Revealing Population Connectivity of the Estuarine Tapertail Anchovy Coilia nasus in the Changjiang River Estuary and Its Adjacent Waters Using Otolith Microchemistry
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Experimental Fish
2.3. Elemental Analysis
2.4. Stable Isotope Analysis
2.5. Statistical Analysis
3. Results
3.1. Elemental Ratios in the Otolith Nucleus Regions of C. nasus
3.2. Stable Isotopic Ratios in the Otolith Nucleus Regions of C. nasus
3.3. Multivariate Statistics Based on Elemental and Stable Isotopic Ratios
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avigliano, E.; Carvalho, B.; Velasco, G.; Tripodi, P.; Vianna, M.; Volpedo, A.V. Nursery areas and connectivity of the adult anadromous catfish (Genidens barbus) revealed by otolith-core microchemistry in the south-western Atlantic Ocean. Mar. Freshw. Res. 2017, 68, 931–940. [Google Scholar] [CrossRef]
- Rohtla, M.; Matetski, L.; Svirgsden, R.; Kesler, M.; Taal, I.; Saura, A.; Vaittinen, M.; Vetemaa, M. Do sea trout Salmo trutta parr surveys monitor the densities of anadromous or resident maternal origin parr, or both? Fish. Manag. Ecol. 2017, 24, 156–162. [Google Scholar] [CrossRef]
- Koeberle, A.L.; Arismendi, I.; Crittenden, W.; Di Prinzio, C.; Gomez-Uchida, D.; Noakes, D.L.G.; Richardson, S. Otolith shape as a classification tool for Chinook salmon (Oncorhynchus tshawytscha) discrimination in native and introduced systems. Can. J. Fish. Aquat. Sci. 2020, 77, 1172–1188. [Google Scholar] [CrossRef]
- Beacham, T.D.; Wallace, C.G.; Jonsen, K.; McIntosh, B.; Candy, J.R.; Horst, K.; Lynch, C.; Willis, D.; Luedke, W.; Kearey, L.; et al. Parentage-based tagging combined with genetic stock identification is a cost-effective and viable replacement for coded-wire tagging in large-scale assessments of marine Chinook salmon fisheries in British Columbia, Canada. Evol. Appl. 2021, 14, 1365–1389. [Google Scholar] [CrossRef] [PubMed]
- McDowall, R.M. Diadromy, diversity and divergence: Implications for speciation processes in fishes. Fish Fish. 2001, 2, 278–285. [Google Scholar] [CrossRef]
- Torniainen, J.; Vuorinen, P.J.; Jones, R.I.; Keinänen, M.; Palm, S.; Vuori, K.A.M.; Kiljunen, M. Migratory connectivity of two Baltic Sea salmon populations: Retrospective analysis using stable isotopes of scales. ICES J. Mar. Sci. 2014, 71, 336–344. [Google Scholar] [CrossRef]
- Alshwairikh, Y.A.; Kroeze, S.L.; Olsson, J.; Stephens-Cardenas, S.A.; Swain, W.L.; Waits, L.P.; Horn, R.L.; Narum, S.R.; Seaborn, T. Influence of environmental conditions at spawning sites and migration routes on adaptive variation and population connectivity in Chinook salmon. Ecol. Evol. 2021, 11, 16890–16908. [Google Scholar] [CrossRef]
- Arai, T. Migration ecology in the freshwater eels of the genus Anguilla Schrank, 1798. Trop. Ecol. 2022, 6, e05176. [Google Scholar] [CrossRef]
- Miller, M.J.; Yoshinaga, T.; Aoyama, J.; Otake, T.; Mochioka, N.; Kurogi, H.; Tsukamoto, K. Offshore spawning of Conger myriaster in the western North Pacific: Evidence for convergent migration strategies of anguilliform eels in the Atlantic and Pacific. Naturwissenschaften 2011, 98, 537–543. [Google Scholar] [CrossRef]
- Thorrold, S.R.; Latkoczy, C.; Swart, P.K.; Jones, C.M. Natal homing in a marine fish metapopulation. Science 2001, 291, 297–299. [Google Scholar] [CrossRef]
- Schulz Mirbach, T.; Ladich, F.; Plath, M.; Heß, M. Enigmatic ear stones: What we know about the functional role and evolution of fish otoliths. Biol. Rev. 2019, 94, 457–482. [Google Scholar] [CrossRef] [PubMed]
- Burton, M.L.; Potts, J.C.; Ostrowski, A.D.; Shertzer, K.W. Age, growth, and natural mortality of Graysby, Cephalophilis cruentata, from the southeastern United States. Fishes 2019, 4, 36. [Google Scholar] [CrossRef]
- Burton, M.L.; Potts, J.C.; Ostrowski, A.D. Preliminary estimates of age, growth and natural mortality of margate, Haemulon album, and black margate, Anisotremus surinamensis, from the Southeastern United States. Fishes 2019, 4, 44. [Google Scholar] [CrossRef]
- Geffen, A.J.; Morales-Nin, B.; Gillanders, B.M. Fish otoliths as indicators in ecosystem based management: Results of the 5th International Otolith Symposium (IOS2014). Mar. Freshw. Res. 2016, 67, i–iv. [Google Scholar] [CrossRef]
- D’Iglio, C.; Albano, M.; Famulari, S.; Savoca, S.; Panarello, G.; Di Paola, D.; Perdichizzi, A.; Rinelli, P.; Lanteri, G.; Spano, N.; et al. Intra- and interspecific variability among congeneric Pagellus otoliths. Sci. Rep. 2021, 11, 16315. [Google Scholar] [CrossRef]
- D’Iglio, C.; Natale, S.; Albano, M.; Savoca, S.; Famulari, S.; Gervasi, C.; Lanteri, G.; Panarello, G.; Spanò, N.; Capillo, G. Otolith analyses highlight morpho-functional differences of three species of mullet (Mugilidae) from transitional water. Sustainability 2022, 14, 398. [Google Scholar] [CrossRef]
- Mitsui, S.; Strüssmann, C.A.; Yokota, M.; Yamamoto, Y. Comparative otolith morphology and species identification of clupeids from Japan. Ichthyol. Res. 2020, 67, 502–513. [Google Scholar] [CrossRef]
- D’Iglio, C.; Albano, M.; Tiralongo, F.; Famulari, S.; Rinelli, P.; Savoca, S.; Spanò, N.; Capillo, G. Biological and ecological aspects of the blackmouth catshark (Galeus melastomus Rafinesque, 1810) in the Southern Tyrrhenian Sea. J. Mar. Sci. Eng. 2021, 9, 967. [Google Scholar] [CrossRef]
- Polito, M.J.; Trivelpiece, W.Z.; Karnovsky, N.J.; Ng, E.; Patterson, W.P.; Emslie, S.D. Integrating stomach content and stable isotope analyses to quantify the diets of pygoscelid penguins. PLoS ONE 2011, 6, e26642. [Google Scholar] [CrossRef]
- Chino, N.; Arai, T. Migratory history of the fourspine sculpin Rheopresbe kazika, a national monument species in Japan. Thalassa 2020, 36, 395–398. [Google Scholar] [CrossRef]
- Tran, N.T.; Labonne, M.; Chung, M.T.; Wang, C.H.; Huang, K.F.; Durand, J.D.; Grudpan, C.; Chan, B.; Hoang, H.D.; Panfili, J. Natal origin and migration pathways of Mekong catfish (Pangasius krempfi) using strontium isotopes and trace element concentrations in environmental water and otoliths. PLoS ONE 2021, 16, e0252769. [Google Scholar] [CrossRef] [PubMed]
- Rohtla, M.; Matetski, L.; Taal, I.; Svirgsden, R.; Kesler, M.; Paiste, P.; Vetemaa, M. Quantifying an overlooked aspect of partial migration using otolith microchemistry. J. Fish Biol. 2020, 97, 1582–1585. [Google Scholar] [CrossRef] [PubMed]
- Avigliano, E.; Pisonero, J.; Mendez, A.; Tombari, A.; Volpedo, A.V. Habitat use of the amphidromous catfish Genidens barbus: First insights at its southern distribution limit. N. Z. J. Mar. Fresh. 2021, 263, 107637. [Google Scholar] [CrossRef]
- Xiong, Y.; Yang, J.; Jiang, T.; Liu, H.B.; Zhong, X.M.; Xiong, Y.; Yang, J.; Jiang, T.; Liu, H.; Zhong, X.; et al. Temporal stability in the otolith Sr:Ca ratio of the yellow croaker, Larimichthys polyactis (Actinopterygii, Perciformes, Sciaenidae), from the southern Yellow Sea. Acta Ichthyol. Piscat. 2021, 51, 59–65. [Google Scholar] [CrossRef]
- Nakamura, M.; Yoneda, M.; Ishimura, T.; Shirai, K.; Tamamura, M.; Nishida, K. Temperature dependency equation for chub mackerel (Scomber japonicus) identified by a laboratory rearing experiment and microscale analysis. Mar. Freshw. Res. 2020, 71, 1384–1389. [Google Scholar] [CrossRef]
- Sanchez, P.; Rooker, J.R.; Zapp Sluis, M.; Pinsky, J.; Dance, M.; Falterman, B.; Allman, R. Application of otolith chemistry at multiple life history stages to assess population structure of Warsaw grouper in the Gulf of Mexico. Mar. Ecol. Prog. Ser. 2020, 651, 111–123. [Google Scholar] [CrossRef]
- Pereira, N.S.; Sial, A.N.; Pinheiro, P.B.; Freitas, F.L.; Silva, A.M.C. Carbon and oxygen stable isotopes of freshwater fish otoliths from the São Francisco River, northeastern Brazil. An. Acad. Bras. Cienc. 2021, 93, e20191050. [Google Scholar] [CrossRef]
- Shiao, J.C.; Hsu, J.; Cheng, C.C.; Tsai, W.Y.; Lu, H.; Tanaka, Y.; Wang, P. Contribution rates of different spawning and feeding grounds to adult Pacific bluefin tuna (Thunnus orientalis) in the northwestern Pacific Ocean. Deep Sea Res. I 2021, 169, 103453. [Google Scholar] [CrossRef]
- Tripp, A.; Murphy, H.M.; Davoren, G.K. Otolith chemistry reveals natal region of larval capelin in coastal Newfoundland, Canada. Front. Mar. Sci. 2020, 7, 258. [Google Scholar] [CrossRef]
- Campana, S.E.; Thorrold, S.R. Otoliths, increments, and elements: Keys to a comprehensive understanding of fish populations? Can. J. Fish. Aquat. Sci. 2001, 58, 30–38. [Google Scholar] [CrossRef]
- Zhang, Z.; Moksness, E. A chemical way of thinning otoliths of adult Atlantic herring (Clupea harengus) to expose the microstructure in the nucleus region. ICES J. Mar. Sci. 1993, 50, 213–217. [Google Scholar] [CrossRef]
- Campana, S.E.; Fowler, A.J. Otolith elemental fingerprinting for stock identification of Atlantic Cod (Gadus morhua) using laser ablation ICPMS. Can. J. Fish. Aquat. Sci. 1994, 51, 1942–1950. [Google Scholar] [CrossRef]
- Itoh, T.; Tsuji, S. Age and growth of juvenile southern bluefin tuna Thunnus maccoyii based on otolith microstructure. Fish. Sci. 1996, 62, 892–896. [Google Scholar] [CrossRef]
- Busbridge, T.A.J.; Marshall, C.T.; Arkhipkin, A.I.; Shcherbich, Z.; Marriott, A.L.; Brickle, P. Can otolith microstructure and elemental fingerprints elucidate the early life history stages of the gadoid southern blue whiting (Micromesistius australis australis)? Fish. Res. 2020, 228, 105572. [Google Scholar] [CrossRef]
- Brickle, P.; Schuchert, P.C.; Arkhipkin, A.I.; Reid, M.R.; Randhawa, H.S. Otolith trace elemental analyses of south American austral hake, Merluccius australis (Hutton, 1872) indicates complex salinity structuring on their spawning/larval grounds. PLoS ONE 2016, 11, e0145479. [Google Scholar] [CrossRef]
- Mu, X.X.; Zhang, C.; Zhang, C.L.; Yang, J.; Ren, Y. Age-structured otolith chemistry profiles revealing the migration of Conger myriaster in China Seas. Fish. Res. 2021, 239, 105938. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chang, P.H.; Shih, C.H.; Shiao, J.C.; Tzeng, T.D.; Chang, W.C. The impact of religious release fish on conservation. Glob. Ecol. Conser. 2021, 27, e01556. [Google Scholar] [CrossRef]
- Lazartigues, A.; Girard, C.; Brodeur, P.; Lecomte, F.; Mingelbier, M.; Sirois, P. Otolith microchemistry to identify sources of larval yellow perch in a fluvial lake: An approach towards freshwater fish management. Can. J. Fish. Aquat. Sci. 2018, 75, 474–487. [Google Scholar] [CrossRef]
- Fitzpatrick, R.M.; Winkelman, D.L.; Johnson, B.M. Using isotopic data to evaluate Esox lucius (Linnaeus, 1758) natal origins in a hydrologically complex river basin. Fishes 2021, 6, 67. [Google Scholar] [CrossRef]
- Izzo, C.; Doubleday, Z.A.; Gillanders, B.M. Where do elements bind within the otoliths of fish? Mar. Freshw. Res. 2016, 67, 1072–1076. [Google Scholar] [CrossRef]
- Thomas, O.R.B.; Ganio, K.; Roberts, B.R.; Swearer, S.E. Trace element-protein interactions in endolymph from the inner ear of fish: Implications for environmental reconstructions using fish otolith chemistry. Metallomics 2017, 9, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Hüssy, K.; Limburg, K.E.; De Pontual, H.; Thomas, O.R.B.; Cook, P.K.; Heimbrand, Y.; Blass, M.; Sturrock, A.M. Trace element patterns in otoliths: The role of biomineralization. Rev. Fish. Sci. Aquac. 2020, 29, 1–33. [Google Scholar] [CrossRef]
- Jessop, B.M.; Wang, C.H.; Tzeng, W.N.; You, C.F.; Shiao, J.C.; Lin, S.H. Otolith Sr:Ca and Ba:Ca may give inconsistent indications of estuarine habitat use for American eels (Anguilla rostrata). Environ. Biol. Fish. 2012, 93, 193–207. [Google Scholar] [CrossRef]
- Menezes, R.; Moura, P.E.S.; Santos, A.C.A.; Moraes, L.E.; Condini, M.V.; Rosa, R.S.; Albuquerque, C.Q. Habitat use plasticity by the dog snapper (Lutjanus jocu) across the Abrolhos Bank shelf, eastern Brazil, inferred from otolith chemistry. Estuar. Coast. Shelf Sci. 2021, 263, 107637. [Google Scholar] [CrossRef]
- Yuan, C.M.; Qin, A.L.; Liu, R.H.; Lin, J.B. On the classification of the anchovies, Coilia, from the lower Yangtze River and the southeast coast of China. J. Nanjing Univ. (Nat. Sci.) 1980, 3, 67–77, (In Chinese with English Abstract). [Google Scholar]
- Khumbanyiwa, D.D.; Li, M.M.; Jiang, T.; Liu, H.B.; Yang, J. Unraveling habitat use of Coilia nasus from Qiantang River of China by otolith microchemistry. Reg. Stud. Mar. Sci. 2018, 18, 122–128. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, H.B.; Shen, X.Q.; Shimasaki, Y.; Ohshima, Y.; Yang, J. Life history variations among different populations of Coilia nasus along the Chinese Coast, inferred from otolith microchemistry. J. Fac. Agric. Kyushu Univ. 2014, 59, 383–389. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, H.B.; Lu, M.J.; Chen, T.T.; Yang, J. A possible connectivity among estuarine tapertail anchovy (Coilia nasus) populations in the Yangtze River, Yellow Sea, and Poyang Lake. Estuar. Coast. 2016, 39, 1762–1768. [Google Scholar] [CrossRef]
- Moura, A.; Dias, E.; López, R.; Antunes, C. Regional population structure of the European eel at the southern limit of its distribution revealed by otolith shape signature. Fishes 2022, 7, 135. [Google Scholar] [CrossRef]
- Li, Y.; Xie, S.; Li, Z.; Gong, W.; He, W. Gonad development of an anadromous fish Coilia ectenes (Engraulidae) in lower reach of Yangtze River, China. Fish. Sci. 2007, 73, 1224–1230. [Google Scholar] [CrossRef]
- Xu, G.C.; Nie, Z.J.; Zhang, C.X.; Wei, G.L.; Xu, P.; Gu, R.B. Histological studies on testis development of Coilia nasus under artificial farming conditions. J. Huazhong Agric. Univ. 2012, 31, 247–252, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Ge, K.K.; Zhong, J.S. Daily-age structure and growth characteristics of Coilia nasus larvae and juveniles in the surf zone of Yangtze River Estuary. Acta Hydro. Sin. 2010, 34, 716–721. [Google Scholar] [CrossRef]
- Dou, S.Z.; Amano, Y.; Yu, X.; Cao, L.; Kotaro, S.; Otake, T.; Tsukamoto, K. Elemental signature in otolith nuclei for stock discrimination of anadromous tapertail anchovy (Coilia nasus) using laser ablation ICPMS. Environ. Biol. Fish. 2012, 95, 431–443. [Google Scholar] [CrossRef]
- Vesanto, J. Neural network tool for data mining: SOM Toolbox. In Proceedings of the 5th International Symposium on Tool Environments and Development Methods for Intelligent Systems (TOOLMET2000), Oulu, Finland, 13–14 April 2000; Oulun Yliopistopaino: Oulu, Finland, 2000; pp. 184–196. [Google Scholar]
- Tsai, W.P.; Huang, S.P.; Cheng, S.T.; Shao, K.T.; Chang, F.J. A data-mining framework for exploring the multi-relation between fish species and water quality through self-organizing map. Sci. Total Environ. 2017, 579, 474–483. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.H.; Lai, Z.N.; Li, Y.F.; Dauta, A.; Lek, S. Patterning and predicting phytoplankton assemblages in a large subtropical river. Fund. Appl. Limnol. 2014, 185, 263–279. [Google Scholar] [CrossRef]
- Schaffler, J.J.; Young, S.P.; Herrington, S.; Ingram, T.; Tannehill, J. Otolith chemistry to determine within-river origins of Alabama Shad in the Apalachicola-Chattahoochee-Flint River basin. Trans. Am. Fish. Soc. 2015, 144, 1–10. [Google Scholar] [CrossRef]
- Aschenbrenner, A.; Ferreira, B.P.; Rooker, J.R. Spatial and temporal variability in the otolith chemistry of the Brazilian snapper Lutjanus alexandrei from estuarine and coastal environments. J. Fish Biol. 2016, 89, 753–769. [Google Scholar] [CrossRef] [PubMed]
- Bouchoucha, M.; Pécheyran, C.; Gonzalez, J.L.; Lenfant, P.; Darnaude, A.M. Otolith fingerprints as natural tags to identify juvenile fish life in ports. Estuar. Coast. Shelf Sci. 2018, 212, 210–218. [Google Scholar] [CrossRef]
- Jiang, T.; Yang, J.; Liu, H.; Shen, X.Q. Life history of Coilia nasus from the Yellow Sea inferred from otolith Sr:Ca ratios. Environ. Biol. Fish. 2012, 95, 503–508. [Google Scholar] [CrossRef]
- Hane, Y.; Kimura, S.; Yokoyama, Y.; Miyairi, Y.; Ushikubo, T.; Ishimura, T.; Ogawa, N.; Aono, T.; Nishida, K. Reconstruction of temperature experienced by Pacific bluefin tuna Thunnus orientalis larvae using SIMS and microvolume CF-IRMS otolith oxygen isotope analyses. Mar. Ecol. Prog. Ser. 2020, 649, 175–188. [Google Scholar] [CrossRef]
- Yuan, C.M.; Qin, A.L. Ecological habits and distribution of Coilia along the Chinese Coast and its changes of output. Mar. Sci. 1984, 5, 35–37, (In Chinese with English Abstract). [Google Scholar]
- Martino, J.C.; Doubleday, Z.A.; Chung, M.T.; Gillanders, B.M. Experimental support towards a metabolic proxy in fish using otolith carbon isotopes. J. Exp. Biol. 2020, 223, jeb217091. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.T.; Trueman, C.N.; Godiksen, J.A.; Grønkjær, P. Otolith δ13C values as a metabolic proxy: Approaches and mechanical underpinnings. Mar. Freshw. Res. 2019, 70, 1747. [Google Scholar] [CrossRef]
- Elsdon, T.S.; Ayvazian, S.; McMahon, K.W.; Thorrold, S.R. Experimental evaluation of stable isotope fractionation in fish muscle and otoliths. Mar. Ecol. Prog. Ser. 2010, 408, 195–205. [Google Scholar] [CrossRef]
- Von Biela, V.R.; Newsome, S.D.; Zimmerman, C.E. Examining the utility of bulk otolith δ13C to describe diet in wild-caught black rockfish Sebastes melanops. Aquat. Biol. 2015, 23, 201–208. [Google Scholar] [CrossRef][Green Version]
- Dittman, A.H.; Quinn, T.P. Homing in Pacific salmon: Mechanisms and ecological basis. J. Exp. Biol. 1996, 199, 83–91. [Google Scholar] [CrossRef]
- Jiang, T.; Yang, J.; Lu, M.J.; Liu, H.B.; Chen, T.T.; Gao, Y.W. Discovery of a spawning area for anadromous Coilia nasus temminck et schlegel, 1846 in Poyang Lake, China. J. Appl. Ichthyol. 2017, 33, 189–192. [Google Scholar] [CrossRef]
- Brennan, S.R.; Zimmerman, C.E.; Fernandez, D.P.; Cerling, T.E.; Mcphee, M.V.; Wooller, M.J. Strontium isotopes delineate fine-scale natal origins and migration histories of Pacific salmon. Sci. Adv. 2015, 1, e1400124. [Google Scholar] [CrossRef]
- Yano, K.; Nakamura, A. Observations on the effect of visual and olfactory ablation on the swimming behavior of migrating adult chum salmon, Oncorhynchus keta. Jpn. J. Ichthyol. 1992, 39, 67–83. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, L.; Tang, W.; Wang, X.; Wang, C. Identification of olfactory receptor genes in the Japanese grenadier anchovy Coilia nasus. Genes Genom. 2017, 39, 521–532. [Google Scholar] [CrossRef]
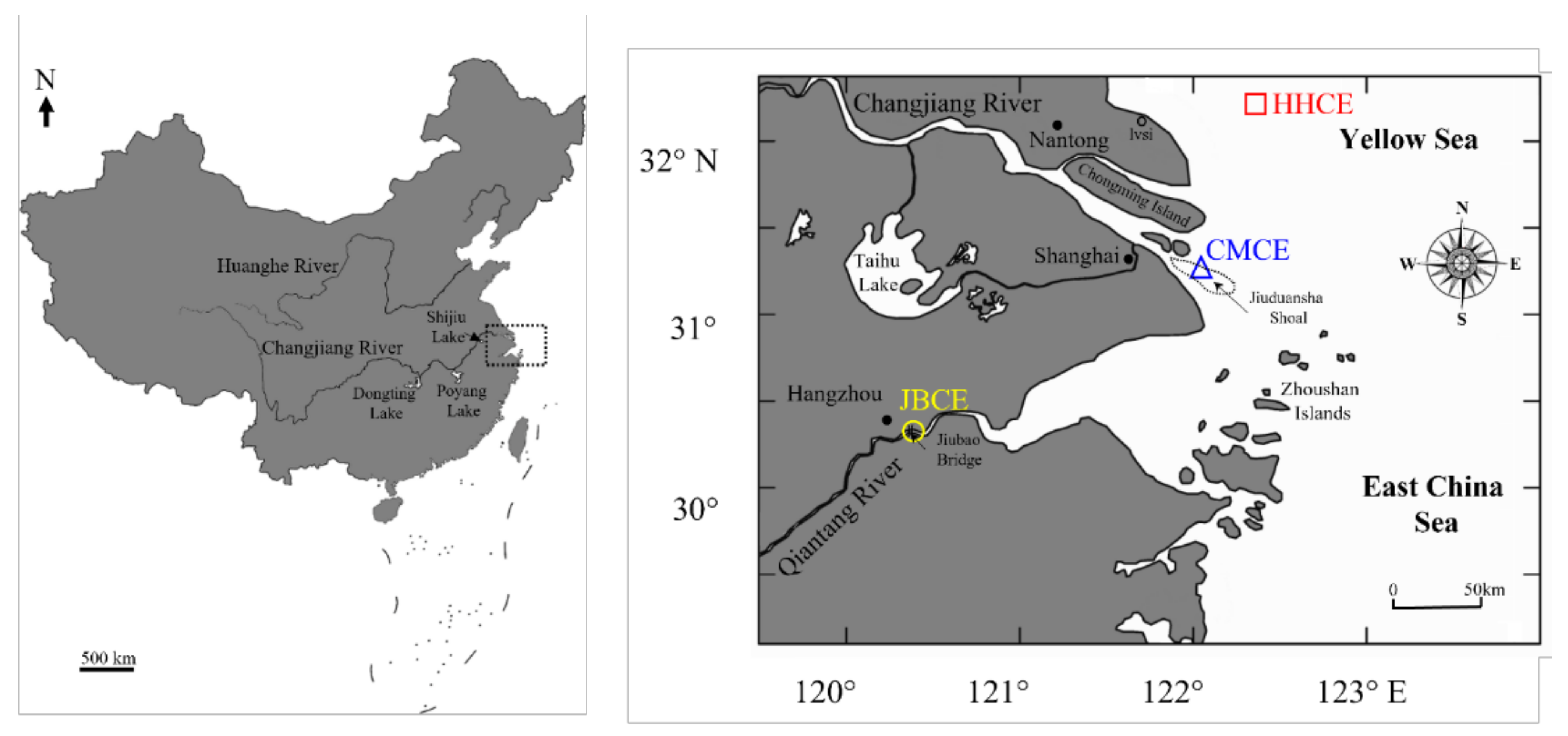

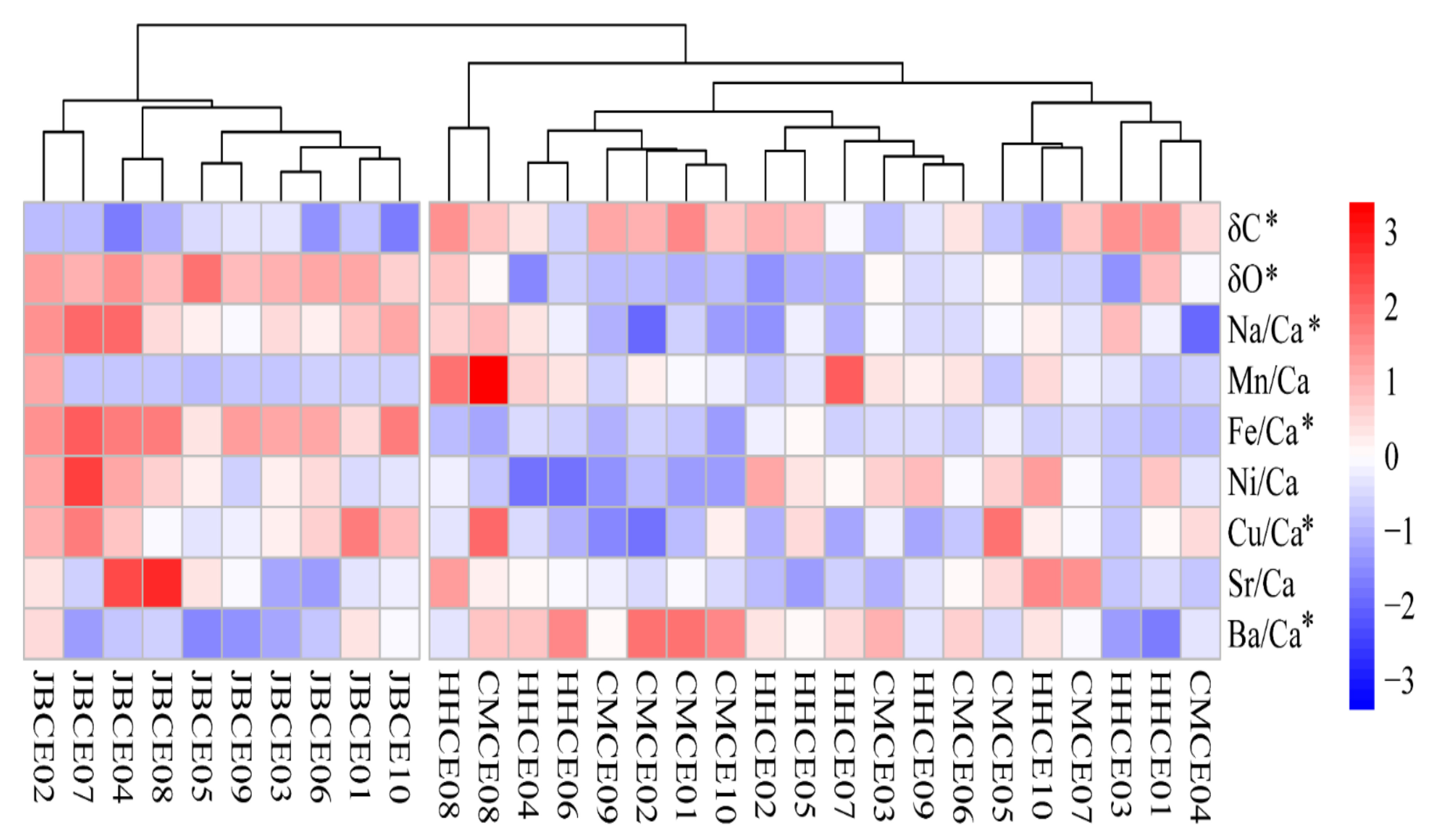
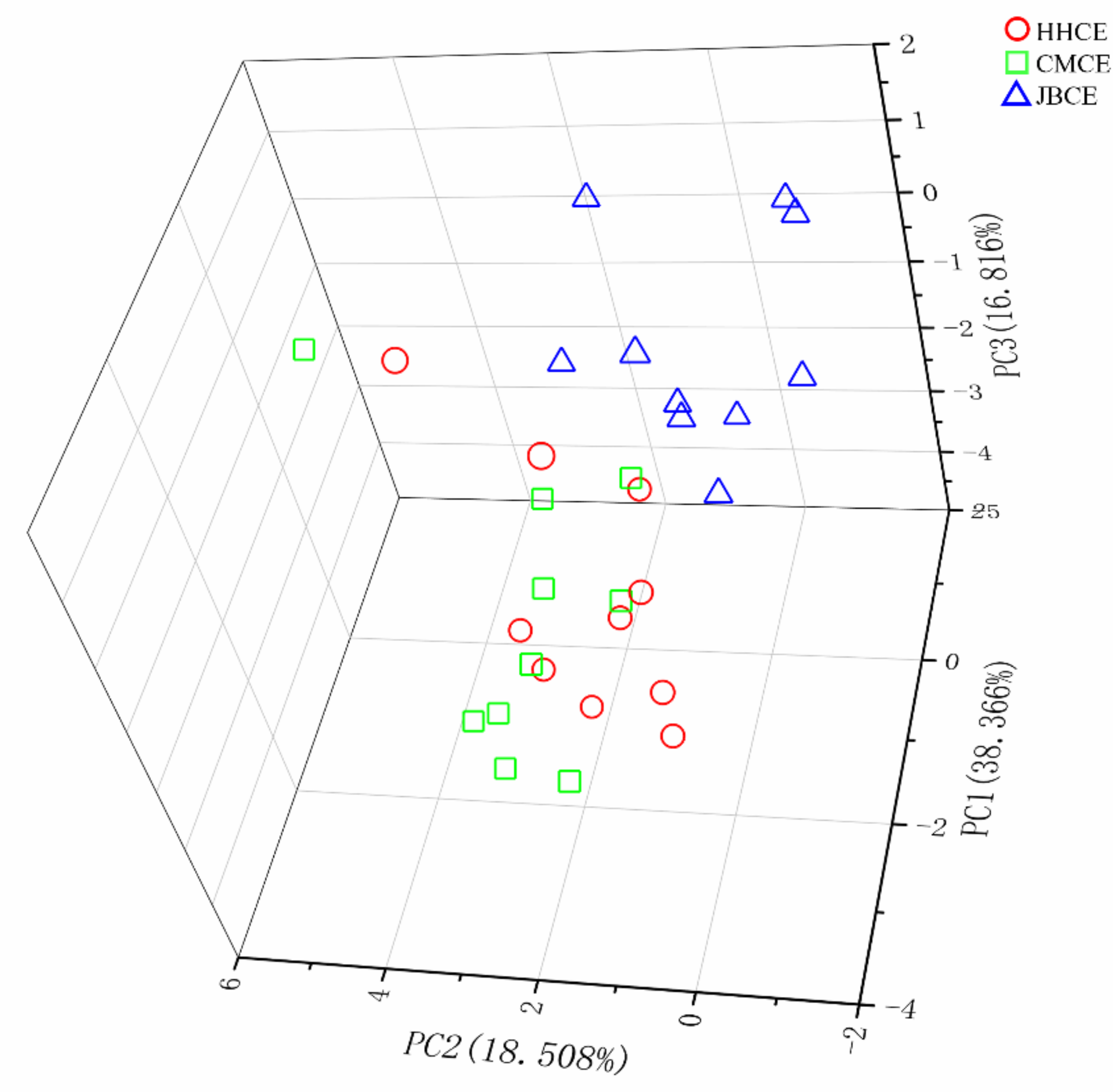
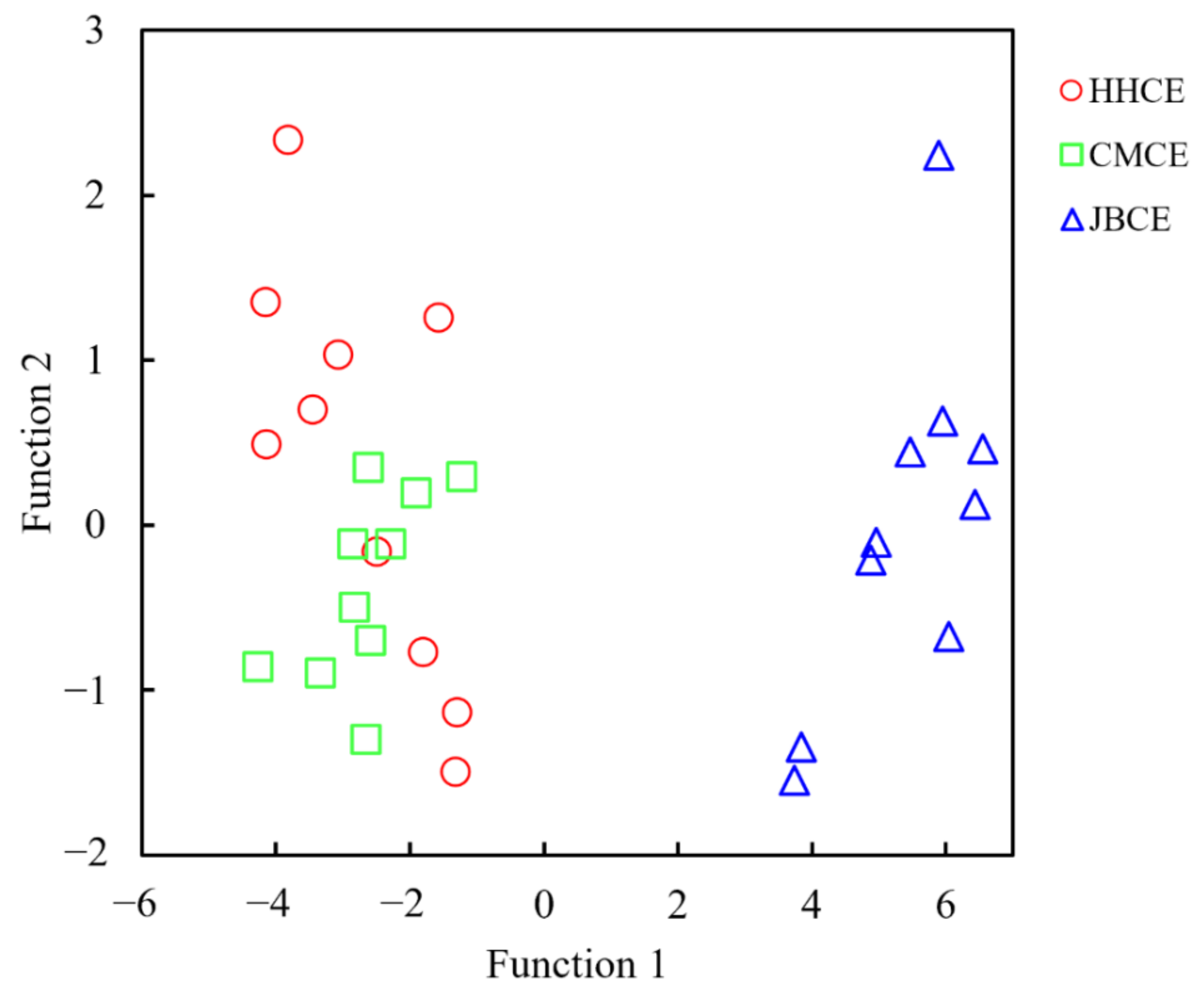
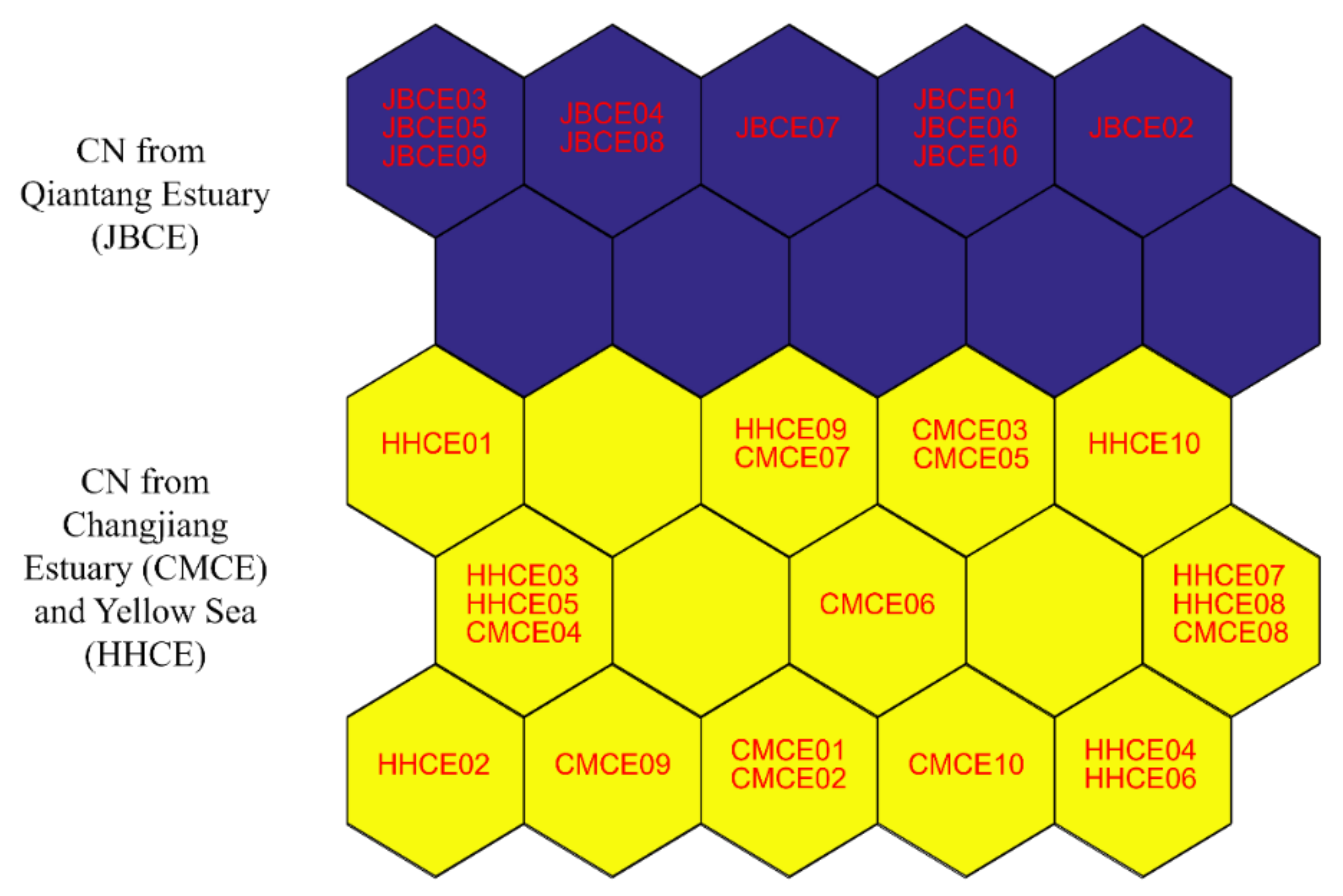
| Sampling Site | Sample Code | Sampling Date | Sample Size (N) | Total Length (mm) | Body Weight (g) | Age a (Year) | Gonad Stage b |
|---|---|---|---|---|---|---|---|
| Coastal water in the Yellow Sea near Nantong City | HHCE | 13 April 2016 | 10 | 261 ± 14 | 49.00 ± 15.56 | 2 | II |
| Changjiang River estuary near Jiuduansha Shoal | CMCE | 1–5 April 2016 | 10 | 284 ± 10 | 64.61 ± 7.75 | 2 | II (2 ind.) III (8 ind.) |
| Qiantang River estuary near Jiubao bridge waters | JBCE | 10 May 2016 | 10 | 271 ± 12 | 46.85 ± 7.79 | 2 | III |
| Item | Elemental Ratio (mmol/mol) | ||||||
|---|---|---|---|---|---|---|---|
| Na/Ca | Mn/Ca | Fe/Ca | Ni/Ca | Cu/Ca | Sr/Ca | Ba/Ca | |
| RSD (%) | 7.33 | 5.42 | 2.31 | 8.50 | 6.25 | 2.05 | 3.14 |
| LOD | 0.031 | 6.20 × 10−4 | 0.0051 | 6.40 × 10−5 | 1.10 × 10−4 | 6.60 × 10−6 | 3.20 × 10−6 |
| HHCE | 8.73 ± 0.3 a | 0.012 ± 0.008 a | 0.43 ± 0.01 a | 0.0077 ± 0.0011 ab | 0.00030 ± 0.00007 a | 0.69 ± 0.14 a | 0.013 ± 0.006 ab |
| CMCE | 8.51 ± 0.36 a | 0.011 ± 0.01 a | 0.42 ± 0.01 a | 0.0072 ± 0.0007 b | 0.00035 ± 0.00015 ab | 0.70 ± 0.10 a | 0.017 ± 0.005 b |
| JBCE | 9.15 ± 0.3 b | 0.005 ± 0.005 a | 0.46 ± 0.01 b | 0.0082 ± 0.0009 a | 0.00043 ± 0.00008 b | 0.74 ± 0.21 a | 0.009 ± 0.004 a |
| Group | Stable Isotopic Ratios (‰, VPDB) | |
|---|---|---|
| δ13C | δ18O | |
| HHCE | −10.42 ± 1.81 a | −7.72 ± 1.79 a |
| CMCE | −10.31 ± 1.49 a | −7.32 ± 0.93 a |
| JBCE | −13.12 ± 1.00 b | −4.08 ± 0.72 b |
| Group | Predicted Group (Original/Cross-Validated) | |||
|---|---|---|---|---|
| HHCE | CMCE | JBCE | ||
| Count | HHCE | 6 (6) | 4 (4) | 0 (0) |
| CMCE | 3 (4) | 7 (6) | 0 (0) | |
| JBCE | 0 (0) | 0 (0) | 10 (10) | |
| % | HHCE | 60 (60) | 40 (40) | 0 (0) |
| CMCE | 30 (40) | 70 (60) | 0 (0) | |
| JBCE | 0 (0) | 0 (0) | 100 (100) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, T.; Liu, H.; Hu, Y.; Chen, X.; Yang, J. Revealing Population Connectivity of the Estuarine Tapertail Anchovy Coilia nasus in the Changjiang River Estuary and Its Adjacent Waters Using Otolith Microchemistry. Fishes 2022, 7, 147. https://doi.org/10.3390/fishes7040147
Jiang T, Liu H, Hu Y, Chen X, Yang J. Revealing Population Connectivity of the Estuarine Tapertail Anchovy Coilia nasus in the Changjiang River Estuary and Its Adjacent Waters Using Otolith Microchemistry. Fishes. 2022; 7(4):147. https://doi.org/10.3390/fishes7040147
Chicago/Turabian StyleJiang, Tao, Hongbo Liu, Yuhai Hu, Xiubao Chen, and Jian Yang. 2022. "Revealing Population Connectivity of the Estuarine Tapertail Anchovy Coilia nasus in the Changjiang River Estuary and Its Adjacent Waters Using Otolith Microchemistry" Fishes 7, no. 4: 147. https://doi.org/10.3390/fishes7040147
APA StyleJiang, T., Liu, H., Hu, Y., Chen, X., & Yang, J. (2022). Revealing Population Connectivity of the Estuarine Tapertail Anchovy Coilia nasus in the Changjiang River Estuary and Its Adjacent Waters Using Otolith Microchemistry. Fishes, 7(4), 147. https://doi.org/10.3390/fishes7040147







