Pre-Hatching Ontogenetic Changes of Morphological Characters of Small-Spotted Catshark (Scyliorhinus canicula)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Morphometry
2.2. Statistics
3. Results
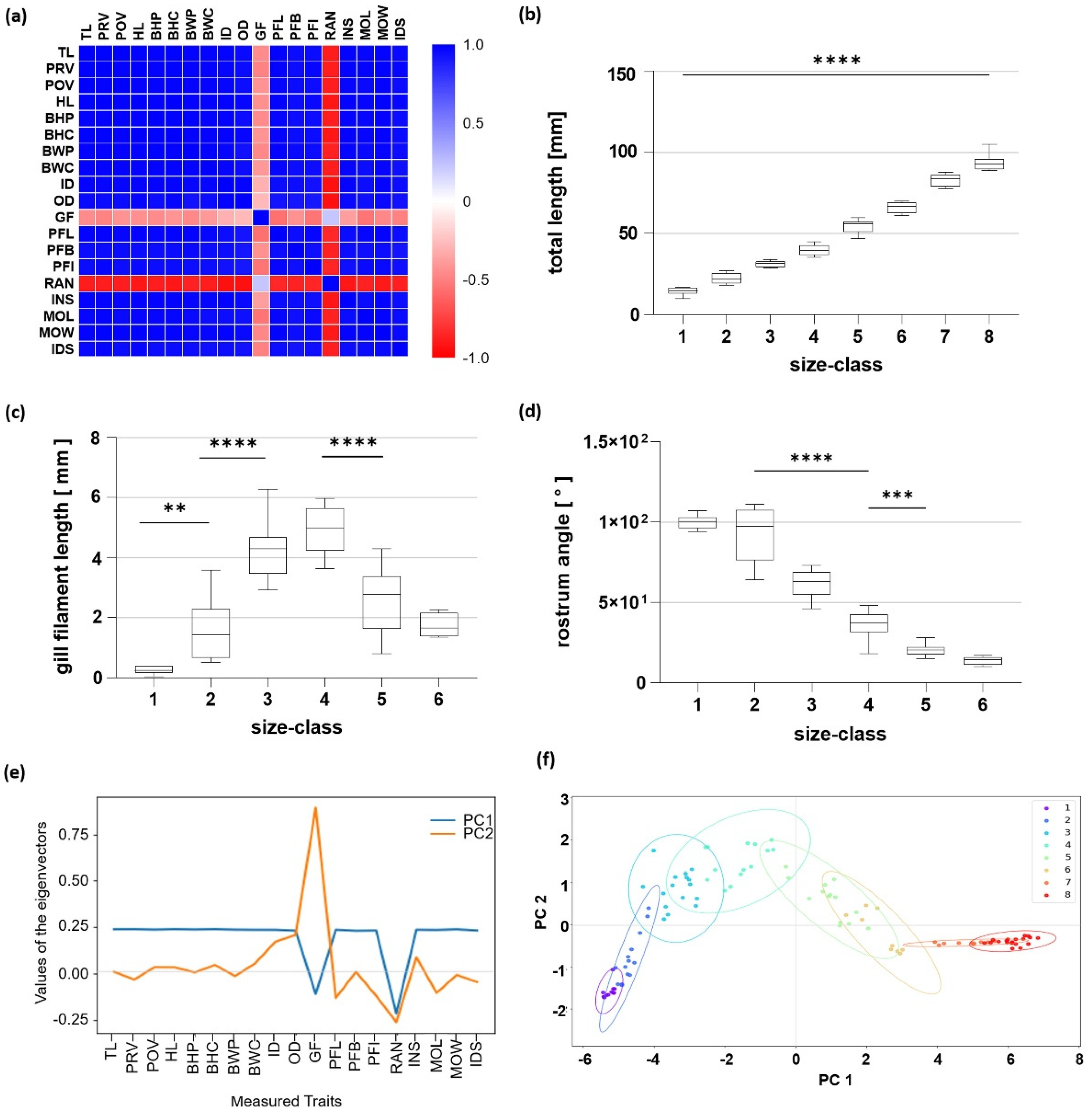
3.1. Morphometric Analysis
3.2. Measurements in Relation to Total Length
4. Discussion
4.1. Stages Leading to Pre-Hatching
4.2. Stages from Pre-Hatching to Hatching
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bibliography Database of Living/Fossil Sharks, Rays and Chimaeras (Chondrichthyes: Elasmobranchii, Holocephali). Available online: www.shark-references.com (accessed on 12 January 2022).
- Coolen, M.; Menuet, A.; Chassoux, D.; Compagnucci, C.; Henry, S.; Lévèque, L.; Da Silva, C.; Gavory, F.; Samain, S.; Wincker, P.; et al. The Dogfish Scyliorhinus canicula: A Reference in Jawed Vertebrates. Cold Spring Harb. Protoc. 2008, 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- López-Unzu, M.A.; Durán, A.C.; Rodríguez, C.; Soto-Navarrete, M.T.; Sans-Coma, V.; Fernández, B. Development of the ventricular myocardial trabeculae in Scyliorhinus canicula (Chondrichthyes): Evolutionary implications. Sci. Rep. 2020, 10, 14434. [Google Scholar] [CrossRef] [PubMed]
- Aicardi, S.; Amaroli, A.; Gallus, L.; Di Blasi, D.; Ghigliotti, L.; Betti, F.; Vacchi, M.; Ferrando, S. Quantification of neurons in the olfactory bulb of the catsharks Scyliorhinus canicula (Linnaeus, 1758) and Galeus melastomus (Rafinesque, 1810). Zoology 2020, 141, 125796. [Google Scholar] [CrossRef] [PubMed]
- Dearden, R.P.; Mansuit, R.; Cuckovic, A.; Herrel, A.; Didier, D.; Tafforeau, P.; Pradel, A. The morphology and evolution of chondrichthyan cranial muscles: A digital dissection of the elephantfish Callorhinchus milii and the catshark Scyliorhinus canicula. J. Anat. 2021, 238, 1082–1105. [Google Scholar] [CrossRef] [PubMed]
- Bernal, D.; Sepulveda, C.; Mathieu-Costello, O.; Graham, J.B. Comparative studies of high performance swimming in sharks I. Red muscle morphometrics, vascularization and ultrastructure. J. Exp. Biol. 2003, 206, 2831–2843. [Google Scholar] [CrossRef] [Green Version]
- Lauriano, E.; Pergolizzi, S.; Aragona, M.; Montalbano, G.; Guerrera, M.; Crupi, R.; Faggio, C.; Capillo, G. Intestinal immunity of dogfish Scyliorhinus canicula spiral valve: A histochemical, immunohistochemical and confocal study. Fish Shellfish Immunol. 2019, 87, 490–498. [Google Scholar] [CrossRef]
- Crouch, K.; Smith, L.E.; Williams, R.; Cao, W.; Lee, M.; Jensen, A.; Dooley, H. Humoral immune response of the small-spotted catshark, Scyliorhinus canicula. Fish Shellfish Immunol. 2013, 34, 1158–1169. [Google Scholar] [CrossRef]
- Pettinello, R.; Redmond, A.; Secombes, C.J.; Macqueen, D.J.; Dooley, H. Evolutionary history of the T cell receptor complex as revealed by small-spotted catshark (Scyliorhinus canicula). Dev. Comp. Immunol. 2017, 74, 125–135. [Google Scholar] [CrossRef]
- Soares, K.D.A.; Carvalho, M.R. The catshark genus Scyliorhinus (Chondrichthyes: Carcharhiniformes: Scyliorhinidae): Taxonomy, morphology and distribution. Zootaxa 2019, 4601, 1–147. [Google Scholar] [CrossRef]
- Ebert, D.A.; Dando, M. Field Guide to Sharks, Rays & Chimaeras of Europe and the Mediterranean; Princeton University Press: Princeton, NJ, USA, 2021; p. 383. [Google Scholar]
- Finucci, B.; Derrick, D.; Neat, F.C.; Pacoureau, N.; Serena, F.; VanderWright, W.J. Scyliorhinus canicula. The IUCN Red List of Threatened Species 2021. 2021. 2021. Available online: www.iucnredlist.org/species/161307554/124478351 (accessed on 12 January 2022).
- Compagno, L.J.V. Bullhead, mackerel and carpet sharks (Heterodontiformes, Lamniformes and Orectolobiformes). In Sharks of the World. An Annotated and Illustrated Catalogue of Shark Species Known to Date; FAO Species Catalogue for Fishery Purposes 2002; FAO: Rome, Italy, 2001; Volume 2, 269p. [Google Scholar]
- Ballard, W.W.; Mellinger, J.; Lechenault, H. A series of normal stages for development of Scyliorhinus canicula, the lesser spotted dogfish (Chondrichthyes: Scyliorhinidae). J. Exp. Zool. 1993, 267, 318–336. [Google Scholar] [CrossRef]
- López-Romero, F.A.; Klimpfinger, C.; Tanaka, S.; Kriwet, J. Growth trajectories of prenatal embryos of the deep-sea shark Chlamydoselachus anguineus (Chondrichthyes). J. Fish Biol. 2020, 97, 212–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grunow, B.; Kirchhoff, T.; Lange, T.; Moritz, T.; Harzsch, S. Histochemistry on vibratome sections of fish tissue: A comparison of fixation and embedding methods. Aquat. Biol. 2015, 23, 251–263. [Google Scholar] [CrossRef] [Green Version]
- Soares, K.D.A.; Gomes, U.L.; De Carvalho, M.R. Taxonomic review of catsharks of the Scyliorhinus haeckelii group, with the description of a new species (Chondrichthyes: Carcharhiniformes: Scyliorhinidae). Zootaxa 2016, 4066, 501–534. [Google Scholar] [CrossRef] [PubMed]
- Franz, G.P.; Lewerentz, L.; Grunow, B. Growth changes during the embryonic-larval-transition of pikeperch (Sander lucioperca). J. Fish Biol. 2021, 99, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res 2011, 12, 2825–2830. [Google Scholar]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Waskom, M.; Botvinnik, O.; Ostblom, J.; Gelbart, M.; Lukauskas, S.; Hobson, P.; Gemperline, D.C.; Augspurger, T.; Yaroslav, H.; Cole, J.B.; et al. Mwaskom/Seaborn: V0.10.1 (April 2020); Version v0.10.1; Zenodo: Geneva, Switzerland, 2020. [Google Scholar] [CrossRef]
- Didier, D.A.; LeClair, E.E.; Vanbuskirk, D.R. Embryonic staging and external features of development of the Chimaeroid fish, Callorhinchus milii (Holocephali, Callorhinchidae). J. Morphol. 1998, 236, 25–47. [Google Scholar] [CrossRef]
- Maxwell, E.E.; Fröbisch, N.B.; Heppleston, A.C. Variability and Conservation in Late Chondrichthyan Development: Ontogeny of the Winter Skate (Leucoraja ocellata). Anat. Rec. 2008, 291, 1079–1087. [Google Scholar] [CrossRef]
- Rodda, K.R.; Seymour, R.S. Functional morphology of embryonic development in the Port Jackson shark Heterodontus portusjacksoni (Meyer). J. Fish Biol. 2008, 72, 961–984. [Google Scholar] [CrossRef]
- Musa, S.M.; Ripley, D.M.; Moritz, T.; Shiels, H.A. Ocean warming and hypoxia affect embryonic growth, fitness and survival of small-spotted catsharks, Scyliorhinus canicula. J. Fish Biol. 2020, 97, 257–264. [Google Scholar] [CrossRef]
- Tanaka, S.; Shiobara, Y.; Hioki, S.; Abe, H.; Nishi, G.; Yano, K.; Suzuki, K. The Reproductive Biology of the Frilled Shark, Chlamydoselachus anguineus, from Suruga Bay, Japan. Jpn. J. Ichthyol. 1990, 37, 273–291. [Google Scholar]
- Onimaru, K.; Motone, F.; Kiyatake, I.; Nishida, K.; Kuraku, S. A staging table for the embryonic development of the brownbanded bamboo shark (Chiloscyllium punctatum). Dev. Dyn. 2018, 247, 712–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hook, S.A.; McMurray, C.; Ripley, D.; Allen, N.; Moritz, T.; Grunow, B.; Shiels, H.A. Recognition software successfully aids the identification of individual small-spotted catsharks Scyliorhinus canicula during their first year of life. J. Fish Biol. 2019, 95, 1465–1470. [Google Scholar] [CrossRef] [Green Version]
- Crooks, N.; Babey, L.; Haddon, W.J.; Love, A.C.; Waring, C.P. Sexual Dimorphisms in the Dermal Denticles of the Lesser-Spotted Catshark, Scyliorhinus canicula (Linnaeus, 1758). PLoS ONE 2013, 8, e76887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, E. A Contribution to Our Knowledge of the Life-Histories of the Dogfishes Landed at Plymouth. J. Mar. Biol. Assoc. 1921, 12, 468–505. [Google Scholar] [CrossRef] [Green Version]
- Filiz, H.; Taskavak, E. Sexual dimorphism in the head, mouth, and body morphology of the small spotted catshark, Scyliorhinus canicula (Linnaeus, 1758) (Chondrichthyes: Scyliorhinidae) from Turkey. Acta Adriat. 2006, 47, 37–47. [Google Scholar]
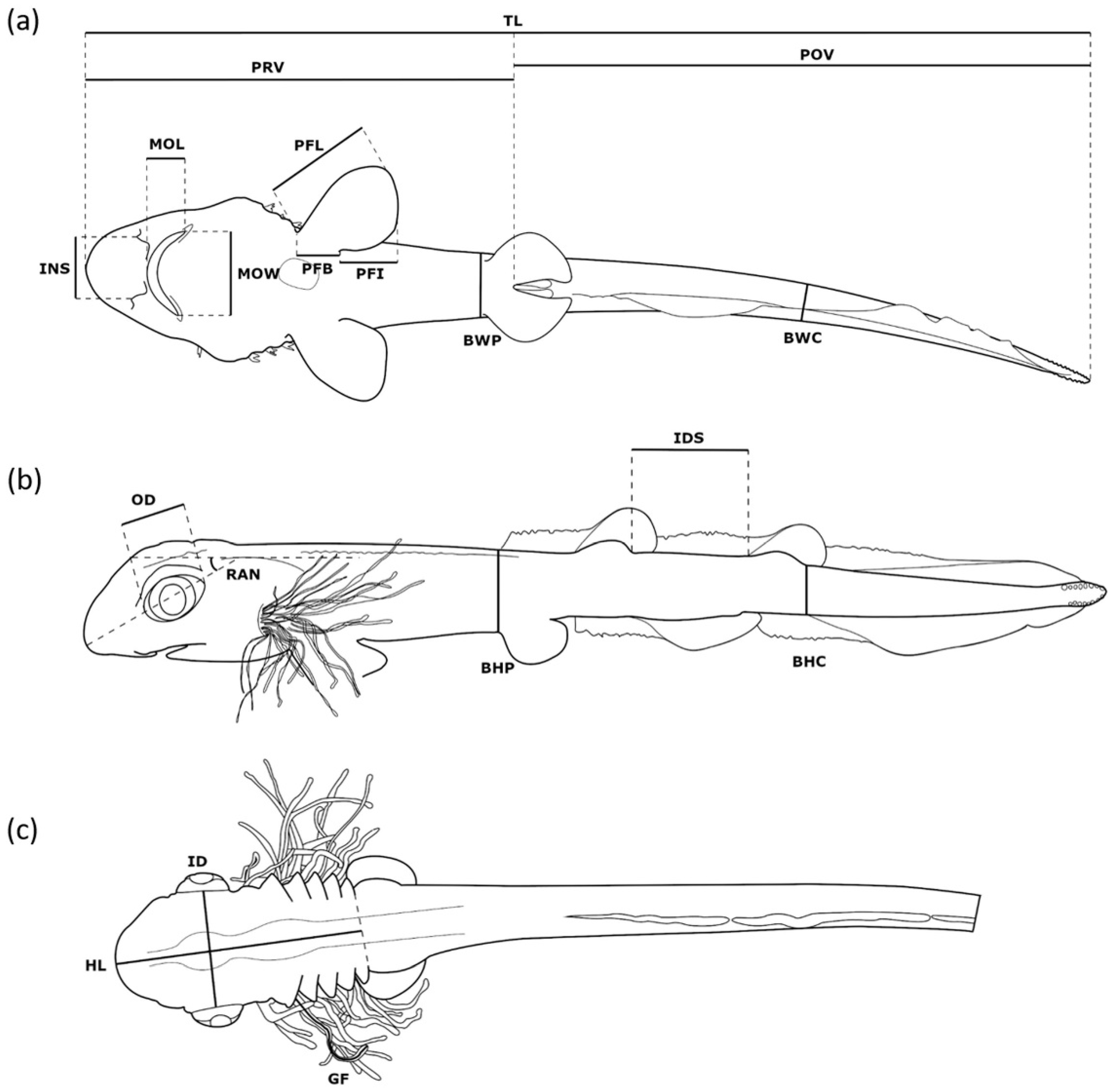

| Abbreviation | Name | Description |
|---|---|---|
| BHC | Body depth at caudal peduncle | Maximal distance dorsal to ventral margin at base of caudal fin 1 |
| BHP | Body depth at pelvic fin | Maximal distance dorsal to ventral at origin of pelvic-fin base 1 |
| BWC | Body width at caudal peduncle | Maximal width at caudal peduncle |
| BWP | Body width at pelvic fin | Maximal width at origin of pelvic-fin base |
| GF | Gill filament length | Estimated average length of external gill filaments of second gill slit |
| HL | Head length | Distance from rostral tip to fifth gill slit |
| ID | Interorbital distance | Width of neurocranium between midlevel of eyes |
| IDS | Interdorsal space | Distance from first dorsal-fin base to second dorsal-fin base |
| INS | Internostril space | Distance between nostrils at base of anterior nasal flaps 2 |
| MOL | Mouth length | Distance from anteriormost point of mouth opening to posterior level of cleft end along body axis |
| MOW | Mouth width | Distance from left to right corner of mouth |
| OD | Orbita diameter | Length of eye along body axis |
| PFB | Pectoral fin base | Width of pectoral-fin base |
| PFI | Pectoral fin inner margin | Length of posterior margin of pectoral fin |
| PFL | Pectoral fin length | Longest distance from anterior base to tip of pectoral fin |
| POV | Post-vent length | Body length from vent to end of tail |
| PRV | Pre-vent length | Body length from rostral tip to vent |
| RAN | Rostrum angle | Angle from rostral tip to body axis going anterior. Body axis is drawn from most distal anterior point over midpoint of otic capsule to midpoint of chorda at height of pectoral fin. |
| TL | Total length | Distance from rostral tip to end of tail |
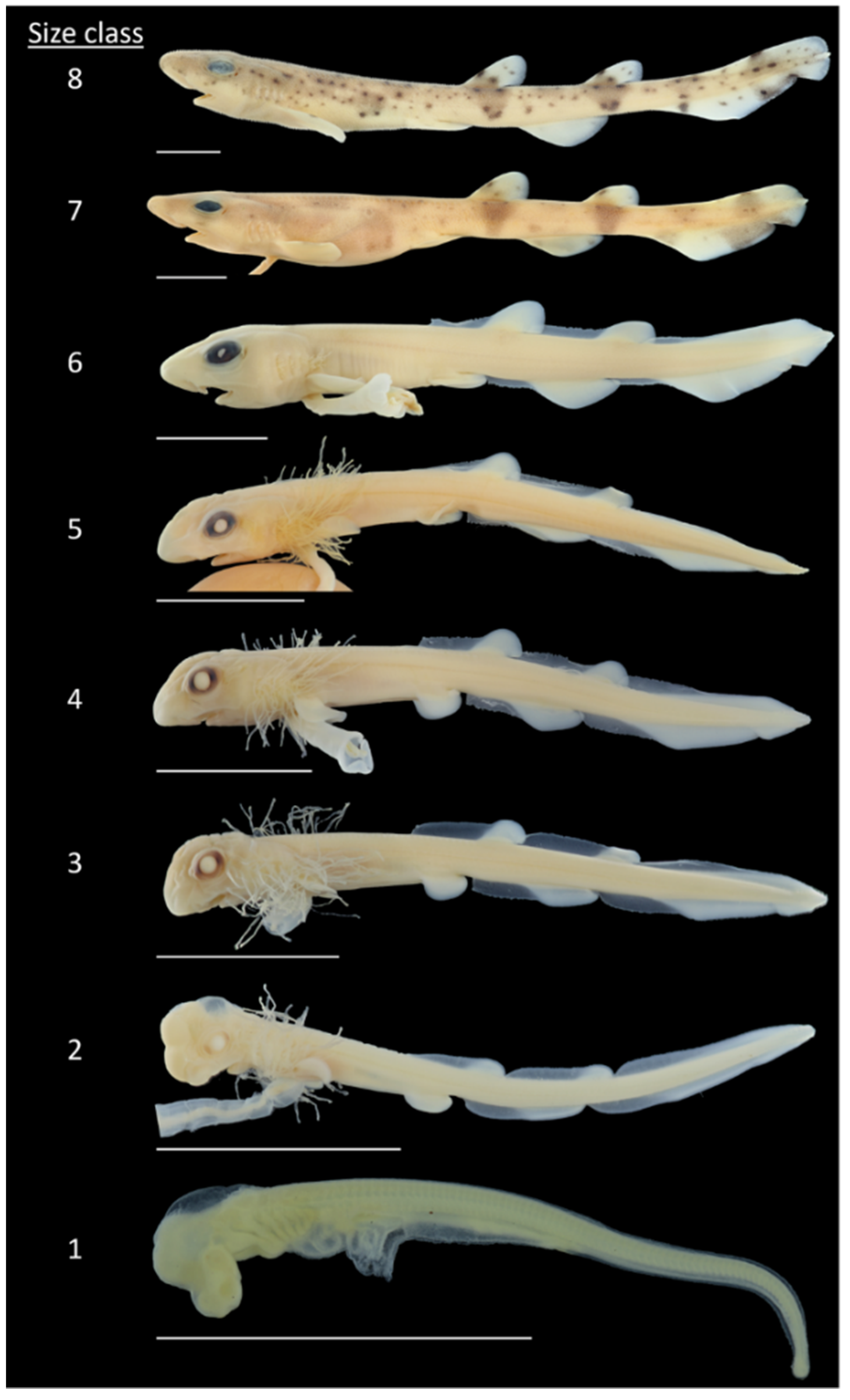
| Size Class | n | Total Length (mm) | Stages According to Ballard et al. (1993) |
|---|---|---|---|
| 1 | 11 | 9.7–16.8 | 24–26 |
| 2 | 13 | 18.0–27.0 | 27 |
| 3 | 16 | 28.6–33.8 | 28–29 |
| 4 | 14 | 35.2–44.8 | 31 |
| 5 | 14 | 46.7–59.6 | 32 |
| 6 | 9 | 61.1–69.9 | 34 |
| 7 | 10 | 77.5–87.7 | Not defined |
| 8 | 20 | 88.9–104.9 | Not defined |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grunow, B.; Reismann, T.; Moritz, T. Pre-Hatching Ontogenetic Changes of Morphological Characters of Small-Spotted Catshark (Scyliorhinus canicula). Fishes 2022, 7, 100. https://doi.org/10.3390/fishes7030100
Grunow B, Reismann T, Moritz T. Pre-Hatching Ontogenetic Changes of Morphological Characters of Small-Spotted Catshark (Scyliorhinus canicula). Fishes. 2022; 7(3):100. https://doi.org/10.3390/fishes7030100
Chicago/Turabian StyleGrunow, Bianka, Theresa Reismann, and Timo Moritz. 2022. "Pre-Hatching Ontogenetic Changes of Morphological Characters of Small-Spotted Catshark (Scyliorhinus canicula)" Fishes 7, no. 3: 100. https://doi.org/10.3390/fishes7030100
APA StyleGrunow, B., Reismann, T., & Moritz, T. (2022). Pre-Hatching Ontogenetic Changes of Morphological Characters of Small-Spotted Catshark (Scyliorhinus canicula). Fishes, 7(3), 100. https://doi.org/10.3390/fishes7030100






