Effect of Dietary Compound Acidifiers Supplementation on Growth Performance, Serum Biochemical Parameters, and Body Composition of Juvenile American Eel (Anguilla rostrata)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Design and Diets
2.2. Trial Fish Management
2.3. Sample Collection and Analysis
2.4. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Serum Biochemical Parameters
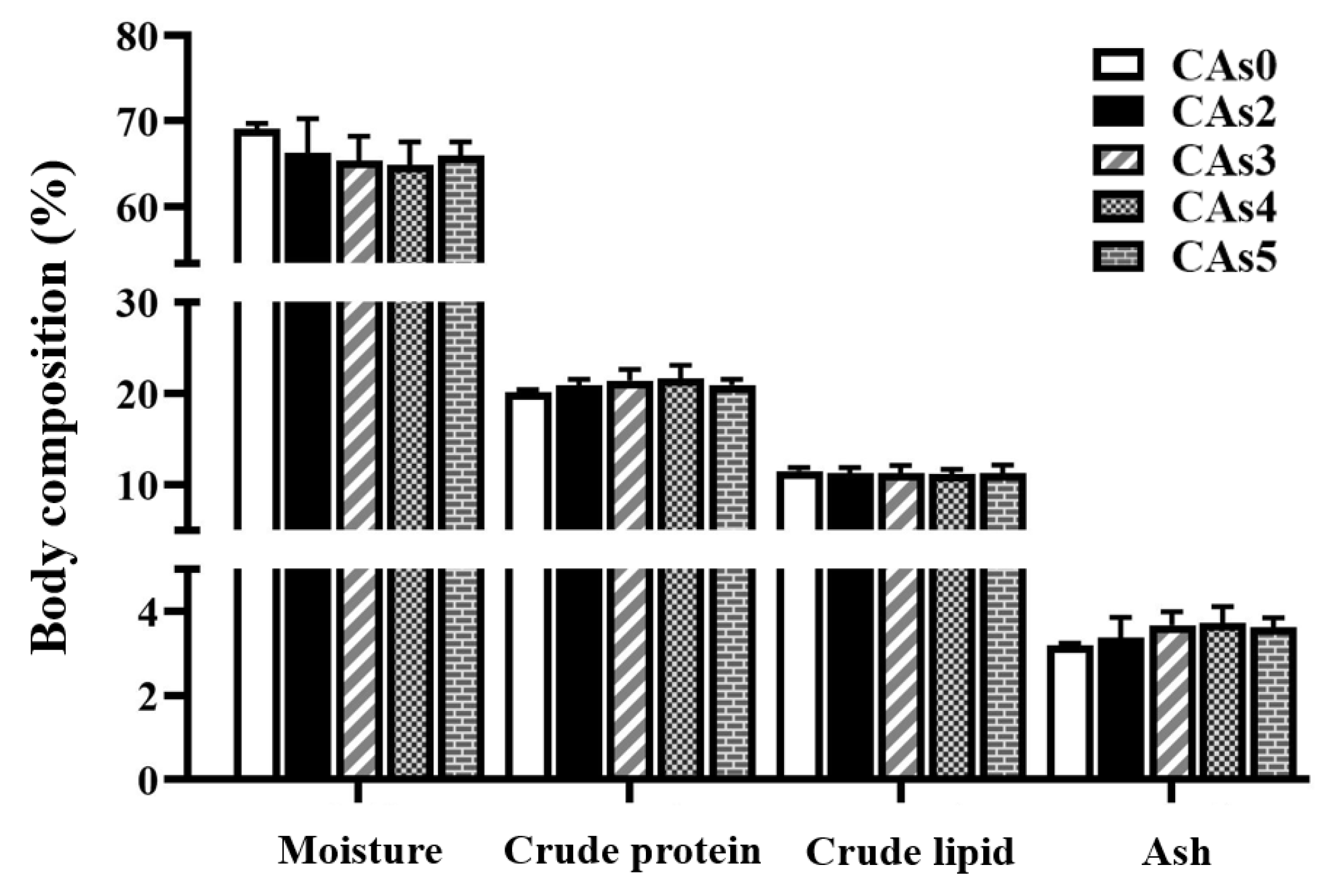
3.3. Body Composition of the Whole Fish
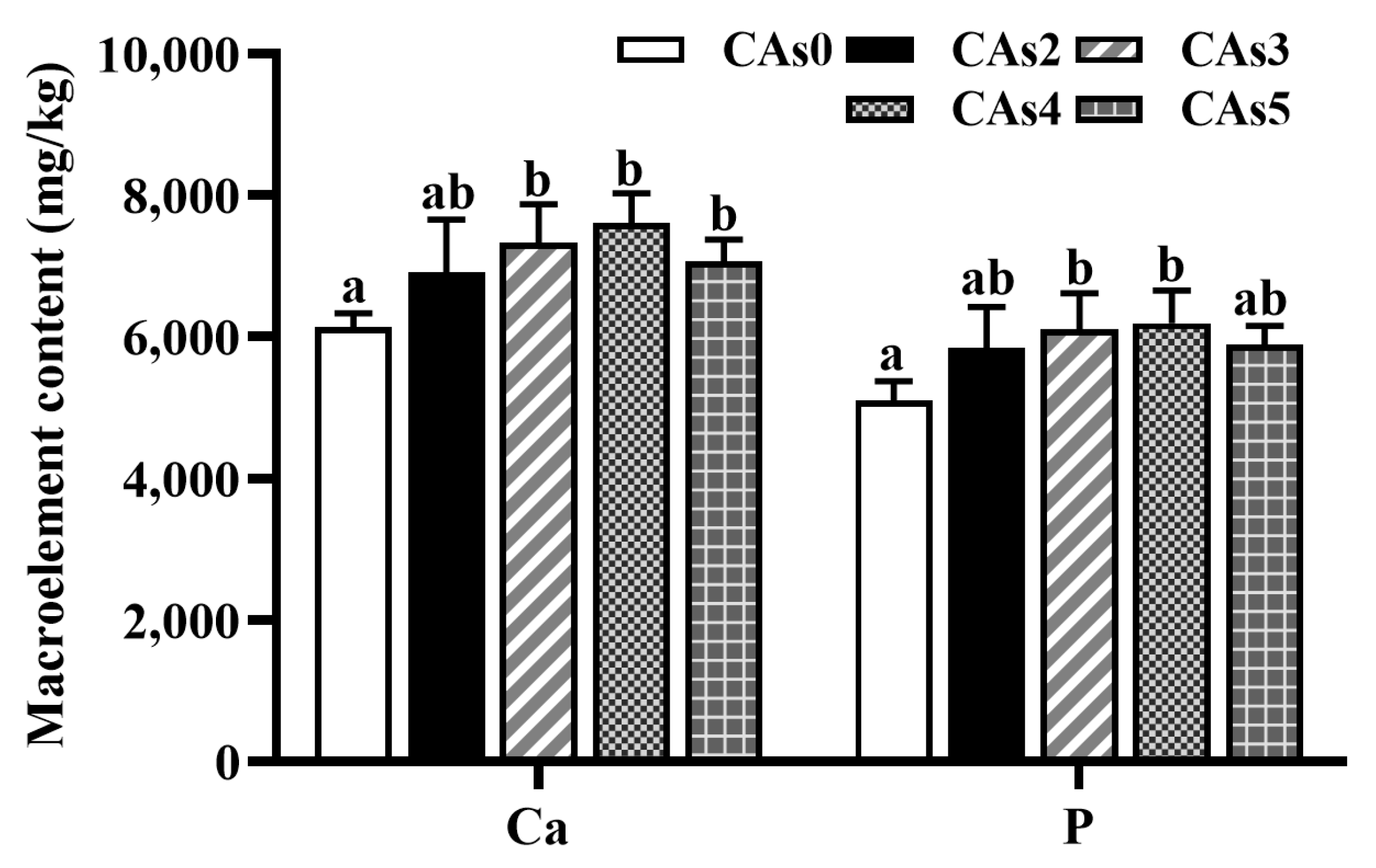
3.4. Macroelement Content in the Whole Fish
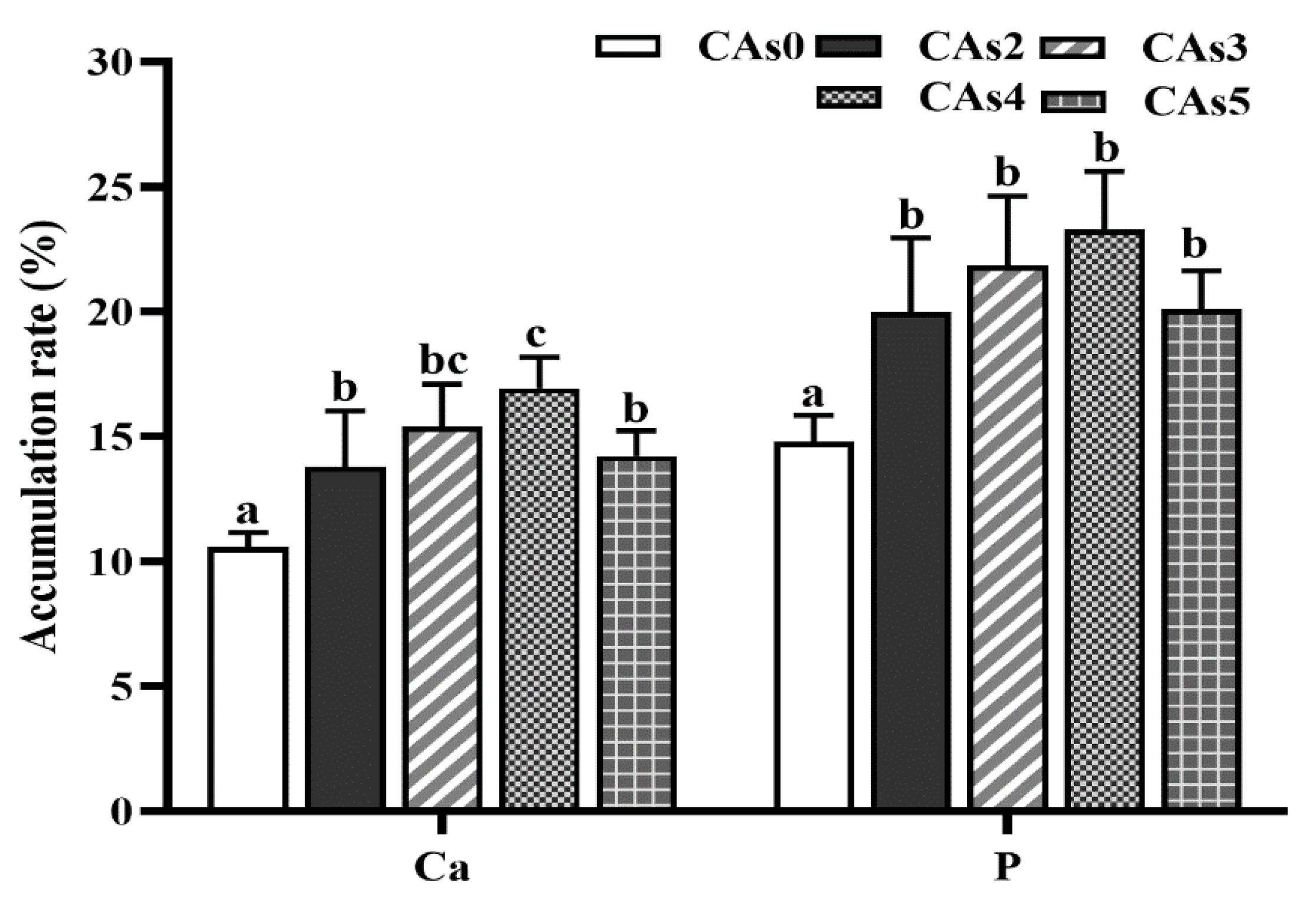
3.5. Accumulation Rate of Dietary Ca and P
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pearlin, B.V.; Muthuvel, S.; Govidasamy, P.; Villavan, M.; Alagawany, M.; Ragab Farag, M.; Dhama, K.; Gopi, M. Role of acidifiers in livestock nutrition and health: A review. J. Anim. Physiol. Anim. Nutr. 2019, 104, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L.; Wang, Y.; Zhai, S.W. Effects of dietary compound acidifiers supplementation on growth performance and intestinal health of juvenile American eels (Anguilla rostrata) cultured in cement tanks. Isr. J. Aquac.-Bamidgeh 2021, 73, 1520998. [Google Scholar] [CrossRef]
- Ng, W.K.; Koh, C.B. The utilization and mode of action of organic acids in the feeds of cultured aquatic animals. Rev. Aquac. 2017, 9, 342–368. [Google Scholar] [CrossRef]
- Huang, Z.F.; Ye, Y.L.; Xu, A.L.; Li, Z.B.; Wang, Z. Dietary supplementation with an acidifier blend (citric, lactic, and phosphoric acids) influences growth, digestive enzymes, and blood chemistry of juvenile Japanese sea-bass (Lateolabrax japonicus). Aquac. Int. 2022, 30, 19–32. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Khattaby, A.R.A.; Monier, M.N. Dietary acidifiers blend enhanced the production of Nile tilapia (Oreochromis niloticus), Striped mullet (Mugil cephalus), and African catfish (Clarias gariepinus) polycultured in earthen ponds. Aquac. Int. 2019, 27, 369–379. [Google Scholar] [CrossRef]
- Sardar, P.; Shamna, N.; Sahu, N.P. Acidifiers in aquafeed as an alternate growth promoter: A short review. Anim. Nutr. Feed. Technol. 2020, 20, 353–366. [Google Scholar] [CrossRef]
- Reda, R.M.; Mahmoud, R.; Selim, K.M.; El-Araby, I.E. Effects of dietary acidifiers on growth, hematology, immune response and disease resistance of Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2016, 50, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Soltan, M.A.; Hassaan, M.S.; Meshrf, R.N. Response of Nile tilapia (Oreochromis niloticus) to diet acidification: Effect on growth performance and feed utilization. J. Applied. Aquac. 2017, 29, 207–219. [Google Scholar] [CrossRef]
- Kumar, P.; Jain, K.K.; Sardar, P.; Sahu, N.P.; Gupta, S. Dietary supplementation of acidifier: Effect on growth performance and haemato-biochemical parameters in the diet of Cirrhinus mrigala juvenile. Aquac. Int. 2017, 25, 2101–2116. [Google Scholar] [CrossRef]
- Katya, K.; Park, G.; Bharadwaj, A.S.; Browdy, C.L.; Vazquez-Anon, M.; Bai, S.C. Organic acids blend as dietary antibiotic replacer in marine fish olive flounder, Paralichthys olivaceus. Aquac. Res. 2018, 49, 2861–2868. [Google Scholar] [CrossRef]
- Sotoudeh, E.; Sangari, M.; Bagheri, D.; Morammazi, S.; Torfi Mozanzadeh, M. Dietary organic acid salts mitigate plant protein induced inflammatory response and improve humoral immunity, antioxidative status and digestive enzyme activities in yellowfin seabream, Acanthopagrus latus. Aquac. Int. 2020, 26, 1669–1680. [Google Scholar] [CrossRef]
- Gao, Y.L.; Storebakken, T.; Shearer, K.D.; Penn, M.; Øverland, M. Supplementation of fishmeal and plant protein-based diets for rainbow trout with a mixture of sodium formate and butyrate. Aquaculture 2011, 311, 233–240. [Google Scholar] [CrossRef]
- Zhu, Y.; Qiu, X.; Ding, Q.L.; Duan, M.M.; Wang, C.F. Combined effects of dietary phytase and organic acid on growth and phosphorus utilization of juvenile yellow catfish Pelteobagrus fulvidraco. Aquaculture 2014, 430, 1–8. [Google Scholar] [CrossRef]
- Yuan, Y.; Yuan, Y.M.; Dai, Y.Y.; Gong, Y.C.; Yuan, Y.Q. Development status and trends in the eel farming industry in Asia. N. Am. J. Aquac. 2022, 84, 3–17. [Google Scholar] [CrossRef]
- Zhai, S.W.; Zhao, P.Y.; Huang, L.X. Dietary bile acids supplementation improves the growth performance with regulation of serum biochemical parameters and intestinal microbiota of growth retarded European eels (Anguilla anguilla) cultured in cement tanks. Isr. J. Aquac.-Bamidgeh 2020, 72, 1217104. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of Official Analytical Chemists International, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Addam, K.G.S.; Pereira, S.A.; Jesus, G.F.A.; Cardoso, L.; Syracuse, N.; Lopes, G.R.; Mouriño, J.L.P. Dietary organic acids blend alone or in combination with an essential oil on the survival, growth, gut/liver structure and de hemato-immunological in Nile tilapia Oreochromis niloticus. Aquac. Res. 2019, 50, 2960–2971. [Google Scholar] [CrossRef]
- Sony, N.M.; Ishikawa, M.; Hossain, M.; Koshio, S.; Yokoyama, S. The effect of dietary fucoidan on growth, immune functions, blood characteristics and oxidative stress resistance of juvenile red sea bream, Pagrus major. Fish Physiol. Biochem. 2019, 45, 439–454. [Google Scholar] [CrossRef]
- Zhao, H.; Peng, K.; Wang, G.; Mo, W.; Huang, Y.; Cao, J. Metabolic changes, antioxidant status, immune response and resistance to ammonia stress in juvenile yellow catfish (Pelteobagrus fulvidraco) fed diet supplemented with sodium butyrate. Aquaculture 2021, 536, 736441. [Google Scholar] [CrossRef]
- Shah, S.Z.H.; Afzal, M.; Khan, S.Y.; Hussain, S.M.; Habib, R.Z. Prospects of using citric acid as fish feed supplement. Int. J. Agric. Biol. 2015, 17, 1–8. [Google Scholar]
- Halver, J.E.; Hardy, R.W. Fish Nutrition; Academic Press: London, UK, 1989; pp. 154–209. [Google Scholar]
- Xun, P.W.; Zhou, C.P.; Huang, X.L.; Huang, Z.; Yu, W.; Yang, Y.K.; Li, T.; Huang, J.B.; Wu, Y.; Lin, H.Z. Effects of dietary potassium diformate on growth performance, fillet quality, plasma indices, intestinal morphology and liver health of juvenile golden pompano (Trachinotus ovatus). Aquac. Rep. 2022, 24, 101110. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, Y.; Liang, X.F.; He, S.; Li, L. Dietary supplementation of exogenous probiotics reduces excessive liver lipid deposition in Chinese perch (Siniperca chuatsi). Aquac. Res. 2021, 52, 5430–5440. [Google Scholar] [CrossRef]
- Alex, S.; Lange, K.; Amolo, T.; Grinstead, J.S.; Haakonsson, A.K.; Szalowska, E.; Koppen, A.; Mudde, K.; Haenen, D.; Al-Lahham, S.; et al. Short-chain fatty acids stimulate angiopoietin-like 4 synthesis in human colon adenocarcinoma cells by activating peroxisome proliferator-activated receptor γ. Mol. Cell Biol. 2013, 33, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; Bleeker, A.; Gerding, A.; Van Eunen, K.; Havinga, R.; Van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.J.; et al. Short-chain fatty acids protect against high-fat diet–induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Wafa, M.A.; Soltan, M.A.; Goda, A.S.; Mogheth, N.M.A. Effect of dietary organic salts on growth, nutrient digestibility, mineral absorption and some biochemical indices of Nile tilapia, Oreochromis niloticus. World Appl. Sci. J. 2014, 29, 47–55. [Google Scholar]
- Zhou, C.P.; Lin, H.Z.; Huang, Z.; Wang, J.; Wang, Y.; Yu, W. Effect of dietary sodium butyrate on growth performance, enzyme activities and intestinal proliferation-related gene expression of juvenile golden pompano Trachinotus ovatus. Aquac. Nutr. 2019, 25, 1261–1271. [Google Scholar] [CrossRef]
- Hu, E.D.; Chen, D.Z.; Wu, J.L.; Lu, F.B.; Chen, L.; Zheng, M.H.; Li, H.; Huang, Y.; Li, J.; Jin, X.Y.; et al. High fiber dietary and sodium butyrate attenuate experimental autoimmune hepatitis through regulation of immune regulatory cells and intestinal barrier. Cell Immunol. 2018, 328, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.C.; Wang, L.G.; Wang, H.L.; Xie, F.J.; Wang, T. Effect of dietary vitamin C on the growth performance and innate immunity of juvenile cobia (Rachycentron canadum). Fish Shellfish Immunol. 2012, 32, 969–975. [Google Scholar] [CrossRef] [PubMed]
- El-Adawy, M.; El-Aziz, M.A.; El-Shazly, K.; Ali, N.G.; El-Magd, M.A. Dietary propionic acid enhances antibacterial and immunomodulatory effects of oxytetracycline on Nile tilapia, Oreochromis niloticus. Environ. Sci. Pollut. Res. 2018, 25, 34200–34211. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Sun, Y.Z.; Caipang, C.M. Short-chain fatty acids as feed supplements for sustainable aquaculture: An updated view. Aquac. Res. 2017, 48, 1380–1391. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.Y.; Fei, W.D.; Ye, Y.Q.; Zhao, M.D.; Zheng, C.H. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit. Rev. Food Sci. 2022, 62, 1–12. [Google Scholar] [CrossRef]
- Tran, N.T.; Li, Z.Z.; Wang, S.Q.; Zheng, H.P.; Aweya, J.J.; Wen, X.B.; Li, S.K. Progress and perspectives of short-chain fatty acids in aquaculture. Rev. Aquac. 2020, 12, 283–298. [Google Scholar] [CrossRef]
- Zhang, H.L.; Yi, L.A.; Sun, R.J.; Zhou, H.H.; Xu, W.; Zhang, W.B.; Mai, K.S. Effects of dietary citric acid on growth performance, mineral status and intestinal digestive enzyme activities of large yellow croaker Larimichthys crocea (Richardson, 1846) fed high plant protein diets. Aquaculture 2016, 453, 147–153. [Google Scholar] [CrossRef]
- Hossain, M.A.; Pandey, A.; Satoh, S. Effects of organic acids on growth and phosphorus utilization in red sea bream Pagrus major. Fish. Sci. 2007, 73, 1309–1317. [Google Scholar]
- Koh, C.B.; Romano, N.; Zahrah, A.S.; Ng, W.K. Effects of a dietary organic acids blend and oxytetracycline on the growth, nutrient utilization and total cultivable gut microbiota of the red hybrid tilapia, Oreochromis sp., and resistance to Streptococcus agalactiae. Aquac. Res. 2016, 47, 357–369. [Google Scholar] [CrossRef]
- Abd El-Naby, A.S.; Khattaby, A.E.R.A.; Samir, F.; Awad, S.M.; Abdel-Tawwab, M. Stimulatory effect of dietary butyrate on growth, immune response, and resistance of Nile tilapia, Oreochromis niloticus against Aeromonas hydrophila infection. Anim. Feed Sci. Technol. 2019, 254, 114212. [Google Scholar] [CrossRef]
- Tian, L.; Zhou, X.Q.; Jiang, W.D.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Sodium butyrate improved intestinal immune function associated with NF-κB and p38MAPK signalling pathways in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2017, 66, 548–563. [Google Scholar] [CrossRef] [PubMed]

| Items | CAs0 Group | CAs2 Group | CAs3 Group | CAs4 Group | CAs5 Group |
|---|---|---|---|---|---|
| IFW (g/fish) | 11.03 ± 0.04 a | 11.01 ± 0.03 a | 11.00 ± 0.01 a | 10.99 ± 0.02 a | 10.99 ± 0.03 a |
| FFW (g/fish) | 23.07 ± 0.50 a | 25.67 ± 0.43 b | 27.73 ± 0.86 c | 30.02 ± 1.19 d | 26.55 ± 0.24 b |
| WGR (%) | 109.26 ± 4.76 a | 133.25 ± 3.75 b | 152.05 ± 7.55 c | 173.20 ± 11.11 d | 139.70 ± 3.96 b |
| SGR (%/d) | 0.88 ± 0.03 a | 1.01 ± 0.02 b | 1.10 ± 0.04 c | 1.20 ± 0.05 d | 1.04 ± 0.02 b |
| FCR | 1.69 ± 0.07 c | 1.54 ± 0.02 b | 1.48 ± 0.02 b | 1.39 ± 0.07 a | 1.54 ± 0.03 b |
| FR (%) | 1.42 ± 0.02 a | 1.46 ± 0.01 ab | 1.52 ± 0.05 c | 1.54 ± 0.02 c | 1.50 ± 0.01 bc |
| FI (g/fish) | 20.32 ± 0.10 a | 22.51 ± 0.47 b | 24.72 ± 1.44 c | 26.42 ± 0.43 d | 23.75 ± 0.18 c |
| PER (%) | 128.44 ± 5.17 a | 141.13 ± 1.61 b | 146.69 ± 1.60 b | 155.98 ± 7.64 c | 141.18 ± 2.89 b |
| SR (%) | 100 ± 0.00 a | 100 ± 0.00 a | 100 ± 0.00 a | 100 ± 0.00 a | 99.17 ± 1.67 a |
| Items | CAs0 Group | CAs2 Group | CAs3 Group | CAs4 Group | CAs5 Group |
|---|---|---|---|---|---|
| BUN (mmol/L) | 10.19 ± 0.58 b | 9.93 ± 0.47 b | 9.33 ± 0.71 ab | 8.52 ± 0.92 a | 9.52 ± 0.34 ab |
| Ca (mmol/L) | 1.59 ± 0.03 a | 1.71 ± 0.09 b | 1.73 ± 0.11 b | 1.76 ± 0.06 b | 1.70 ± 0.07 ab |
| P (mmol/L) | 4.00 ± 0.29 a | 4.37 ± 0.48 ab | 4.57 ± 0.27 b | 4.71 ± 0.31 b | 4.68 ± 0.08 b |
| TG (mmol/L) | 7.50 ± 0.14 d | 4.86 ± 0.53 b | 4.29 ± 0.08 a | 4.16 ± 0.08 a | 6.36 ± 0.24 c |
| TC (mmol/L) | 30.87 ± 2.23 b | 26.09 ± 3.39 a | 22.58 ± 2.07 a | 22.40 ± 1.15 a | 25.15 ± 3.05 a |
| HDL-C (mmol/L) | 5.26 ± 0.35 a | 6.12 ± 0.66 b | 6.45 ± 0.60 b | 6.40 ± 0.37 b | 5.66 ± 0.48 ab |
| LDL-C (mmol/L) | 8.39 ± 0.97 b | 6.99 ± 0.87 a | 6.04 ± 0.83 a | 5.69 ± 0.91 a | 6.30 ± 0.74 a |
| GPT (U/L) | 7.34 ± 0.24 e | 5.22 ± 0.45 d | 3.72 ± 0.27 b | 1.37 ± 0.54 a | 4.55 ± 0.42 c |
| GOT (U/L) | 14.91 ± 0.58 d | 8.08 ± 0.48 b | 6.37 ± 0.33 a | 6.13 ± 0.83 a | 10.95 ± 1.48 c |
| AKP (U/L) | 3.15 ± 0.47 a | 3.29 ± 0.34 a | 3.32 ± 0.41 a | 4.54 ± 0.40 b | 4.04 ± 0.39 b |
| ACP (U/L) | 7.68 ± 0.59 ab | 7.72 ± 0.19 ab | 8.81 ± 0.48 bc | 9.02 ± 0.94 c | 7.24 ± 1.29 a |
| LZM (U/mL) | 167.59 ± 5.14 a | 204.24 ± 10.88 b | 258.96 ± 7.57 c | 276.06 ± 12.09 d | 215.96 ± 10.16 b |
| C3 (μg/mL) | 2417.33 ± 102.53 a | 2561.25 ± 54.23 b | 2565.33 ± 13.02 b | 2732.04 ± 17.91 c | 2599.58 ± 106.76 b |
| IgM (μg/mL) | 868.75 ± 67.99 a | 1016.97 ± 39.73 b | 1236.36 ± 81.50 c | 1381.47 ± 62.85 d | 1214.36 ± 134.93 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Wu, X.; Zhai, S. Effect of Dietary Compound Acidifiers Supplementation on Growth Performance, Serum Biochemical Parameters, and Body Composition of Juvenile American Eel (Anguilla rostrata). Fishes 2022, 7, 203. https://doi.org/10.3390/fishes7040203
Zhang M, Wu X, Zhai S. Effect of Dietary Compound Acidifiers Supplementation on Growth Performance, Serum Biochemical Parameters, and Body Composition of Juvenile American Eel (Anguilla rostrata). Fishes. 2022; 7(4):203. https://doi.org/10.3390/fishes7040203
Chicago/Turabian StyleZhang, Mingliang, Xinyi Wu, and Shaowei Zhai. 2022. "Effect of Dietary Compound Acidifiers Supplementation on Growth Performance, Serum Biochemical Parameters, and Body Composition of Juvenile American Eel (Anguilla rostrata)" Fishes 7, no. 4: 203. https://doi.org/10.3390/fishes7040203
APA StyleZhang, M., Wu, X., & Zhai, S. (2022). Effect of Dietary Compound Acidifiers Supplementation on Growth Performance, Serum Biochemical Parameters, and Body Composition of Juvenile American Eel (Anguilla rostrata). Fishes, 7(4), 203. https://doi.org/10.3390/fishes7040203






