] [CrossRef]
- Sousa-Santos, C.; Matono, P.; Da Silva, J.; Ilheu, M. Evaluation of Potential Hybridization between Native Fishes and the Invasive Bleak, Alburnus Alburnus (Actinopterygii: Cypriniformes: Cyprinidae). Acta Ichthyol. Et Piscat. 2018, 48, 109–122. [Google Scholar] [CrossRef]
- Collares-Pereira, M.; Matos, I.; Morgado-Santos, M.; Coelho, M. Natural Pathways towards Polyploidy in Animals: The Squalius Alburnoides Fish Complex as a Model System to Study Genome Size and Genome Reorganization in Polyploids. Cytogenet. Genome Res. 2013, 140, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Morgado-Santos, M.; Magalhães, M.; Vicente, L.; Collares-Pereira, M. Mate Choice Driven by Genome in an Allopolyploid Fish Complex. Behav. Ecol. 2018, 29, 1359–1370. [Google Scholar] [CrossRef]
- Gante, H.F.; Collares-Pereira, M.J.; Coelho, M.M. Introgressive hybridisation between two Iberian Chondrostoma species (Teleostei, Cyprinidae) revisited: New evidence from morphology, mitochondrial DNA, allozymes and NOR-phenotypes. Folia Zool. 2004, 53, 423–432. [Google Scholar]
- Aboim, M.A.; Mavárez, J.; Bernatchez, L.; Coelho, M.M. Introgressive hybridization between two Iberian endemic cyprinid fish: A comparison between two independent hybrid zones. J. Evol. Biol. 2010, 23, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Santos, C.; Robalo, J.I.; Collares-Pereira, M.-J.; Almada, V.C. Heterozygous Indels as Useful Tools in the Reconstruction of DNA Sequences and in the Assessment of Ploidy Level and Genomic Constitution of Hybrid Organisms. DNA Seq. 2005, 16, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, N.V.; Zemlak, T.S.; Hanner, R.H.; Hebert, P.D. Universal Primer Cocktails for Fish DNA Barcoding. Mol. Ecol. Notes 2007, 7, 544–548. [Google Scholar] [CrossRef]
- Rubidge, E.; Corbett, P.; Taylor, E.B. A Molecular Analysis of Hybridization between Native Westslope Cutthroat Trout and Introduced Rainbow Trout in Southeastern British Columbia, Canada. J. Fish Biol. 2001, 59, 42–54. [Google Scholar] [CrossRef]
- Meldgaard, T.; Crivelli, A.J.; Jesensek, D.; Poizat, G.; Rubin, J.-F.; Berrebi, P. Hybridization Mechanisms between the Endangered Marble Trout (Salmo Marmoratus) and the Brown Trout (Salmo Trutta) as Revealed by in-Stream Experiments. Biol. Conserv. 2007, 136, 602–611. [Google Scholar] [CrossRef]
- Meraner, A.; Venturi, A.; Ficetola, G.; Rossi, S.; Candiotto, A.; Gandolfi, A. Massive Invasion of Exotic B Arbus Barbus and Introgressive Hybridization with Endemic B Arbus Plebejus in N Orthern I Taly: Where, How and Why? Mol. Ecol. 2013, 22, 5295–5312. [Google Scholar] [CrossRef]
- Buonerba, L.; Zaccara, S.; Delmastro, G.B.; Lorenzoni, M.; Salzburger, W.; Gante, H.F. Intrinsic and Extrinsic Factors Act at Different Spatial and Temporal Scales to Shape Population Structure, Distribution and Speciation in Italian Barbus (Osteichthyes: Cyprinidae). Mol. Phylogenetics Evol. 2015, 89, 115–129. [Google Scholar] [CrossRef]
- Doadrio, I. Atlas y Libro Rojo de Los Peces Continentales de España; Dirección General de Conservación de la Naturaleza: Madrid, Spain, 2001. [Google Scholar]
- Cabral, M.J.; Almeida, J.; Almeida, P.R.; Dellinger, T.; Ferrand de Almeida, N.; Oliveira, M.; Palmeirim, J.; Queirós, A.; Rogado, L.; Santos-Reis, M. Livro Vermelho Dos Vertebrados de Portugal; Instituto da Conservação da Natureza e das Florestas: Lisbon, Portugal, 2005. [Google Scholar]
- Morgado-Santos, M.; Carona, S.; Magalhaes, M.; Vicente, L.; Collares-Pereira, M. Reproductive Dynamics Shapes Genomotype Composition in an Allopolyploid Complex. Proc. R. Soc. B Biol. Sci. 2016, 283, 20153009. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.M.; Cowx, I.G.; Coelho, M.M. Life History Strategy of Leuciscus Pyrenaicus (Cyprinidae) in Intermittent Streams of the Guadiana Basin (Portugal). Cybium 2000, 24, 287–297. [Google Scholar]
- Alexandre, C.M.; Ferreira, M.T.; Almeida, P.R. Life-Cycle Responses of a Mediterranean Non-Migratory Cyprinid Species, the Northern Iberian Chub (Squalius Carolitertii Doadrio, 1988), to Streamflow Regulation. Ecohydrology 2018, 11, e1998. [Google Scholar] [CrossRef]
- Rinchard, J.; Kestemont, P. Comparative Study of Reproductive Biology in Single-and Multiple-Spawner Cyprinid Fish. I. Morphological and Histological Features. J. Fish Biol. 1996, 49, 883–894. [Google Scholar] [CrossRef]
- Masó, G.; Latorre, D.; Tarkan, A.S.; Vila-Gispert, A.; Almeida, D. Inter-Population Plasticity in Growth and Reproduction of Invasive Bleak, Alburnus Alburnus (Cyprinidae, Actinopterygii), in Northeastern Iberian Peninsula. Folia Zool. 2016, 65, 10–14. [Google Scholar] [CrossRef]
- Morgado-Santos, M.; Pereira, H.M.; Vicente, L.; Collares-Pereira, M.J. Mate Choice Drives Evolutionary Stability in a Hybrid Complex. PLoS ONE 2015, 10, e0132760. [Google Scholar] [CrossRef][Green Version]
- Perea, S.; Böhme, M.; Zupančič, P.; Freyhof, J.; Šanda, R.; Özuluğ, M.; Abdoli, A.; Doadrio, I. Phylogenetic Relationships and Biogeographical Patterns in Circum-Mediterranean Subfamily Leuciscinae (Teleostei, Cyprinidae) Inferred from Both Mitochondrial and Nuclear Data. BMC Evol. Biol. 2010, 10, 265. [Google Scholar] [CrossRef]
- Echelle, A.A.; Connor, P.J. Rapid, Geographically Extensive Genetic Introgression after Secondary Contact between Two Pupfish Species (Cyprinodon, Cyprinodontidae). Evolution 1989, 43, 717–727. [Google Scholar]
- Kovach, R.P.; Muhlfeld, C.C.; Boyer, M.C.; Lowe, W.H.; Allendorf, F.W.; Luikart, G. Dispersal and Selection Mediate Hybridization between a Native and Invasive Species. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142454. [Google Scholar] [CrossRef]
- Haynes, G.; Gongora, J.; Gilligan, D.; Grewe, P.; Moran, C.; Nicholas, F. Cryptic Hybridization and Introgression between Invasive C Yprinid Species C Yprinus Carpio and C Arassius Auratus in A Ustralia: Implications for Invasive Species Management. Anim. Conserv. 2012, 15, 83–94. [Google Scholar] [CrossRef]
- Hohenlohe, P.A.; Amish, S.J.; Catchen, J.M.; Allendorf, F.W.; Luikart, G. Next-Generation RAD Sequencing Identifies Thousands of SNPs for Assessing Hybridization between Rainbow and Westslope Cutthroat Trout. Mol. Ecol. Resour. 2011, 11, 117–122. [Google Scholar] [CrossRef] [PubMed]
- von Thaden, A.; Nowak, C.; Tiesmeyer, A.; Reiners, T.E.; Alves, P.C.; Lyons, L.A.; Mattucci, F.; Randi, E.; Cragnolini, M.; Galián, J.; et al. Applying Genomic Data in Wildlife Monitoring: Development Guidelines for Genotyping Degraded Samples with Reduced Single Nucleotide Polymorphism Panels. Mol. Ecol. Resour. 2020, 20, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Curto, M.; Winter, S.; Seiter, A.; Schmid, L.; Scheicher, K.; Barthel, L.M.; Plass, J.; Meimberg, H. Application of a SSR-GBS Marker System on Investigation of European Hedgehog Species and Their Hybrid Zone Dynamics. Ecol. Evol. 2019, 9, 2814–2832. [Google Scholar] [CrossRef] [PubMed]
| ID | Nuclear Genome | Mitochondrial Genome | Parentage | |
|---|---|---|---|---|
| Beta-actin Gene SNP | COI BOLD Systems Blast | Mother | Father | |
| Squalius carolitertii (consensus sequence) | … TAAACGTTTTA … … TAAACGTTTTA … | - | - | - |
| Alburnus alburnus (consensus sequence) | … TAAACATTTTA … … TAAACATTTTA … | - | - | - |
| V2H1 | … TAAACGTTTTA … … TAAACATTTTA … | S. carolitertii (95.22–100%) | Squalius carolitertii | Alburnus alburnus |
| V4H1 | … TAAACGTTTTA … … TAAACATTTTA … | S. carolitertii (94.91–100%) | Squalius carolitertii | Alburnus alburnus |
| V4H1_21 | … TAAACGTTTTA … … TAAACATTTTA … | S. carolitertii (95.17–100%) | Squalius carolitertii | Alburnus alburnus |
| V4H2 | … TAAACGTTTTA … … TAAACATTTTA … | S. carolitertii (94.91–100%) | Squalius carolitertii | Alburnus alburnus |
| V4H3_21 | … TAAACGTTTTA … … TAAACATTTTA … | S. carolitertii (94.97–100%) | Squalius carolitertii | Alburnus alburnus |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curto, M.; Morgado-Santos, M.; Alexandre, C.M.; Alves, M.J.; Gante, H.F.; Gkenas, C.; Medeiros, J.P.; Pinheiro, P.J.; Almeida, P.R.; Magalhães, M.F.; et al. Widespread Hybridization between Invasive Bleak (Alburnus alburnus) and Iberian Chub (Squalius spp.): A Neglected Conservation Threat. Fishes 2022, 7, 247. https://doi.org/10.3390/fishes7050247
Curto M, Morgado-Santos M, Alexandre CM, Alves MJ, Gante HF, Gkenas C, Medeiros JP, Pinheiro PJ, Almeida PR, Magalhães MF, et al. Widespread Hybridization between Invasive Bleak (Alburnus alburnus) and Iberian Chub (Squalius spp.): A Neglected Conservation Threat. Fishes. 2022; 7(5):247. https://doi.org/10.3390/fishes7050247
Chicago/Turabian StyleCurto, Manuel, Miguel Morgado-Santos, Carlos M. Alexandre, Maria Judite Alves, Hugo F. Gante, Christos Gkenas, João P. Medeiros, Paulo J. Pinheiro, Pedro R. Almeida, Maria Filomena Magalhães, and et al. 2022. "Widespread Hybridization between Invasive Bleak (Alburnus alburnus) and Iberian Chub (Squalius spp.): A Neglected Conservation Threat" Fishes 7, no. 5: 247. https://doi.org/10.3390/fishes7050247
APA StyleCurto, M., Morgado-Santos, M., Alexandre, C. M., Alves, M. J., Gante, H. F., Gkenas, C., Medeiros, J. P., Pinheiro, P. J., Almeida, P. R., Magalhães, M. F., & Ribeiro, F. (2022). Widespread Hybridization between Invasive Bleak (Alburnus alburnus) and Iberian Chub (Squalius spp.): A Neglected Conservation Threat. Fishes, 7(5), 247. https://doi.org/10.3390/fishes7050247










