Abstract
Brown trout of non-native lineages have been stocked into Croatian streams and rivers primarily to meet angler demand. The diet of brown trout in the Black Sea Basin of Croatia is poorly understood, and there are no studies examining feeding competition between the Atlantic (AT) and Danube (DA) lineages of brown trout and their hybrids (HY). The aim of this study was to examine the natural diet of brown trout of both lineages and their hybrids and to compare feeding overlap. Canonical correspondence analysis was used to investigate the relationships between feeding habits of fish from different streams and of different genetic origin. The differences in variation of the consumed prey items were analysed by canonical variate analysis, and diet overlap was assessed by the Schoener index. The results indicate that stocked brown trout (AT) adapt rapidly to new habitat and food, as revealed by the consumption of a wide range of available food items and competition for food and space by taking on the feeding behaviour of wild native conspecifics. Diet overlap was also detected between brown trout of the DA and AT lineages. This study highlights the need to implement control measures to preserve and protect the native diversity of this species.
1. Introduction
The Western Balkans is a region known for prominent diversity of trout (Salmo spp.), although the taxonomy of this group remains unresolved [1,2,3]. In Croatia, trout species are distributed in both the Adriatic and Black Sea Basins. At least four distinct species of the genus Salmo can be considered native to the karst watersheds of the Adriatic Basin in Croatia [1], whereas native Danubian (DA) salmon Salmo labrax (Pallas, 1814) tentatively occur in the Black Sea Basin [3,4] in coexistence with the introduced Atlantic (AT) lineage of S. trutta [5].
Brown trout of the AT lineage have been stocked into Croatian streams and rivers primarily to meet angler demand [6,7], which is also the case elsewhere in the Western Balkans [3] and worldwide [8,9]. The AT lineage of brown trout inhabits the whole European Atlantic basin, from the Barents Sea and Iceland in the north to the Atlas Mountains of Morocco in the south [10]. This lineage has been assessed as invasive in the Balkan region, posing a threat to native populations [11] due to introgression into the gene pool of the native DA lineage of brown trout [12] and strong competition for food resources [13,14,15].
The genetic effect of introduced brown trout lineages on native brown trout populations has been studied intensively [12,16,17], although less attention has been given to their ecological impacts [18]. Brown trout is an opportunistic feeder [19], feeding on a wide range of available prey in the aquatic environment [20] and on food items captured near the surface [18,19].
Several studies have been performed on diet overlap between hatchery-reared, stocked brown trout specimens of unknown genetic status and wild specimens in the recipient, i.e., stocked native brown trout populations in inland waters [13,18,21,22]. Stocked brown trout start to consume wild prey immediately after their release and, within one week of their release, feed on wild prey just like wild fish [21,22], indicating possible feeding competition. In the streams and rivers of northeast Portugal, stocked fish showed a nearly exclusive preference for adult insects, emergent pupae and subimago insects with aquatic life periods as food items captured near the surface, and there was an absence of evident competition for food with wild trout specimens [18]. On the contrary, diet overlap of stocked and native trout was reported in a subarctic lake in northern Finland, indicating that stocked trout compete for food and space with native, i.e., wild, trout and possibly suppress native trout populations [13].
With the exception of a study by Trožić-Borovac [20], the diet of brown trout in the Black Sea Basin of Croatia is poorly understood, and no studies have been conducted to date on feeding competition between the AT and DA lineages of brown trout and their hybrids. Thus, the aim of this study was to examine the natural diets of brown trout of both AT and DA lineages and their hybrids and to determine any overlap between them.
2. Materials and Methods
2.1. Study Area and Fish Sampling
A total of ten streams and rivers in the Gorski Kotar (Bresni potok, Mala Lešnica, Curak), Žumberak (Kupčina, Slapnica) (Jankovački potok, Brzaja, Veličanka, Toplica and Orljava) Mountains (Figure 1) were sampled during daylight hours using an electrofishing device (Hans Grassl 2.2 kW). The Curak and Slapnica streams are under the concession of the local angling club and may have been restocked with juvenile brown trout of unknown origin during the winter. Sampling was performed several months later (May), when the new juveniles were assumed to have fully adapted to consume natural food. The upper part of the Kupčina River is the location of an operational brown trout farm (farming Denmark AT brown trout lineage). No active fishing associations or brown trout farms have been identified on other streams, and therefore, the collected specimens can be assumed to be of fully wild origin. Sampling was performed with the permission of the national authorities. Samples from Gorski Kotar were collected in April 2017, from Žumberak in May 2017 and from Papuk in May 2018. In the Gorski Kotar and Žumberak regions, Trichoptera represented ≈50% and Diptera ≈30% of the total macroinvertebrate biomass in spring [23,24,25]. The dominant benthic macroinvertebrates in the Papuk region are Amphipoda, Coleoptera, Ephemeroptera and Diptera, with the highest abundances in spring [26,27].

Figure 1.
Sampling locations: Gorski Kotar region: 1–Mala Lešnica, 2–Curak; 3–Bresni Potok; Mt. Žumberak area: 4–Kupčina, 5–Slapnica; Mt. Papuk area: 6–Orljava, 7–Toplica, 8–Brzaja, 9–Jankovački potok, 10–Veličanka.
2.2. Gut Content Analysis, Diet Overlap and Prey Importance
After sampling, fish specimens were stored at −20 °C. In the laboratory, each specimen was measured for total length (TL, in cm) and weight (W, in g). The gut content was removed and weighed, and prey items were determined to the lowest possible systematic category. Brown trout specimens were classified into three groups (see Supplementary Material File S1 and Kanjuh et al. [5]): two phylogenetic lineages, Danubian (DA), Atlantic (AT) sensu Bernatchez [28]; and a hybrid (HY) group: Papuk n = 33 (DA = 8, AT = 1, HY = 22, undeterminable = 2), Gorski Kotar n = 28 (DA = 11, AT = 6, HY = 11), Žumberak n = 21 (DA = 3, AT = 0, HY = 18) (Table 1). Two specimens were unclassified and removed from further analysis related to genetic origin due to inconclusive results of the genetic analysis (Table 2).

Table 1.
Descriptive statistics of sampled brown trout specimens from 10 streams with affiliation to lineages determined by molecular methods (sensu Kanjuh et al. [5]), (n = number of specimens; x = average; W = weight (g); TL = total length (cm); DA = Danube lineage; AT = Atlantic lineage; UD—undeterminable; HY = hybrids; M = male; F = female; J = juveniles).

Table 2.
Descriptive statistics of sampled brown trout specimens classified by identified lineages (n = number of specimens; W = weight (g); TL = total length (cm); DA = Danube lineage; AT = Atlantic lineage; M = male; F = female; J = juveniles) (two unclassified specimens excluded).
Assessment of the fish diet was based on the numerical (N%) and mass (W%) proportion and frequency of occurrence (F%) of different diet components [29] using the following formulae:
where ni is the total number of a particular prey item, and Σn is the total number of prey items consumed by the fish;
where wi is the total mass of a single prey item, and Σw is the total mass of prey items consumed by the fish;
where fi is the number of guts containing each prey item, and Σf is the total number of guts containing food [30].
Analysis of changes in feeding habits was performed using the following indices [29]:
The indices of absolute (IAIα) and relative importance (IRI) for each prey category were calculated according to Cortés [31]:
where α is a specific prey category, and n is the number of different prey categories.
Data of the specimen affiliation to haplotype were taken from Kanjuh et al. [5,32], and diet overlap between DA and AT lineages and their hybrids was calculated separately for each region using the index proposed by Schoener [33] based on IRI:
where n is the number of prey items, PVxi is the IRI percentage of prey item i in species x and PVyi is the IRI percentage of prey item i in species y. The values range from 0 (no feeding overlap) to 1 (total feeding overlap), and values >0.60 are considered a significant overlap.
2.3. Statistical Analysis of Fish Feeding Habits
For statistical analyses of fish feeding habits, all specimens with empty stomachs were removed as follows: Bresni potok (1 HY), Curak (2 Da, 1 Hy), Mala Lešnica (3 HY) and Slapnica (1 DA). The determined prey items used in all statistical analysis were based on IRI values.
The difference in variation of consumed prey types from regions sampled in April, at the beginning and end of May were analysed by linear discriminant analysis used as canonical variate analysis (CVA) and filtered by stepwise selection of discriminating variables (for selection of the best predictors, p < 0.05), and unrestricted permutations of test parameters were applied. The region variable was expressed as the factor for group classification. Prey types were expressed as IRI values and represented predictors.
The relationships between fish feeding habits (response variables) from streams at all sampling locations, streams within each region, genetic origin (AT; DA; HY) and two specimen sizes (<20 cm; >20 cm TL), as explanatory variables, were analysed using canonical correspondence analysis (CCA). The sex effect in the Papuk region was excluded due to the insignificant result. Streams, region and specimen size were expressed as factors, whereas genetic origin was a dummy variable. The specimen sizes were chosen according to Čanak-Atlagić [34] with the assumption that specimens > 20 cm TL shift their diet toward larger prey. Data were log-transformed, centred and standardised by species (prey types); both above tests were performed; and unrestricted permutations were applied. The Monte Carlo permutation test was carried out using 499 permutations to test for the significance of the eigenvalues of all canonical axes. The conditional and simple effect of the explanatory variables were tested by summarising the effect of explanatory variables. The false-discovery rate was applied to perform p-value correction. CVA and CCA analyses were performed using the CANOCO 5 software package [35].
3. Results
In total, 82 brown trout specimens were analysed. TL ranged from 10.0 to 30.3 cm and W from 10.19 to 323.52 g (TLAT = 17.6–24.7 cm; WAT = 57.92–155.92 g; TLDA = 11.6–27.6 cm; WDA = 16.22–233.06 g; TLHY = 10.0–30.3 cm; WHY = 10.19–323.52 g). The overall sex ratio was skewed heavily, with more than five times more females (75:14:3; females: males: juveniles) and only females detected in the Žumberak and Gorski Kotar streams. According to the a priori classification based on mtDNA, 7, 22 and 51 specimens of AT, DA and HY, respectively, were identified, whereas the origin of two specimens remained unknown. The native DA group was found in all but one stream in the Žumberak region, whereas six of the seven AT specimens were found in Gorski Kotar streams. HY specimens were found in all analysed water bodies (Table 1 and Table 2).
The frequency of occurrence (F%), numerical frequency (N%), mass frequency (W%), index of relative importance (IRI), vacuity coefficient (VI%) and fullness index (FI%) of the food items consumed by brown trout caught in the Gorski Kotar, Papuk and Žumberak regions are presented in detail in Appendix A and Appendix B. The FI index was highest in the Bresni Potok stream (FI% = 3.1). Most analysed specimens contained full guts, whereas empty guts were found only in Curak (VI% = 37.5) and Slapnica (VI% = 10.0) specimens (Appendix A and Appendix B). The highest FI was detected in the AT lineage (FI% = 3.02), and no specimens had empty guts. Empty guts were found in both DA and HY specimens (VI% = 13.6 and 3.9, respectively) (Appendix C).
CVA analysis indicated differences in prey intake within regions. The best predictors in the Žumberak region were Trichoptera and Vespidae (p < 0.05), as opposed to Coleoptera, Formicidae and Muscidae (Diptera) in the Papuk region (p < 0.05) and Trichoptera in Gorski Kotar (p < 0.05). Discrimination of consumed prey items between lineages and within regions was not indicated, except the grouping of AT specimens in Gorski Kotar (Figure 2; Appendix D).
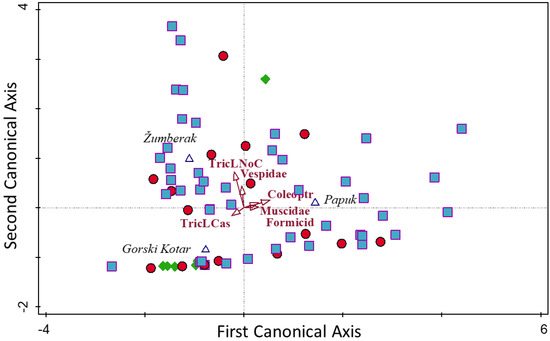
Figure 2.
Canonical variate analysis plot of consumed prey items (→) within regions Δ (Gorski Kotar, Žumberak and Papuk) and between Danube (DA =  ), Atlantic (AT =
), Atlantic (AT =  ) and hybrid (HY =
) and hybrid (HY =  ) specimens of brown trout (See Appendix C for abbreviations).
) specimens of brown trout (See Appendix C for abbreviations).
 ), Atlantic (AT =
), Atlantic (AT =  ) and hybrid (HY =
) and hybrid (HY =  ) specimens of brown trout (See Appendix C for abbreviations).
) specimens of brown trout (See Appendix C for abbreviations).
CCA analysis suggests that statistically different groups of prey items were consumed by brown trout caught in the Kupčina and Brzaja streams (p < 0.01) (Figure 3A; Appendix E.1). In the Mala Lešnica and Bresni Potok streams, larger specimen sizes (>20 cm TL) were detected (p > 0.05), and larger prey was found in the guts (e.g., the bullhead Cottus gobio and native stone crayfish Austropotamobius torrentium) (Figure 3A,B; Appendix E.1 and Appendix E.2). In the Papuk region, individuals of smaller size (<20 cm TL) and of the HY group and DA lineage were recorded (p > 0.05), whereas AT lineage are associated with larger specimen size (Figure 3A,C; Appendix E.1 and Appendix E.3). Plecoptera, Caelifera, Psychodidae and Amphipoda were the important prey items for specimens from the Kupčina stream, and Vespidae, Trichoptera and Bivalvia were the important prey items for specimens from the Slapnica stream in the Žumberak region (Figure 3A,D; Appendix E.1 and Appendix E.4). In the Papuk region, Amphipoda was associated with the DA lineage, whereas Coleoptera, Hymenoptera and Formicidae were associated with HY specimens (Figure 3A,C; Appendix E.1 and Appendix E.3). The brown trout of AT lineage in the Gorski Kotar and Papuk regions are associated with larger prey, such as Plecoptera and stone crayfish (Figure 3A–C; Appendix A and Appendix B). Trichoptera was associated with both lineages in the Gorski Kotar and Žumberak regions (Figure 3B,C, Appendix E.2 and Appendix E.3), which corroborates the results of the CVA (Figure 2).
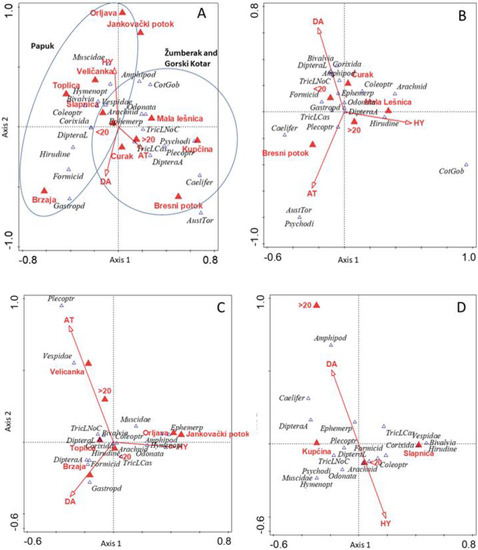
Figure 3.
Canonical correspondence analysis ordination diagram representing the IRI of the main prey items of brown trout at each sampling site. (A) Total variation is 6.79, and explanatory variables account for 25.0% of the variation; Monte Carlo permutation test results on all axes: pseudo-F = 2.0, p = 0.002. (B) Gorski Kotar: total variation is 6.06, and explanatory variables account for 32.3% of the variation; Monte Carlo permutation test results on all axes: pseudo-F = 1.4, p = 0.024. (C) Papuk: total variation is 4.50, and explanatory variables account for 36.1% of the variation; Monte Carlo permutation test results on all axes: pseudo-F = 1.9, p = 0.002. (D) Žumberak: total variation is 5.09, and explanatory variables account for 25.0% of the variation; Monte Carlo permutation test results on all axes: pseudo-F = 1.2, p = 0.198; (→—dummy explanatory variables  —nominal explanatory variables,
—nominal explanatory variables,  —prey item) (See Appendix C for abbreviations).
—prey item) (See Appendix C for abbreviations).
 —nominal explanatory variables,
—nominal explanatory variables,  —prey item) (See Appendix C for abbreviations).
—prey item) (See Appendix C for abbreviations).
According to the IRI coefficient, the main prey items of brown trout of both lineages were aquatic Coleoptera, Trichoptera, Diptera and terrestrial Formicidae (Figure 4; Appendix C). Terrestrial Formicidae, Gastropoda and Corixidae appear to be important for the DA lineage, whereas Coleoptera, Amphipoda and bullhead are important for the HY group. Cased Trichoptera larvae, adult Diptera, Psychodidae and larger prey, such as the native stone crayfish, were more important prey for the AT than the DA lineage and HY group (Figure 4). According to the Schoener index (α), statistically significant diet overlap was detected between DA and AT specimens (α = 0.66, p < 0.05) (Table 3) within the Gorski Kotar region, where the most of AT lineage were detected.
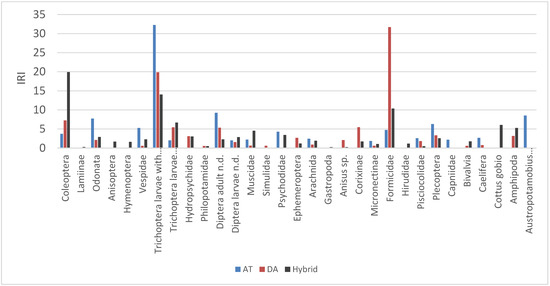
Figure 4.
IRI prey importance for the AT and DA lineages and hybrids of brown trout from the Žumberak, Gorski Kotar and Papuk Mountains.

Table 3.
Schoener index (α) results based on the IRI of feeding overlap between brown trout of Atlantic (AT) and Danube (DA) lineages and their hybrids (HY) (* p < 0.05).
4. Discussion
Stocking inland waters with non-native lineages of brown trout is currently the main threat to the original diversity of wild brown trout stocks in part of the Black Sea Basin in the Western Balkans [11]. The highest number of brown trout belonging to the non-native AT lineage was detected in the streams of Gorski Kotar, suggesting strong recent stocking pressure there [5]. Hybrid specimens were found in all streams, which may be either the result of long-lasting stocking pressure or recent escapes from trout farms [5,36]. For example, there is a trout fish farm that rears brown trout exclusively of the AT lineage in the upper Kupčina River, and most hybrid specimens were found there. Other streams do not have trout farms, and data on stocking activity were not available. Brown trout of the DA lineage were found in eight of ten streams, although their presence does not confirm their native origin due to the possibility of translocation from another region or river basin [5].
The food composition of brown trout in this study revealed dietary flexibility, likely due to prey availability in the drift and benthos [19,20,37]. Seasonal changes in food availability and seasonal diet preferences were considered as three periods in late spring, when different prey items were expected to be the most available in the environment [21]. The main prey items of specimens from Gorski Kotar (Bresni potok, Mala Lešnica and Curak) and Žumberak (Kupčina, Slapnica) were various Trichoptera species, which are abundant and distributed throughout the region [25] and indicate that in the sampling period of this study, Trichoptera taxa were well developed in both seasons, late April and early May. The guts of specimens originating from Mt. Papuk streams (Jankovački Potok, Brzaja, Orljava, Veličanka and Toplica) showed the prevalence of Coleoptera and Amphipoda, which corresponds to previously recorded entomofauna and macroinvertebrate communities in the streams of that region [26,27] and coincides with the most consumed prey taxa by brown trout from this study. In addition to the most abundant prey, a wide range of food items was consumed, similarly to previous reports by Cada et al. [19] and Trožić-Borovac [20].
The size structure of the analysed brown trout specimens differed between the lineages. The size range (TL, cm) of DA and HY individuals was larger than that of AT individuals. The specimens were divided into larger and smaller than 20 cm TL, although no statistical differences were found in consumed prey items between these size classes. Therefore, the consumption of prey by brown trout is likely dependent on relative prey abundance, i.e., brown trout exploits the most abundant prey regardless of brown trout and prey size structure, as suggested by Rincón and Lobón-Cerviá [38]. However, the lack of brown trout specimens smaller than 17 cm TL and the small number of analysed specimens in the AT lineage may have affected the result.
The results of this study show that various Trichoptera larvae, Coleoptera and Diptera are important in the diet of both genetic lineages and HY. Similar results were obtained for other native stream-dwelling, resident brown trout distributed throughout Europe [20,39]. probably due to the similarity of brown trout taste preferences regulated by orosensory control [40]. A preference for larger prey (e.g., Plecoptera, Decapoda) was found only in brown trout of the AT lineage, where only large specimens were captured, which might have skewed the results. The examined AT specimens originated from a fish farm, as confirmed by Špelić et al. [36] based on morphological traits. Kahilainen and Lehtonen [13] demonstrated that resource utilisation (both habitat and food) of stocked and native trout becomes similar during the first summer after stocking. It appears that the brown trout of the AT lineage analysed here were released recently (in the same year or the year before sampling), as AT individuals of only 17 to 24 cm TL were already well adapted to natural food.
Brown trout are opportunistic feeders, consuming prey in proportion to its abundance in the environment [19]. In order to ensure stability of brown trout populations in streams with relatively low freshwater food production, their feeding habits likely shift to prey items of terrestrial origin [19]. Preliminary data have implied that brown trout of the AT lineage may prefer terrestrial prey [15], although no significant consumption of terrestrial prey items in either brown trout lineage or the hybrids was revealed in this study. Such a result may be affected by the differing number of individuals from each lineage and HY or from a seasonal effect. Furthermore, piscivory was observed only in hybrid specimens in the Mala Lešnica stream, although this was not found to be significant. In general, the investigated region belongs to the upper rhithron type of fish communities represented by low diversity, with brown trout Salmo cf. trutta (here denoted as S. labrax), European bullhead Cottus gobio and minnow Phoxinus phoxinus as the most common fish species [41,42]. Brown trout was observed to be less piscivorous in lotic than lentic ecosystems due to the absence of appropriately sized fish prey [43], which may cause a diet shift of larger specimens toward available larger prey and more abundant food items [38]. Additionally, brown trout specimens in this study did not exceed 30 cm TL, when brown trout are reported to shift to piscivory [44,45], although Čanak-Atlagić et al. [34] revealed that even those of only 19–21.5 cm TL can prey on small fish, such as minnows (Phoxinus sp.). Furthermore, the possibility that larger specimens had been removed through angling activities in the Žumberak and Gorski Kotar regions should not be neglected.
In the Gorski Kotar region, significant diet overlap was found between the DA and AT lineages, supporting the findings of Kahilainen and Lehtonen [13] on feeding competition between stocked and native brown trout. In the other localities of the study area, no sufficient samples of AT lineage were found to further confirm this finding. In this field experiment, especially in the small streams of the study area, it is difficult to obtain a perfectly balanced sample that would give clear and reliable results, and in cases where differences were found, this could be caused by the limited productivity of the investigated streams [46,47].
5. Conclusions
This study confirmed that brown trout consume a wide range of prey types. Results revealed significant feeding overlap between brown trout of the DA lineage and AT brown trout lineages. The results imply that stocked brown trout of the AT lineage can quickly adapt to a new habitat by means of efficient competition for food by acquiring the feeding behaviour of wild conspecifics. Because of this rapid adaptation to natural food sources, their continued restocking could disburse existing food resources available to the pure, native ichthyofauna when present in the same habitat. Furthermore, the large number of hybrid specimens found in the study area indicates that the native character of brown trout populations is additionally disturbed, raising the need to apply conservation measures to preserve and protect the genetic diversity of this species. Such measures might include the use of Whitlock–Vibert boxes to assist native stock enhancement [48,49] or encouraging farm production of native lineages for inland water stocking.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes7040179/s1, File S1: Brown trout haplogroups determination. Reference [50] is cited in the supplementary materials.
Author Contributions
All authors contributed extensively to the work presented in this paper. M.P. designed the study. M.P., P.S., I.Š., T.K., L.V. and I.L. collected the data. M.P., I.M. and P.S. prepared the first draft of the manuscript. M.P., I.Š., L.V., I.L. and T.R. analysed and interpreted the results. M.P., T.K. and A.M. finalised the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from the Croatian Science Foundation (CLINEinBIOta—IP-2016–06-2563), by the Croatia-Serbia bilateral programme 2019–2022 and by the EIFAAC/FAO Project “Management/Threat of Aquatic Invasive Species in Europe”.
Institutional Review Board Statement
All applicable international, national and institutional guidelines for the care and use of animals were followed.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available because no valid data repositories exist.
Acknowledgments
We thank Linda Zanella for language editing and constructive comments.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Appendix A
Frequency of occurrence (F%), numerical proportion (N%), mass proportion (W%), index of relative importance (IRI), vacuity coefficient (VI) and fullness index (FI) of the food items consumed by brown trout caught in the Žumberak (Kupčina, Slapnica) and Gorski Kotar (Bresni Potok, Mala Lešnica, Curak) streams (n—number of analysed specimens; n.d.—not determined). Bold values represent the most important food items.
| Food Item | Curak FI = 1.6; VI = 37.5; n = 8 | Kupčina FI = 2.7; VI = 0; n = 11 | Mala Lešnica FI = 1.82; VI = 18.2; n = 10 | Slapnica FI = 2.6; VI = 10.0; n = 10 | Bresni Potok FI = 3.1; VI = 0; n = 10 | |||||||||||||||
| N% | W% | F% | IRI | N% | W% | F% | IRI | N% | W% | F% | IRI | N% | W% | F% | IRI | N% | W% | F% | IRI | |
| Coleoptera | 3.62 | 2.48 | 7.14 | 4.83 | 1.37 | 1.37 | 6.38 | 3.29 | 5.56 | 4.13 | 9.09 | 7.05 | 4.33 | 4.94 | 9.09 | 6.89 | 2.53 | 0.89 | 6.45 | 4.18 |
| Odonata | 14.49 | 10.92 | 7.14 | 11.88 | 3.56 | 3.49 | 4.26 | 4.08 | 1.44 | 1.81 | 4.55 | 2.93 | 1.27 | 0.55 | 3.23 | 2.14 | ||||
| Anisoptera | 10.68 | 6.18 | 3.45 | 7.33 | ||||||||||||||||
| Hymenoptera | 0.27 | 0.49 | 1.72 | 0.89 | ||||||||||||||||
| Vespidae | 8.17 | 17.36 | 11.36 | 13.84 | ||||||||||||||||
| Trichoptera larvae with case n.d. | 24.64 | 29.28 | 14.29 | 24.89 | 4.11 | 17.10 | 6.90 | 10.14 | 36.11 | 34.84 | 22.73 | 35.19 | 51.92 | 17.93 | 11.36 | 30.47 | 60.76 | 43.36 | 22.58 | 53.69 |
| Trichoptera larvae without case n.d. | 5.80 | 4.42 | 10.71 | 7.64 | 28.22 | 22.84 | 18.97 | 25.26 | 2.78 | 0.43 | 4.55 | 2.91 | 4.33 | 7.84 | 9.09 | 7.98 | 2.53 | 0.33 | 3.23 | 2.58 |
| Hydropsychidae | 1.64 | 2.56 | 5.17 | 3.38 | 1.27 | 0.46 | 3.23 | 2.10 | ||||||||||||
| Diptera adult n.d. | 16.67 | 11.99 | 7.14 | 13.06 | 17.81 | 7.97 | 3.45 | 10.54 | 5.56 | 0.85 | 4.55 | 4.12 | 0.00 | 5.06 | 0.23 | 3.23 | 3.61 | |||
| Diptera larvae n.d. | 2.90 | 1.39 | 3.57 | 2.87 | 1.10 | 0.48 | 1.72 | 1.19 | 0.96 | 3.19 | 4.55 | 3.26 | ||||||||
| Muscidae | 0.55 | 2.23 | 1.72 | 1.62 | ||||||||||||||||
| Psychodidae | 19.45 | 13.70 | 8.62 | 15.07 | ||||||||||||||||
| Ephemeroptera | 0.55 | 0.74 | 3.45 | 1.71 | 0.96 | 2.50 | 4.55 | 3.01 | 2.53 | 0.25 | 3.23 | 2.55 | ||||||||
| Arachnida | 2.17 | 2.55 | 7.14 | 4.33 | 0.27 | 0.24 | 1.72 | 0.80 | 5.56 | 0.10 | 4.55 | 3.84 | 0.48 | 0.44 | 2.27 | 1.20 | ||||
| Gastropoda | ||||||||||||||||||||
| Anisus sp. | 1.27 | 0.20 | 3.23 | 1.99 | ||||||||||||||||
| Corixidae | ||||||||||||||||||||
| Corixinae | 9.42 | 9.17 | 7.14 | 9.39 | 2.78 | 0.08 | 4.55 | 2.78 | 12.50 | 8.22 | 6.82 | 10.33 | ||||||||
| Formicidae | 15.22 | 15.38 | 7.14 | 13.77 | 0.55 | 0.49 | 1.72 | 1.00 | 3.37 | 2.29 | 6.82 | 4.68 | 12.66 | 3.68 | 9.68 | 11.03 | ||||
| Hirudinea | ||||||||||||||||||||
| Pisciocolidae | 2.16 | 1.01 | 3.57 | 2.46 | ||||||||||||||||
| Hirudidae | 0.48 | 2.76 | 2.27 | 2.07 | ||||||||||||||||
| Plecoptera | 2.90 | 1.37 | 3.57 | 2.86 | 4.38 | 6.72 | 8.62 | 7.11 | 5.56 | 5.15 | 9.09 | 7.44 | 1.44 | 1.40 | 4.55 | 2.77 | 6.33 | 11.23 | 12.90 | 12.91 |
| Bivalvia | 2.78 | 0.08 | 4.55 | 2.78 | 9.62 | 16.31 | 2.27 | 10.58 | ||||||||||||
| Caelifera | 1.10 | 1.31 | 1.72 | 1.49 | ||||||||||||||||
| Pisces | ||||||||||||||||||||
| Cottus gobio | 8.33 | 46.47 | 4.55 | 22.29 | ||||||||||||||||
| Crustacea | ||||||||||||||||||||
| Amphipoda | 0.01 | 1.94 | 3.57 | 2.01 | 4.38 | 4.54 | 5.17 | 5.08 | 25.00 | 1.32 | 4.55 | 11.60 | 2.53 | 1.83 | 3.23 | 3.22 | ||||
| Austropotamobius torrentium | 1.27 | 24.21 | 3.23 | 12.17 | ||||||||||||||||
| Digested prey | 8.08 | 17.86 | 6.49 | 17.24 | 6.53 | 27.27 | 13.01 | 20.45 | 12.80 | 22.58 | ||||||||||
Appendix B
Frequency of occurrence (F%), numerical proportion (N%), mass proportion (W%), index of relative importance (IRI), vacuity coefficient (VI) and fullness index (FI) of the food items consumed by brown trout caught in Papuk streams (n—number of analysed specimens; n.d.—not determined). Bold values represent the most important food items.
| Food Item | Brzaja FI = 2.5; VI = 0; n = 10 | Veličanka FI = 2.2; VI = 0; n = 4 | Toplica FI = 2.8; VI = 0; n = 10 | Orljava FI = 1.6; VI = 0; n = 4 | Orljava FI = 2.4; VI = 0; n = 5 | |||||||||||||||
| N% | W% | F% | IRI | N% | W% | F% | IRI | N% | W% | F% | IRI | N% | W% | F% | IRI | N% | W% | F% | IRI | |
| Coleoptera | 20.72 | 19.89 | 10.42 | 18.04 | 13.68 | 36.07 | 21.05 | 28.94 | 83.14 | 28.55 | 20.00 | 49.00 | 8.75 | 11.46 | 16.67 | 12.90 | 25.55 | 18.09 | 10.00 | 17.88 |
| Lamiinae | 0.35 | 0.68 | 2.04 | 1.09 | ||||||||||||||||
| Odonata | 0.35 | 1.95 | 2.08 | 1.55 | 6.25 | 22.92 | 16.67 | 16.03 | 5.11 | 1.83 | 10.00 | 5.65 | ||||||||
| Hymenoptera | 1.40 | 1.16 | 2.08 | 1.64 | 6.25 | 6.30 | 0.00 | 4.39 | 1.46 | 1.79 | 5.00 | 2.75 | ||||||||
| Vespidae | 12.63 | 3.62 | 10.53 | 10.95 | 0.29 | 0.47 | 2.00 | 1.03 | ||||||||||||
| Trichoptera larvae with case n.d. | 1.40 | 8.20 | 8.16 | 6.28 | 1.43 | 4.94 | 8.00 | 5.35 | 4.38 | 30.09 | 16.67 | 17.88 | 0.73 | 0.48 | 5.00 | 2.07 | ||||
| Trichoptera larvae without case | ||||||||||||||||||||
| Hydropsychidae | 0.81 | 3.81 | 4.08 | 3.08 | 8.42 | 6.09 | 10.53 | 10.24 | 2.29 | 11.30 | 8.00 | 8.03 | 1.46 | 2.32 | 10.00 | 4.59 | ||||
| Philopotamidae | 0.12 | 2.12 | 2.04 | 1.51 | 2.19 | 2.67 | 10.00 | 4.95 | ||||||||||||
| Diptera adult n.d. | 0.58 | 5.14 | 4.08 | 3.46 | 0.57 | 0.78 | 2.00 | 1.25 | ||||||||||||
| Diptera larvae n.d. | 0.93 | 0.58 | 6.12 | 2.70 | 28.42 | 2.60 | 10.53 | 16.99 | 1.43 | 4.37 | 4.00 | 3.65 | ||||||||
| Muscidae | 0.35 | 0.28 | 4.08 | 1.66 | 2.11 | 1.70 | 5.26 | 3.71 | 2.00 | 3.93 | 6.00 | 4.44 | 63.75 | 17.19 | 16.67 | 34.13 | 0.73 | 1.94 | 5.00 | 2.56 |
| Simuliidae | 0.23 | 0.19 | 2.04 | 0.87 | ||||||||||||||||
| Ephemeroptera | 5.63 | 1.15 | 8.33 | 5.28 | 0.73 | 0.97 | 5.00 | 2.23 | ||||||||||||
| Arachnida | 0.12 | 2.89 | 2.04 | 1.79 | 0.86 | 2.68 | 6.00 | 3.55 | 1.88 | 2.29 | 8.33 | 4.37 | ||||||||
| Gastropoda | 0.12 | 0.68 | 2.04 | 1.00 | ||||||||||||||||
| Anisus sp. | 0.81 | 1.75 | 6.12 | 3.07 | 4.82 | 10.00 | 4.94 | |||||||||||||
| Corixidae | ||||||||||||||||||||
| Corixinae | 0.12 | 0.28 | 2.04 | 0.86 | 1.14 | 1.59 | 4.00 | 2.50 | ||||||||||||
| Micronectinae | 0.58 | 2.37 | 6.12 | 3.21 | 1.05 | 1.36 | 5.26 | 3.14 | 0.29 | 1.04 | 2.00 | 1.24 | 0.73 | 0.97 | 5.00 | 2.23 | ||||
| Formicidae | 70.43 | 32.74 | 18.37 | 42.96 | 6.32 | 1.80 | 5.26 | 5.47 | 5.14 | 4.84 | 14.00 | 8.92 | ||||||||
| Hirudinea | ||||||||||||||||||||
| Pisciocolidae | 0.47 | 5.83 | 6.12 | 4.39 | 0.29 | 0.76 | 2.00 | 1.13 | ||||||||||||
| Hirudidae | 0.29 | 16.87 | 2.00 | 7.13 | ||||||||||||||||
| Plecoptera | ||||||||||||||||||||
| Capniidae | 1.05 | 3.41 | 5.26 | 3.97 | ||||||||||||||||
| Bivalvia | 0.57 | 0.45 | 4.00 | 1.87 | ||||||||||||||||
| Amphipoda | 0.12 | 0.24 | 2.04 | 0.85 | 26.32 | 9.03 | 5.26 | 16.60 | 0.29 | 0.19 | 2.00 | 0.92 | 3.13 | 2.87 | 8.33 | 5.01 | 61.31 | 64.14 | 25.00 | 50.15 |
| Digested prey | 9.23 | 8.16 | 34.31 | 21.05 | 17.25 | 14.00 | 5.73 | 8.33 | 4.82 | 10.0 | ||||||||||
Appendix C
Frequency of occurrence (F%), numerical proportion (N%), mass proportion (W%), index of relative importance (IRI), vacuity coefficient (VI) and fullness index (FI) of the food items consumed by different brown trout haplogroups and hybrids (n—number of analysed specimens; n.d.—not determined). Bold values represent the most important food items.
| Food Item | Code | AT Haplogroup FI = 3.02; VI = 0; n = 7 | DA Haplogroup FI = 2.02; VI = 13.6; n = 22 | Hybrids FI = 2.54; VI = 3.9; n = 51 | |||||||||
| N% | W% | F% | IRI | N% | W% | F% | IRI | N% | W% | F% | IRI | ||
| Coleoptera | Coleoptr | 1.75 | 1.18 | 7.69 | 3.73 | 5.21 | 4.85 | 10.81 | 7.25 | 30.05 | 13.35 | 13.66 | 19.91 |
| Lamiinae | - | 0.18 | 0.10 | 0.55 | 0.29 | ||||||||
| Odonata | Odonata | 9.65 | 4.66 | 7.69 | 7.72 | 2.51 | 0.89 | 2.70 | 2.12 | 1.89 | 3.16 | 3.28 | 2.91 |
| Anisoptera | - | 2.48 | 1.30 | 1.09 | 1.70 | ||||||||
| Hymenoptera | Hymenopt | 1.48 | 0.98 | 2.19 | 1.62 | ||||||||
| Vespidae | Vespidae | 9.65 | 1.47 | 3.85 | 5.25 | 0.18 | 0.07 | 1.35 | 0.56 | 1.06 | 2.19 | 3.28 | 2.28 |
| Trichoptera larvae with case n.d. | TrichLCas | 39.47 | 33.27 | 19.20 | 32.26 | 9.16 | 31.73 | 16.22 | 19.84 | 8.68 | 20.03 | 11.48 | 14.03 |
| Trichoptera larvae without case n.d. | TrchLNoC | 0.88 | 0.97 | 3.85 | 2.00 | 2.69 | 4.86 | 8.11 | 5.44 | 6.32 | 5.13 | 7.65 | 6.67 |
| Hydropsychidae | - | 2.15 | 2.70 | 4.05 | 3.09 | 1.12 | 2.15 | 5.46 | 3.05 | ||||
| Philopotamidae | - | 0.18 | 0.02 | 1.35 | 0.54 | 0.06 | 0.81 | 0.55 | 0.50 | ||||
| Diptera adult n.d. | DipteraA | 15.79 | 6.74 | 3.85 | 9.26 | 3.59 | 6.32 | 5.41 | 5.32 | 3.60 | 1.26 | 1.64 | 2.27 |
| Diptera larvae n.d. | DipteraL | 1.75 | 0.15 | 3.85 | 2.02 | 0.90 | 0.86 | 2.70 | 1.55 | 2.54 | 1.29 | 4.37 | 2.86 |
| Muscidae | Muscidae | 1.75 | 0.74 | 3.85 | 2.22 | 0.18 | 0.32 | 1.35 | 0.64 | 6.67 | 2.52 | 3.83 | 4.54 |
| Simulidae | - | 0.36 | 0.07 | 1.35 | 0.62 | ||||||||
| Psychodidae | Psychodi | 2.63 | 5.70 | 3.85 | 4.27 | 4.19 | 2.88 | 2.73 | 3.42 | ||||
| Ephemeroptera | Ephemerp | 0.44 | 1.80 | 5.41 | 2.66 | 0.77 | 0.44 | 2.19 | 1.19 | ||||
| Arachnida | Arachnid | 2.63 | 0.51 | 3.85 | 2.45 | 0.10 | 1.15 | 1.35 | 0.90 | 0.65 | 1.01 | 3.83 | 1.92 |
| Gastropoda | Gastropd | 0.06 | 0.10 | 0.55 | 0.25 | ||||||||
| Anisus sp. | - | 1.26 | 0.67 | 4.05 | 2.08 | 0.06 | 0.04 | 0.55 | 0.23 | ||||
| Corixidae | Corixida | ||||||||||||
| Corixinae | - | 3.05 | 5.89 | 6.76 | 5.45 | 1.65 | 1.07 | 2.19 | 1.71 | ||||
| Micronectinae | - | 0.88 | 0.59 | 3.85 | 1.87 | 0.18 | 0.16 | 1.35 | 0.59 | 0.35 | 0.46 | 2.19 | 1.05 |
| Formicidae | Formicid | 4.39 | 1.40 | 7.69 | 4.73 | 64.27 | 16.20 | 10.81 | 31.71 | 17.83 | 3.63 | 8.20 | 10.35 |
| Hirudinea | Hirudine | ||||||||||||
| Hirudidae | - | 0.12 | 2.11 | 1.09 | 1.16 | ||||||||
| Pisciocolidae | - | 2.63 | 0.84 | 3.85 | 2.57 | 0.54 | 1.98 | 2.70 | 1.81 | 0.12 | 0.18 | 1.09 | 0.49 |
| Plecoptera | Plecoptr | 6.14 | 4.06 | 7.69 | 6.28 | 0.54 | 5.04 | 4.05 | 3.34 | 1.18 | 1.82 | 4.37 | 2.57 |
| Capniidae | - | 0.88 | 1.47 | 3.85 | 2.18 | ||||||||
| Bivalvia | Bivalvia | 0.18 | 0.08 | 1.35 | 0.56 | 1.30 | 2.05 | 1.64 | 1.74 | ||||
| Caelifera | Caelifer | 0.88 | 2.92 | 3.85 | 2.68 | 0.18 | 0.71 | 1.35 | 0.78 | ||||
| Pisces | |||||||||||||
| Cottus gobio | CotGob | 0.18 | 16.60 | 0.55 | 6.05 | ||||||||
| Crustacea | |||||||||||||
| Amphipoda | Amphipod | 2.15 | 1.54 | 5.41 | 3.16 | 5.43 | 4.74 | 4.92 | 5.27 | ||||
| Austropotamobius torrentium | AustTor | 0.88 | 19.57 | 3.85 | 8.53 | ||||||||
| Digested prey | - | 13.77 | 3.85 | 11.31 | 0.00 | 9.39 | 4.92 | ||||||
Appendix D
Canonical variate analysis (CVA) of the consumed prey items by different lineages between the regions (summary statistics for Figure 1). See Appendix C for abbreviations.
Canonical Variate Analysis in ‘Discriminant-analysis’.
Discriminant axes summary:
| Statistic | Axis 1 | Axis 2 |
| Eigenvalue | 1.33 | 0.51 |
| Percent variance | 72.2 | 100.0 |
Discriminant functions (p < 0.05):
| Species | Mean | c1 | c2 |
| ColeopteraM | 0.377813 | 1.486190 | 0.314247 |
| Vespidae | 0.242137 | −0.254234 | 0.897252 |
| TricLCase | 0.487970 | −0.834828 | −0.052515 |
| TricLNoCas | 0.454475 | −0.469598 | 1.521430 |
| Muscidae | 0.193654 | 1.002860 | 0.415385 |
| Formicidae | 0.278082 | 1.000940 | 0.141494 |
Class centroids:
| Class | Axis 1 | Axis 2 |
| Gorski Kotar | −0.77173 | −0.85066 |
| Žumberak | −1.10159 | 0.98720 |
| Papuk | 1.44329 | 0.09959 |
Appendix E
Appendix E.1
Canonical correspondence analysis (CCA) summary of the results at all sampling locations, as well as simple and conditional term effects of explanatory variables (P(adj) = p values correction by False discovery rate). The results support Figure 3A.
Summary Table:
| Summary Statistic | Axis 1 | Axis 2 | Axis 3 | Axis 4 |
| Eigenvalues | 0.3924 | 0.2825 | 0.2582 | 0.1841 |
| Explained variation (cumulative) | 5.78 | 9.94 | 13.74 | 16.45 |
| Pseudo-canonical correlation | 0.8869 | 0.7883 | 0.8086 | 0.6898 |
| Explained fitted variation (cumulative) | 23.14 | 39.80 | 55.02 | 65.88 |
Simple and conditional term effects:
| Explanatory Variable | Explains % | Pseudo-F | p | P(adj) |
| Simple Term Effects | ||||
| Location.Brzaja | 4.1 | 3.0 | 0.002 | 0.015 |
| Location.Kupcina | 4.0 | 2.9 | 0.002 | 0.015 |
| Location.Orljava | 2.8 | 2.0 | 0.050 | 0.250 |
| Location.Slapnica | 2.6 | 1.9 | 0.072 | 0.258 |
| Location.Toplica | 2.2 | 1.6 | 0.086 | 0.258 |
| Location.Jankovacki potok | 2.1 | 1.5 | 0.118 | 0.295 |
| Location.Bresni potok | 2.0 | 1.4 | 0.148 | 0.317 |
| HY | 1.6 | 1.2 | 0.260 | 0.384 |
| AT | 1.6 | 1.1 | 0.246 | 0.384 |
| Location.Velicanka | 1.6 | 1.1 | 0.254 | 0.384 |
| DA | 1.5 | 1.1 | 0.312 | 0.390 |
| Location.Mala Lesnica | 1.5 | 1.1 | 0.282 | 0.384 |
| Size > 20 | 1.2 | 0.8 | 0.66 | 0.660 |
| Size < 20 | 1.2 | 0.8 | 0.626 | 0.66 |
| Location.Curak | 1.1 | 0.7 | 0.636 | 0.66 |
| Conditional Term Effects | ||||
| Location.Brzaja | 4.1 | 3.0 | 0.002 | 0.015 |
| Location.Kupcina | 3.5 | 2.6 | 0.002 | 0.015 |
| Location.Orljava | 2.7 | 2.0 | 0.068 | 0.252 |
| Location.Bresni potok | 2.3 | 1.8 | 0.106 | 0.252 |
| Location.Jankovacki potok | 2.3 | 1.8 | 0.084 | 0.252 |
| Location.Toplica | 1.9 | 1.5 | 0.090 | 0.252 |
| Location.Curak | 1.5 | 1.2 | 0.216 | 0.405 |
| Location.Mala Lesnica | 1.8 | 1.4 | 0.118 | 0.252 |
| DA | 1.4 | 1.1 | 0.288 | 0.48 |
| Size > 20 | 1.2 | 1.0 | 0.458 | 0.687 |
| Size < 20 | 1.2 | 1.0 | unknown | unknown |
| AT | 1.0 | 0.8 | 0.572 | 0.715 |
| Location.Slapnica | 1.1 | 0.9 | 0.546 | 0.715 |
| TL | 2.0 | 1.7 | 0.044 | 0.123 |
Appendix E.2
Canonical correspondence analysis (CCA) summary of the results of the Gorski Kotar region, as well as simple and conditional term effects of explanatory variables (P(adj) = p values correction by False discovery rate). The results support Figure 3B.
Summary Table:
| Summary Statistic | Axis 1 | Axis 2 | Axis 3 | Axis 4 |
| Eigenvalues | 0.5622 | 0.4997 | 0.3648 | 0.3182 |
| Explained variation (cumulative) | 9.27 | 17.51 | 23.53 | 28.78 |
| Pseudo-canonical correlation | 0.9178 | 0.8050 | 0.8519 | 0.7829 |
| Explained fitted variation (cumulative) | 28.68 | 54.16 | 72.77 | 89.00 |
Simple and conditional term effects:
| Explanatory Variable | Explains % | Pseudo-F | p | P(adj) |
| Simple Term Effects: | ||||
| AT | 6.8 | 1.4 | 0.104 | 0.272 |
| Location.Bresni potok | 6.6 | 1.3 | 0.042 | 0.272 |
| Location.Curak | 6.3 | 1.3 | 0.202 | 0.272 |
| HY | 6.1 | 1.2 | 0.180 | 0.272 |
| Location.Mala Lesnica | 5.9 | 1.2 | 0.196 | 0.272 |
| DA | 5.9 | 1.2 | 0.214 | 0.272 |
| Size > 20 | 5.4 | 1.1 | 0.272 | 0.272 |
| Size < 20 | 5.4 | 1.1 | 0.240 | 0.272 |
| Conditional Term Effects: | ||||
| AT | 6.8 | 1.4 | 0.136 | 0.320 |
| Location.Curak | 6.3 | 1.3 | 0.166 | 0.320 |
| Size < 20 | 6.2 | 1.3 | 0.164 | 0.320 |
| Size > 20 | 6.2 | 1.3 | unknown | unknown |
| Location.Mala Lesnica | 5.9 | 1.3 | 0.200 | 0.320 |
| Location.Bresni potok | 5.9 | 1.3 | unknown | unknown |
| HY | 7.1 | 1.6 | 0.074 | 0.320 |
Appendix E.3
Canonical correspondence analysis (CCA) summary of the results of the Papuk region, as well as simple and conditional term effects of explanatory variables (P(adj) = p values correction by False discovery rate). The results support Figure 3C.
Summary Table:
| Summary Statistic | Axis 1 | Axis 2 | Axis 3 | Axis 4 |
| Eigenvalues | 0.4798 | 0.4065 | 0.2550 | 0.2271 |
| Explained variation (cumulative) | 10.66 | 19.69 | 25.36 | 30.40 |
| Pseudo-canonical correlation | 0.9164 | 0.9052 | 0.7958 | 0.7746 |
| Explained fitted variation (cumulative) | 29.51 | 54.50 | 70.19 | 84.15 |
Simple and conditional term effects:
| Explanatory Variable | Explains % | Pseudo-F | p | P(adj) |
| Simple Term Effects: | ||||
| AT | 8.0 | 2.5 | 0.020 | 0.060 |
| Location.Brzaja | 7.6 | 2.4 | 0.002 | 0.020 |
| Location.Velicanka | 7.0 | 2.2 | 0.006 | 0.030 |
| Location.Orljava | 6.4 | 2.0 | 0.024 | 0.060 |
| Location.Jankovacki potok | 5.6 | 1.7 | 0.062 | 0.088 |
| HY | 5.3 | 1.6 | 0.054 | 0.088 |
| Location.Toplica | 5.1 | 1.6 | 0.058 | 0.088 |
| DA | 4.9 | 1.5 | 0.114 | 0.142 |
| Size > 20 | 4.7 | 1.4 | 0.134 | 0.149 |
| Size < 20 | 4.7 | 1.4 | 0.152 | 0.152 |
| Conditional Term Effects: | ||||
| AT | 8.0 | 2.5 | 0.030 | 0.100 |
| Location.Brzaja | 7.3 | 2.4 | 0.002 | 0.020 |
| Location.Toplica | 6.7 | 2.3 | 0.004 | 0.020 |
| Location.Orljava | 4.6 | 1.6 | 0.052 | 0.130 |
| Location.Velicanka | 3.0 | 1.1 | 0.332 | 0.551 |
| Location.Jankovacki potok | 3.0 | 1.1 | unknown | unknown |
| Size > 20 | 3.6 | 1.3 | 0.214 | 0.428 |
| Size < 20 | 3.6 | 1.3 | unknown | unknown |
| DA | 2.9 | 1.1 | 0.386 | 0.551 |
Appendix E.4
Canonical correspondence analysis (CCA) summary of the results of the Žumberak region, simple and conditional term effects of explanatory variables (P(adj)= p values correction by False discovery rate). The results support Figure 3D.
Summary Table:
| Summary Statistic | Axis 1 | Axis 2 | Axis 3 | Axis 4 |
| Eigenvalues | 0.5580 | 0.2451 | 0.1481 | 0.9861 |
| Explained variation (cumulative) | 10.96 | 15.77 | 18.68 | 38.05 |
| Pseudo-canonical correlation | 0.9543 | 0.7766 | 0.7346 | 0.0000 |
| Explained fitted variation (cumulative) | 58.66 | 84.43 | 100.00 |
Simple and conditional term effects:
| Name | Explains % | Pseudo-F | p | P(adj) |
| Simple Term Effects | ||||
| Location.Slapnica | 10.4 | 2.1 | 0.002 | 0.006 |
| Location.Kupcina | 10.4 | 2.1 | 0.002 | 0.006 |
| Size < 20 | 5.4 | 1.0 | 0.332 | 0.54 |
| Size > 20 | 5.4 | 1.0 | 0.36 | 0.54 |
| DA | 3.7 | 0.7 | 0.684 | 0.722 |
| HY | 3.7 | 0.7 | 0.722 | 0.722 |
| Conditional Term Effects: | ||||
| Location.Slapnica | 10.4 | 2.1 | 0.002 | 0.006 |
| Location.Kupcina | 10.4 | 2.1 | 0.002 | 0.006 |
| Size < 20 | 4.8 | 1.0 | 0.422 | 0.844 |
| Size > 20 | 4.8 | 1.0 | unknown | unknown |
| DA | 3.5 | 0.7 | 0.602 | 0.903 |
References
- Buj, I.; Ivić, L.; Raguz, L.; Ćaleta, M.; Marčić, Z.; Duplić, A.; Zanella, D.; Tomašić, A.; Horvatić, S.; Karlović, R.; et al. Trouts in Karstic Watersheds—Diversity, Origin and Perspective. Available online: https://www.frontiersin.org/10.3389%2fconf.fmars.2019.07.00114/event_abstract (accessed on 15 July 2022).
- Buj, I.; Raguž, L.; Marčić, Z.; Ćaleta, M.; Duplić, A.; Zanella, D.; Mustafić, P.; Ivić, L.; Horvatić, S.; Karlović, R. Plitvice Lakes National park harbors ancient, yet endangered diversity of trout (genus Salmo). J. Appl. Ichthyol. 2020, 37, 20–37. [Google Scholar] [CrossRef]
- Jurlina, D.; Marić, A.; Mrdak, D.; Kanjuh, T.; Špelić, I.; Nikolić, V.; Piria, M.; Simonović, P. Alternative Life-History in Native Trout (Salmo spp.) Suppresses the Invasive Effect of Alien Trout Strains Introduced Into Streams in the Western Part of the Balkans. Front. Ecol. Evol. 2020, 8, 188. [Google Scholar] [CrossRef]
- Ivić, L.; Buj, I.; Raguz, L. Impact of Glacial Refugia on Present-Day Diversity of the Black Sea Trout (Salmo labrax Pallas, 1814) in Croatia. Available online: https://www.frontiersin.org/10.3389%2fconf.fmars.2019.07.00052/event_abstract (accessed on 15 July 2022).
- Kanjuh, T.; Marić, A.; Piria, M.; Špelić, I.; Maguire, I.; Simonović, P. Diversity of brown trout, Salmo trutta (Actinopterygii: Salmoniformes: Salmonidae), in the Danube River basin of Croatia revealed by mitochondrial DNA. Acta Ichthyol. Piscat. 2020, 50, 291–300. [Google Scholar] [CrossRef]
- Jadan, M.; Strunjak-Perović, I.; Popović, N.T.; Čož-Rakovac, R. Three major phylogenetic lineages of brown trout (Salmo trutta Linnaeus, 1758) in the Krka River system (Croatia) revealed by complete mitochondrial DNA control region sequencing. J. Appl. Ichthyol. 2014, 31, 192–196. [Google Scholar] [CrossRef]
- Piria, M.; Špelić, I.; Rezić, A.; Šprem, N. Morphological traits and condition of brown trout Salmo trutta from Žumberak and Samobor mountain streams. J. Cent. Eur. Agric. 2020, 21, 231–245. [Google Scholar] [CrossRef]
- Mezzera, M.; Largiader, C.R. Evidence for selective angling of introduced trout and their hybrids in a stocked brown trout population. J. Fish Biol. 2001, 59, 287–301. [Google Scholar] [CrossRef]
- Almodovar, A.; Nicola, G.G. Angling impact on conservation of Spanish stream-dwelling brown trout Salmo trutta. Fish. Manag. Ecol. 2004, 11, 173–182. [Google Scholar] [CrossRef]
- Schöffmann, J.; Sušnik, S.; Snoj, A. Phylogenetic origin of Salmo trutta L. 1758 from Sicily, based on mitochondrial and nuclear DNA analyses. Hydrobiologia 2006, 575, 51–55. [Google Scholar] [CrossRef]
- Simonović, P.; Vidović, Z.; Tošić, A.; Škraba, D.; Čanak Atlagić, J.; Nikolić, V. Risks to stocks of native trout of the genus Salmo (Actinopterygii: Salmoniformes: Salmonidae) of Serbia and management for their recovery. Acta Ichthyol. Piscat. 2015, 45, 161–173. [Google Scholar] [CrossRef]
- Simonović, P.; Mrdak, D.; Tošić, A.; Škraba, D.; Grujić, S.; Nikolić, V. Effects of stocking with brood fish to manage resident stream dwelling brown trout Salmo cf. trutta stock. J. Fish. Sci. 2014, 8, 139–152. [Google Scholar] [CrossRef]
- Kahilainen, K.; Lehtonen, H. Resource use of native and stocked brown trout Salmo trutta L., in a subarctic lake. Fish. Manag. Ecol. 2001, 8, 83–94. [Google Scholar] [CrossRef]
- Ward, D.L.; Morton-Starner, R.; Vaage, B. Are Hatchery-Reared Rainbow Trout and Brown Trout Effective Predators on Juvenile Native Fish? N. Am. J. Fish. Manag. 2018, 38, 1105–1113. [Google Scholar] [CrossRef]
- Piria, M.; Špelić, I.; Velagić, L.; Lisica, I.; Kanjuh, T.; Marić, A.; Simonović, P. Feeding preferences and diet overlap of introduced Atlantic and native Danubian lineages of brown trout (Salmo trutta) from Croatia. In Proceedings of the XVI European Congress of Ichthyology, Lausanne, Switzerland, 2–6 September 2019. [Google Scholar] [CrossRef]
- Sønstebø, J.H.; Borgstrøm, R.; Heun, M. Genetic structure of brown trout (Salmo trutta L.) from the Hardangervidda mountain plateau (Norway) analyzed by microsatellite DNA: A basis for conservation guidelines. Conserv. Genet. 2006, 8, 33–44. [Google Scholar] [CrossRef]
- Horváth, A.; Hoitsy, G.; Kovács, B.; Sipos, D.K.; Ősz, A.; Bogataj, K.; Urbányi, B. The effect of domestication on a brown trout (Salmo trutta m fario) broodstock in Hungary. Aquac. Int. 2013, 22, 5–11. [Google Scholar] [CrossRef]
- Teixeira, A.; Cortes, R. Diet of stocked and wild trout, Salmo trutta: Is there competition for resources? Folia Zool. 2006, 55, 61–73. [Google Scholar]
- Cada, G.F.; Loar, J.M.; Cox, D.K. Food and Feeding Preferences of Rainbow and Brown Trout in Southern Appalachian Streams. Am. Midl. Nat. 1987, 117, 374. [Google Scholar] [CrossRef]
- Trožić-Borovac, S. Prehrana potočne pastrve, Salmo trutta morfo fario L., u rijeci Uni (The nutrition of a brown trout Salmo trutta m. fario in the river Una). Ribarstvo 2002, 60, 83–104. (In Croatian) [Google Scholar]
- Johnsen, B.O.; Ugedal, O. Feeding by hatchery-reared and wild brown trout, Salmo trutta L., in a Norwegian stream. Aquac. Res. 1986, 17, 281–287. [Google Scholar] [CrossRef]
- Johnsen, B.O.; Ugedal, O. Feeding by hatchery-reared brown trout, Salmo trutta L. released in lakes. Aquac. Res. 1989, 20, 97–104. [Google Scholar] [CrossRef]
- Belinić, I.; Tavčar, V.; Habdija, I. Trophic importance of dipteran larvae in macrobenthic communities of a karstic river. Arch. Hydrobiol. 1993, 127, 239–252. [Google Scholar] [CrossRef]
- Habdija, I.; Radanović, I.; Primc-Habdija, B.; Špoljar, M. Vegetation Cover and Substrate Type as Factors Influencing the Spatial Distribution of Trichopterans along a Karstic River. Int. Rev. Hydrobiol. 2002, 87, 423–437. [Google Scholar] [CrossRef]
- Kučinić, M.; Cerjanec, D.; Vučković, I.; Mihoci, I.; Perović, F.; Kutnjak, H.; Ibrahimi, H.; Fixa, D.P.; Žalac, S.; Vojvoda, A.M.; et al. Some new and interesting species of caddisflies (Insecta, Trichoptera) found in Croatia. Nat. Croat. 2015, 24, 293–310. [Google Scholar] [CrossRef]
- Špoljar, M.; Dražina, T.; Ostojić, A.; Miliša, M.; Udovič, M.G.; Štafa, D. Bryophyte communities and seston in a karst stream (Jankovac Stream, Papuk Nature Park, Croatia). Ann. Limnol.—Int. J. Limnol. 2012, 48, 125–138. [Google Scholar] [CrossRef][Green Version]
- Špoljar, M.; Šneller, D.; Miliša, M.; Lajtner, J.; Sertić Perić, M.; Radanović, I. Entomofauna of submerged macrophyte stands in reservoirs (Papuk nature park). Entomol. Croat. 2012, 16, 7–20. [Google Scholar]
- Bernatchez, L.; Guyomard, R.; Bonhomme, F. DNA sequence variation of the mitochondrial control region among geographically and morphologically remote European brown trout Saltno trutta populations. Mol. Ecol. 1992, 1, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Hyslop, E.J. Stomach contents analysis-a review of methods and their application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef]
- Holden, M.J.; Raitt, D.F.S. 1974: Methods of Resource Investigation and their Application. In Manual of Fisheries Science; FAO: Rome, Italy, 1974. [Google Scholar]
- Cortes, E. A critical review of methods of studying fish feeding based on analysis of stomach contents: Application to elasmobranch fishes. Can. J. Fish. Aquat. Sci. 1997, 54, 726–738. [Google Scholar] [CrossRef]
- Kanjuh, T.; Tošić, A.; Špelić, I.; Piria, M.; Simonović, P.; Maguire, I. Human influence on the native brown trout (Salmo trutta) genepool in the continental western Croatia. In Proceedings of the 3rd International Congress on Applied Ichthyology & Aquatic Environment (HydroMediT 2018), Volos, Greece, 8–11 November 2018; pp. 555–558. [Google Scholar]
- Schoener, T.W. Nonsynchronous Spatial Overlap of Lizards in Patchy Habitats. Ecology 1970, 51, 408–418. [Google Scholar] [CrossRef]
- Atlagić, J.; Marić, A.; Tubić, B.; Andjus, S.; Đuknić, J.; Marković, V.; Paunović, M.; Simonović, P. What’s on the Menu for the Resident Brown Trout in a Rich Limestone Stream? Water 2021, 13, 2492. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, 5th ed.; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Špelić, I.; Rezić, A.; Kanjuh, T.; Marić, A.; Maguire, I.; Simonović, P.; Radočaj, T.; Piria, M. Application of the geometric morphometrics approach in the discrimination of morphological traits between brown trout lineages in the Danube Basin of Croatia. Knowl. Manag. Aquat. Ecosyst. 2021, 422, 22. [Google Scholar] [CrossRef]
- Elliott, J.M. The Food of Trout (Salmo trutta) in a Dartmoor Stream. J. Appl. Ecol. 1967, 4, 59. [Google Scholar] [CrossRef]
- Rincón, P.A.; Lobón-Cerviá, J. Prey-size selection by brown trout (Salmo trutta L.) in a stream in northern Spain. Can. J. Zool. 1999, 77, 755–765. [Google Scholar] [CrossRef]
- Kara, C.; Alp, A. Feeding habits and diet composition of brown trout (Salmo trutta) in the upper streams of River Ceyhan and River Euphrates in Turkey. Turk. J. Vet. Anim. Sci. 2005, 29, 417–428. [Google Scholar]
- Kasumyan, A.O.; Sidorov, S.S. Taste preferences of the brown trout Salmo trutta from three geographically isolated populations. J. Ichthyol. 2005, 45, 111–123. [Google Scholar]
- Simonović, P.; Povž, M.; Piria, M.; Treer, T.; Adrović, A.; Škrijelj, R.; Nikolić, V.; Simić, V. Ichthyofauna of the River Sava System. In The Sava River; The Handbook of Environmental Chemistry 31; Milačič, R., Ščančar, J., Paunović, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 361–400. [Google Scholar] [CrossRef]
- Simonović, P.; Piria, M.; Zuliani, T.; Ilić, M.; Marinković, N.; Kračun-Kolarević, M.; Paunović, M. Characterization of sections of the Sava River based on fish community structure. Sci. Total Environ. 2017, 574, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, J. Drivers of piscivory in a globally distributed aquatic predator (brown trout): A meta-analysis. Sci. Rep. 2020, 10, 11258. [Google Scholar] [CrossRef] [PubMed]
- Larscheid, J.G.; Hubert, W.A. Factors Influencing the Size Structure of Brook Trout and Brown Trout in Southeastern Wyoming Mountain Streams. N. Am. J. Fish. Manag. 1992, 12, 109–117. [Google Scholar] [CrossRef]
- Jensen, H.; Kiljunen, M.; Amundsen, P.-A. Dietary ontogeny and niche shift to piscivory in lacustrine brown trout Salmo trutta revealed by stomach content and stable isotope analyses. J. Fish Biol. 2012, 80, 2448–2462. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The River Continuum Concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Simonović, P.; Marić, A.; Jurlina, D.; Kanjuh, T.; Nikolić, V. Determination of resident brown trout Salmo trutta features by their habitat characteristics in streams of Serbia. Biologia 2020, 75, 103–114. [Google Scholar] [CrossRef]
- Barlaup, B.T.; Moen, V. Planting of Salmonid eggs for stock enhancement—A review of the most commonly used methods. Nord. J. Freshw. Res. 2001, 75, 7–19. [Google Scholar]
- Turković, M.; Hrašovec, B.; Šprem, N. Supplemental stocking of eyed brown trout eggs (Salmo trutta m. fario L., 1758) with the use of Whitlock–Vibert boxes. Ribarstvo 2006, 64, 113–123. [Google Scholar]
- Bernatchez, L. The Evolutionary History of Brown Trout (Salmo trutta L.) Inferred From hylogeographic, Nested Clade, and Mismatch Analyses of Mitochondrial DNA Variation. Evolution 2001, 55, 351–379. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).