An Overview of Interlocation Sexual Shape Dimorphism in Caquetaia kraussi (Perciformes: Cichlidae): A Geometric Morphometric Approach
Abstract
1. Introduction
2. Materials and Methods
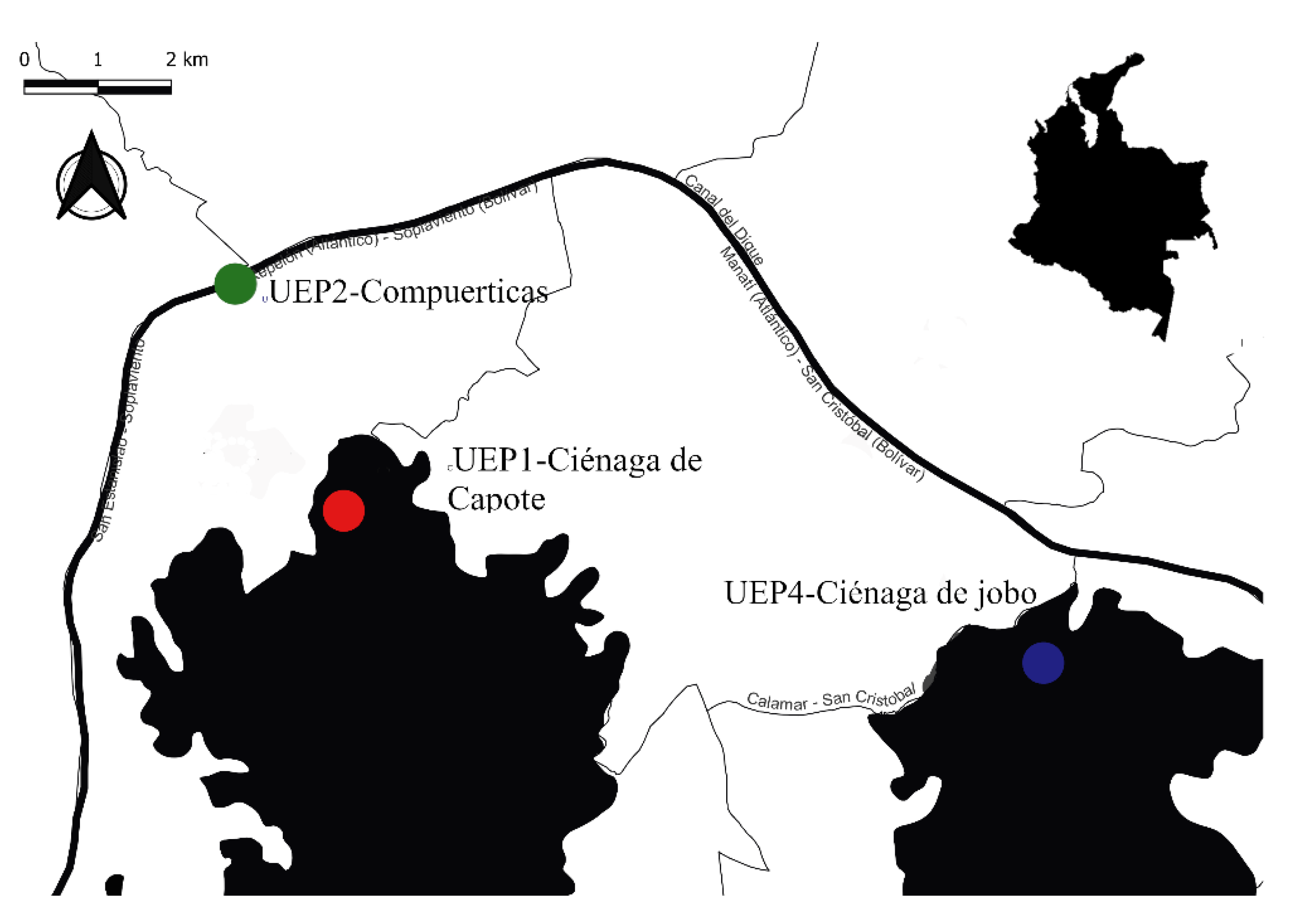
2.1. Study Area
2.2. Field Work
2.3. Geometric Morphometrics
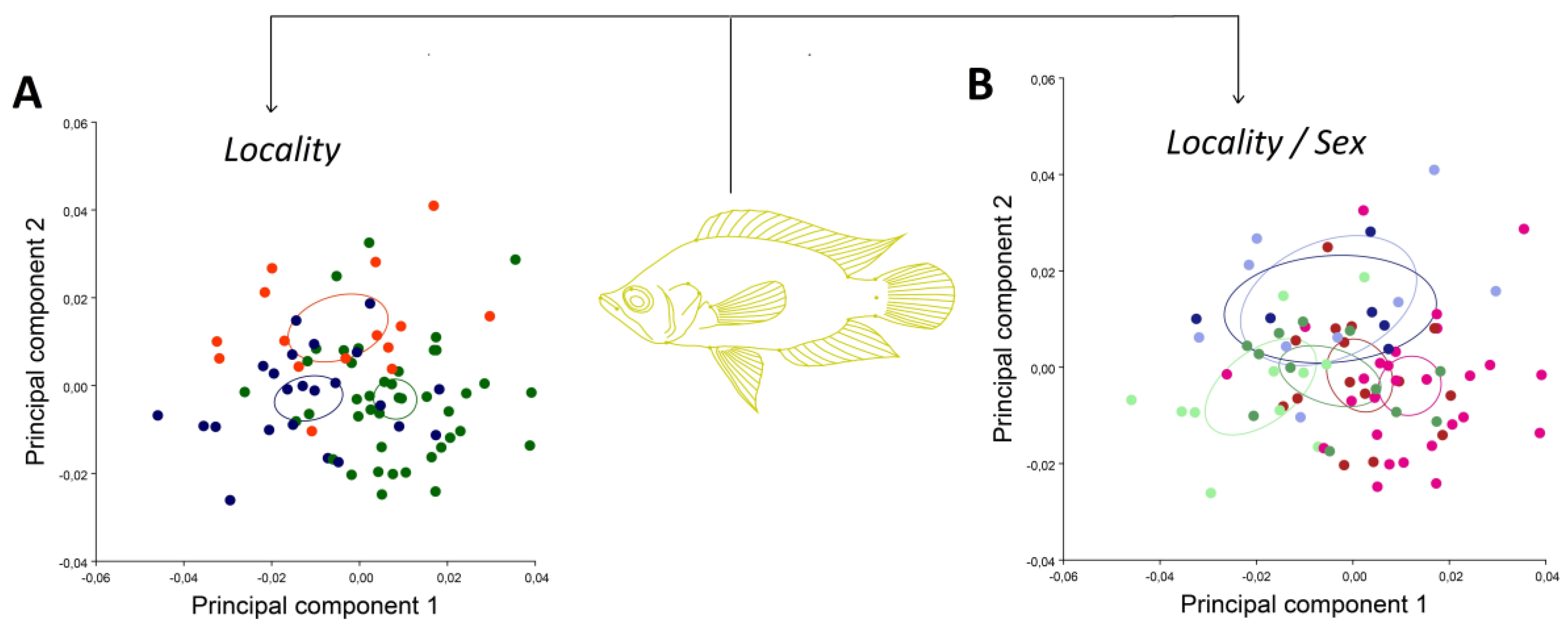
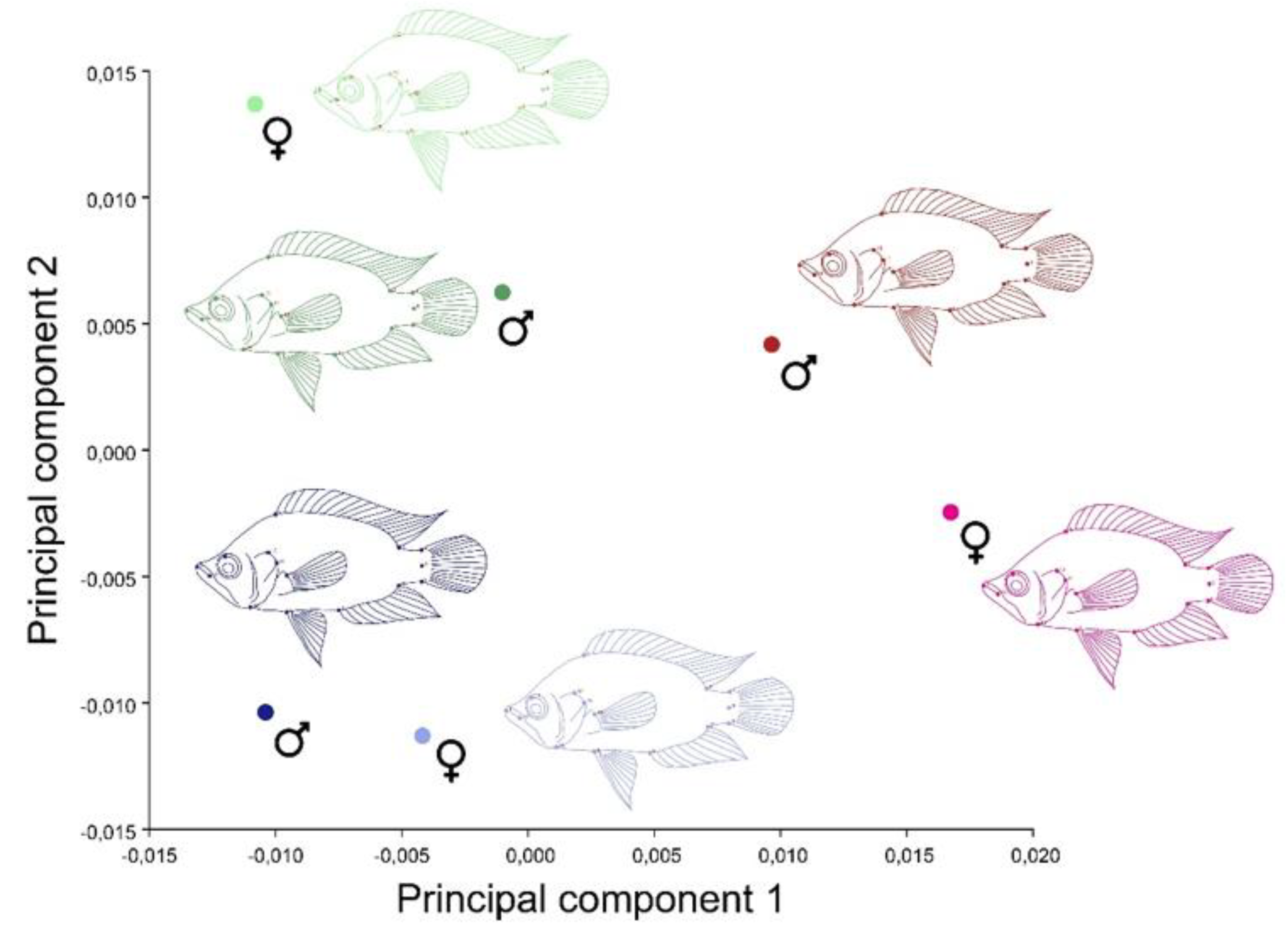
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersson, M. Sexual Selection; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Fairbairn, D.J.; Preziosi, R.F. Sexual Selection and the Evolution of Sexual Size Dimorphism in the Water Strider. Aquar. Remigis. Evol. 1996, 50, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Rojas, G.C.; Castro, K.R. Selección de pareja y comportamiento sexual de los guppys (Poecilia reticulata). Orinoquia 2005, 9, 38–44. [Google Scholar]
- Lima-Filho, P.A.; Bidau, C.J.; Alencar, C.E.R.D.; Molina, W.F. Latitudinal influence on the sexual dimorphism of the marine fish Bathygobius soporator (Gobiidae: Teleostei). Evol. Biol. 2017, 44, 374–385. [Google Scholar] [CrossRef]
- Herler, J.; Kerschbaumer, M.; Mitteroecker, P.; Postl, L.; Sturmbauer, C. Sexual dimorphism and population divergence in the Lake Tanganyika cichlid fish genus Tropheus. Front. Zool. 2010, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, D.J. Allometry for sexual size dimorphism: Pattern and process in the coevolution of body size in males and females. Annu. Rev. Ecol. Syst. 1997, 28, 659–687. [Google Scholar] [CrossRef]
- Horne, C.R.; Hirst, A.G.; Atkinson, D. Selection for increased male size predicts variation in sexual size dimorphism among fish species. Proc. R. Soc. B 2020, 287, 20192640. [Google Scholar] [CrossRef]
- Benítez, H.A.; Sukhodolskaya, R.A.; Órdenes-Clavería, R.; Avtaeva, T.A.; Kushalieva, S.A.; Saveliev, A.A. Measuring the Inter and Intraspecific Sexual Shape Dimorphism and Body Shape Variation in Generalist Ground Beetles in Russia. Insects 2020, 11, 361. [Google Scholar] [CrossRef]
- Benítez, H.A. Sexual dimorphism using geometric morphometric approach. In Sexual Dimorphism; Moriyama, H., Ed.; Intech: Rijeka, Croatia, 2013; pp. 35–50. [Google Scholar]
- Serrano-Meneses, M.A.; Reyes Hernández, M.; Carrillo Muñoz, A.; Rivas, M. La Conducta Reproductiva y La Evolución Del Dimorfismo Sexual En Tamaño; R.-AJ Martínez-Gómez, M., Lucio, R.A., Eds.; Biología del Comportamiento: Aportaciones desde la Fisiología: Tlaxcala, México, 2014; pp. 285–298. [Google Scholar]
- Uba, K.I.N. Sexual shape dimorphism in the monomorphic fish Decapterus macrosoma (Teleostei: Carangidae). Comput. Ecol. Softw. 2019, 9, 134. [Google Scholar]
- Benítez, H.A.; Sanzana, M.-J.; Jerez, V.; Parra, L.E.; Hernandez, C.E.; Canales-Aguirre, C.B. Sexual Shape and Size Dimorphism in Carabid Beetles of the Genus Ceroglossus: Is Geometric Body Size Similar Between Sexes Due to Sex Ratio? Zool. Sci. 2013, 30, 289–295. [Google Scholar] [CrossRef]
- Adams, D.C.; Rohlf, F.J.; Slice, D.E. Geometric morphometrics: Ten years of progress following the ‘revolution’. Ital. J. Zool. 2004, 71, 5–16. [Google Scholar] [CrossRef]
- Rohlf, F.J.; Slice, D. Extensions of the Procustes methods for the optimal superimposition of landmarks. Syst. Zool. 1990, 39, 40–59. [Google Scholar] [CrossRef]
- Slice, D.E. Geometric morphometrics. In Annual Review of Anthropology; Annual Reviews: Palo Alto, CA, USA, 2007; Volume 36, pp. 261–281. [Google Scholar]
- Adams, D.C.; Rohlf, F.J.; Slice, D.E. A field comes of age: Geometric morphometrics in the 21st century. Hystrix-Ital. J. Mammal. 2013, 24, 7–14. [Google Scholar] [CrossRef]
- Villalobos-Leiva, A.; Benítez, H.A. Morfometría Geométrica y sus Nuevas Aplicaciones en Ecología y Biología Evolutiva. Parte 2. Int. J. Morphol. 2020, 38, 1818–1836. [Google Scholar] [CrossRef]
- Espinosa-Santana, N. Selección sexual en machos de Poecilia reticulata (gupis) ante hembras emparentadas. An. Univ. De Etol. 2010, 4, 54–62. [Google Scholar]
- Berns, C.M. The evolution of sexual dimorphism: Understanding mechanisms of sexual shape differences. In Sexual Dimorphism; Moriyama, H., Ed.; Intech: Rijeka, Croatia, 2013; pp. 1–16. [Google Scholar]
- Benítez, H.A.; Avaria-Llautureo, J.; Canales-Aguirre, C.B.; Jerez, V.; Parra, L.E.; Hernandez, C.E. Evolution of sexual size dimorphism and its relationship with sex ratio in carabid beetles of Genus Ceroglossus Solier. Curr. Zool. 2013, 59, 769–777. [Google Scholar] [CrossRef]
- Parker, G. The evolution of sexual size dimorphism in fish. J. Fish Biol. 1992, 41, 1–20. [Google Scholar] [CrossRef]
- Oliveira, R.F.; Ros, A.F.; Gonçalves, D.M. Intra-sexual variation in male reproduction in teleost fish: A comparative approach. Horm. Behav. 2005, 48, 430–439. [Google Scholar] [CrossRef]
- González, R.; Bermúdez Tobón, A. Determinación de dimorfismo sexual usando técnicas morfométricas en Rachycentron canadum (Perciformes: Rachycentridae) cultivados en cautiverio. Bol. Investig. Mar. Costeras. 2021, 50, 71–90. [Google Scholar]
- Brzozowski, F.; Roscoe, J.; Parsons, K.; Albertson, C. Sexually Dimorphic Levels of Color Trait Integration and the Resolution of Sexual Conflict in L ake M alawi Cichlids. J. Exp. Zool. Part B Mol. Dev. Evol. 2012, 318, 268–278. [Google Scholar]
- López Álvarez, L.E.; Benavides Morales, Y.C. Biología reproductiva de la Cachegua Trachelyopterus insignis (Steindachner, 1878) en la Ciénaga de Ayapel, Colombia; Laboratorio de Investigación Biológico Pesquera-LIBP-FMVZ-Dpto. Ciencias Acuícolas: Bogota, Colombia, 2021. [Google Scholar]
- Pastana, M.; Dagosta, F.; Esguícero, A. A new sexually dichromatic miniature Hyphessobrycon (Teleostei: Characiformes: Characidae) from the Rio Formiga, upper Rio Juruena basin, Mato Grosso, Brazil, with a review of sexual dichromatism in Characiformes. J. Fish Biol. 2017, 91, 1301–1318. [Google Scholar] [CrossRef]
- Solano-Peña, D.; Segura-Guevara, F.; Olaya-Nieto, C. Crecimiento y reproducción de la mojarra amarilla (Caquetaia kraussii Steindachner, 1878) en el embalse de Urrá, Colombia. Rev. MVZ Córdoba 2013, 18, 3525–3533. [Google Scholar] [CrossRef][Green Version]
- Olaya-Nieto, C.; Martínez-González, Á.; Díaz-Sánchez, D.; Pérez-Doria, W.; Segura-Guevara, F.; Tordecilla-Petro, G. Relación longitud-peso de la mojarra amarilla Caquetaia kraussii en la Ciénaga de Ayapel, Colombia. Neiva: V Congreso Colombiano de Acuicultura. Braz. J. Anim. Environ. Res. 2021, 4, 6469–6484. [Google Scholar] [CrossRef]
- Vanega, H.D.G. Algunos aspectos biológicos y pesqueros de Caquetaia kraussii (Steindachner, 1878) en la cuenca media y baja del río Atrato, Chocó. Rev. Biodivers. Neotrop. 2017, 7, 14–21. [Google Scholar] [CrossRef][Green Version]
- Olaya-Nieto, C.W.; Ubarnes-Coronado, G.M.; Ensuncho-Morales, J.E. Crecimiento y mortalidad de mojarra amarilla Caquetaia kraussii en la ciénaga Grande de Lorica, Colombia. Rev. Logos Cienc. Tecnol. 2014, 5, 202–212. [Google Scholar] [CrossRef]
- Maldonado-Ocampo, J.A.; Ortega-Lara, A.; Usma, J.S.; Galvis, G.; Villa-Navarro, F.A.; Vásquez, L.; Prada-Pedreros, S.; Ardila, C. Peces de los Andes de Colombia. Instituto de Investigación de Recursos Biológicos “Alexander Von Humboldt”. Bogotá Colomb. 2005, 1, 346. [Google Scholar]
- Guzmán, M.Á.; Olaya, M.; Lasso, C.A.; Morales Betancourt, M.A.; Santos Calderón, J.M.; Uribe Botero, B.; Castaño Uribe, C.; Sanclemente, X.; Fajardo Rodríguez, Z.; Ponce de León Chaux, E. Catálogos de los Recursos Pesqueros Continentales de Colombia; Ministerio de Ambiente, Vivienda y Desarrollo Territorial-Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2011. [Google Scholar]
- Ahumada Lagares, G.A.; Penso Martínez, L.D. Caracterización socioeconómica de la subregión del Canal del Dique. Aguaita 2014, 26, 37–61. [Google Scholar]
- Lasso, C.; Agudelo-Córdoba, E.; Jiménez-Segura, L.; Ramírez-Gil, H.; Morales-Betancourt, M.; Ajiaco-Martínez, R.; Paula-Gutiérrez, F.; Usma-Oviedo, J.; Muñoz, S.; Sanabria, A.I. Catálogo de los recursos pesqueros continentales de Colombia. Serie Editorial Recursos Hidrobiológicos y Pesqueros Amazonian migratory catfish Brachyplatystoma rousseauxii in the Iquitos Región, Peru. J. Fish Biol. 2011, 75, 2527–2551. [Google Scholar]
- Agudelo, E.; Ajiaco, R.; Álvarez, L.; Barreto, C.; Bessudo, S.; Borda, C.; Bustamante, C.; Caldas, J.; De la Hoz, J.; Díazgranados, M. Protocolo De Captura De Información Pesquera, Biológica y Socio-Económica En Colombia; MADR, DPA: Bogotá, Colombia, 2011; 80p. [Google Scholar]
- Corti, M.; Crosetti, D. Geographic variation in the grey mullet: A geometric morphometric analysis using partial warp scores. J. Fish Biol. 1996, 48, 255–269. [Google Scholar] [CrossRef]
- Soria-Barreto, M.; Rodiles-Hernández, R.; González-Díaz, A.A. Morfometría de las especies de Vieja (Cichlidae) en ríos de la cuenca del Usumacinta, Chiapas, México. Rev. Mex. De Biodivers. 2011, 82, 569–579. [Google Scholar] [CrossRef]
- Aguirre, W.E.; Prado, P.J. Guía Práctica de Morfometría Geométrica: Aplicaciones En La Ictiología; Pontificia Universidad Católica del Ecuador Sede Esmeraldas (PUCESE): Esmeraldas, Ecuador, 2018; 104p. [Google Scholar]
- Rohlf, F.J. TPSdig, v. 2.17. Digitize Landmarks and Outlines; Department of Ecology and Evolution, State University at Stony Brook: New York, NY, USA, 2013. [Google Scholar]
- Klingenberg, C.P. Morphometrics and the role of the phenotype in studies of the evolution of developmental mechanisms. Gene 2002, 287, 3–10. [Google Scholar] [CrossRef]
- Toro-Ibacache, M.V.; Soto, G.M.; Galdames, I.S. Geometric Morphometry and the Biologic Shapes Study: From the Descriptive Morphology to the Quantitative Morphology. Int. J. Morphol. 2010, 28, 977–990. [Google Scholar]
- Benítez, H.A.; Püschel, T.A. Modelando la Varianza de la Forma: Morfometría Geométrica Aplicaciones en Biología Evolutiva. Int. J. Morphol. 2014, 32, 998–1008. [Google Scholar] [CrossRef]
- Arnqvist, G.; Martensson, T. Measurement error in geometric morphometrics: Empirical strategies to assess and reduce its impact on measures of shape. Acta Zool. Acad. Sci. Hung. 1998, 44, 73–96. [Google Scholar]
- Fruciano, C. Measurment error in geometric morphometrics. Dev. Genes Evol. 2016, 226, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez, J.; Cubillos, E.; Forero, G. Balance hídrico y sedimentológico del Canal del Dique y sus efectos sobre la sedimentación de la Bahía de Cartagena. In Proceedings of the Third Regional Symposium in Hydraulics on Rivers, Universidad Nacional de Colombia, Sede Bógota-Laboratorios de Ensayos Hidraulicos, Ottawa, ON, Canada, 8–10 November 2022; p. 18. [Google Scholar]
- Perazzo, G.X.; Corrêa, F.; Salzburger, W.; Gava, A. Morphological differences between an artificial lentic and adjacent lotic environments in a characid species. Rev. Fish Biol. Fish. 2019, 29, 935–949. [Google Scholar] [CrossRef]
- Hedrick, A.V.; Temeles, E.J. The evolution of sexual dimorphism in animals—hypotheses and tests. Trends Ecol. Evol. 1989, 4, 136–138. [Google Scholar] [CrossRef]
- Kodric-Brown, A.; Nicoletto, P.F. Female choice in the guppy (Poecilia reticulata): The interaction between male color and display. Behav. Ecol. Sociobiol. 2001, 50, 346–351. [Google Scholar] [CrossRef]
- Ramos Salazar, A. Selección sexual en Gambusia holbrooki (Agassiz, 1859) (Pisces: Poeciliidae). An. Univ. De Etol. 2007, 1, 90–95. [Google Scholar]
- Selz, O.; Thommen, R.; Pierotti, M.; Anaya-Rojas, J.M.; Seehausen, O. Differences in male coloration are predicted by divergent sexual selection between populations of a cichlid fish. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160172. [Google Scholar] [CrossRef]
- Maan, M.E.; Sefc, K.M. Colour variation in cichlid fish: Developmental mechanisms, selective pressures and evolutionary consequences. Semin. Cell Dev. Biol. 2013, 24, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Bolnick, D.I.; Doebeli, M. Sexual dimorphism and adaptive speciation: Two sides of the same ecological coin. Evolution 2003, 57, 2433–2449. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.M.; Calsbeek, R. Sexually Antagonistic Selection, Sexual Dimorphism, and the Resolution of Intralocus Sexual Conflict. Am. Nat. 2009, 173, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Badyaev, A.V. Origin of the fittest: Link between emergent variation and evolutionary change as a critical question in evolutionary biology. Proc. R. Soc. B Biol. Sci. 2011, 278, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, D.J. Allometry for sexual size dimorphism: Testing two hypotheses for Rensch’s rule in the water strider Aquarius remigis. Am. Nat. 2005, 166, S69–S84. [Google Scholar] [CrossRef]
- Blanckenhorn, W.U.; Stillwell, R.C.; Young, K.A.; Fox, C.W.; Ashton, K.G. When Rensch meets Bergmann: Does sexual size dimorphism change systematically with latitude? Evolution 2006, 60, 2004–2011. [Google Scholar] [CrossRef]
- Stillwell, R.C.; Fox, C.W. Environmental effects on sexual size dimorphism of a seed-feeding beetle. Oecologia 2007, 153, 273–280. [Google Scholar] [CrossRef]
- Laporte, M.; Berrebi, P.; Claude, J.; Vinyoles, D.; Pou-Rovira, Q.; Raymond, J.-C.; Magnan, P. The ecology of sexual dimorphism in size and shape of the freshwater blenny Salaria fluviatilis. Curr. Zool. 2018, 64, 183–191. [Google Scholar] [CrossRef]
- Parvis, E.S.; Coleman, R.M. Sexual Dimorphism and Size-Related Changes in Body Shape in Tule Perch (Family: Embiotocidae), a Native California Live-Bearing Fish. Copeia 2020, 108, 12–18. [Google Scholar] [CrossRef]
- Saura, R.B.; Falcasantos, G.; Munda, L.; Alimorong, M.; Hernando, B.J. Evaluation of fluctuating asymmetry and sexual dimorphism of Channa striata using landmark-based geometric morphometric analysis from Agusan Marsh and Lake Mainit in Caraga Region, Philippines. Nusant. Biosci. 2021, 13, 100–110. [Google Scholar] [CrossRef]

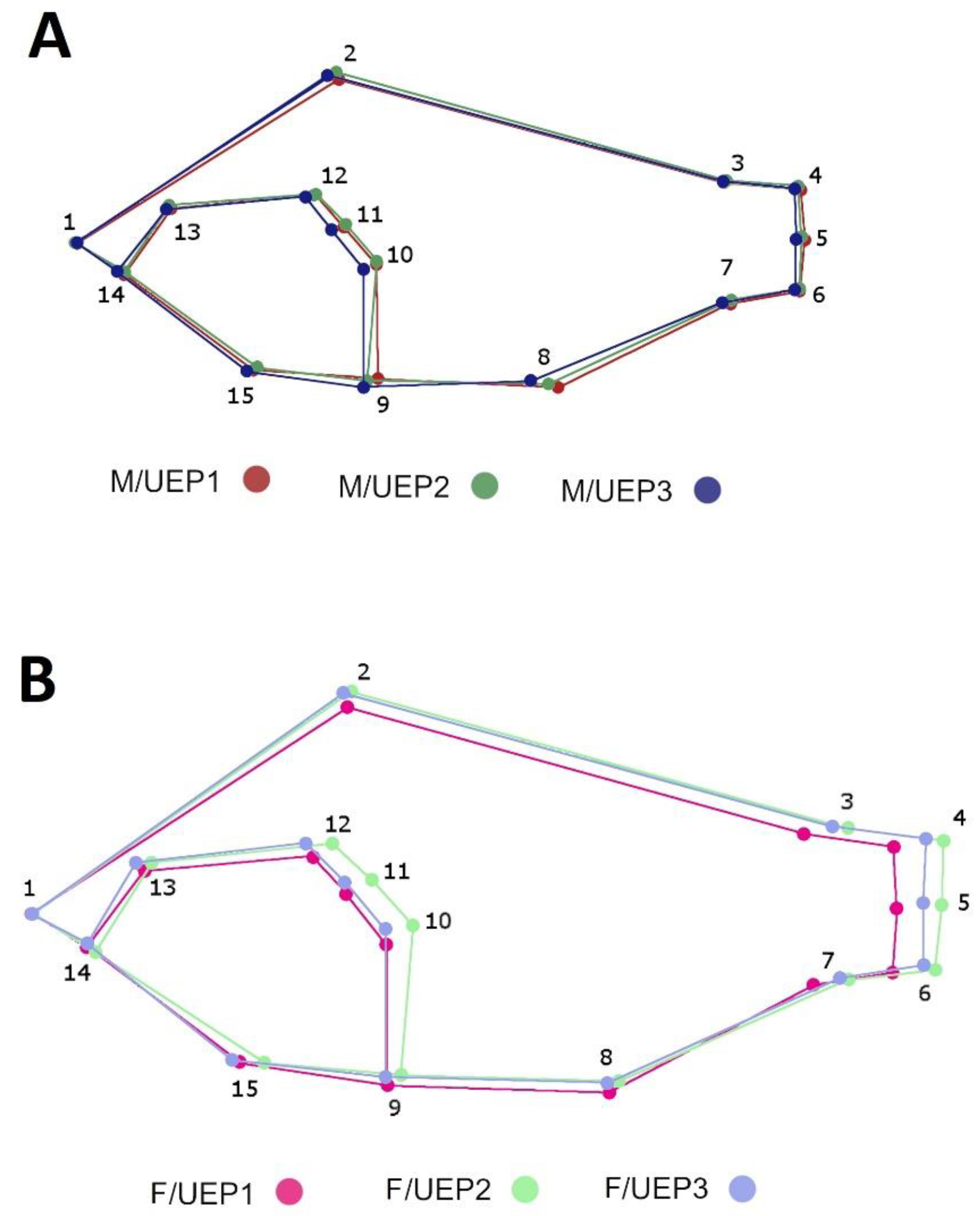

| N° Landmarks | Position |
|---|---|
| 1 | Upper tip of the mouth |
| 2 | First spine of the dorsal region |
| 3 | Posterior insertion of dorsal fin |
| 4 | Dorsal base of caudal fin |
| 5 | Ventral insertion of caudal fin |
| 6 | Ventral base of caudal fin |
| 7 | Posterior insertion of anal fin |
| 8 | First spine anal |
| 9 | Anterior base of first pelvic fin ray |
| 10 | Superior insertion of pectoral fin |
| 11 | Posterior tip of operculum |
| 12 | Dorsal border of preoperculum |
| 13 | Middle of the eye |
| 14 | Cleft of the upper lip |
| 15 | Anterior margin of the cleithrum |
| Centroid Size | |||||||
| Effect | SS | MS | df | F | P (param.) | ||
| Individual | 186.8477 | 5.049938 | 37 | 12.95 | <0.0001 | ||
| Error 1 | 14.430189 | 0.390005 | 37 | ||||
| Shape, Procrustes ANOVA | |||||||
| Effect | SS | MS | df | F | P (param.) | Pillai tr. | P (param.) |
| Individual | 0.07439725 | 0.000077336 | 962 | 11.28 | <0.0001 | 19.65 | <0.0001 |
| Error 1 | 0.00659669 | 0.000006857 | 962 | ||||
| H/UEP1 | H/UEP2 | H/UEP4 | M/UEP1 | M/UEP2 | |
|---|---|---|---|---|---|
| H/UEP2 | 3.1459 | ||||
| p-values: | <0.0001 | ||||
| H/UEP4 | 3.3519 | 3.6005 | |||
| p-values: | <0.0001 | <0.0001 | |||
| M/UEP1 | 2.2344 | 3.2729 | 3.9664 | ||
| p-values: | 0.0001 | <0.0001 | <0.0001 | ||
| M/UEP2 | 2.5726 | 2.014 | 3.6465 | 2.7917 | |
| p-values: | <0.0001 | 0.4495 | <0.0001 | 0.0002 | |
| M/UEP4 | 4.4469 | 5.0323 | 4.2434 | 5.1425 | 4.2772 |
| p-values: | <0.0001 | <0.0001 | 0.002 | <0.0001 | 0.0002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez, J.; Villalobos-Leiva, A.; Bermúdez, A.; Ahumada-Cabarcas, D.; Suazo, M.J.; Benítez, H.A. An Overview of Interlocation Sexual Shape Dimorphism in Caquetaia kraussi (Perciformes: Cichlidae): A Geometric Morphometric Approach. Fishes 2022, 7, 146. https://doi.org/10.3390/fishes7040146
Hernandez J, Villalobos-Leiva A, Bermúdez A, Ahumada-Cabarcas D, Suazo MJ, Benítez HA. An Overview of Interlocation Sexual Shape Dimorphism in Caquetaia kraussi (Perciformes: Cichlidae): A Geometric Morphometric Approach. Fishes. 2022; 7(4):146. https://doi.org/10.3390/fishes7040146
Chicago/Turabian StyleHernandez, Jordan, Amado Villalobos-Leiva, Adriana Bermúdez, Daniela Ahumada-Cabarcas, Manuel J. Suazo, and Hugo A. Benítez. 2022. "An Overview of Interlocation Sexual Shape Dimorphism in Caquetaia kraussi (Perciformes: Cichlidae): A Geometric Morphometric Approach" Fishes 7, no. 4: 146. https://doi.org/10.3390/fishes7040146
APA StyleHernandez, J., Villalobos-Leiva, A., Bermúdez, A., Ahumada-Cabarcas, D., Suazo, M. J., & Benítez, H. A. (2022). An Overview of Interlocation Sexual Shape Dimorphism in Caquetaia kraussi (Perciformes: Cichlidae): A Geometric Morphometric Approach. Fishes, 7(4), 146. https://doi.org/10.3390/fishes7040146







