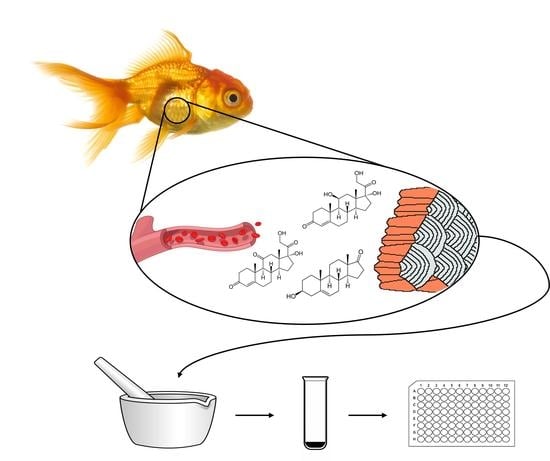
First Look into the Use of Fish Scales as a Medium for Multi-Hormone Stress Analyses
Abstract
:1. Introduction
2. Materials and Methods
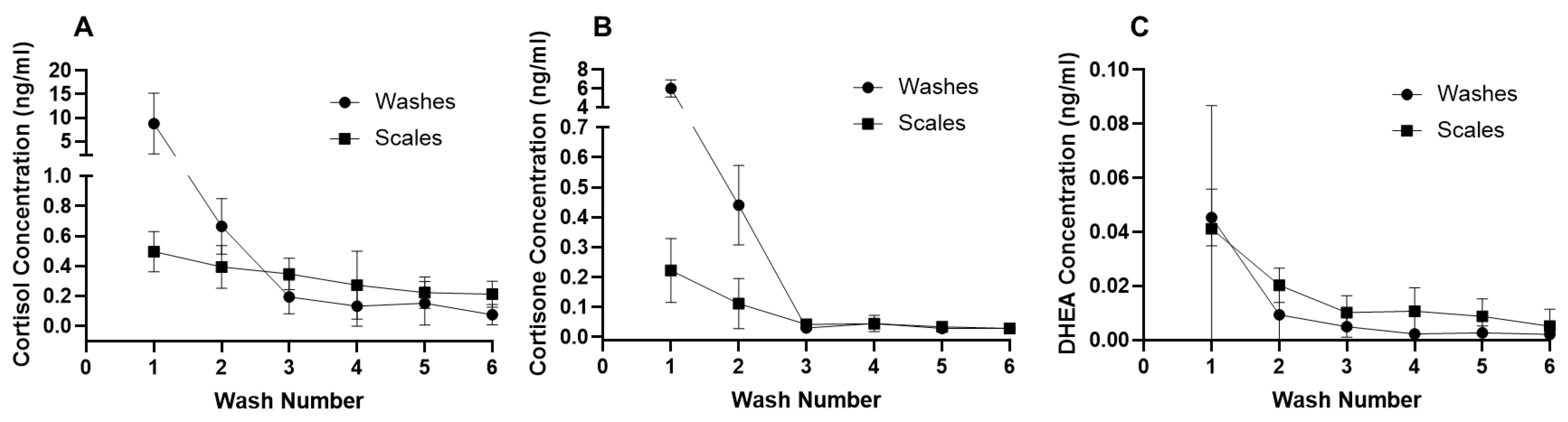
2.1. Wash Protocol Validation
2.2. Stressor Exposure
2.3. Sample Collection
2.4. Hormone Extraction and Quantitation
2.5. Statistical Analyses
3. Results
3.1. Wash Protocol Validation
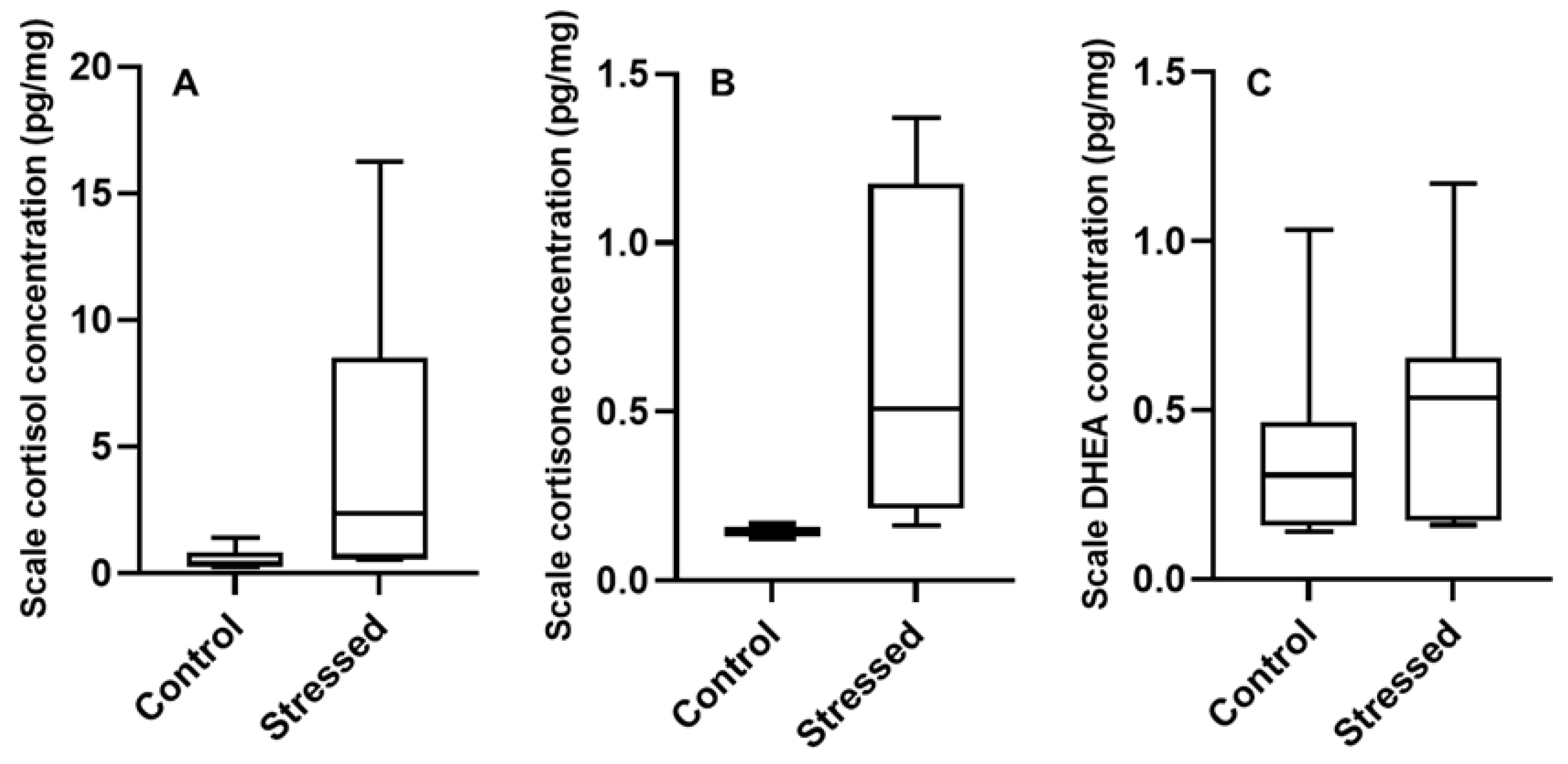
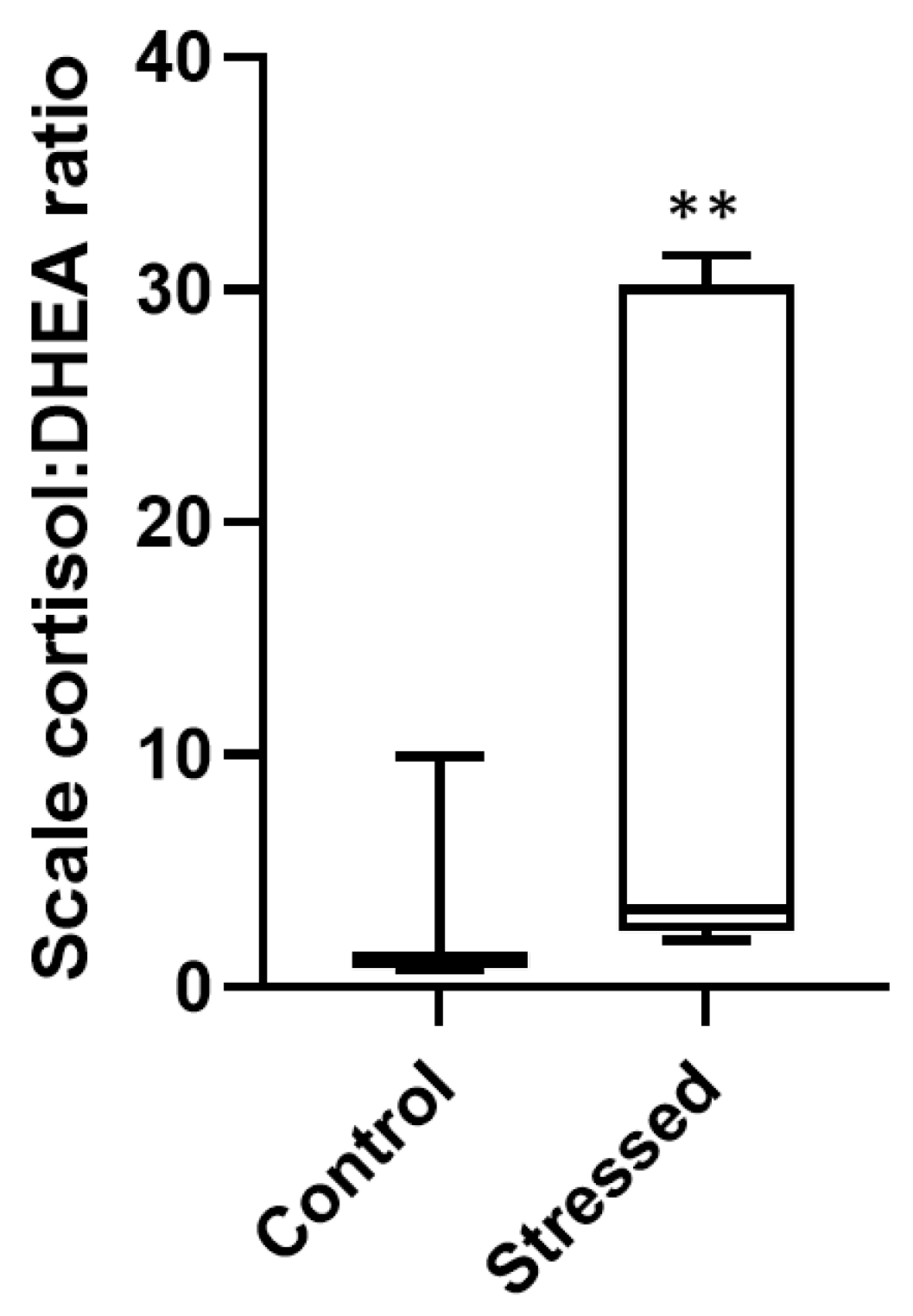
3.2. Scale Hormone Concentrations
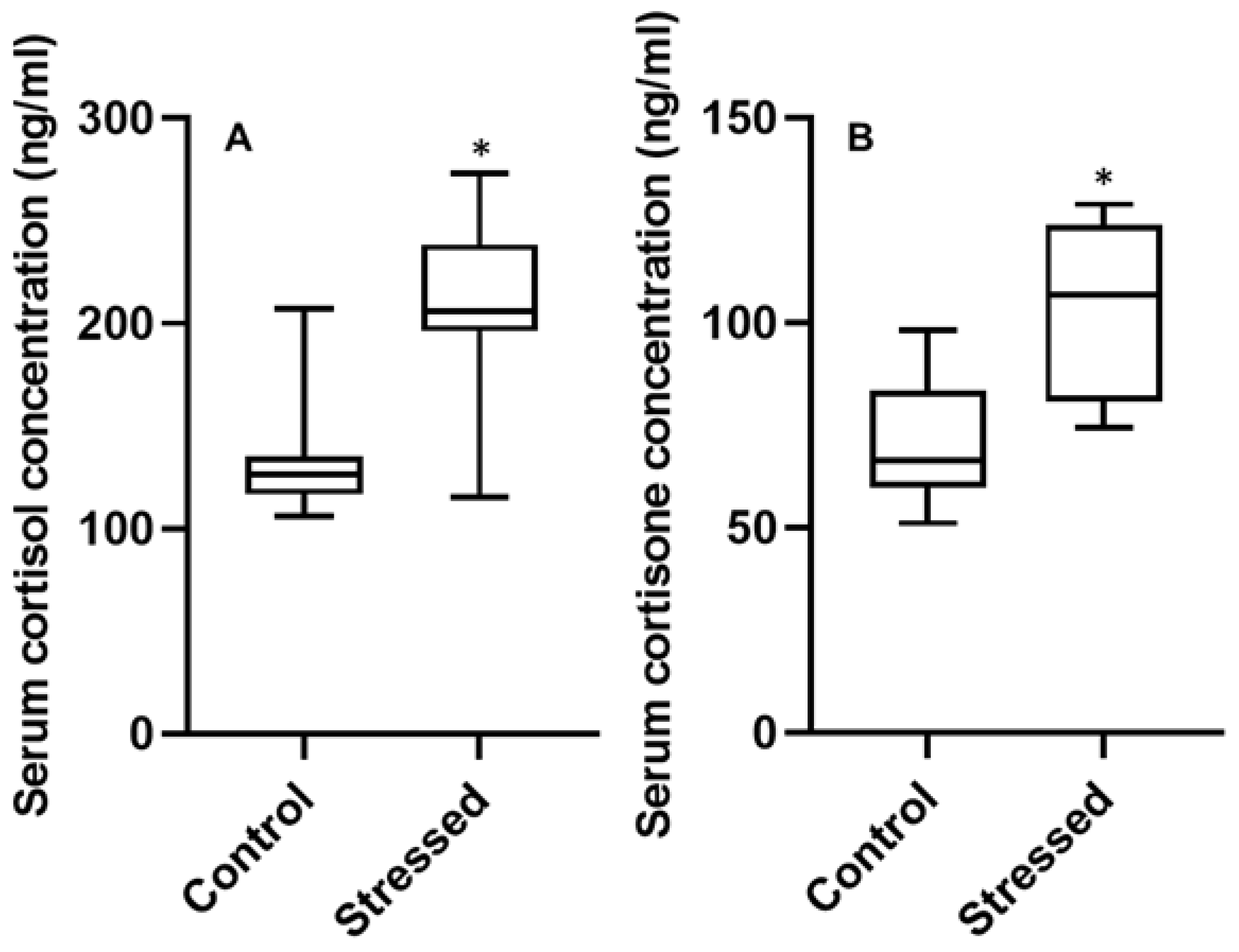
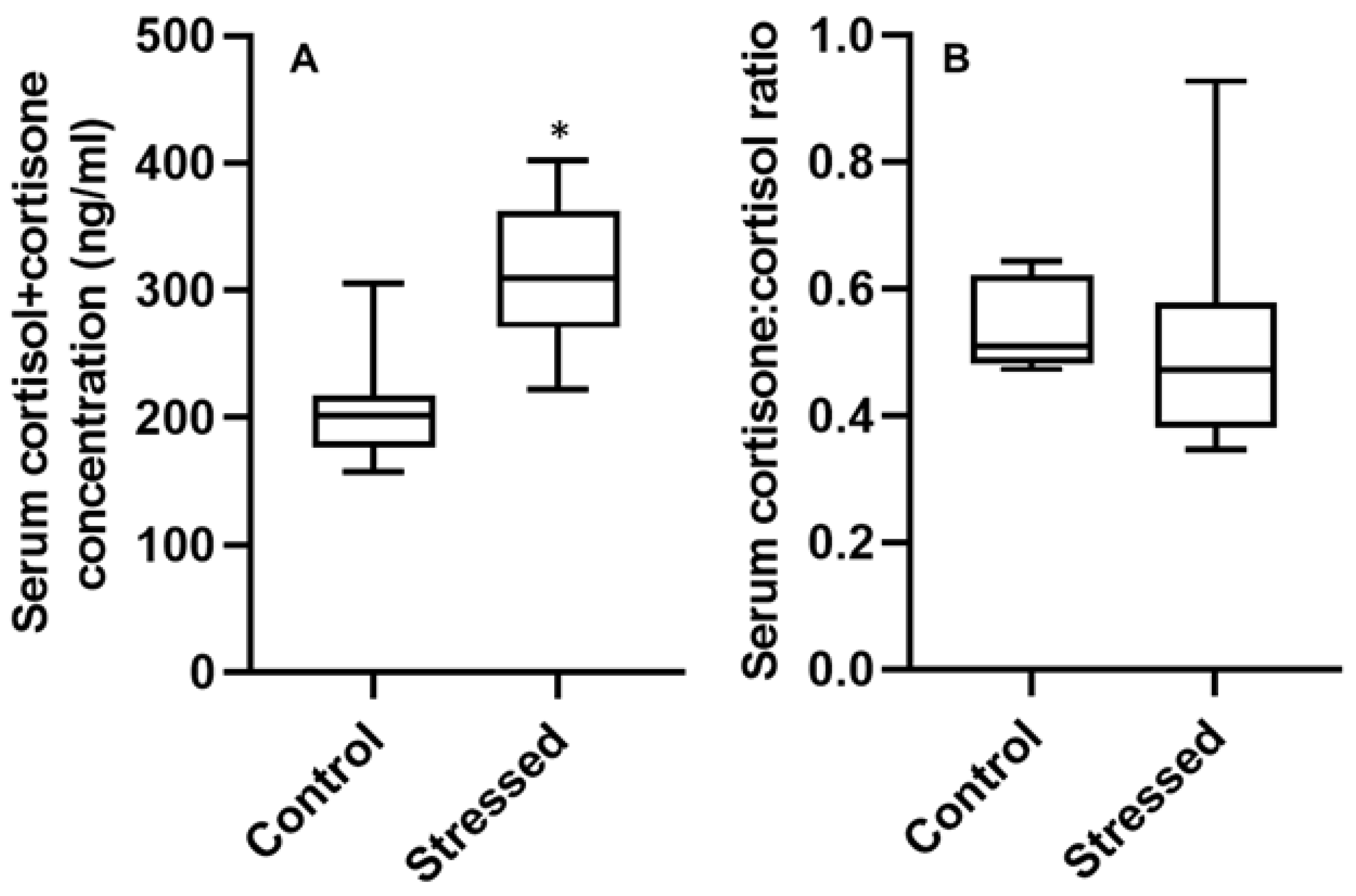
3.3. Serum Hormone Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Ellis, T.; Sanders, M.B.; Scott, A.P. Non-invasive monitoring of steroids in fishes. Vet. Med. Austria 2013, 100, 255–269. [Google Scholar]
- Sheriff, M.J.; Dantzer, B.; Delehanty, B.; Palme, R.; Boonstra, R. Measuring stress in wildlife: Techniques for quantifying glucocorticoids. Oecologia 2011, 166, 869–887. [Google Scholar] [CrossRef] [PubMed]
- Sadoul, B.; Geffroy, B. Measuring cortisol, the major stress hormone in fishes. J. Fish Biol. 2019, 94, 540–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Alacid, L.; Sanahuja, I.; Ordóñez-Grande, B.; Sánchez-Nuño, S.; Viscor, G.; Gisbert, E.; Herrera, M.; Ibarz, A. Skin mucus metabolites in response to physiological challenges: A valuable non-invasive method to study teleost marine species. Sci. Total Environ. 2018, 644, 1323–1335. [Google Scholar] [CrossRef]
- Aerts, J.; Metz, J.R.; Ampe, B.; Decostere, A.; Flik, G.; De Saeger, S. Scales tell a story on the stress history of fish. PLoS ONE 2015, 10, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Laberge, F.; Yin-Liao, I.; Bernier, N.J. Temporal profiles of cortisol accumulation and clearance support scale cortisol content as an indicator of chronic stress in fish. Conserv. Physiol. 2019, 7, coz052. [Google Scholar] [CrossRef] [PubMed]
- Carbajal, A.; Monclús, L.; Tallo-Parra, O.; Sabes-Alsina, M.; Vinyoles, D.; Lopez-Bejar, M. Cortisol detection in fish scales by enzyme immunoassay: Biochemical and methodological validation. J. Appl. Ichthyol. 2018, 34, 967–970. [Google Scholar] [CrossRef] [Green Version]
- Carbajal, A.; Tallo-Parra, O.; Monclús, L.; Vinyoles, D.; Solé, M.; Lacorte, S.; Lopez-Bejar, M. Variation in scale cortisol concentrations of a wild freshwater fish: Habitat quality or seasonal influences? Gen. Comp. Endocrinol. 2019, 275, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Hanke, I.; Hassenrück, C.; Ampe, B.; Kunzmann, A.; Gärdes, A.; Aerts, J. Chronic stress under commercial aquaculture conditions: Scale cortisol to identify and quantify potential stressors in milkfish (Chanos chanos) mariculture. Aquaculture 2020, 526, 735352. [Google Scholar] [CrossRef]
- Hanke, I.; Ampe, B.; Kunzmann, A.; Gärdes, A.; Aerts, J. Thermal stress response of juvenile milkfish (Chanos chanos) quantified by ontogenetic and regenerated scale cortisol. Aquaculture 2019, 500, 24–30. [Google Scholar] [CrossRef]
- Lorenzi, V.; Earley, R.; Rodgers, E.; Pepper, D.; Grober, M. Diurnal patterns and sex differences in cortisol, 11-ketotestosterone, testosterone, and 17b-estradiol in the bluebanded goby (Lythrypnus dalli). Gen. Comp. Endocrinol. 2008, 155, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Faught, E.; Aluru, N.; Vijayan, M.M. The Molecular Stress Response; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 35, ISBN 9780128027288. [Google Scholar]
- Gorissen, M.; Flik, G. The Endocrinology of the Stress Response in Fish: An Adaptation-Physiological View; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 35, ISBN 9780128027288. [Google Scholar]
- Weirup, L.; Schulz, C.; Seibel, H.; Aerts, J. Scale cortisol is positively correlated to fin injuries in rainbow trout (Oncorhynchus mykiss) reared in commercial flow through systems. Aquaculture 2021, 543, 736924. [Google Scholar] [CrossRef]
- Azevedo, A.; Wauters, J.; Kirschbaum, C.; Serra, R.; Rivas, A.; Jewgenow, K. Sex steroids and glucocorticoid ratios in Iberian lynx hair. Conserv. Physiol. 2020, 8, coaa075. [Google Scholar] [CrossRef] [PubMed]
- Sollberger, S.; Ehlert, U. How to use and interpret hormone ratios. Psychoneuroendocrinology 2016, 63, 385–397. [Google Scholar] [CrossRef]
- Kamin, H.S.; Kertes, D.A. Cortisol and DHEA in development and psychopathology. Horm. Behav. 2017, 89, 69–85. [Google Scholar] [CrossRef]
- Maninger, N.; Wolkowitz, O.M.; Reus, V.I.; Epel, E.S.; Mellon, S.H. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front. Neuroendocrinol. 2009, 30, 65–91. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Cardounel, A.; Gursoy, E.; Anderson, P.; Kalimi, M. Anti-stress effects of dehydroepiandrosterone: Protection of rats against repeated immobilization stress-induced weight loss, glucocorticoid receptor production, and lipid peroxidation. Biochem. Pharm. 2000, 59, 753–762. [Google Scholar] [CrossRef]
- Kalimi, M.; Shafagoj, Y.; Loria, R.; Padgett, D.; Regelson, W. Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA). Mol. Cell. Biochem. 1994, 131, 99–104. [Google Scholar] [CrossRef]
- Mueller, J.W.; Gilligan, L.C.; Idkowiak, J.; Arlt, W.; Foster, P.A. The Regulation of Steroid Action by Sulfation and Desulfation. Endocr. Rev. 2015, 36, 526–563. [Google Scholar] [CrossRef] [PubMed]
- Wolkowitz, O.M.; Epel, E.S.; Reus, V.I. Stress hormone-related psychopathology: Pathophysiological and treatment implications. World J. Biol. Psychiatry 2001, 2, 115–143. [Google Scholar] [CrossRef]
- Balazs, Z.; Schweizer, R.A.S.; Frey, F.J.; Rohner-Jeanrenaud, F.; Odermatt, A. DHEA induces 11β-HSD2 by acting on CCAAT/enhancer-binding proteins. J. Am. Soc. Nephrol. 2008, 19, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Li, J.; Zhang, J.; Xing, X.; Gao, W.; Lu, Z.; Deng, H. Simultaneous determination of hair cortisol, cortisone and DHEAS with liquid chromatography–electrospray ionization-tandem mass spectrometry in negative mode. J. Chromatogr. B 2013, 929, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, M.; Nakamura, I.; Young, G. Enzymatic activity of 11β-hydroxysteroid dehydrogenase in rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2003, 28, 197–198. [Google Scholar] [CrossRef] [Green Version]
- Raul, J.S.; Cirimele, V.; Ludes, B.; Kintz, P. Detection of physiological concentrations of cortisol and cortisone in human hair. Clin. Biochem. 2004, 37, 1105–1111. [Google Scholar] [CrossRef]
- Davison, B.; Singh, G.R.; McFarlane, J. Hair cortisol and cortisone as markers of stress in indigenous and non-indigenous young adults. Stress 2019, 22, 210–220. [Google Scholar] [CrossRef]
- Musana, J.W.; Cohen, C.R.; Kuppermann, M.; Gerona, R.; Wanyoro, A.; Aguilar, D.; Santos, N.; Temmerman, M.; Weiss, S.J. Association of differential symptoms of stress to hair cortisol and cortisone concentrations among pregnant women in Kenya. Stress 2020, 23, 556–566. [Google Scholar] [CrossRef]
- Kapoor, A.; Schultz-Darken, N.; Ziegler, T.E. Radiolabel validation of cortisol in the hair of rhesus monkeys. Psychoneuroendocrinology 2018, 97, 190–195. [Google Scholar] [CrossRef]
- Sopinka, N.M.; Donaldson, M.R.; O′Connor, C.M.; Suski, C.D.; Cooke, S.J. Stress Indicators in Fish; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 35, ISBN 9780128027288. [Google Scholar]
- Rege, J.; Garber, S.; Conley, A.J.; Elsey, R.M.; Turcu, A.F.; Auchus, R.J.; Rainey, W.E. Circulating 11-oxygenated androgens across species. J. Steroid Biochem. Mol. Biol. 2019, 190, 242–249. [Google Scholar] [CrossRef]
- Gauberg, J.; Kolosov, D.; Kelly, S.P. Claudin tight junction proteins in rainbow trout (Oncorhynchus mykiss) skin: Spatial response to elevated cortisol levels. Gen. Comp. Endocrinol. 2017, 240, 214–226. [Google Scholar] [CrossRef]
- Ito, N.; Ito, T.; Paus, R. The human hair follicle has established a fully functional peripheral equivalent of the hypothamalic-pituitary-adrenal-axis (HPA). Exp. Dermatol. 2005, 14, 158. [Google Scholar] [CrossRef]
- Hein, A.; Baumgartner, K.; von Fersen, L.; Bechshoft, T.; Woelfing, B.; Kirschbaum, C.; Mastromonaco, G.; Greenwood, A.D.; Siebert, U. Analysis of hair steroid hormones in polar bears (Ursus maritimus) via liquid chromatography–tandem mass spectrometry: Comparison with two immunoassays and application for longitudinal monitoring in zoos. Gen. Comp. Endocrinol. 2021, 310, 113837. [Google Scholar] [CrossRef]
- Mugiya, Y.; Watabe, N. Studies on fish scale formation and resorption-II. Effect of estradiol on calcium homeostasis and skeletal tissue resorption in the goldfish, Carassius auratus, and the killifish, Fundulus heteroclitus. Comp. Biochem. Physiol. A—Physiol. 1977, 57, 197–202. [Google Scholar] [CrossRef]
- Ozaki, Y.; Higuchi, M.; Miura, C.; Yamaguchi, S.; Tozawa, Y.; Miura, T. Roles of 11β-hydroxysteroid dehydrogenase in fish spermatogenesis. Endocrinology 2006, 147, 5139–5146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokarz, J.; Mindnich, R.; Norton, W.; Möller, G.; Hrabé de Angelis, M.; Adamski, J. Discovery of a novel enzyme mediating glucocorticoid catabolism in fish: 20beta-Hydroxysteroid dehydrogenase type 2. Mol. Cell Endocrinol. 2012, 349, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, M.; Nakamura, I.; Young, G. 11β-Hydroxysteroid dehydrogenase complementary deoxyribonucleic acid in rainbow trout: Cloning, sites of expression, and seasonal changes in gonads. Endocrinology 2003, 144, 2534–2545. [Google Scholar] [CrossRef] [Green Version]
- Staufenbiel, S.M.; Penninx, B.W.J.H.; de Rijke, Y.B.; van den Akker, E.L.T.; van Rossum, E.F.C. Determinants of hair cortisol and hair cortisone concentrations in adults. Psychoneuroendocrinology 2015, 60, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kennedy, E.K.C.; Janz, D.M. First Look into the Use of Fish Scales as a Medium for Multi-Hormone Stress Analyses. Fishes 2022, 7, 145. https://doi.org/10.3390/fishes7040145
Kennedy EKC, Janz DM. First Look into the Use of Fish Scales as a Medium for Multi-Hormone Stress Analyses. Fishes. 2022; 7(4):145. https://doi.org/10.3390/fishes7040145
Chicago/Turabian StyleKennedy, Emily K. C., and David M. Janz. 2022. "First Look into the Use of Fish Scales as a Medium for Multi-Hormone Stress Analyses" Fishes 7, no. 4: 145. https://doi.org/10.3390/fishes7040145
APA StyleKennedy, E. K. C., & Janz, D. M. (2022). First Look into the Use of Fish Scales as a Medium for Multi-Hormone Stress Analyses. Fishes, 7(4), 145. https://doi.org/10.3390/fishes7040145