Comparative Otolith Morphology of Two Morphs of Schizopygopsis thermalis Herzenstein 1891 (Pisces, Cyprinidae) in a Headwater Lake on the Qinghai-Tibet Plateau
Abstract
:1. Introduction
2. Materials and Methods
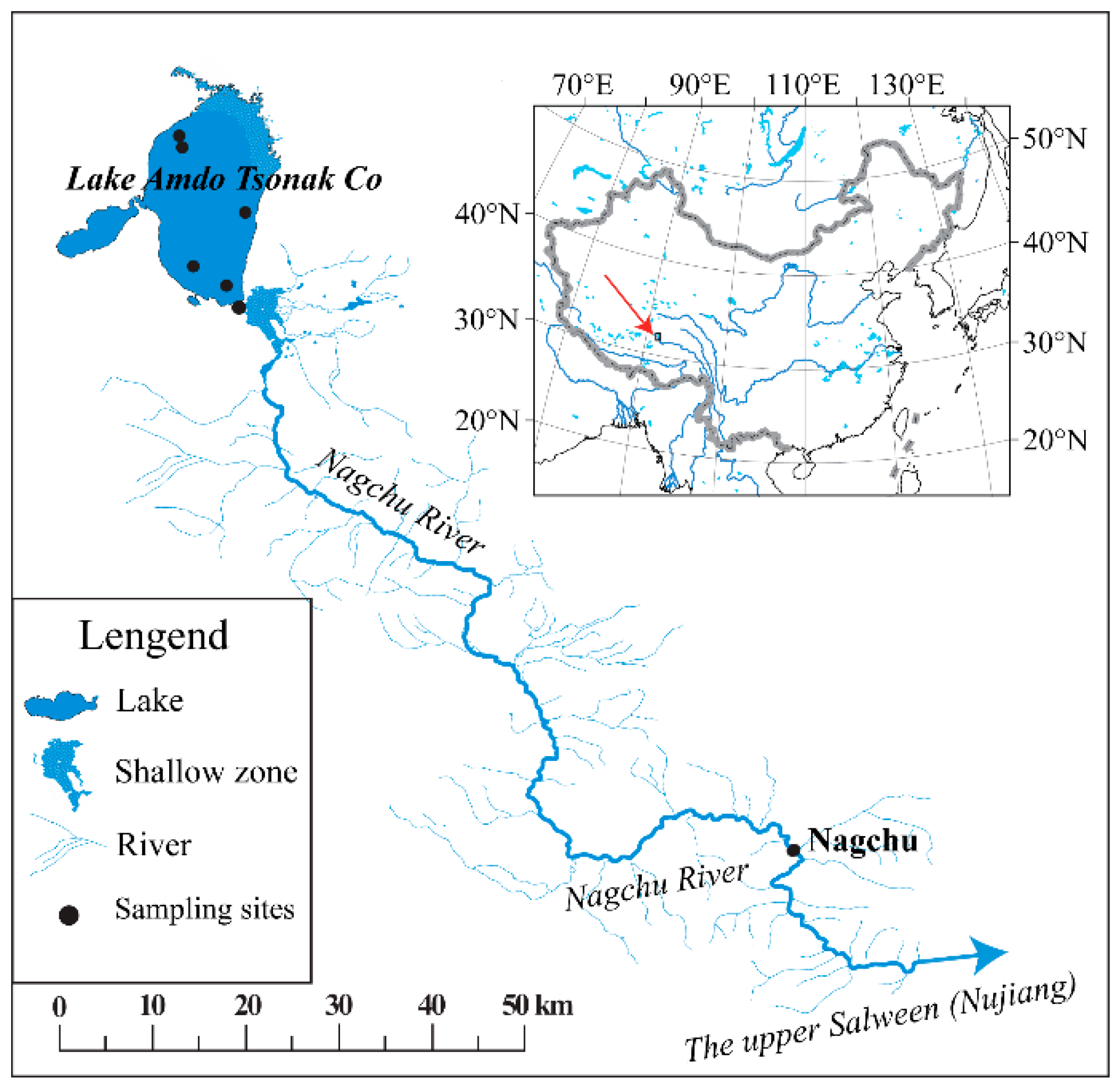
2.1. Study Area and Field Sampling
2.2. Total Length-Based Group Divisions
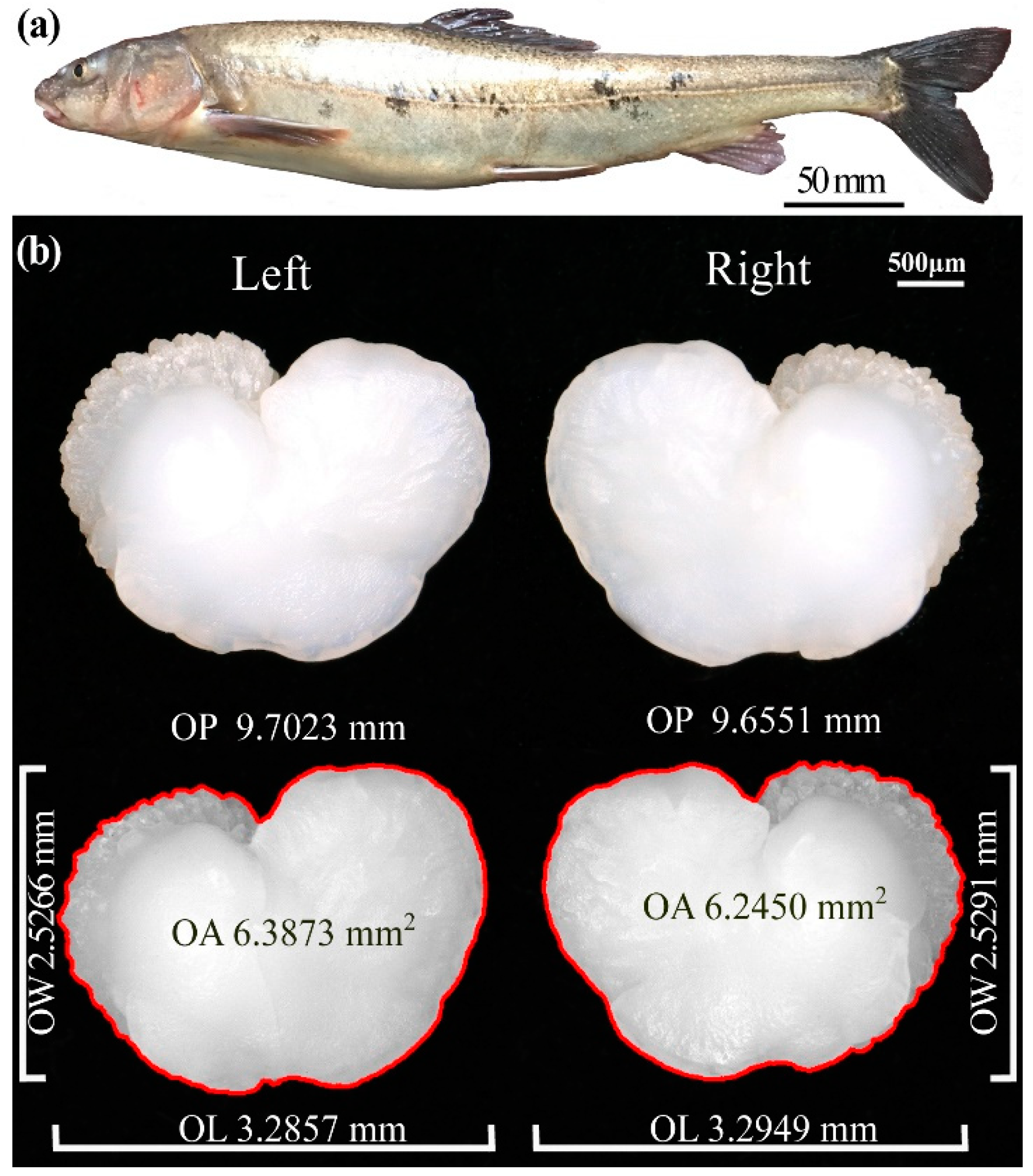
2.3. Otolith Morphometry
2.4. Statistical Analysis
3. Results
3.1. Length Frequency Distributions of Fish Samples
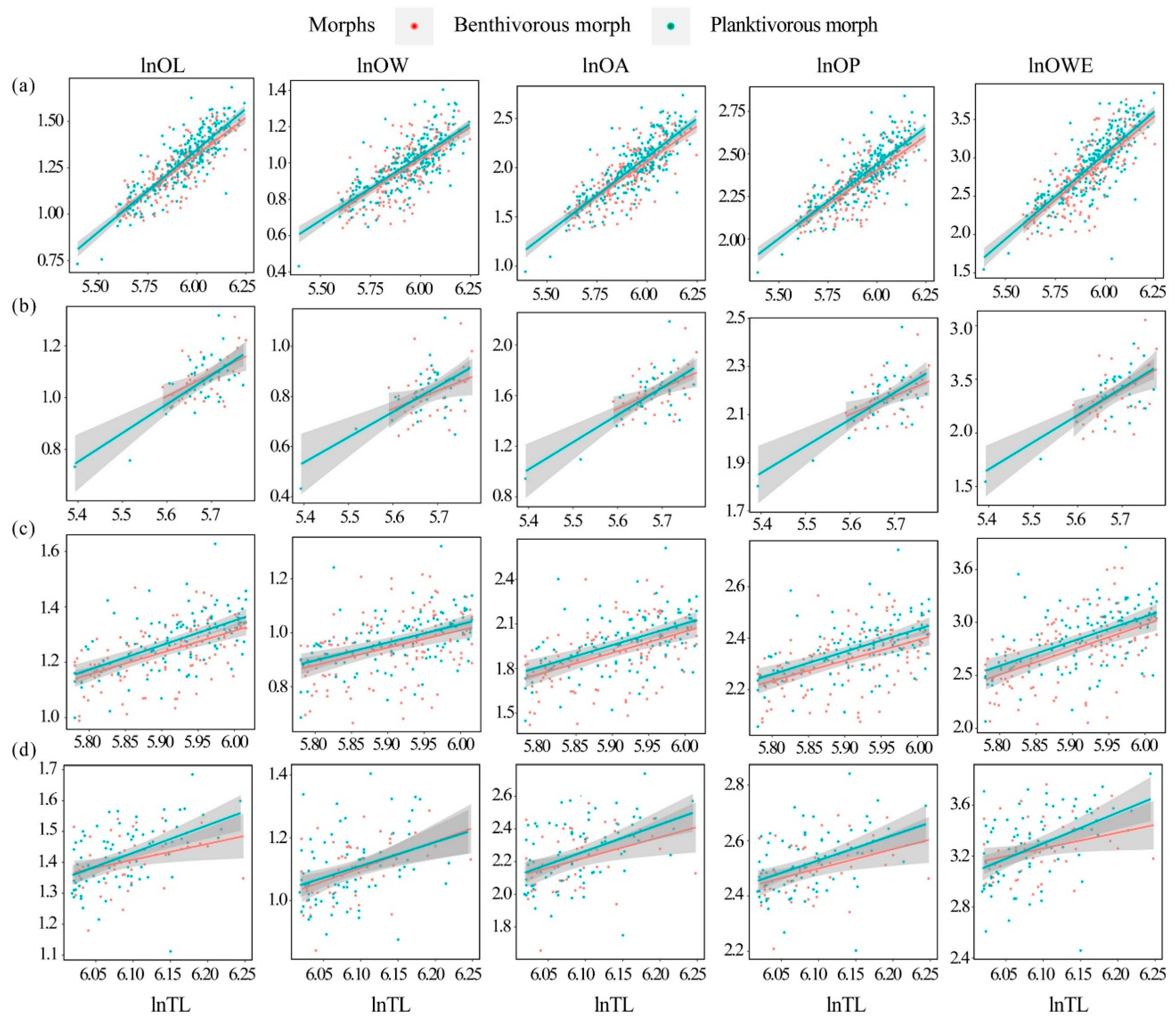
3.2. Relationships between Shape Indices and TL
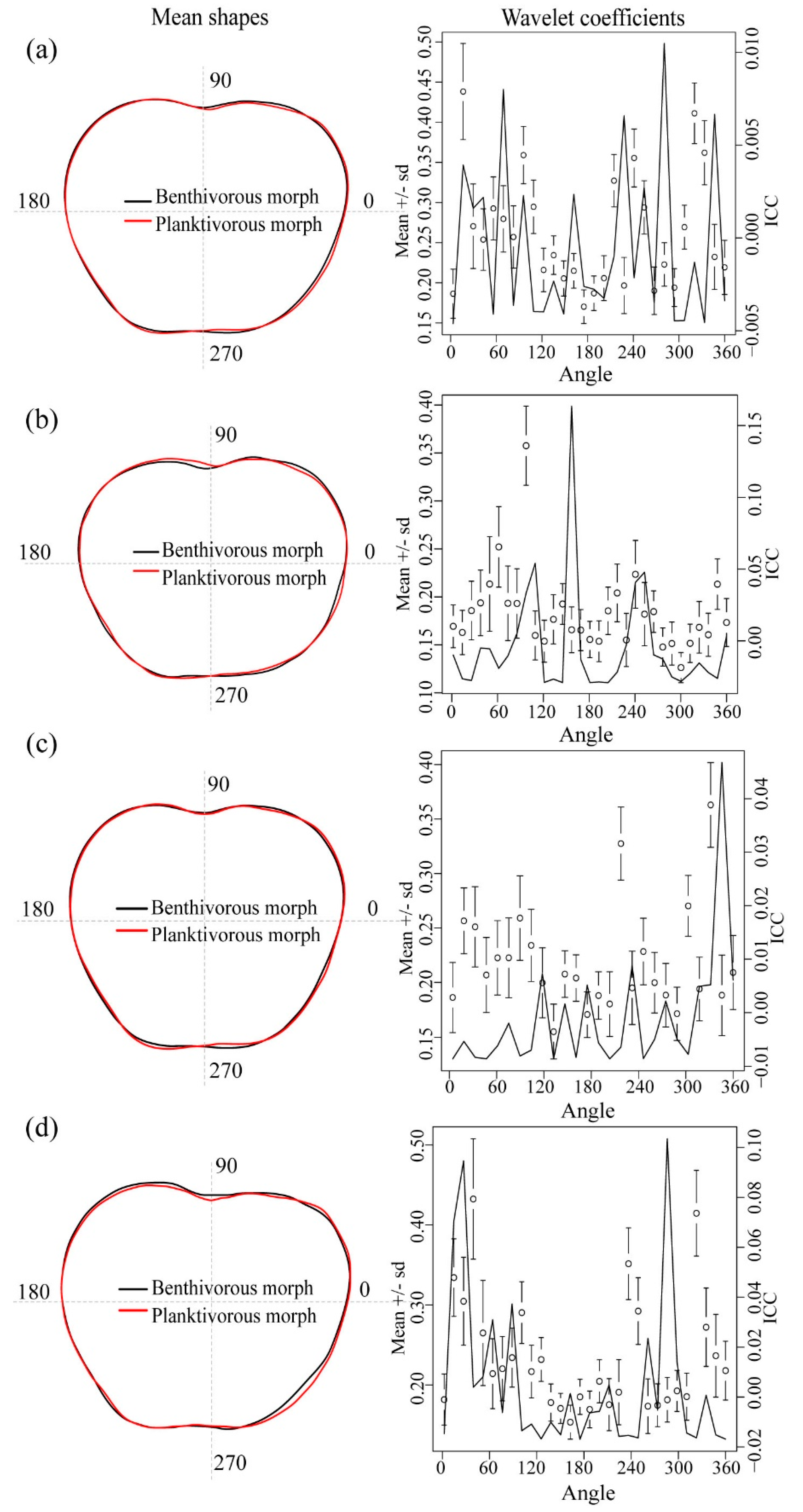
3.3. Otolith Morphometry
4. Discussion
4.1. Relationships between Shape Indices and TL
4.2. Otolith Morphometry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Popper, A.N.; Ramcharitar, J.; Campana, S.E. Why otoliths? Insights from inner ear physiology and fisheries biology. Mar. Freshw. Res. 2005, 56, 497–504. [Google Scholar] [CrossRef]
- Moreira, C.; Froufe, E.; Vaz-Pires, P.; Correia, A.T. Otolith shape analysis as a tool to infer the population structure of the blue jack mackerel, Trachurus picturatus, in the NE Atlantic. Fish. Res. 2019, 209, 40–48. [Google Scholar] [CrossRef]
- Schulz-Mirbach, T.; Ladich, F.; Plath, M.; He, M. Enigmatic ear stones: What we know about the functional role and evolution of fish otoliths. Biol. Rev. 2019, 94, 457–482. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.; Silva, A.A.; Moreno, A.; Veríssimo, A.; Santos, A.M.; Garrido, S. Population structure of the European sardine Sardina pilchardus from Atlantic and Mediterranean waters based on otolith shape analysis. Fish. Res. 2021, 243, 106050. [Google Scholar] [CrossRef]
- Secor, D.H.; Dean, J.M.; Curtis, T.A.; Sessions, F.W. Effect of female size and propagation methods on larval production at a South Carolina striped bass (Marone saxatilis) hatchery. Can. J. Fish. Aquat. Sci. 1992, 49, 1778–1787. [Google Scholar] [CrossRef]
- Thresher, R.E.; Proctor, C.H.; Gunn, J.S.; Harrowfield, I.R. An evaluation of electron-probe microanalysis of otoliths for stock delineation and identification of nursery areas in a Southern Temperate Groundfish, Nemadactylus Macropterus (Cheilodactylidae). Fish. Bull. 1994, 92, 817–840. Available online: https://fisherybulletin.nmfs.noaa.gov/content/evaluation-electron-probe-microanalysis-otoliths-stock-delineation-and-identification (accessed on 22 April 2022).
- Fablet, R. Des Otolithes aux Satellites: Méthodes et Applications du Traitement du Signal et des Images Pour l’observation de l’océan; Université de Bretagne Occidentale: Brest, France, 2012; pp. 14–15. [Google Scholar]
- Campana, S.E.; Neilson, J.D. Microstructure of fish otoliths. Can. J. Fish. Aquat. Sci. 1985, 42, 1014–1032. [Google Scholar] [CrossRef]
- Campana, S.E.; Thorrold, S.R. Otoliths, increments, and elements: Keys to a comprehensive understanding of fish populations? Can. J. Fish. Aquat. Sci. 2001, 58, 30–38. [Google Scholar] [CrossRef]
- Morat, F. Influence des Apports Rhodaniens Sur les Traits D’histoire de vie de la Sole Commune (Solea solea): Apports de l’étude Minéralogique et Chimique des Otolithes; Thèse de doctorat, Université Aix Marseille II: Marseille, France, 2011; p. 308. [Google Scholar]
- Kever, L.; Colleye, O.; Herrel, A.; Romans, P.; Parmentier, E. Hearing capacities and otolith size in two ophidiiform species (ophidion rochei and carapus acus). J. Exp. Biol. 2014, 217, 2517–2525. [Google Scholar] [CrossRef] [Green Version]
- Inoue, M.; Tanimoto, M.; Oda, Y. The role of ear stone size in hair cell acoustic sensory transduction. Sci. Rep. 2013, 3, 2114. [Google Scholar] [CrossRef]
- Schellart, N.A.; Popper, A.N. Functional Aspects of the Evolution of the Auditory System of Actinopterygian Fish. In The Evolutionary Biology of Hearing; Webster, D.B., Popper, A.N., Fay, R.R., Eds.; Springer: New York, NY, USA, 1992. [Google Scholar] [CrossRef]
- Campana, S.E. Photographic Atlas of Fish Otoliths of the Northwest Atlantic Ocean; NRC Research Press: Ottawa, ON, Canada, 2004; p. 284. [Google Scholar] [CrossRef]
- Tuset, V.M.; Lombarte, A.; Assis, C.A. Otolith atlas for the western Mediterranean, north and central Eastern Atlantic. Sci. Mar. 2008, 72, 7–198. [Google Scholar] [CrossRef]
- Beyer, S.G.; Szedlmayer, S.T. The use of otolith shape analysis for ageing juvenile red snapper, Lutjanus campechanus. Environ. Biol. Fishes 2010, 89, 333–340. [Google Scholar] [CrossRef]
- Dou, S.; Yu, X.; Cao, L. Otolith analysis and its application in fish stock discrimination: A case study. Oceanol. Et Limnol. Sin. 2012, 43, 702–712. [Google Scholar] [CrossRef]
- Bose, A.P.; Adragna, J.B.; Balshine, S. Otolith morphology varies between populations, sexes and male alternative reproductive tactics in a vocal toadfish Porichthys notatus. J. Fish Biol. 2017, 90, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Kristjansson, B.K.; Leblanc, C.A.; Skúlason, S.; Snorrason, S.S.; Noakes, D.L. Phenotypic plasticity in the morphology of small benthic Icelandic Arctic charr (Salvelinus alpinus). Ecol. Freshw. Fish 2018, 27, 636–645. [Google Scholar] [CrossRef]
- Bano, F.; Serajuddin, M. Sulcus and outline morphometrics of sagittal otolith variability in freshwater fragmented populations of dwarf gourami, Trichogaster lalia (Hamilton, 1822). Limnologica 2021, 86, 125842. [Google Scholar] [CrossRef]
- Cerda, J.M.; Palacios-Fuentes, P.; Díaz-Santana-Iturrios, M.; Ojeda, F.P. Description and discrimination of sagittae otoliths of two sympatric labrisomid blennies Auchenionchus crinitus and A. microcirrhis using morphometric analyses. J. Sea Res. 2021, 173, 102063. [Google Scholar] [CrossRef]
- Cardinale, M.; Doering-Arjes, P.; Kastowsky, M.; Mosegaard, H. Effects of sex, stock, and environment on the shape of known-age Atlantic cod (Gadus morhua) otoliths. Can. J. Fish. Aquat. Sci. 2004, 61, 158–167. [Google Scholar] [CrossRef]
- Basusta, N.; Khan, U. Sexual dimorphism in the otolith shape of shi drum, Umbrina cirrosa (L.), in the eastern Mediterranean Sea: Fish size-otolith size relationships. J. Fish Biol. 2021, 99, 164–174. [Google Scholar] [CrossRef]
- Chanthran, S.S.D.; Lim, P.E.; Poong, S.; Du, J.; Loh, K. Relationships between sagittal otolith size and body size of Terapon jarbua (Teleostei, Terapontidae) in Malaysian waters. J. Oceanol. Limnol. 2021, 39, 372–381. [Google Scholar] [CrossRef]
- Parson, P.A. Fluctuating asymmetry: An epigenetic measure of stress. Biol Rev Camb Philos Soc. 1990, 65, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Gauldie, R.; Crampton, J. An eco-morphological explanation of individual variability in the shape of the fish otolith: Comparison of the otolith of Hoplostethus atlanticus with other species by depth. J. Fish Biol. 2002, 60, 1204–1221. [Google Scholar] [CrossRef]
- Gagliano, M.; Mccormick, M.I. Feeding history influences otolith shape in tropical fish. Mar. Ecol. Prog. Ser. 2004, 278, 291–296. [Google Scholar] [CrossRef]
- Holmberg, R.J.; Wilcox-Freeburg, E.; Rhyne, A.L.; Tlusty, M.F.; Stebbins, A., Jr.; Ney, S.W., Jr.; Honig, A.; Johnston, A.E.; Antonio, C.M.S.; Bourque, B.; et al. Ocean acidification alters morphology of all otolith types in Clark’s anemonefish (Amphiprion clarkii). PeerJ 2019, 7, e6152. [Google Scholar] [CrossRef] [Green Version]
- Fey, D.P.; Greszkiewicz, M. Effects of temperature on somatic growth, otolith growth, and uncoupling in the otolith to fish size relationship of larval northern pike, Esox lucius L. Fish. Res. 2021, 236, 105843. [Google Scholar] [CrossRef]
- L’Abée-Lund, J.H.; Jensen, A.J. Otoliths as natural tags in the systematics of salmonids. Environ. Biol. Fishes 1993, 36, 389–393. [Google Scholar] [CrossRef]
- Strelcheck, A.J.; Fitzhugh, G.R.; Coleman, F.C.; Koenig, C.C. Otolith–fish size relationship in juvenile gag (Mycteroperca microlepis) of the eastern Gulf of Mexico: A comparison of growth rates between laboratory and field populations. Fish. Res. 2003, 60, 255–265. [Google Scholar] [CrossRef]
- D’Iglio, C.; Albano, M.; Famulari, S.; Savoca, S.; Panarello, G.; Di Paola, D.; Perdichizzi, A.; Rinelli, P.; Lanteri, G.; Spanò, N.; et al. Intra- and interspecific variability among congeneric Pagellus otoliths. Sci. Rep. 2021, 11, 16315. [Google Scholar] [CrossRef]
- D’Iglio, C.; Natale, S.; Albano, M.; Savoca, S.; Famulari, S.; Gervasi, C.; Lanteri, G.; Panarello, G.; Spanò, N.; Capillo, G. Otolith Analyses Highlight Morpho-Functional Differences of Three Species of Mullet (Mugilidae) from Transitional Water. Sustainability 2022, 14, 398. [Google Scholar] [CrossRef]
- Avigliano, E.; Velasco, G.; Volpedo, A.V. Use of lapillus otolith microchemistry as an indicator of the habitat of Genidens barbus from different estuarine environments in the southwestern Atlantic Ocean. Environ. Biol. Fishes 2015, 98, 1623–1632. [Google Scholar] [CrossRef]
- Ding, L.; Tao, J.; Ding, C.; Chen, L.; Zhang, C.; Xiang, Q.; Sun, J. Hydrogeomorphic factors drive differences in otolith morphology in fish from the Nu-Salween River. Ecol. Freshw. Fish 2018, 28, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.F.; Wu, C.Z. The Fishes of the Qinghai Xizang Plateau; Sichuan Publishing House of Science and Technology: Chengdu, China, 1992. [Google Scholar]
- Chen, Y.F.; Cao, W.X. Schizothoracinae. In Fauna Sinica, Osteichthyes: Cypriniformes III; Yue, P.Q., Ed.; Science Press: Beijing, China, 2000; pp. 275–390. [Google Scholar]
- Qiao, J.; Hu, J.; Xia, Q.; Zhu, R.; Chen, K.; Zhao, J.; Yan, Y.; Chu, L.; He, D. Pelagic–benthic resource polymorphism in Schizopygopsis thermalis Herzenstein 1891 (Pisces, Cyprinidae) in a headwater lake in the Salween River system on the Tibetan Plateau. Ecol. Evol 2020, 10, 7431–7444. [Google Scholar] [CrossRef] [PubMed]
- Campana, S.E.; Casselman, J.M. Stock discrimination using otolith shape analysis. Can. J. Fish. Aquat. Sci. 1993, 50, 1062–1083. [Google Scholar] [CrossRef]
- Chen, F.; Chen, Y.; He, D. Age and growth of Schizopygopsis younghusbandi in the Yarlung Zangbo River in Tibet, China. Environ. Biol. Fishes 2009, 86, 155. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, Y. Otolith microstructure of Oxygymnocypris stewartii (Cypriniformes, Cyprinidae, Schizothoracinae) in the Lhasa River in Tibet, China. Environ. Biol. Fishes 2009, 86, 45–52. [Google Scholar] [CrossRef]
- Ding, C.; He, D.; Chen, Y.; Jia, Y.; Tao, J. Otolith microstructure analysis based on wild young fish and its application in confirming the first annual increment in tibetan. gymnocypris selincuoensis. Fish. Res. 2020, 221, 105386. [Google Scholar] [CrossRef]
- Chen, K.; He, D.; Ding, C.; Jia, Y.; Chen, Y. Evaluation of the lapillar otolith shape as a tool for discrimination of stock of naked carp, gymnocypris selincuoensis in the QTP. Pak. J. Zool. 2021, 53, 1–13. [Google Scholar] [CrossRef]
- Libungan, L.A.; Pálsson, S. ShapeR: An R package to study otolith shape variation among fish populations. PLoS ONE 2015, 10, 1–12. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation, for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 8 April 2021).
- Tuset, V.M.; Lozano, I.J.; González, J.; Pertusa, J.F.; García-Díaz, M.M. Shape indices to identify regional differences in otolith morphology of comber, Serranus cabrilla (L., 1758). J. Appl. Ichthyol. 2003, 19, 88–93. [Google Scholar] [CrossRef]
- Lombarte, A.; Lleonart, J. Otolith size changes related with body growth, habitat depth and temperature. Environ. Biol. Fishes 1993, 37, 297–306. [Google Scholar] [CrossRef]
- Kikuchi, E.; Garcia, S.; Costa, P.A.; Cardoso, L.G.; Haimovici, M. Discrimination of red porgy Pagrus pagrus (Sparidae) potential stocks in the south-western Atlantic by otolith shape analysis. J. Fish Biol. 2021, 98, 548–556. [Google Scholar] [CrossRef]
- Bose, A.P.; Mccallum, E.S.; Raymond, K.; Marentette, J.R.; Balshine, S.J. Growth and otolith morphology vary with alternative reproductive tactics and contaminant exposure in the round goby Neogobius melanostomus. J. Fish Biol. 2018, 93, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Wiecaszek, B.; Nowosielski, A.; Dabrowski, J.; Gorecka, K.; Keszka, S.; Strzelczak, A. Fish size effect on sagittal otolith outer shape variability in round goby Neogobius melanostomus (Pallas 1814). J. Fish Biol. 2020, 97, 1520–1541. [Google Scholar] [CrossRef] [PubMed]
- Waessle, J.A.; Lasta, C.A.; Favero, M. Otolith morphology and body size relationships for juvenile Sciaenidae in the Río de la Plata estuary (35–36°S). Sci. Mar. 2003, 67, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Hüssy, K. Otolith shape in juvenile cod (Gadus morhua): Ontogenetic and environmental effects. J. Exp. Mar. Biol. Ecol. 2008, 364, 35–41. [Google Scholar] [CrossRef]
- Capoccioni, F.; Costa, C.; Aguzzi, J.; Menesatti, P.; Lombarte, A.; Ciccotti, E. Ontogenetic and environmental effects on otolith shape variability in three Mediterranean European eel (Anguilla anguilla, L.) local stocks. J. Exp. Mar. Biol. Ecol. 2011, 397, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Aguirre, H.; Lombarte, A. Ecomorphological comparisons of sagittae in Mullus barbatus and M. surmuletus. J. Fish Biol. 1999, 55, 105–114. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1095-8649.1999.tb00660.x (accessed on 22 April 2022). [CrossRef]
- Randon, M.; Le Pape, O.; Ernande, B.; Mahe, K.; Volckaert, F.M.; Petit, E.J.; Reveillac, E. Complementarity and discriminatory power of genotype and otolith shape in describing the fine-scale population structure of an exploited fish, the common sole of the Eastern English Channel. PLoS ONE 2020, 15, e0241429. [Google Scholar] [CrossRef]
- Parmentier, E.; Boistel, R.; Bahri, M.A.; Plenevaux, A.; Schwarzhans, W. Sexual dimorphism in the sonic system and otolith morphology of Neobythites gilli (Ophidiiformes). J. Zool. 2018, 305, 274–280. [Google Scholar] [CrossRef] [Green Version]
- Morales-Nin, B. Review of the growth regulation processes of otolith daily increment formation. Fish. Res. 2000, 46, 53–67. [Google Scholar] [CrossRef]
- Vignon, M. Ontogenetic trajectories of otolith shape during shift in habitat use: Interaction between otolith growth and environment. J. Exp. Mar. Biol. Ecol. 2012, 420, 26–32. [Google Scholar] [CrossRef]
- Gutiérrez, E.; Morales-Nin, B. Time series analysis of daily growth in Dicentrarchus labrax L. otoliths. J. Exp. Mar. Biol. Ecol. 1986, 103, 163–179. [Google Scholar] [CrossRef]
- Maillet, G.L.; Checkley, D.M. Storm-related variation in the growth rate of otoliths of larval Atlantic menhaden Brevoortia tyrannus: A time series analysis of biological and physical variables and implications for larva growth and mortality. Mar. Ecol. Prog. Ser. 1991, 79, 1–16. [Google Scholar] [CrossRef]
- Bani, A.; Poursaeid, S.; Tuset, V.M. Comparative morphology of the sagittal otolith in three species of south Caspian gobies. J. Fish Biol. 2013, 82, 1321–1332. [Google Scholar] [CrossRef]
- Flock, A. Structure of the macula utriculi with special reference to directional interplay of sensory responses as revealed by morphological polarization. J. Cell Biol. 1964, 22, 413–431. [Google Scholar] [CrossRef]
- Hawkins, A.D. Underwater Sound and Fish Behaviour. In Behaviour of Teleost Fishes; Pitcher, T.J., Ed.; Chapman and Hall: London, UK, 1993; pp. 129–169. [Google Scholar] [CrossRef]
- Gauldie, R.W.; Nelson, D. Otolith growth in fishes. Comp. Biochem. Physiol. Part A Physiol. 1990, 97, 119–135. [Google Scholar] [CrossRef]
- Devries, D.A.; Grimes, C.B.; Prager, M.H. Using otolith shape analysis to distinguish eastern gulf of mexico and atlantic ocean stocks of king mackerel. Fish. Res. 2002, 57, 51–62. [Google Scholar] [CrossRef]
- Stransky, C. Geographic variation of golden redfish (Sebastes marinus) and deep-sea redfish (S. mentella) in the North Atlantic based on otolith shape analysis. ICES J. Mar. Sci. 2005, 62, 1691–1698. [Google Scholar] [CrossRef] [Green Version]

| Morphs | N | Total Length (mm) | Weight (g) | Sex Ratio | |||
|---|---|---|---|---|---|---|---|
| Range | Mean ± SD | Range | Mean ± SD | Male:Female | |||
| All | Benthivorous morph | 206 | 268–517 | 372.35 ± 52.67 | 109.2–1036.2 | 440.88 ± 181.49 | 2.07:1 |
| Planktivorous morph | 226 | 220–515 | 383.52 ± 56.70 | 80.1–974.6 | 450.44 ± 177.87 | 1.86:1 | |
| Group 1 | Benthivorous morph | 34 | 268–323 | 293.97 ± 14.83 | 109.2–280.8 | 204.38 ± 42.07 | 3.25:1 |
| Planktivorous morph | 37 | 220–321 | 292.41 ± 18.98 | 80.1–280.0 | 201.47 ± 36.57 | 3.6:1 | |
| Group 2 | Benthivorous morph | 123 | 324–410 | 366.04 ± 25.48 | 238.9–706.4 | 413.42 ± 99.86 | 1.37:1 |
| Planktivorous morph | 111 | 324–410 | 372.33 ± 24.91 | 140.4–649.4 | 404.71 ± 90.82 | 0.85:1 | |
| Group 3 | Benthivorous morph | 49 | 412–517 | 442.59 ± 23.57 | 412.4–1036.2 | 673.94 ± 130.85 | 6:1 |
| Planktivorous morph | 78 | 411–515 | 442.64 ± 25.09 | 409.0–974.6 | 633.63 ± 115.07 | 6.09:1 | |
| Independent Variables | Dependent Variables | Morphs | Equation | a | b | R2 | p | |
|---|---|---|---|---|---|---|---|---|
| All | TL | OL | Benthivorous morph | y = −3.45 + 0.795x | −3.45 | 0.8 | 0.67 | <0.001 *** |
| Planktivorous morph | y = −3.95 + 0.883x | −3.95 | 0.88 | 0.73 | <0.001 *** | |||
| TL | OW | Benthivorous morph | y = −3.19 + 0.702x | −3.19 | 0.7 | 0.49 | <0.001 *** | |
| Planktivorous morph | y = −3.21 + 0.708x | −3.21 | 0.71 | 0.58 | <0.001 *** | |||
| TL | OA | Benthivorous morph | y = − 6.55 + 1.44x | −6.55 | 1.44 | 0.59 | <0.001 *** | |
| Planktivorous morph | y = −7.22 + 1.55x | −7.22 | 1.55 | 0.68 | <0.001 *** | |||
| TL | OP | Benthivorous morph | y = −2.42 + 0.804x | −2.42 | 0.8 | 0.61 | <0.001 *** | |
| Planktivorous morph | y = −2.80 + 0.873x | −2.80 | 0.87 | 0.68 | <0.001 *** | |||
| TL | OWE | Benthivorous morph | y = −10.30 + 2.21x | −10.30 | 2.21 | 0.61 | <0.001 *** | |
| Planktivorous morph | y = −10.40+ 2.24x | −10.40 | 2.24 | 0.64 | <0.001 *** | |||
| Group 1 | TL | OL | Benthivorous morph | y = −3.87 + 0.87x | −3.87 | 0.87 | 0.26 | =0.002 ** |
| Planktivorous morph | y = −5.72 + 1.11x | −5.72 | 1.11 | 0.51 | <0.001 *** | |||
| TL | OW | Benthivorous morph | y = −3.26 + 0.716x | −3.26 | 0.72 | 0.13 | =0.04 * | |
| Planktivorous morph | y = −4.91 + 1.01x | −4.91 | 1.01 | 0.41 | <0.001 *** | |||
| TL | OA | Benthivorous morph | y = −7.48 + 1.60x | −7.48 | 1.6 | 0.23 | =0.004 ** | |
| Planktivorous morph | y = −10.60 + 2.16x | −10.60 | 2.16 | 0.51 | <0.001 *** | |||
| TL | OP | Benthivorous morph | y = −2.24 + 0.775x | −2.24 | 0.78 | 0.2 | =0.009 ** | |
| Planktivorous morph | y = −4.15 + 1.11x | −4.15 | 1.11 | 0.46 | <0.001 *** | |||
| TL | OWE | Benthivorous morph | y = −11.70 + 2.48x | −11.70 | 2.48 | 0.23 | =0.004 ** | |
| Planktivorous morph | y = −12.00 + 2.53x | −12.00 | 2.53 | 0.53 | <0.001 *** | |||
| Group 2 | TL | OL | Benthivorous morph | y = −3.41 + 0.788x | −3.41 | 0.79 | 0.31 | <0.001 *** |
| Planktivorous morph | y = −3.96 + 0.884x | −3.96 | 0.88 | 0.34 | <0.001 *** | |||
| TL | OW | Benthivorous morph | y = −2.88 + 0.649x | −2.88 | 0.65 | 0.15 | <0.001 *** | |
| Planktivorous morph | y = −2.99 + 0.67x | −2.99 | 0.67 | 0.21 | <0.001 *** | |||
| TL | OA | Benthivorous morph | y = −6.49+ 1.42x | −6.49 | 1.42 | 0.24 | <0.001 *** | |
| Planktivorous morph | y = −6.67 + 1.46x | −6.67 | 1.46 | 0.27 | <0.001 *** | |||
| TL | OP | Benthivorous morph | y = −2.36 + 0.792x | −2.36 | 0.79 | 0.25 | <0.001 *** | |
| Planktivorous morph | y = −2.81 + 0.875x | −2.81 | 0.88 | 0.3 | <0.001 *** | |||
| TL | OWE | Benthivorous morph | y = −11.20 + 2.36x | −11.20 | 2.36 | 0.28 | <0.001 *** | |
| Planktivorous morph | y = −10.90 + 2.32x | −10.90 | 2.32 | 0.29 | <0.001 *** | |||
| Group 3 | TL | OL | Benthivorous morph | y = −1.74 + 0.516x | −1.74 | 0.52 | 0.11 | =0.021 * |
| Planktivorous morph | y = −4.01 + 0.891x | −4.01 | 0.89 | 0.25 | <0.001 *** | |||
| TL | OW | Benthivorous morph | y = −4.06 + 0.846x | −4.06 | 0.85 | 0.22 | <0.001 *** | |
| Planktivorous morph | y = −3.50 + 0.756x | −3.50 | 0.76 | 0.14 | <0.001 *** | |||
| TL | OA | Benthivorous morph | y = −5.41 + 1.25x | −5.41 | 1.25 | 0.14 | =0.009 ** | |
| Planktivorous morph | y = −7.65 + 1.63x | −7.65 | 1.63 | 0.22 | <0.001 *** | |||
| TL | OP | Benthivorous morph | y = −1.75 + 0.697x | −1.75 | 0.7 | 0.14 | =0.007 ** | |
| Planktivorous morph | y = −3.00 + 0.907x | −3.00 | 0.91 | 0.2 | <0.001 *** | |||
| TL | OWE | Benthivorous morph | y = −4.26 + 1.23x | −4.26 | 1.23 | 0.09 | =0.038 * | |
| Planktivorous morph | y = −11.40 + 2.41x | −11.40 | 2.41 | 0.21 | <0.001 *** |
| Shape Indices | Benthivorous Morph | Planktivorous Morph | |||||
|---|---|---|---|---|---|---|---|
| t | df | p | t | df | p | ||
| All | Otolith length | −1.923 | 204 | 0.056 | 1.422 | 224 | 0.156 |
| Aspect ratio | 0.872 | 204 | 0.384 | 2.036 | 224 | 0.043 * | |
| Circularity | −1.512 | 204 | 0.132 | −0.439 | 224 | 0.661 | |
| Surface density | −4.15 | 204 | 0.000 *** | −2.815 | 224 | 0.005 ** | |
| Group 1 | Otolith length | −1.622 | 32 | 0.115 | −0.937 | 35 | 0.355 |
| Aspect ratio | 1.885 | 32 | 0.069 | 1.152 | 35 | 0.257 | |
| Circularity | −2.168 | 32 | 0.038 * | −2.316 | 35 | 0.027 * | |
| Surface density | −0.17 | 32 | 0.866 | 0.51 | 35 | 0.614 | |
| Group 2 | Otolith length | −1.434 | 121 | 0.154 | 1.249 | 109 | 0.214 |
| Aspect ratio | 0.376 | 121 | 0.707 | 0.983 | 109 | 0.328 | |
| Circularity | −0.17 | 121 | 0.865 | 2.71 | 109 | 0.008 ** | |
| Surface density | −4.195 | 121 | 0.000 *** | −0.914 | 109 | 0.363 | |
| Group 3 | Otolith length | −0.02 | 47 | 0.984 | 1.335 | 76 | 0.186 |
| Otolith width | 0.369 | 47 | 0.714 | 0.345 | 76 | 0.731 | |
| Aspect ratio | −0.504 | 47 | 0.617 | 0.876 | 76 | 0.384 | |
| Circularity | −0.437 | 47 | 0.664 | −0.493 | 76 | 0.623 | |
| Surface density | −2.034 | 47 | 0.048 * | −0.951 | 76 | 0.345 | |
| Shape Indices | Benthivorous Morph | Planktivorous Morph | t | df | p | |
|---|---|---|---|---|---|---|
| All | Otolith length | 3.57 ± 0.29 | 3.64 ± 0.30 | −2.613 | 430 | 0.009 ** |
| Aspect ratio | 1.34 ± 0.09 | 1.35 ± 0.09 | −0.798 | 430 | 0.425 | |
| Circularity | 15.42 ± 0.84 | 15.75 ± 0.99 | −3.672 | 430 | 0.000 *** | |
| Surface density | 2.43 ± 0.83 | 2.65 ± 0.95 | −2.545 | 430 | 0.011 * | |
| Group 1 | Otolith length | 2.94 ± 0.22 | 2.91 ± 0.22 | 0.578 | 69 | 0.565 |
| Aspect ratio | 1.31 ± 0.07 | 1.28 ± 0.09 | 1.327 | 69 | 0.189 | |
| Circularity | 14.91 ± 0.52 | 15.17 ± 0.75 | −1.713 | 69 | 0.091 | |
| Surface density | 2.11 ± 0.36 | 2.13 ± 0.40 | −0.225 | 69 | 0.823 | |
| Group 2 | Otolith length | 3.48 ± 0.29 | 3.58 ± 0.31 | −2.57 | 232 | 0.011 * |
| Aspect ratio | 1.34 ± 0.09 | 1.35 ± 0.07 | −0.928 | 232 | 0.354 | |
| Circularity | 15.24 ± 0.83 | 15.51 ± 0.72 | −2.681 | 232 | 0.008 ** | |
| Surface density | 2.33 ± 0.48 | 2.48 ± 0.49 | −2.489 | 232 | 0.014 * | |
| Group 3 | Otolith length | 4.09 ± 0.31 | 4.17 ± 0.35 | −1.395 | 125 | 0.165 |
| Otolith width | 3.01 ± 0.25 | 3.03 ± 0.32 | −0.518 | 125 | 0.606 | |
| Aspect ratio | 1.36 ± 0.09 | 1.38 ± 0.10 | −1.066 | 125 | 0.289 | |
| Circularity | 16.04 ± 0.99 | 16.36 ± 1.35 | −1.417 | 125 | 0.159 | |
| Surface density | 2.85 ± 0.43 | 2.81 ± 0.59 | 0.332 | 125 | 0.740 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, J.; Zhu, R.; Chen, K.; Zhang, D.; Yan, Y.; He, D. Comparative Otolith Morphology of Two Morphs of Schizopygopsis thermalis Herzenstein 1891 (Pisces, Cyprinidae) in a Headwater Lake on the Qinghai-Tibet Plateau. Fishes 2022, 7, 99. https://doi.org/10.3390/fishes7030099
Qiao J, Zhu R, Chen K, Zhang D, Yan Y, He D. Comparative Otolith Morphology of Two Morphs of Schizopygopsis thermalis Herzenstein 1891 (Pisces, Cyprinidae) in a Headwater Lake on the Qinghai-Tibet Plateau. Fishes. 2022; 7(3):99. https://doi.org/10.3390/fishes7030099
Chicago/Turabian StyleQiao, Jialing, Ren Zhu, Kang Chen, Dong Zhang, Yunzhi Yan, and Dekui He. 2022. "Comparative Otolith Morphology of Two Morphs of Schizopygopsis thermalis Herzenstein 1891 (Pisces, Cyprinidae) in a Headwater Lake on the Qinghai-Tibet Plateau" Fishes 7, no. 3: 99. https://doi.org/10.3390/fishes7030099
APA StyleQiao, J., Zhu, R., Chen, K., Zhang, D., Yan, Y., & He, D. (2022). Comparative Otolith Morphology of Two Morphs of Schizopygopsis thermalis Herzenstein 1891 (Pisces, Cyprinidae) in a Headwater Lake on the Qinghai-Tibet Plateau. Fishes, 7(3), 99. https://doi.org/10.3390/fishes7030099







