Abstract
The influence of water hardness on copper (Cu) toxicity in Daphnia magna was studied using gene expression analysis. Exposing D. magna to Cu in water with increasing levels of hardness decreased the acute toxicity. Hardness did not affect the predicted Cu complexation. After 24 h, D. magna showed an increased level of genes related to metal homeostasis (mt) following exposure to 25 μg Cu/L in hard water. Daphnids in soft and medium water responded to 25 μg Cu/L by upregulation of antioxidant defense and mt genes, revealing oxidative stress as a mechanism of Cu toxicity in D. magna. D. magna exposed to 25 μg Cu/L in soft water did not survive for 96 h. In contrast, those exposed to 25 μg Cu/L in medium and hard water survived for 96 h with significantly higher levels of mt genes. The genes related to oxidative damage (heat shock protein and glutathione S-transferase) in these groups did not deviate from control levels, indicating the protective effect of hardness. Metallothionein genes were upregulated at 17 μg Cu/L at both 24 h and 96 h. The expression of catalase and ferritin increased in this group in soft and hard water at 96 h. The protective effect of hardness (in the tested range) on survival was also observed at a concentration of 25 μg/L. The results suggest metallothionein (A and B), catalase, and ferritin genes, as potential biomarkers for copper exposure in D. magna regardless of hardness.
1. Introduction
Copper (Cu) is an essential trace metal required for the growth, development, and maintenance, of the health of all biotas [1]. Cu is involved in several metabolic and physiological processes, including cellular respiration, antioxidant defense, immune system, neurotransmitter function, and connective tissue biosynthesis, etc. [2,3]. Cu ions also are essential as a catalytic cofactor in numerous critical enzymes, such as antioxidant enzyme copper-zinc superoxide dismutase, tyrosinase, and cytochrome c oxidase [4]. However, Cu can induce toxicity at concentrations higher than the normal physiological range. In 2007, the United States Environmental Protection Agency classified Cu as an essential trace element for all animals and plants at low concentrations, and a toxic heavy metal to aquatic organisms in excessive amounts [5].
The annual world production and use of Cu are rising yearly and rank in the top three metals after Zn and Pb [6]. Thus, the amount of Cu released into surface waters due to anthropogenic activities is considerable, and aquatic organisms would consequently be at risk of being exposed to a toxic concentration of Cu. Accordingly, the Cu level in natural environments and its biological availability are important factors to protect aquatic life from deficiency and the elevated Cu concentration in their ambient environment.
High water hardness has been observed to reduce the toxicity of metals in aquatic organisms, resulting in guideline values of these metals for site-specific hardness conditions [7,8]. The protective effect of hardness is interpreted either as competition between Ca and Mg and other divalent metals for organisms’ binding sites, or changes in speciation of metals in the water [9].
It is generally accepted that Cu2+ and CuOH+ are the main toxic forms of Cu as they can pass across biological membranes and reach biochemical targets [10]. In an earlier study, it was observed that there was a direct correlation between the mortality rate of D. magna and the Cu2+ and CuOH+ concentrations. At the same time, the toxicity was negatively related to soluble CuCO3 [11].
The ameliorating effect of hardness on Cu toxicity varies between different studies. In some studies, no significant effects of water hardness on toxicity have been observed [12,13,14], whereas other studies indicate decreased Cu toxicity with increased water hardness [15,16]. De Schamphelaere and co-workers observed a linear increase between 48 h Cu EC50 and Ca2+ and Mg2+ concentrations, suggesting competition between these ions with Cu ions in D. magna [17]. However, De Schamphelaere and Janssen (2002) observed no protective effects of hardness on the chronic Cu toxicity to D. magna in synthetic or natural freshwater containing dissolved organic carbon with a hardness ranging from 25 to 500 mg/L as CaCO3 [12]. Similarly, chronic exposure of zebrafish to Cu in the presence of Ca2+ reduced the accumulation of Cu in gills with no reduction in oxidative damage [18]. Together, these observations suggest the possibility of physiological protection of Ca in Cu toxicity, rather than simply a function of reduced uptake through binding sites, underlining the need for more research into the mechanisms of Cu toxicity. Mechanism-based toxicity studies give us a better understanding of the ecological relevance of risk assessment guidelines for Cu in the aquatic environment.
In this study, we used gene expression analysis to provide a mechanistic basis for predicting the toxicity of Cu to D. magna as a function of water hardness. D. magna was exposed to Cu in three different harnesses: 50 (soft), 100 (medium), and 200 (hard) mg/L as CaCO3. The mortality of daphnids was compared and, accordingly, four concentrations of Cu were chosen to study the expression of genes related to stress response and development after 24 h and 96 h exposure.
2. Materials and Methods
2.1. Preparation of Test Media and Chemical Analysis
Reconstructed laboratory water was prepared in Milli-Q (Millipore) water by adding KCl and NaHCO3 to a final concentration of 3 mg K/L and 18 mg Na/L, respectively. Water hardness was adjusted to 50 (soft), 100 (medium), and 200 (hard) mg/L as CaCO3 by adding appropriate amounts of CaCl2 and MgSO4 (Ca: Mg = 2:1). The test water was prepared once before the experiment. Stock solution CuCl2.2H2O salt (1 mg/L) was used during the trials to prepare Cu-spiked solutions. All tests were carried out using polypropylene vessels to decrease the effects of the adsorption of Cu. Sub-samples were taken from each concentration at the beginning and before water exchange (24 h) to confirm Cu concentration in the test media. The concentrations of metals in the prepared media were confirmed using Inductively Coupled Plasma Quadrupole Mass Spectroscopy (ICP-QMS; Agilent 7500 cx). Before the analysis, the water samples were diluted (1:20) and acidified with 1% nitric acid in deionized water (18.2 MΩ). External calibration was performed with diluted solutions of the Merck 10,580 multi-element standard VI, and 103 Rh was added as an internal standard. Isotopes prone to suffer from di- and polyatomic interferences, i.e., 39 K, 51 V, 56 Fe, 63 Cu, 75 As, 82 Se, were analyzed with the Octopole Reaction System operating in collision mode. Helium was used as the collision gas with a flow rate of 5 mL/min during analysis. The deviation from the nominal concentration was always less than 20% (Appendix A: Table A1 and Table A2).
2.2. Acute Toxicity Test
Ephippia of D. magna (Daphtoxkit, MicroBioTests Inc., Belgium) were incubated for 72 h in standard freshwater (67.75 mg/L NaHCO3, 294 mg/L CaCl2, 123.25 mg/L MgSO4, and 5.75 mg/L KCl) at a temperature of 20–22 °C under continuous illumination of 6000 lx. Newly hatched D. magna neonates (>24 h) were exposed to six Cu-spiked solutions (7.5, 11.2, 16.8, 25.3, 37.9, 59.9 µg/L) and a control (no-added Cu) in soft, medium, and hard waters. Fifteen daphnids were used in each test vessel filled with 30 mL of test solution. The experiment was carried out in four replicates at 22 ± 2 °C with a 16:8 h light: dark photoperiod for 96 h. The Cu-spiked solutions were prepared 24 h before use and stored at room temperature. Test media were refreshed every 24 h. Animals were fed once a day with a mixture of Spirulina microalgae and yeast.
2.3. Speciation Modeling of Cu in Different Hardness Conditions
The chemical equilibrium modeling software Visual MINTEQ® 3.1 was used to predict the speciation of Cu under different hardness conditions. The input parameters were either measured (pH and temperature) or calculated according to theoretical concentrations of cation and anion in the test water.
2.4. Gene Expression Analysis
To study the effect of hardness on the gene expression in D. magna exposed to Cu, we analyzed the transcript level of genes associated with stress response and development following 24 h and 96 h exposure to sublethal concentrations of Cu, acquired from the survival curve (7, 11, 17, and 25 µg/L) in different hardness conditions. Animals were fed as survival assay once per day. Each test vessel contained 20 neonates in a 40 mL test solution. Six replicates were used for each treatment. At the end of each exposure, animals were collected from each vessel into plastic micro-centrifuge tubes and snap-frozen in liquid nitrogen till further analysis. We pooled 20 neonates as one sample to yield enough tissue for gene expression analysis.
Total RNA was extracted using Direct-zol Kit (Zymo Research) according to the manufacturer’s instructions. The RNA concentration and purity were measured using a DeNovix DS-11 spectrophotometer (Wilmington DE, USA). One μg of RNA was used to synthesize cDNA using qScript cDNA synthesis kit (Quanta Biosciences) following the manufacturer’s instructions. Primers were designed (Appendix A, Table A3) or culled from other previously published literature. The PCR efficiency was determined with a dilution series (in triplicate) of pooled cDNA. Primer pairs were only considered when the efficiency of the PCR was between 90–100% (corresponding to −3.6 ≥ slope of regression ≥ −3.3). qRT-PCR was performed using qPCRBIO SyGreen Mix Lo-ROX (PCR Biosystems) on CFX384 Real-time PCR detection system (Bio-Rad). The thermocycling profile was 2 min initial denaturation step at 95 °C followed by 35 cycles (5 s at 95 °C and 30 s at 60 °C).
2.5. Data Process and Statistical Analysis
β-actin, elongation factor 1 alpha, glyceraldehyde-3-phosphate dehydrogenase, and Alpha-tubulin, were candidate housekeeping genes. According to the geNorm calculation, β-actin was the most stable housekeeping gene in our experiment. Therefore, the Ct values of the target genes were normalized using β-actin as the normalizing control. Gene expression was analyzed using the comparative Ct (ΔΔCt) method [19]. In each hardness, the ΔCt value from the control group (no-added Cu) was used to calculate ΔΔCt values. The mean values of relative gene expression expressed as the n-fold increase in mRNA levels ± standard deviation (SD) compared to their relevant control groups. Data were tested for normality and homogeneity of variance by the Shapiro-Wilk and Levene’s test, respectively. If data meet the required assumption of normality and homogeneity of variance, One-way ANOVA and Tukey’s HSD post-hoc test, and in the case of violation of the assumptions, Kruskal-Wallis and post hoc Dunn’s test, were performed to determine significant differences among treatments in each hardness level. p-values < 0.05 were considered significantly different. Pearson rank correlations were computed to evaluate relationships between Cu concentration and the expression of target genes. The significance level was set at p-values < 0.01. Principal components analysis (PCA) of correlated genes with Cu was performed on continuous dosage experiments in different hardness conditions.
The statistical analysis and graphs generation were performed using R version 4.0.2 [20,21]. The PCA analysis and plots were performed using functions from the FactoMineR package [22]. Heatmaps of the average expression of genes were produced using the GraphPad Prism 8.0.2 software (GraphPad Software, San Diego, USA).
3. Results
3.1. Effect of Water Hardness on Cu Speciation and 96 h Survival
The 96 h acute toxicity decreased by increasing water hardness. Daphnids exposed to Cu in soft water showed a lower LC50 value compared to daphnids exposed to Cu in medium and hard water (Figure 1). A chemical equilibrium model was applied to determine whether the protective effects of hardness on the survival of daphnids were related to changes in the speciation of Cu. Thus, increasing the water hardness from 50 to 200 mg/L as CaCO3 did not change the speciation of Cu (Appendix A, Table A4).
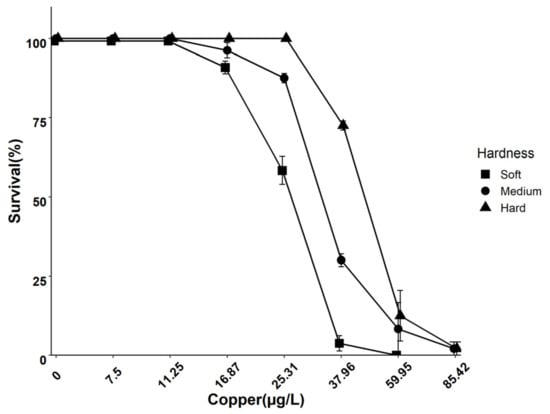
Figure 1.
Concentration–response curves of D. magna survival during 96 h exposures to copper in different hardness levels (50 (soft), 100 (medium), and 200 (hard) mg/L as CaCO3).
3.2. Relative Gene Expression in D. magna Exposed to Cu in Soft Water
After 24 h exposure to Cu in soft water, the expression levels of genes related to metal homeostasis, such as metallothioneins (mtA, mtB, mtC), oxidative stress response genes such as glutathione-S-transferase (gst), catalase (cat), ferritin 3 (fer3), cytochrome P450 314 family (cyp314) and heat shock protein 70 (hsp70), significantly increased in the highest Cu concentration (25 µg Cu/L), compared to the control group (Figure 2A). Daphnids exposed to 17 µg Cu/L for 24 h showed increased expression levels of mtA, mtB, mtC, while mtC did not deviate from control levels at 96 h. The cat and fer3 genes were upregulated in 17 µg Cu/L after 96 h of exposure to Cu (Figure 3A). The bar plots of the expression levels of target genes are shown in Appendix B, Figure A1.
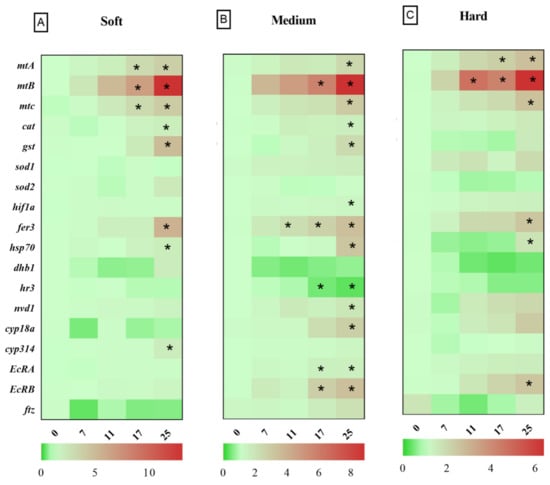
Figure 2.
Heat map of gene expression in D. magna after 24 h exposure to copper in different hardness levels (A) 50 (soft); (B) 100 (medium); (C) 200 (hard) mg/L as CaCO3. Asterisks in each row indicate statistically significant differences compared to control group (p < 0.05).
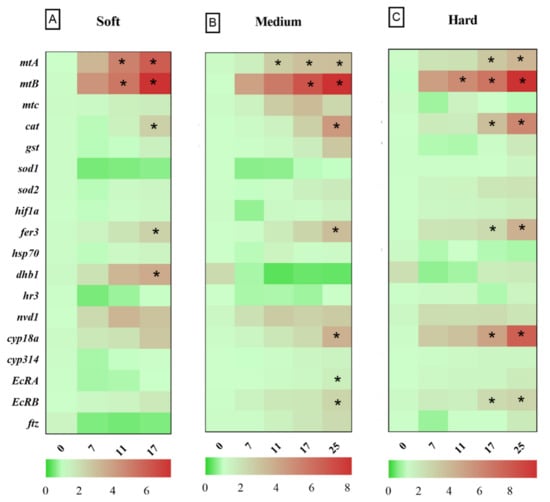
Figure 3.
Heat map of gene expression response of D. magna to copper after 96 h exposure under varying hardness levels. (A) 50 (soft); (B) 100 (medium); (C) 200 (hard) mg/L as CaCO3. Asterisks in each row indicate statistically significant differences compared to control (p < 0.05).
3.3. Relative Gene Expression in D. magna Exposed to Cu in Medium Water
Daphnids exposed to 25 µg Cu/L in medium hardness water upregulated mtA, mtB, mtC, gst, fer3, cat, hsp70, nuclear receptors HR3 (hr3), ecdysteroid receptor (EcRA, EcRB), cyp18a, and neverland1 (nvd1) genes after 24 h (Figure 2B). However, gst, hsp70, nvd1, and hr3 expression did not deviate from the control group by 96 h (Figure 3B). The expression levels of mtB, fer3, hr3, EcRA, and EcRB, were increased in groups exposed to 17 µg Cu/L at 24 h (Figure 2B) while only mtA and mtB showed increased expression at 96 h (Figure 3B). The expression levels of target genes are shown in bar graphs (Appendix B, Figure A1).
3.4. Relative Gene Expression in D. magna Exposed to Cu in Hard Water
Daphnids exposed to Cu in hard water expressed higher levels of mtA, mtB, mtC, gst, fer3, hsp70, and EcRB, in response to 25 µg Cu/L at 24 h (Figure 2C). However, hsp70 and gst did not significantly differ from the control group after 96 h exposure (Figure 3C). Following exposure to 17 µg Cu/L, the expression levels of mtA and mtB increased at both 24 and 96 h (Figure 2C and Figure 3C). The upregulation of cat, fer3, and cyp18a was observed by 96 h in this group (Figure 3C). The results are illustrated in bar charts (Appendix B, Figure A1).
3.5. Principal Component Analysis
mt, cat, and gst genes showed a strong positive correlation in all hardness conditions after 24 h (Table 1). After 96 h exposure, mtC did not correlate with Cu levels in any group, and gst did not correlate to Cu in soft water. The hif-1α correlated with Cu in medium and hard water at 24 h and 96 h (Table 1). In medium water, genes related to development showed a strong correlation with Cu.

Table 1.
Pearson correlation coefficient (r) between the expression level of target genes and copper concentrations in each hardness levels (50 (soft), 100 (medium), and 200 (hard) mg/L as CaCO3.
The PCA revealed a separation of target genes in groups exposed to 25 µg Cu/L in medium (Figure 4C) and hard water (Figure 4E) at 24 h. This separation is mostly driven by genes such as fer3, mtB, mtC, and gst. After 96 h exposure, separation was observed between daphnids exposed to the highest Cu concentration in soft (17 µg/L) (Figure 4B) and medium (25 µg/L) water (Figure 4D), compared to the other groups. The genes contributing to this grouping included fer3, mtB, gst, cat, and hif1a for medium condition, and mtA, mtB, cat, and fer3 for soft water. A positive correlation can be observed between mtA, mtB, fer3, and cat under different hardness conditions.
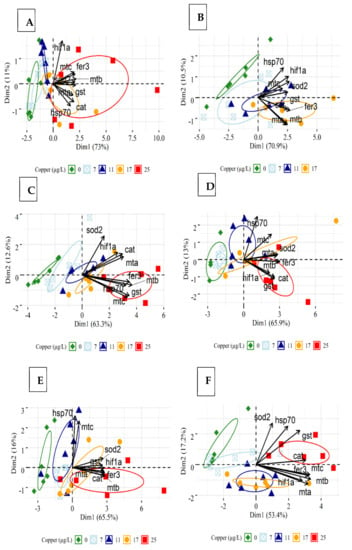
Figure 4.
Principal component biplots displaying the relationship between all investigated genes in different hardness levels: (A) Daphnids exposed to Cu in soft water for 24 h. (B) Daphnids exposed to Cu in soft water for 96 h. (C) Daphnids exposed to Cu in medium water for 24 h. (D) Daphnids exposed to Cu in medium water for 96 h. (E) Daphnids exposed to Cu in hard water for 24 h. (F) Daphnids exposed to Cu in hard water for 96 h. (Hardness levels: 50 (soft), 100 (medium), and 200 (hard) mg/L as CaCO3. Ellipses indicate the 95% confidence intervals. A small angle between 2 variables (arrows) (<90) indicates a positive correlation between them. Overall, a positive correlation is observed between variables such as mtA, mtB, fer3, and cat, in different hardness levels.
4. Discussion
The survival decreased in a dose-dependent manner under all three hardness levels tested in the current study. The 96 h LC50 was therefore inversely related to the hardness level as water hardness is considered as a protective factor against toxicity of most heavy metal. This is in line with current literature suggesting the protective effect of hardness on the acute toxicity of Cu to D. magna [17]. It is considered that ameliorating effects of hardness on the acute toxicity of Cu to freshwater organisms is due to competition of positively charged multivalent ions (e.g., Ca2+ and Mg2+) and Cu2+ for binding to biotic ligands [23]. The decreased gill permeability to ions caused by Ca ions is believed to be another factor for the mitigating effects of hardness on the acute toxicity of metals [24]. Ca ions bind to the gill surface, inducing a positive charge that repels other cations from approaching their relevant binding sites [25].
Moreover, hardness may be protective by changing the metal speciation in the water, resulting in reduced toxicity. However, in the present study, the predicted Cu speciation was not altered by the hardness of the test water. This is in line with a previous study where a minor change was observed in the calculated speciation of Cu in both reconstituted and natural waters where water hardness increased from 44 to 374 mg/L as CaCO3 [26]. They found a negligible effect on the toxicity of Cu to Ceriodaphnia cf. dubia in both reconstituted and natural waters of varying hardness (44–375 mg/L as CaCO3). Species variations in susceptibility to Cu may explain the inconsistency regarding protective effects of hardness in Cu toxicity between our and their study. In synthetic freshwater, the 48 h EC50 observed for C. cf. dubia was 1.6 (1.4–1.7) µg Cu/L. The lowest 96 h LC50 observed in our study was 24.81 µg Cu/L in soft water. We also observed that hardness had no significant effect on the survival of daphnids exposed to the lower concentrations of Cu.
The present study indicates that genes related to development (hr3, EcRa, EcRb, cyp18a, and nvd1) of D. magna were regulated in response to Cu in medium and hard water but not in soft water. Previous studies have observed that hardness can influence the growth of D. magna at early life stages because the neonates have higher Ca demand during juvenile stages due to higher surface-to-volume ratios and shorter molt cycles [27,28]. Thus, the expression of genes related to the development is most likely expressed differently depending on the hardness of the water and the developmental stage.
After 24 h, daphnids responded to 17 and 25 µg Cu/L by increasing the expression of genes related to metal homeostasis (mtA, mtB, mtC) in all hardness levels. Induction of mt genes following Cu exposure has been observed in previous studies for D. magna [29] and D. pulex [30]. The mt genes were also induced under excess Cu conditions in fish [31] and mammals [32]. The induction of the mt genes under Cu stress protects against the Cu insult as metallothionein protein (MT) is known to play an essential role in both homeostasis and detoxification of excess amounts of metals [33,34,35]. In addition, induction of MT has been observed in response to oxidative inducers, which proposed it as a free radical scavenger [36,37,38,39,40]. Accordingly, their antioxidant properties might play a role in Cu toxicity along with their metal scavenging function in D. magna.
After 24 h exposure, the expression of fer3 and hsp70 were upregulated at the highest concentration (25 µg/L) in all hardness levels. The upregulation of gst and cat was observed in daphnids exposed to the highest concentration in medium and soft water but not in hard water. Considering the contribution of induced genes (cat, gst, fer3, and hsp70) to the protection against oxidative stress, this indicates an antioxidant defense system response to high concentrations of Cu. Heavy metals can generate reactive oxygen species (ROS), which initiate the response of the antioxidant defense system and cause oxidative damage if cellular antioxidant capacity is exceeded [41]. Previous studies also observed an increased glutathione S-transferase activity and expression in daphnids exposed to Cu [42,43]. Co-expression of gst and cat in medium and soft water indicates the responsiveness of the antioxidant systems. cat is an antioxidant enzyme, and gst is a downstream antioxidant defense detoxifying the reactive aldehyde species [44]. The cytoprotective effect of gst against reactive metabolites produced during oxidative stress has also been observed [45]. However, the unchanged expression of cat and gst in hard water likely demonstrates that daphnids experienced lower stress due to Cu excess. The expression of oxidative stress-related genes did not change in daphnids exposed to 17 µg/L in medium water. However, cat and fer3 were upregulated in daphnids exposed to 17 µg Cu/L in soft and hard conditions at 96 h.
Upregulation of fer3 expression by Cu exposure has also been documented previously in D. magna [46]. Ferritin is regulated by inducers of oxidative stress and has an important function in protecting against oxidative stress [47]. Hence, the increased expression of fer3 may contribute to the detoxification mechanism against oxidative stress caused by Cu excess.
The increased expression of hsp70 observed in the highest concentration is probably linked to stress-induced denaturation of other proteins due to oxidative stress. This is supported by a previous study where increased expression of mitochondrial hsp70, and high levels of oxidative stress were observed in liver mitochondria of grey mullet fish collected from a polluted estuary [48]. Metal-induced expression of hsp70 has been observed in freshwater organisms, including D. magna [49,50,51,52]. The changes in expression of the inducible forms of hsp70 gene family have been proposed as a general response to environmental stressors [53].
The expression of mtc, gst, and hsp70 in groups exposed to 25 µg Cu/L did not deviate from the control levels at 96 h in medium and hard water, suggesting that their antioxidant system succeeded in neutralizing the oxidative stress induced by Cu exposure and prevent evoking major cell damage and death within the tested periods. High mortality (58.3 ± 10.8) was observed in daphnids exposed to 25 µg Cu/L in soft water, suggesting that the antioxidant defense systems did not protect against induced oxidative stress. This group may experience higher levels of ROS because of the higher uptake of Cu compared to daphnids exposed to 25 µg Cu/L in medium and hard water. The daphnids exposed to Cu in soft water might also have lower Ca levels. The previous studies observed that the Ca concentrations in D. magna are determined by the Ca concentration in their ambient water [54,55]. If so, this could be another contributing factor to the higher vulnerability to Cu toxicity in soft water. This is supported by a study where pre-exposure to Ca reduced the toxicity of Cu in juvenile Nile tilapia [56].
We observed that the expression of hif-1α did not correlate with Cu in soft water, while it showed a correlation in medium and hard water. It has been suggested that hif-1α mediates an adaptive response to oxidative stress [57], and its induction under increased ROS production due to cadmium exposure has been shown in D. magna [58]. Other studies also reported that Ca play a role in hif-1α regulation [59]. Therefore, hif-1α might be involved in the acute acclimation of daphnids to Cu exposure in medium and hard water.
The PCA results confirmed that exposure to a concentration of 25 µg Cu/L significantly affects the expression of target genes in medium and hard water. In soft water, we observed high individual variability in general stress response. For each gene, however, the mean fold change expression significantly differed from controls. More than 50% of daphnids could not survive within 96 h in this group. Generally, at the sublethal levels, animals respond in a similar manner. However, when they face extreme stress, such as Cu concentration close to the LC50 where the homeostatic system may fail to regulate Cu concentration, the magnitude of the individual’s response varies highly. As seen in bioplots, mtA, mtB, cat, and fer3, are significant contributors to separating the highest exposed group from others in all different hardness conditions and can be proposed as potential biomarkers for sublethal exposure of Cu in D. magna.
5. Conclusions
The present results indicate that water hardness mitigates the acute toxicity of Cu in D. magna. The gene expression profiles indicate activation of antioxidant defense responses as a mechanism of response to Cu excess. The Cu levels required to induce significant expression in the most stress response genes studied here did not vary among different hardness after 24 h exposure. This indicates that the major Cu stress response acts similarly in different hardness conditions. Under prolonged exposure (96 h), daphnids exposed to the high concentration in soft water did not survive. Obviously, a hardness-modified copper guideline value is required for the protection of aquatic biota in terms of water quality guidelines. Our study provides mechanistically based information that can improve the ecological relevance of risk assessment guidelines for Cu in the aquatic environment. Integration of gene biomarkers into risk assessments can provide a valuable complement to existing methods used in evaluating Cu toxicity.
Author Contributions
L.C.—formal analysis, investigation, writing original draft; V.S.—formal analysis, writing-reviewing and editing; J.J.—conceptualization, writing-reviewing and editing, project administration, funding acquisition; P.-E.O.—conceptualization, resources, writing-reviewing and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed by the Knowledge Foundation Sweden, grants 20170118 and 20180027, and Örebro University.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Table A1.
The chemical characteristics of three test media. The deviation from the theoretical concentration was always less than 20%.
Table A1.
The chemical characteristics of three test media. The deviation from the theoretical concentration was always less than 20%.
| Na | Mg | K | Ca | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hardness mg/L as CaCO3 | Replicates | Theoretical (μg/L) | Measured (μg/L) | Recovery (%) | Theoretical (μg/L) | Measured (μg/L) | Recovery (%) | Theoretical (μg/L) | Measured (μg/L) | Recovery (%) | Theoretical (μg/L) | Measured (μg/L) | Recovery (%) |
| 50 (Soft) | 1 | 18,530 | 18,060.5 | 97.5 | 5500 | 5463.2 | 99.3 | 3000 | 3516.4 | 117.2 | 11,000 | 9749.1 | 88.6 |
| 2 | 18,425.7 | 99.4 | 5542.6 | 100.8 | 3120.3 | 104.0 | 9807.9 | 89.2 | |||||
| 3 | 18,029.0 | 97.3 | 5418.9 | 98.5 | 2986.2 | 99.5 | 9431.4 | 85.7 | |||||
| 100 (Medium) | 1 | 18,530 | 18,966.4 | 102.4 | 11,000 | 11,578.0 | 105.3 | 3000 | 3427.2 | 114.2 | 22,000 | 19,957.4 | 90.7 |
| 2 | 18,894.6 | 102.0 | 11,268.6 | 102.4 | 3279.4 | 109.3 | 19,983.5 | 90.8 | |||||
| 3 | 18,616.9 | 100.5 | 11,308.3 | 102.8 | 3237.9 | 107.9 | 19,772.7 | 89.9 | |||||
| 200 (Hard) | 1 | 18,530 | 18,957.3 | 102.3 | 22,000 | 22,574.1 | 102.6 | 3000 | 3260.5 | 108.7 | 44,000 | 40,153.2 | 91.3 |
| 2 | 18,752.9 | 101.2 | 22,500.3 | 102.3 | 3126.0 | 104.2 | 39,374.6 | 89.5 | |||||
| 3 | 18,728.2 | 101.1 | 22,836.2 | 103.8 | 3224.4 | 107.5 | 39,467.6 | 89.7 | |||||

Table A2.
Copper concentration in test media at times 0 h (before exposure) and 24 h (before water exchange).
Table A2.
Copper concentration in test media at times 0 h (before exposure) and 24 h (before water exchange).
| Soft | Medium | Hard | |||||
|---|---|---|---|---|---|---|---|
| Theoretical (μg/L) | Measured (μg/L) | Recovery (%) | Measured (μg/L) | Recovery (%) | Measured (μg/L) | Recovery (%) | |
| Time 0 h | 0 | 1.2 | 1.4 | 1.1 | |||
| 7.5 | 6.8 | 90.53 | 6.6 | 87.91 | 7.1 | 94.89 | |
| 11.25 | 11.7 | 103.79 | 11.1 | 98.39 | 11.4 | 101.59 | |
| 16.87 | 17.3 | 102.56 | 17.3 | 102.65 | 17.2 | 102.06 | |
| 25.313 | 24.9 | 98.20 | 23.5 | 92.99 | 25.4 | 100.35 | |
| Time 24 h | Control | 0.8 | 3.1 | 1.7 | |||
| 7.5 | 7.6 | 101.04 | 7.1 | 94.76 | 7.7 | 102.94 | |
| 11.25 | 10.7 | 95.44 | 10.5 | 93.49 | 9.0 | 80 | |
| 16.87 | 16.1 | 95.70 | 18.0 | 106.69 | 14.7 | 87.10 | |
| 25.313 | 26.2 | 103.3 | 22.2 | 87.65 | 23.6 | 93.41 | |

Table A3.
List of primers used for qRT-PCR analysis.
Table A3.
List of primers used for qRT-PCR analysis.
| Gene Name | Gene Symbol | Forward (5′→3′) | Reverse (5′→3′) | |
|---|---|---|---|---|
| Actin | act | CCCCACGCCATCCTCCGTCT | GGTGGAGGCAGCAGCAGTGG | |
| Catalase | cat | TGGCGGAGAAAGCGGTTCAGC | GTGCGTGGTCTCTGGGCGAA | |
| Cu/zn-superoxide dismutase | sod1 | TCGTCGATACCGTCGTCTCACTTAGCG | ATTGCCCGTCTTGGTGCTGCCTTCGTT | |
| Cytochrome P450 18A1 family | cyp18A1 | TACCCGATCGTCGGTTACCT | GAGCGCCGTCAGCTCTTC | |
| Cytochrome P450 314 family (CYP314) | cyp314 | TCTTGGGTCGGCGTCTGGGA | TCGCGGGTGTCAACGCCTTC | |
| Ecdysone receptor A | EcRA | CAGGCACATCAACATCAACAA | GGCGACATGGAATCGACA | |
| Ecdysone receptor B | EcRB | CACCACAACCAACTGCATTTAC | CCATTAATGTCAAGATCCCACA | |
| Ferritin | fer3 | GCTGGTATCGCCAAGAACCTCAAA | TGGCGAAGAATTTCTGGATGCGGG | |
| Glutathione-s-transferase | gst | TCAGGCTGGTGTTGAGTTTG | GAGCAAGCATTTGTCCATCA | |
| Heat shock protein 70 | hsp70 | CGACGGCGGGAGATACGCAC | CCACGGAAAAGGTCGGCGCA | |
| HR3 nuclear hormone receptor | hr3 | AGTCATCACCTGCGAGGGC | GAACTTTGCGACCGCCG | |
| Hypoxia Inducible Factor 1 Alpha | hif1α | GGTCCAGACCCAAGCAGCCAGGC | GTCCAGGAGCAGCAGCCAGC | |
| Metallothionein A | mtA | GAGCGCCATGCCAAAATCCC | TCGTCGTTGTAAAATCCGCCT | |
| Metallothionein B | mtB | TGGAACCGAATGCAAATGCG | CGGACTTGCATGGACAACTG | |
| Metallothionein C | mtC | AAAGTGTGCCCTCGTTGTCA | CTTACAGTCGTCCCCACACG | |
| Zn-superoxide dismutase | sod2 | CCTAATGGAGGTGAACCAGAGGGAGAG | CCTAGCCAACCCCATCCTGAACCTTGA | |
| Hemoglobin 1 | dhb1 | GCTGGTATCGCCAAGAACCTCAAA | TGGCGAAGAATTTCTGGATGCGGG | |
| Fushi tarazu factor-1 | ftz | GTTGCACAATCACCTGCCTG | TAACGTCGTGAAGGGGTTCG | |
| Gene name | Gene symbol | Forward (5′→3′) | Reverse (5′→3′) | Reference |
| Neverland 1 | ndv1 | AGCACAAGGCGGGAAGAGT | GCTTCCCATTTCACCTTCCA | Sumiya et al., 2016 [60] |

Table A4.
Predicted copper speciation in different hardness levels (50 (soft), 100 (medium), and 200 (hard) mg/L as CaCO3.
Table A4.
Predicted copper speciation in different hardness levels (50 (soft), 100 (medium), and 200 (hard) mg/L as CaCO3.
| Species Name (% of total concentration) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hardness | Copper (µg/L) | Cu+2 | CuOH+ | Cu(OH)2 (aq) | Cu2(OH)2+2 | CuCl+ | CuSO4 (aq) | CuCO3 (aq) | CuHCO3+ | Cu(CO3)2−2 |
| Soft | 7 | 11.76 | 11.89 | 0.73 | 0.01 | 0.01 | 0.36 | 74.48 | 0.43 | 0.34 |
| Medium | 12.47 | 12.05 | 0.73 | 0.01 | 0.02 | 0.65 | 73.29 | 0.43 | 0.34 | |
| Hard | 12.18 | 12.48 | 0.83 | 0.01 | 0.04 | 1.02 | 72.65 | 0.39 | 0.41 | |
| Soft | 11 | 11.76 | 11.89 | 0.73 | 0.02 | 0.01 | 0.36 | 74.47 | 0.43 | 0.34 |
| Medium | 12.47 | 12.05 | 0.73 | 0.02 | 0.02 | 0.65 | 73.28 | 0.43 | 0.34 | |
| Hard | 12.18 | 12.48 | 0.83 | 0.02 | 0.04 | 1.02 | 72.64 | 0.39 | 0.41 | |
| Soft | 17 | 11.75 | 11.89 | 0.73 | 0.03 | 0.01 | 0.36 | 74.46 | 0.43 | 0.34 |
| Medium | 12.47 | 12.05 | 0.73 | 0.03 | 0.02 | 0.65 | 73.28 | 0.43 | 0.34 | |
| Hard | 12.18 | 12.48 | 0.83 | 0.03 | 0.04 | 1.02 | 72.64 | 0.39 | 0.41 | |
| Soft | 25 | 11.75 | 11.89 | 0.73 | 0.04 | 0.01 | 0.36 | 74.45 | 0.43 | 0.34 |
| Medium | 12.47 | 12.05 | 0.73 | 0.04 | 0.02 | 0.65 | 73.26 | 0.43 | 0.34 | |
| Hard | 12.18 | 12.48 | 0.83 | 0.05 | 0.04 | 1.02 | 72.62 | 0.39 | 0.41 | |
Appendix B
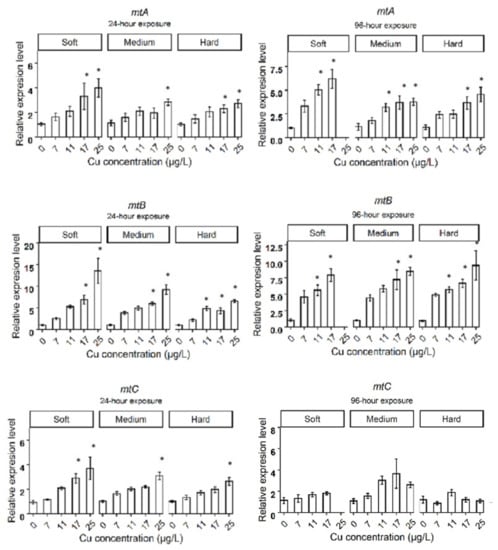
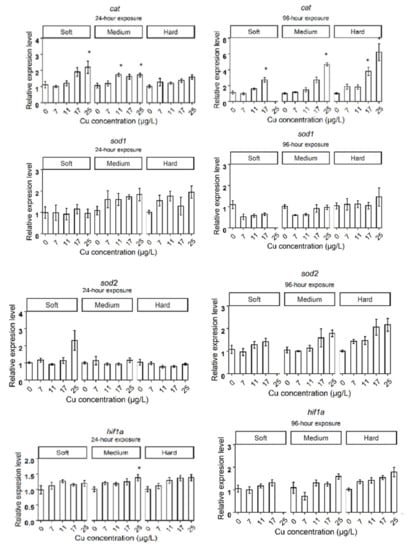
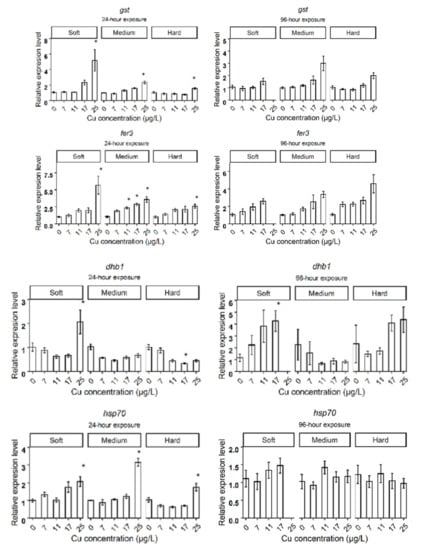
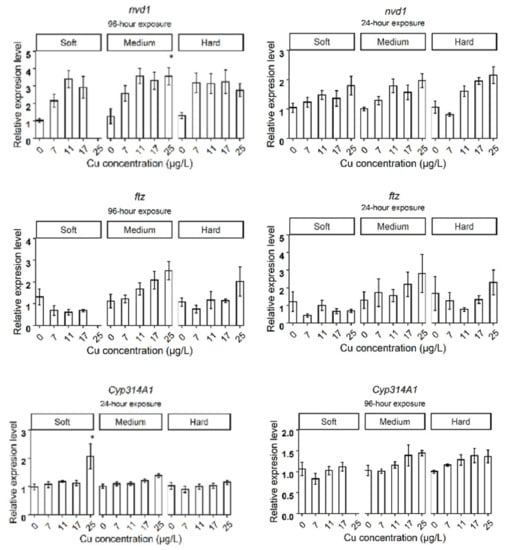
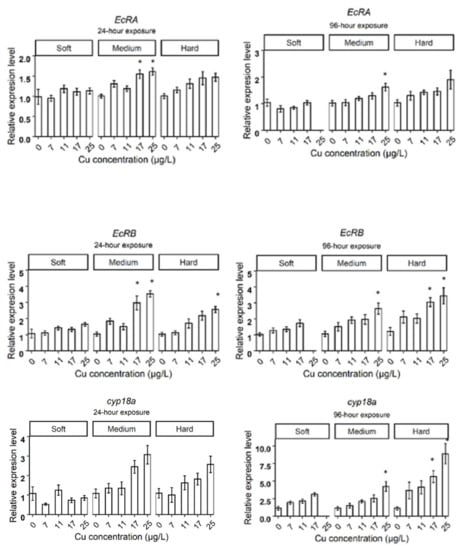
Figure A1.
The expression levels of target genes in different water hardness (50 (soft), 100 (medium), and 200 (hard) mg/L as CaCO3). The asterisks show a significant differences (p < 0.05) between Control and treatments in each hardness level.
References
- Puig, S.; Thiele, D.J. Molecular mechanisms of copper uptake and distribution. Curr. Opin. Chem. Biol. 2002, 6, 171–180. [Google Scholar] [CrossRef]
- De Oliveira-Filho, E.C.; Lopes, R.M.; Paumgartten, F.J.R. Comparative study on the susceptibility of freshwater species to copper-based pesticides. Chemosphere 2004, 56, 369–374. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Collaboration between the World Health Organization and the National Institute of Environmental Health Sciences: Highlights from 30 Years of Partnership; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Stern, B.R. Essentiality and toxicity in copper health risk assessment: Overview, update and regulatory considerations. J. Toxicol. Environ. 2010, 73, 114–127. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency (US EPA). Aquatic Life Ambient Freshwater Quality Criteria-Copper. 2007 Revision. EPA-822-R-07-001. Available online: https://www.federalregister.gov/documents/2007/02/22/E7-3007/aquatic-life-ambient-freshwater-quality-criteria-copper-2007-revision (accessed on 10 June 2021).
- Fu, Z.; Wu, F.; Chen, L.; Xu, B.; Feng, C.; Bai, Y.; Liao, H.; Sun, S.; Giesy, J.P.; Guo, W. Copper and zinc, but not other priority toxic metals, pose risks to native aquatic species in a large urban lake in Eastern China. Environ. Pollut. 2016, 219, 1069–1076. [Google Scholar] [CrossRef]
- Roux, D.; Jooste, S.; MacKay, H.M. Substance-specific water quality criteria for the protection of South African freshwater ecosystems: Methods for derivation and initial results for some inorganic toxic substances. S. Afr. J. Sci. 1996, 92, 198–206. [Google Scholar]
- Borgmann, U.; Couillard, Y.; Doyle, P.; Dixon, D.G. Toxicity of sixty-three metals and metalloids to Hyalella azteca at two levels of water hardness. Environ. Toxicol. 2005, 24, 641–652. [Google Scholar] [CrossRef]
- Rai, L.; Gaur, J.; Kumar, H.D. Phycology and heavy-metal pollution. Biol. Rev. 1981, 56, 99–151. [Google Scholar] [CrossRef]
- Kramer, K.J.; Jak, R.G.; van Hattum, B.; Hooftman, R.N.; Zwolsman, J.J.G. Copper toxicity in relation to surface water-dissolved organic matter: Biological effects to Daphnia magna. Environ. Toxicol. 2004, 23, 2971–2980. [Google Scholar] [CrossRef]
- Andrew, R.; Biesinger, K.; Glass, G.E. Effects of inorganic complexing on the toxicity of copper to Daphnia magna. Water Res. 1977, 11, 309–315. [Google Scholar] [CrossRef]
- De Schamphelaere, K.A.C.; Janssen, C.R. Effects of dissolved organic carbon concentration and source, pH, and water hardness on chronic toxicity of copper to Daphnia magna. Environ. Sci. Technol. 2004, 23, 1115–1122. [Google Scholar] [CrossRef]
- Park, E.J.; Jo, H.J.; Jung, J. Combined effects of pH, hardness and dissolved organic carbon on acute metal toxicity to Daphnia magna. Ind. Eng. Chem. 2009, 15, 82–85. [Google Scholar] [CrossRef]
- Okamoto, A.; Yamamuro, M.; Tatarazako, N. Acute toxicity of 50 metals to Daphnia magna. J. Appl. Toxicol. 2015, 35, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.H.; Arbildua, J.; Villavicencio, G.; Mejías, R.; Urrestarazu, P.; Jiménez, M. Copper acute and chronic toxicity to D. magna: Sensitivity at three different hardness at pH 6.3 (MES buffered) in the presence of 2 mg/L DOC. In Chilean Mining and Metallurgy Research Center, Laboratory of Ecotoxicology and Chemistry of Trace Metals; Centro de Investigación Minera y Metalúrgica: Santiago, Chile, 2012; Available online: http://hdl.handle.net/1854/LU-5902885 (accessed on 10 June 2021).
- Bui, T.-K.L.; Do-Hong, L.C.; Dao, T.-S.; Hoang, T.C. Copper toxicity and the influence of water quality of Dongnai River and Mekong River waters on copper bioavailability and toxicity to three tropical species. Chemosphere 2016, 144, 872–878. [Google Scholar] [CrossRef] [PubMed]
- De Schamphelaere, K.A.; Janssen, C.R. A biotic ligand model predicting acute copper toxicity for Daphnia magna: The effects of calcium, magnesium, sodium, potassium, and pH. Environ. Sci. Technol. 2002, 36, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Craig, P.M.; Wood, C.M.; McClelland, G.B. Water chemistry alters gene expression and physiological end points of chronic waterborne copper exposure in zebrafish, Danio rerio. Environ. Sci. Technol. 2010, 44, 2156–2162. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, version 4.0.2; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org (accessed on 10 June 2021).
- RStudio Team. RStudio: Integrated Development for R, version 1.2.5042; PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com (accessed on 10 June 2021).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kozlova, T.; Wood, C.M.; McGeer, J.C. The effect of water chemistry on the acute toxicity of nickel to the cladoceran Daphnia pulex and the development of a biotic ligand model. Aquat. Toxicol. 2009, 91, 221–228. [Google Scholar] [CrossRef]
- De Paiva Magalhães, D.; da Costa Marques, M.R.; Baptista, D.F.; Buss, D.F. Metal bioavailability and toxicity in freshwaters. Environ. Chem. Lett. 2015, 13, 69–87. [Google Scholar] [CrossRef]
- McWilliams, P.G.; Potts, W.T.W. The effects of pH and calcium concentrations on gill potentials in the brown trout, Salmo trutta. Comp. Biochem. Physiol. B Biochem. 1978, 126, 277–286. [Google Scholar] [CrossRef]
- Markich, S.; Batley, G.; Stauber, J.; Rogers, N.; Apte, S.; Hyne, R.; Bowles, K.; Wilde, K.; Creighton, N.M. Hardness corrections for copper are inappropriate for protecting sensitive freshwater biota. Chemosphere 2005, 60, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hessen, D.O.; Alstad, N.E.; Skardal, L. Calcium limitation in Daphnia magna. J. Plankton Res. 2000, 22, 553–568. [Google Scholar] [CrossRef]
- Jesus, F.T.; Martins, C.; Nogueira, A.J.A. Changes in life-history parameters of Daphnia longispina (Cladocera, Crustacea) as a function of water chemistry. J. Limnol. 2014, 73, 340–349. [Google Scholar] [CrossRef]
- Poynton, H.C.; Loguinov, A.V.; Varshavsky, J.R.; Chan, S.; Perkins, E.J.; Vulpe, C.D. Gene expression profiling in Daphnia magna part I: Concentration-dependent profiles provide support for the no observed transcriptional effect level. Environ. Sci. Technol. 2008, 42, 6250–6256. [Google Scholar] [CrossRef] [PubMed]
- Asselman, J.; Shaw, J.R.; Glaholt, S.P.; Colbourne, J.K.; De Schamphelaere, K.A.C. Transcription patterns of genes encoding four metallothionein homologs in Daphnia pulex exposed to copper and cadmium are time-and homolog-dependent. Aquat. Toxicol. 2013, 142, 422–430. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, G.; Ngo, T.T.H.; Van Campenhout, K.; Blust, R. Differential metallothionein induction patterns in three freshwater fish during sublethal copper exposure. Aquat. Toxicol. 2003, 65, 413–424. [Google Scholar] [CrossRef]
- Coyle, P.; Philcox, J.C.; Carey, L.C.; Rofe, A.M. Metallothionein: The multipurpose protein. Cell. Mol. Life Sci. 2002, 59, 627–647. [Google Scholar] [CrossRef]
- Olsson, P.E.; Kling, P.; Hogstrand, C. Mechanisms of heavy metal accumulation and toxicity in fish. In Metal Metabolism in Aquatic Environments; Bebianno, W.J., Langston, M.J., Eds.; Chapman and Hall: London, UK, 1998; pp. 321–350. [Google Scholar] [CrossRef]
- Mayer, G.D.; Leach, A.; Kling, P.; Olsson, P.E.; Hogstrand, C. Activation of the rainbow trout metallothionein-A promoter by silver and zinc. Comp. Biochem. Physiol. B Biochem. 2003, 134, 181–188. [Google Scholar] [CrossRef]
- Amiard, J.-C.; Amiard-Triquet, C.; Barka, S.; Pellerin, J.; Rainbow, P.S. Metallothioneins in aquatic invertebrates: Their role in metal detoxification and their use as biomarkers. Aquat. Toxicol. 2006, 76, 160–202. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Vašák, M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim. Biophys. Acta 1985, 827, 36–44. [Google Scholar] [CrossRef]
- Iszard, M.B.; Liu, J.; Klaassen, C.D. Effect of several metallothionein inducers on oxidative stress defense mechanisms in rats. Toxicology 1995, 104, 25–33. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, Z.; Kang, J. Attenuation of doxorubicin chronic toxicity in metallothionein-overexpressing transgenic mouse heart. Cancer Res. 2001, 61, 3382–3387. [Google Scholar] [PubMed]
- Kling, P.; Olsson, P.E. Metallothionein: Structure and regulation. In Biochemistry and Molecular Biology of Fishes; Mommsen, T.W.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 6, pp. 289–302. [Google Scholar] [CrossRef]
- Mosleh, Y.Y.; Paris-Palacios, S.; Ahmed, M.T.; Mahmoud, F.; Osman, M.; Biagianti-Risbourg, S. Effects of chitosan on oxidative stress and metallothioneins in aquatic worm Tubifex tubifex (Oligochaeta, Tubificidae). Chemosphere 2007, 67, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Barata, C.; Varo, I.; Navarro, J.C.; Arun, S.; Porte, C. Antioxidant enzyme activities and lipid peroxidation in the freshwater cladoceran Daphnia magna exposed to redox cycling compounds. Comp. Biochem. Physiol. C Toxicol. 2005, 140, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Chain, F.J.; Finlayson, S.; Crease, T.; Cristescu, M. Variation in transcriptional responses to copper exposure across Daphnia pulex lineages. Aquat. Toxicol. 2019, 210, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; McLellan, L.I. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic. Res. 1999, 31, 273–300. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Pulford, D.J. The glutathione S-transferase supergene family: Regulation of GST and the contribution of the lsoenzymes to cancer chemoprotection and drug resistance part I. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 445–520. [Google Scholar] [CrossRef] [PubMed]
- Poynton, H.C.; Varshavsky, J.R.; Chang, B.; Cavigiolio, G.; Chan, S.; Holman, P.S.; Loguinov, A.V.; Bauer, D.J.; Komachi, K.; Theil, E.C.; et al. Daphnia magna ecotoxicogenomics provides mechanistic insights into metal toxicity. Environ. Sci. Technol. 2007, 41, 1044–1050. [Google Scholar] [CrossRef]
- Tsuji, Y.; Ayaki, H.; Whitman, S.P.; Morrow, C.S.; Torti, S.V.; Torti, F.M. Coordinate transcriptional and translational regulation of ferritin in response to oxidative stress. Mol. Cell. Biol. 2000, 20, 5818–5827. [Google Scholar] [CrossRef]
- Padmini, E.; Geetha, B.V. Impact of season on liver mitochondrial oxidative stress and the expression of HSP70 in grey mullets from contaminated estuary. Ecotoxicology 2009, 18, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Efremova, S.M.; Margulis, B.A.; Guzhova, I.V.; Itskovich, V.B.; Lauenroth, S.; Müller, W.E.; Schröder, H.C. Heat shock protein Hsp70 expression and DNA damage in Baikalian sponges exposed to model pollutants and wastewater from Baikalsk Pulp and Paper Plant. Aquat. Toxicol. 2002, 57, 267–280. [Google Scholar] [CrossRef]
- Connon, R.; Hooper, H.L.; Sibly, R.M.; Lim, F.L.; Heckmann, L.H.; Moore, D.J.; Watanabe, H.; Soetaert, A.; Cook, K.; Maund, S.J.; et al. Linking molecular and population stress responses in Daphnia magna exposed to cadmium. Environ. Sci. Technol. 2008, 42, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Mini, J.; Munuswamy, N. Effects of heavy metals on antioxidants and expression of HSP70 in different tissues of Milk fish (Chanos chanos) of Kaattuppalli Island, Chennai, India. Ecotoxicol. Environ. Saf. 2013, 98, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.S.; Kim, P.J.; Won, E.J.; Lee, Y.M. Response of antioxidant enzymes to Cd and Pb exposure in water flea Daphnia magna: Differential metal and age—Specific patterns. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 209, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef] [PubMed]
- Cowgill, U.; Emmel, H.; Hopkins, D.; Applegath, S.; Takahashi, I.T. The influence of water on reproductive success and chemical composition of laboratory reared populations of Daphnia magna. Water Res. 1986, 20, 317–323. [Google Scholar] [CrossRef]
- Alstad, N.E.; Skardal, L.; Hessen, D.O. The effect of calcium concentration on the calcification of Daphnia magna. Limnol. Oceanogr. 1999, 44, 2011–2017. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Mousa, M.A.; Ahmad, M.H.; Sakr, S.F.M. The use of calcium pre-exposure as a protective agent against environmental copper toxicity for juvenile Nile tilapia, Oreochromis niloticus (L.). Aquaculture 2007, 264, 236–246. [Google Scholar] [CrossRef]
- Li, H.S.; Zhou, Y.N.; Li, L.; Li, S.F.; Long, D.; Chen, X.L.; Zhang, J.B.; Feng, L.; Li, Y.P. HIF-1α protects against oxidative stress by directly targeting mitochondria. Redox Biol. 2019, 25, 101109. [Google Scholar] [CrossRef]
- Chen, S.; McKinney, G.J.; Nichols, K.M.; Colbourne, J.K.; Sepulveda, M.S. Novel cadmium responsive microRNAs in Daphnia pulex. Environ. Sci. Technol. 2015, 49, 14605–14613. [Google Scholar] [CrossRef]
- Mottet, D.; Michel, G.; Renard, P.; Ninane, N.; Raes, M.; Michiels, C. Role of ERK and calcium in the hypoxia-induced activation of HIF-1. J. Cell. Physiol. 2002, 94, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Sumiya, E.; Ogino, Y.; Toyota, K.; Miyakawa, H.; Miyagawa, S.; Iguchi, T. Neverland regulates embryonic moltings through the regulation of ecdysteroid synthesis in the water flea Daphnia magna, and may thus act as a target for chemical disruption of molting. J. Appl. Toxicol. 2016, 36, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).