Abstract
To study the effects of Bacillus subtilis supplementation in a high-fat diet on the gut microbiota and nonalcoholic fatty liver disease in grass carp (Ctenopharyngodon idella), juveniles (60 ± 5 g) were fed three diets: (a) a control diet (CON), (b) a high-fat diet (HFD) and (c) a high-fat diet supplemented with B. subtilis (HFD + BS). After 8 weeks of feeding, fish growth, serum biochemical indices and total liver lipid content were measured, and gut microbiota analysis was performed using the MiSeq250 high-throughput sequencing platform. The results of this study showed that B. subtilis could improve growth and blood serum indices and reduce lipid deposition in the fish liver, preventing fatty liver disease. A grass carp model of fatty liver induced by a high-fat diet was successfully established. Moreover, B. subtilis altered the intestinal microbiota of HFD-fed grass carp, making it more similar to that of the control group. This study revealed the important effects of B. subtilis on grass carp with fatty liver induced by a high-fat diet and provides the foundation for the application of probiotics in grass carp farming.
1. Introduction
Fatty liver disease is a common metabolic disease in fish. It is characterized by histomorphological features such as the vacuolar degeneration of stem cells and the deposition of lipid droplets, which seriously affect the metabolic capacity of the liver and reduce the disease resistance of the animals, making the diseased fish susceptible to exogenous pathogenic infections and causing mass mortality and huge economic losses [1,2]. Abnormal hepatic lipid accumulation is the basis for the development of fatty liver disease. The classical “second strike” theory suggests that an initial excessive lipid deposition causes insulin resistance (IR) and abnormal glucose metabolism, resulting in hepatic lipid deposition and increased susceptibility to a second strike—oxidative stress and abnormal levels of cytokines—ultimately leading to liver fibrosis and cirrhosis [3,4].
The liver and intestine are closely related in anatomy and function, and the concept of a “gut–liver axis” in mammals has been widely recognized and intensively studied to explain the relationship between fatty liver and the intestinal microbiota [5]. The intestinal microbiota has established a very close relationship with the host over evolution and participates in the metabolic processes of the host by secreting digestive enzymes and stimulating the maturation of the immune system, thus playing an important role in host immunity [6]. Homeostasis of the intestinal microbiota is important to enhance the host health and promote the host growth and development. Dysbiosis of the intestinal microbiota causes tight junctions of the affected intestinal mucosal cells to become more permeable. Thus, it weakens the barrier function of the intestinal mucosa, leading to the appearance of ectopic endotoxins and causing enterogenic endotoxemia and aggravation or complications of liver diseases [7].
Probiotic preparations are considered an important treatment for fatty liver and have been proven to ameliorate liver damage in humans and mice [8,9,10,11,12,13]. Wang found that three Bacillus amyloliquefaciens strains could improve glucose–insulin homeostasis and hepatic steatosis in mice with metabolic syndrome induced by a high-fat diet (HFD) [14]. Studies in fish have also shown the potential of probiotics to attenuate metabolic disorders associated with a high-fat diet [15,16]. Among the many probiotics used in fish, Bacillus has the advantages of high resistance, easy storage and the ability to inhibit pathogen multiplication [17]. In our previous study, B. subtilis was found to have regulatory effects on liver lipid metabolism in grass carp, and the addition of B. subtilis to the feed enhanced the antioxidant capacity of grass carp, alleviated liver damage caused by Aeromonas hydrophila infection, ameliorated the intestinal microbiota and promoted the digestion and absorption of nutrients [18]. However, the regulatory effects of B. subtilis on the intestinal microbiota of grass carp with fatty liver are unclear.
This study investigated the changes in the intestinal microbiota of grass carp with fatty liver induced by high-fat feed and further explored the regulatory effect of B. subtilis on the intestinal microbiota of grass carp with non-alcoholic fatty liver based on the previous work. It provides theoretical guidance for the use of microbial agents for the prevention and control of fatty liver in grass carp.
2. Materials and Methods
2.1. Experimental Animals and Bacteria
The experimental grass carp were collected from Bairong Aquatic Breeding Co., Ltd. in Hubei, China. A total of 120 healthy grass carp (60 ± 5 g) were domesticated for 2 weeks in a recirculating aquaculture system (RAS) at a water depth of 60 ± 5 cm and a water temperature of 25 ± 1 °C. The commercial pellet feed was fed twice a day at 4% of the body weight, and the experimental fish were fasted for 1 d before the experiment.
The B. subtilis Ch9 strain was isolated from the intestines of grass carp and preserved in the microbiology laboratory of the College of Fisheries, Huazhong Agricultural University. The strain was inoculated on LB plates and incubated at 37 °C for 24 h. Single colonies were inoculated in LB broth and incubated at 37 °C for 2 days. The supernatant was removed by centrifugation at 4000 r/min for 15 min, and a bacterial suspension was obtained by resuspending the pellet with sterile phosphate buffer (PBS, pH = 7.4). The bacteria were added to powder feed, and then, the mixture was pressed into pellets. The number of viable bacteria in the pellet feed was 107 CFU/g, as determined every week by the plate counting method.
2.2. Feed Preparation and Feeding
A total of 108 grass carp were randomly assigned to three groups: (a) a control group (Con), fed a normal pellet diet with 4% fat content; (b) a high-fat diet group (HFD), fed a high-fat pellet diet with 8% fat content; and (c) a high-fat diet with B. subtilis group (BS.HFD), fed a high-fat pellet diet supplemented with B. subtilis (Table 1). Following an 8-week feeding period, the growth indices of the grass carp in each group were measured; blood was drawn from the caudal vein to measure serum biochemical indices; liver and intestine samples were removed; and oil red O staining and high-throughput sequencing of the intestinal microbiota were performed.

Table 1.
(a) Design of the three experimental diets; (b) compositions of the experimental diets.
2.3. Growth Indices
A total of 12 grass carp were selected randomly from each group. The standard length (L), body weight (W), initial weight (Wi) and final weight (Wf) of the grass carp in each group were determined to calculate the weight gain rate (WGR), the specific growth rate (SGR) and the condition factor (CF). The liver and the remaining visceral organs were isolated from the fish, and the liver weight (Wl) and viscera weight (Wv) were measured separately to calculate the hepatosomatic index (HSI) and the visceral index (VSI).
2.4. Serum Biochemical Indices
A total of 6 grass carp were selected randomly from each group. The blood was randomly collected from the tail vein of the grass carp, centrifuged at 3000 r/min for 10 min at 4 °C and placed in a refrigerator at 4 °C for 1 h. The upper serum was taken to measure the serum biochemical indices, including the levels of cholesterol (CHO), albumin (ALB), total protein (TP), triglyceride (TG), aspartate aminotransferase (AST), alanine aminotransferase (ALT), high-density lipoprotein (HDL) and low-density lipoprotein (LDL) through a biochemical analyzer (VITALAB Selectra Junior), using kits purchased from Biosino Bio-Technology and Science Inc., Beijing, China.
2.5. Oil Red O Staining
Grass carp liver samples were isolated and kept in 4% paraformaldehyde for 36 h. Then, the tissues were trimmed for smoothness and successively placed in 15% and 30% sucrose solutions at 4 °C for gradient dehydration. The liquid on the surface of the dehydrated tissues was dried, and the tissues were embedded with OCT embedding agent, then cut into slices (thickness 8 μm) using a cryotome and stained with Oil Red O. Photographs were taken under a microscope (Leica DM4 B, Wetzlar, Germany). A total of 10 fields of view were randomly selected from each sample, and the relative areas of lipid droplets that were stained red were calculated using Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA).
2.6. Total Liver Lipid
A total of 6 grass carp were selected randomly from each group. The liver samples were freeze-dried at −50 °C for 24 h, and then the crude lipid content of the liver was determined through Soxhlet extraction. The crude fat content of the liver was calculated according to the following formula: crude fat (%) = 100% × sample fat mass/sample mass.
2.7. Intestinal Microbiota
A total of 6 grass carp were sampled randomly from each group. The body surface of the grass carp was washed and disinfected using 75% ethanol. Then, the intact hindguts were isolated with sterile forceps and scissors under aseptic operation conditions. Total genomic DNA was isolated from the samples according to the instructions of the Fecal DNA Extraction Kit (Aidlab Biotech, Beijing, China). The V3/V4 region of the 16S rRNA gene was amplified using the primers 341F and 806R (341F, 5′-CCTAYGGGRBGCASCAG-3′; 806R, 5′-GGACTACNNGGGTATCTAAT-3′). The PCR amplification reaction was performed as follows: 0.25 μL Q5 high-fidelity DNA polymerase, 5 μL 5 × Reaction Buffer, 5 μL 5 × High GC Buffer, 0.5 μL dNTP Mix (10 mM), 2 μL Template DNA, 1 μL Forward primer (10 μM), 1 μL Reverse primer (10 μM) and 10.25 μL ddH2O. The PCR conditions were: denaturation at 94 °C for 5 min, followed by 30 cycles consisting of 94 °C for 30 s, 54 °C for 30 s and 72 °C for 30 s, and a final elongation step at 72 °C for 10 min. The amplification products were analyzed via electrophoresis in 1.2% agarose gels. Then, the DNA in each band was excised and purified using the To PureTM Gel Extraction Kit (Gene Tech, Shanghai, China). Each purified PCR product was subjected to high-throughput sequencing using the MiSeq250 high-throughput sequencing platform (BGI, Shenzhen, China).
2.8. Bioinformatic Analysis
To obtain more accurate and reliable results in the subsequent bioinformatic analysis [19], the data were preprocessed. According to the overlap between paired-end reads, the paired-end sequencing data obtained by sequencing were merged into raw tags using FLASH v1.2.7 (Magoc, T. USA). Next, the clean tags were filtered from the raw tags using Trimmomatic v0.33 (Bolger, A. M. Germany). Sequencing errors and chimeras were detected and removed, and the reads that could not be assembled were discarded. The clean tags were clustered into operational taxonomic units (OTUs) by USEARCH v10.0 (Edgar, R. C. USA) at 97% similarity levels [20]. The OTUs were compared with the database to evaluate the species, and complexity analysis and intergroup difference analysis were based on the OTU and species annotation results.
Rarefaction curves were plotted for each sample to determine the abundance of communities and sequencing data of each sample. The alpha diversity index was determined using Chao1 (total species richness), ACE (abundance-based coverage estimator), the Shannon index and the Simpson index in Mothur v1.31.2(Schloss, P. D. USA) [21]. Community bar plot analysis was carried out to show the relative abundance of the gut bacterial communities among the samples at the phylum and genus levels. Beta diversity measurements, including principal component analysis (PCA) and partial least-squares discriminant analysis (PLS-DA), were performed.
2.9. Statistical Analysis
The results are presented as the mean ± SEM. Differences between the groups were analyzed by one-way ANOVA, followed by Duncan multiple comparison tests using SPSS v26.0; p < 0.05 was considered statistically significant.
3. Result
3.1. Role of B. subtilis in the Effect of High-Fat Diet on Growth Index
The growth indices of grass carp are shown in Table 2. Several indices, including final body weight (FBW), body weight gain rate (WGR) and specific growth rate (SGR), were significantly higher in the HFD group compared with the control group (p < 0.05). For the BS.HFD group, their FBW, SGR and liver fat content were not significantly different from those of the CON group (p > 0.05), but they were significantly lower than those of the HFD group (p < 0.05). The WGR of the BS.HFD group was higher than that of the CON group (p < 0.05) but significantly lower than that of the HFD group (p < 0.05); the HSI and VSI of the BS.HFD group were significantly lower than those of the CON and HFD groups (p < 0.05), showing a suppressing effect of B. subtilis on lipid deposition in grass carp.

Table 2.
Growth indices of C. idella.
3.2. Role of B. subtilis in the Effect of High-Fat Diet on Serum Biochemical Indices
The serum biochemical indices of grass carp are presented in Table 3. Compared with the CON group, TP, ALT, HDL and LDL levels were significantly higher in the HFD group (p < 0.05), and there was no significant difference between the various serum indices in the BS.HFD and CON groups (p > 0.05). CHO, ALB, TG and AST levels were not significantly different between the groups (p > 0.05).

Table 3.
Serum biochemical indices of C. idella.
3.3. Role of B. subtilis in the Effect of High-Fat Diet on Liver Lipid Content
The liver tissues were stained with oil red O, and their lipid content was determined (Figure 1). The results showed that the liver lipid droplets in the HFD group were large and dense, to a significantly greater extent than those in the CON group; the lipid content was also significantly higher than in the CON group (p < 0.05), liver lipid deposition being caused by the high-fat diet. A grass carp model with fatty liver induced by a high-fat diet was successfully established. The liver lipid droplets in the BS.HFD group were greatly reduced compared with the HFD group, and the lipid content was significantly lower than that in the HFD group (p < 0.05) and not significantly different from that in the CON group (p > 0.05), indicating the suppression of high-fat diet-induced liver lipid deposition by B. subtilis.
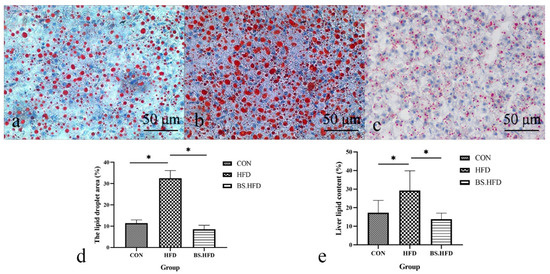
Figure 1.
Oil red O staining and lipid content of C. idella liver; (a) CON, (b) HFD, (c) BS.HFD, (d) lipid droplet area proportion, (e) liver lipid content determined by Soxlet extraction. “*” means significant differences between groups (p < 0.05).
3.4. Role of B. subtilis in the Effect of High-Fat Diet on the Intestinal Microbiota
3.4.1. General Analysis of High-Throughput Sequencing
The intestinal microbiota of the four groups was sequenced, subsumed and divided into OTUs according to a 97% similarity level, and a total of 2842 OTUs were obtained. The rarefaction curves were plotted based on OTUs, and the curves of each group plateaued, indicating that the sequencing depth of all samples was sufficient to reflect the composition of the intestinal microbiota (Figure 2).
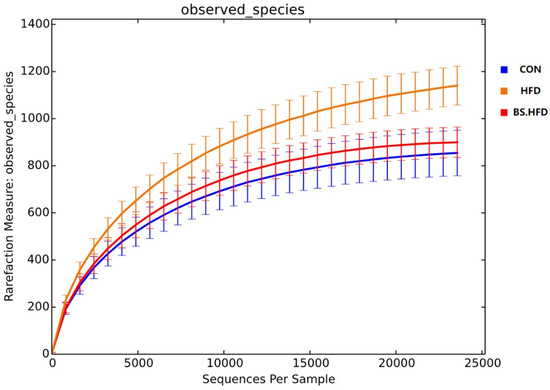
Figure 2.
Rarefaction curve of the intestinal microbiota in C. idella.
Good coverage estimates indicated that the completeness for each sample was over 98.88%, and sample richness was calculated using Chao and ACE indices. The Shannon and Simpson indices were calculated for diversity (Table 4). The results showed that the HFD group had significantly higher richness than the CON and BS.HFD groups, while there was no significant difference among the groups in the Simpson and Shannon indices. The above results show that the high-fat diet elevated the alpha diversity of the gut microbiota in grass carp, while B. subtilis restored it to the normal level.

Table 4.
Alpha diversity of the intestinal microbiota in C. idella.
3.4.2. Comparison and Structure of the Intestinal Microbiota
The relative abundance histogram of each sample was plotted at the phylum and genus levels (Figure 3, Table 5). At the phylum level, the dominant phyla in the intestinal microbiota in the CON group were Bacteroidetes (32.77%), Firmicutes (30.45%), Proteobacteria (18.28%), Fusobacteria (13.23%), Verrucomicrobia (3.72%), etc. The dominant phyla in the HFD group included Fusobacteria (51.76%), Proteobacteria (16.61%), Firmicutes (15.48%), Bacteroidetes (14.46%) and Verrucomicrobia (1.28%). The dominant phyla in the BS.HFD group were Fusobacteria (45.66%), Bacteroidetes (23.48%), Proteobacteria (12.32%), Firmicutes (9.41%), Verrucomicrobia (6.89%), Tenericutes (1.95%), etc.
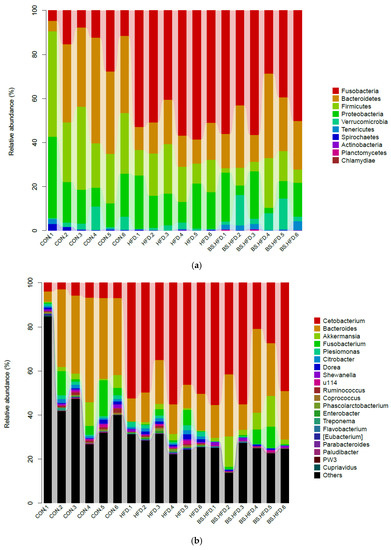
Figure 3.
Composition of the intestinal microbiota of C. idella at phylum (a) and genus levels (b).

Table 5.
(a) Relative abundance of bacteria in the intestinal microbiota of C. idella at the phylum level; (b) The relative abundance of bacteria in the intestinal microbiota of C. idella at the genus level.
At the genus level, the dominant genera in the CON group included Bacteroides (32.33%), an unclassified genus of Erysipelotrichaceae (23.65%), an unclassified genus of Enterobacteriaceae (13.04%), Fusobacterium (6.02%), Cetobacterium (5.54%), Akkermansia (3.72%), an unclassified genus of Lachnospiraceae (2.10%), Plesiomonas (1.24%), an unclassified genus of Aeromonadaceae (1.21%), an unclassified genus of Ruminococcaceae (1.15%), Citrobacter (1.11%) and an unclassified genus of Fusobacteriaceae (1.11%). In the HFD group, the dominant intestinal genera were Cetobacterium (48.14%), Bacteroides (14.04%), an unclassified genus of Enterobacteriaceae (11.34%), an unclassified genus of Erysipelotrichaceae (8.78%), an unclassified genus of Ruminococcaceae (3.04%), Fusobacterium (1.89%), Plesiomonas (1.70%), Akkermansia (1.28%), Citrobacter (1.22%), an unclassified genus of Fusobacteriaceae (1.17%) and Dorea (1.09%). The dominant genera in the BS.HFD group were Cetobacterium (41.57%), Bacteroides (23.09%), an unclassified genus of Enterobacteriaceae (9.45%), Akkermansia (6.89%), an unclassified genus of Erysipelotrichaceae (6.23%), Fusobacterium (3.11%), an unclassified genus of CK-1C4-19 (1.93%), an unclassified genus of Peptostreptococcaceae (1.11%) and an unclassified genus of Aeromonadaceae (1.07%).
3.4.3. Analysis of the Differences in the Intestinal Microbiota of the Three Groups
The OTU composition was analyzed through PCA to compare the differences in the intestinal microbiota (Figure 4). The results showed that the CON, HFD and BS.HFD groups clustered separately according and varied mainly on the PC1 axis, while the differences on the PC2 axis were not significant. This indicated that the high-fat diet changed the grass carp intestinal microbiota dramatically, while the addition of B. subtilis made the intestinal microbiota more similar to that of the CON group and attenuated the effects of the high-fat diet on the intestinal microbiota.
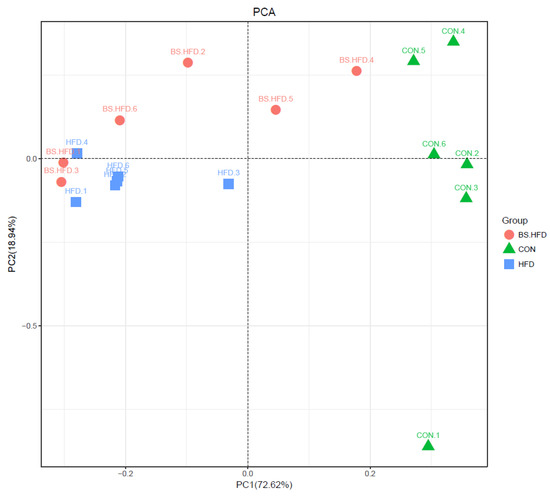
Figure 4.
PCA analysis of intestinal microbiota Beta diversity in C. idella.
A PLS-DA (partial least-squares discriminant analysis) model was constructed based on the species abundance matrix and sample grouping data through R software (Figure 5). The results showed that the samples separately clustered according to their group; the CON and HFD groups were similarly distributed on the PLS1 axis and varied mainly on the PLS2 axis, while the BS.HFD group differed from the CON and HFD groups on the PLS1 axis, and distributed between the CON and HFD groups on the PLS2 axis.
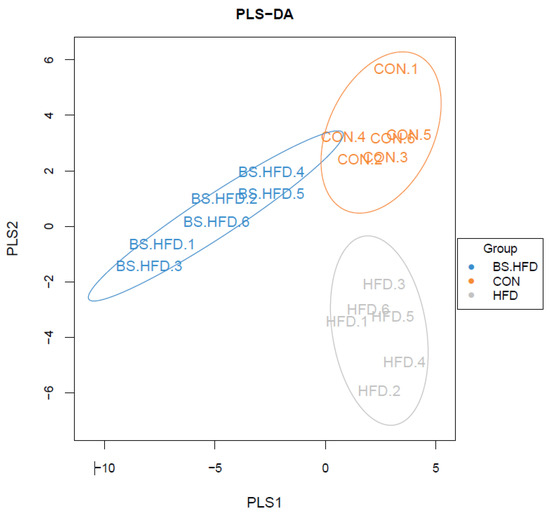
Figure 5.
PLS-DA (partial least-squares discriminant analysis) of the intestinal microbiota of C. idella.
4. Discussion
There are many factors affecting lipid deposition in fish liver, mainly linked to nutrition, physiology, species and the environment. Liver fat deposition is related not only to the amount of dietary lipids, but also to lipid quality and FA profile [22,23,24,25,26]. Many studies have shown that dietary lipid content and source are the main factors causing lipid deposition in the liver. It has been reported that the VSI, HSI and liver lipid content of mullet (Chelon haematocheilus) increased significantly with the increase in lipid content in the feed [27]. In addition, increases in feed lipids have been reported to cause fatty liver in many fish, such as Cyprinus carpio and Lateolabrax japonicus [28,29]. A high-fat diet is the most common method to establish fatty liver models. The creation of these models requires a long time, but they have the advantage of a high replication rate and allow the dynamic tracking of the fatty liver model establishment [30]. In this study, grass carp were fed with a high-fat diet with 8% lipid content and B. subtilis, and the growth indices and liver lipid content were measured. The results showed that the growth indices of the HFD group were significantly higher than those of the CON group, especially the liver lipid content. Moreover, oil red O staining showed a significant increase in lipid droplets in the HFD group, indicating that the high-fat diet promoted the growth of the host, but also caused lipid deposition in the liver, which could trigger fatty liver disease. In this study, a high-fat diet-induced fatty liver grass carp model was successfully established.
The WGR of the BS.HFD group was significantly higher than that of the CON group, but there was no difference in the liver lipid content. The HSI and VSI of the BS.HFD group were significantly lower than those of the CON group, and the oil red O staining showed that the number of liver lipid droplets in the BS.HFD group was significantly lower than that in the HFD group. The above results showed that the high-fat diet with B. subtilis promoted the growth of grass carp and reduced the high-fat diet-induced liver lipid deposition. The effect of probiotics on reducing lipid accumulation in the liver has been reported in many studies in both mammals and fish [15,16,31,32,33,34,35]. Many studies have also verified that B. subtilis had a repairing effect on liver damage caused by A. hydrophila infection, promoting the maintenance of the normal lipid metabolism in the liver of grass carp [36].
The addition of B. subtilis to feed has been reported to promote fish growth, digestive enzyme activity and antioxidant capacity [37,38,39,40,41]. Previous studies have shown that supplementation with B. subtilis improved the reduction in lipids and decreased serum AST and ALT activities caused by A. hydrophila infection, and this modulatory effect has also been reported in other fish, including tilapia [36,42]. In this study, serum biochemical indices were measured in all groups, and the results showed that total protein (TP), alanine aminotransferase (ALT), high-density lipoprotein (HDL) and low-density lipoprotein (LDL) were significantly higher in the HFD group compared to the CON group (p < 0.05), further validating our fatty liver model. There was no significant difference between the BS.HFD and the CON groups (p > 0.05), indicating that B. subtilis significantly ameliorated the abnormal serum biochemical indices induced by a high-fat diet. It has been shown that B. subtilis can significantly reduce glucose and triglyceride levels and aspartate aminotransferase and glutamate transaminase activities in the serum of mice fed high-fat diets and also downregulate the expression of genes related to lipid synthesis in the liver, as well as upregulate the expression of some genes related to lipolysis, which is consistent with the results of the present study [43].
The intestinal microbiota homeostasis is closely related to the host health, as it can promote nutrient absorption and metabolism as well as the development of the immune system of the host. In this study, high-throughput sequencing was performed on the intestinal microbiota of grass carp fed high-fat diets supplemented with B. subtilis. In mammals, the healthy and mature intestinal microbiota tend to be abundant and stable [44,45]. However, in aquatic animals, many studies have shown that the alpha diversity of the intestinal microbiota in adult animals is lower than that in juveniles with incomplete gut development, and the high alpha diversity of juveniles may be related to the instability and susceptibility of their gut microbiota [46,47,48]. In this study, the high-fat diet elevated the alpha diversity of the gut microbiota of grass carp, possibly suggesting an unstable and disturbed state of their gut microbiota. Additionally, B. subtilis suppressed the abnormal rise in alpha diversity of the gut microbiota induced by a high-fat diet. At the phylum level, the relative abundance of Fusobacteria was significantly increased in the HFD group compared with the control group, and the relative abundance of the originally dominant phyla, Bacteroidetes and Firmicutes, significantly decreased (p < 0.05). The most dominant phylum in the BS.HFD group was also Fusobacteria, but its relative abundance was lower than in the HFD group, and the proportion of Bacteroidetes was greatly higher in the BS.HFD group (p < 0.05). Firmicutes and Bacteroidetes are important for fermenting polysaccharides and establish a symbiotic relationship that promotes the absorption or storage of energy in the host; changes in their ratios affect the metabolic potential of the host intestinal microbiota. Various studies have investigated the important factors influencing obesity in the host. It has been shown that obesity is correlated with the relative abundance of these two major phyla in the gut and that the ratio of Firmicutes to Bacteroidetes is higher in obese mice than in normal healthy mice. The increase in Firmicutes, decrease in Bacteroidetes, and their higher ratio promote obesity in the host. These microbiota structures are more capable of breaking down food and promoting energy absorption in the host [49]. The Firmicutes-to-Bacteroidetes ratio in this study was 0.93 (F:B = 0.93) in the CON group, 1.07 (F:B = 1.07) in the HFD group and 0.40 (F:B = 0.40) in the BS.HFD group, indicating that the supplementation of B. subtilis significantly inhibited the potential of a high-fat diet to induce obesity. At the genus level, the relative abundance of Cetobacterium in the HFD group increased significantly (P<0.05), and this genus became the most dominant, reaching 48.14%, while the abundance of Bacteroides decreased significantly (it was the most abundant in the CON group, P<0.05). The most dominant genus in the BS.HFD group was still Cetobacterium, but its relative abundance was lower than in the HFD group, and the proportion of Bacteroides was also significantly higher in the BS.HFD group than in the HFD group (p < 0.05). Akkermansia, which has received large attention in recent years, can improve several metabolic indicators in overweight/obese insulin-resistant individuals. With a good safety profile, it is an important candidate for probiotic therapy for obesity [50]. In this study, the relative abundance of Akkermansia was 3.72% in the CON group and was significantly reduced to 1.28% in the HFD group, while in the BS.HFD group, the relative abundance of Akkermansia was as high as 6.89%, making it an important dominant genus in the intestinal microbiota. These results showed the important effect of B. subtilis on the regulation of the intestinal microbiota. In PCA and PLS-DA analyses, the CON, HFD and BS.HFD groups clustered separately according and varied mainly on one axis, while the difference on the other axis was not significant, indicating the dramatical changes in the intestinal microbiota induced by high-fat diets, while the supplement of B. subtilis made the intestinal microbiota more similar to the CON group, suggesting a regulatory effect of B. subtilis on the abnormal changes in the intestinal microbiota induced by a high-fat diet in grass carp. The mechanism of the interaction between B. subtilis and the original microbiota needs further study. The supplementation of B. subtilis in the normal diet also needs further study to fully determine the effect of B. subtilis on grass carp and the potential of B. subtilis as a probiotic applied in the farming of grass carp.
5. Conclusions
In this study, a grass carp model of fatty liver induced by a high-fat diet was successfully established. By measuring growth indices, serum biochemical indices and total liver lipid content, we found that a diet with B. subtilis reduced the potential of the high-fat diet to induce liver lipid deposition, while promoting the growth of grass carp. Moreover, we studied the intestinal microbiota of the examined groups through high-throughput sequencing and found that the diet containing B. subtilis regulated the disorders in the intestinal microbiota of grass carp caused by the high-fat diet.
Author Contributions
Data curation, D.G. and M.X.; Methodology, D.G. and M.X.; Supervision, Z.W.; Writing—original draft, D.G. and M.X.; Writing—review and editing, H.X., L.X., S.Z. and X.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key R&D Program of China (grant No. 31472310, 31972819).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Huazhong Agricultural University (protocol code: HZAUFI-2018-024, and date of approval: 20 February 2018).
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA788089 (accessed on 2 April 2022).
Acknowledgments
The authors thank the microbiology laboratory of the College of Fisheries, Huazhong Agricultural University, for providing the B. subtilis Ch9 strain. The authors also thank Yan-er Luo and Hui Zhao (College of Fisheries, Huazhong Agricultural University) for helping with collecting the samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gao, H.M.; Wang, X.M.; Zhang, G.H. Nutritional metabolic diseases of fish. J. Hydroecol. 2004, 24, 67–69. [Google Scholar]
- Zhang, H.T.; Wang, A.L.; Li, G.L.; Sun, C.C. Effect of nutrient on the fatty liver disease of fish. Marin. Sci. Bull. 2004, 23, 82–89. [Google Scholar]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Chen, W.X.; Yu, Z.H. The relationship between nonalcoholic fatty liver and insulin resistance with abnormal glucose metabolism. Chin. J. Hepatol. 2000, 8, 76–77. [Google Scholar]
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The gut–liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.Q.; Zhang, S.Y.; Xu, L.L.; Wu, Z.X.; Yuan, J.F.; Chen, X.X. Comparison of the intestinal microbiota during the different growth stages of red swamp crayfish (Procambarus clarkii). Front. Microbiol. 2021, 12, 696281. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.C. The gut as a potential trigger of exercise-induced inflammatory responses. Can. J. Physiol. Pharmacol. 1998, 76, 479–484. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Salminen, S.; Isolauri, E. Positive interactions with the microbiota: Probiotics. Adv. Exp. Med. Biol. 2008, 635, 57–66. [Google Scholar]
- Velayudham, A.; Dolganiuc, A.; Ellis, M.; Petrasek, J.; Kodys, K.; Mandrekar, P.; Szabo, G. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology 2010, 49, 989–997. [Google Scholar]
- Verdam, F.J.; Rensen, S.S.; Driessen, A.; Greve, J.W.; Buurman, W.A. Novel evidence for chronic exposure to endotoxin in human nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 2011, 45, 149–152. [Google Scholar] [CrossRef]
- Feng, L.Y.; Shen, Y.J.; Yu, S.L. Effect of enteric Bifid-triple viable capsule on no-alcoholic fatty liver disease in rats and its mechanism. Chin. J. Microecol. 2013, 25, 793–796. [Google Scholar]
- Zhao, H.Y.; Jin, H.L.; Yang, X.Y.; Zhang, F.R. Application of microbiotics in non-alcoholic fatty liver disease. Harbin Med. J. 2013, 33, 190–191. [Google Scholar]
- Alisi, A.; Bedogni, G.; Baviera, G.; Giorgio, V.; Porro, E.; Paris, C.; Giammaria, P.; Reali, L.; Anania, F.; Nobili, V. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment. Pharm. Ther. 2014, 39, 1276–1285. [Google Scholar]
- Wang, J.; Zhao, Y.; Ruan, Y. Effects of bio-organic fertilizers produced by four Bacillus amyloliquefaciens strains on banana fusarium wilt disease. Compost. Sci. Util. 2015, 23, 185–198. [Google Scholar] [CrossRef]
- Falcinelli, S.; Rodiles, A.; Hatef, A.; Picchietti, S.; Cossignani, L.; Merrifield, D.L.; Unniappan, S.; Carnevali, O. Dietary lipid content reorganizes gut microbiota and probiotic L-rhamnosus attenuates obesity and enhances catabolic hormonal milieu in zebrafish. Sci. Rep. 2017, 7, 5512. [Google Scholar] [CrossRef] [PubMed]
- Banda, I.; Lobo, C.; León-Rubio, J.M.; Tapia-Paniagua, S.; Balebona, M.C.; Moriñigo, M.A.; Moreno-Ventas, X.; Lucas, L.M.; Linares, F.; Arce, F.; et al. Influence of two closely related probiotics on juvenile Senegalese sole (Solea senegalensis, Kaup 1858) performance and protection against Photobacterium damselae subsp. Piscicida. Aquac. 2010, 306, 281–288. [Google Scholar] [CrossRef]
- Li, W.F.; Shen, T.; Chen, N.N.; Deng, B.; Fu, L.Q.; Zhou, X.X. Effect of dietary Bacillus subtilis on digestive enzyme activity and intestinal microflora in grass carp Ctenopharyngodon idella. J. Dalian Fish Univ. 2012, 27, 221–225. [Google Scholar] [CrossRef]
- Luo, Y.E.; Zhao, H.; Guo, D.Y.; Wang, H.; Chen, X.X. Effect of Bacillus subtilis on the hepatic lipid metabolism of Ctenopharyngodon idella. Acta Hydrobiol. Sin. 2020, 44, 485–493. [Google Scholar] [CrossRef]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Btotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randazzo, B.; Zarantoniello, M.; Gioacchini, G.; Cardinaletti, G.; Belloni, A.; Giorgini, E.; Faccenda, F.; Cerri, R.; Tibaldi, E.; Olivotto, I. Physiological response of rainbow trout (Oncorhynchus mykiss) to graded levels of Hermetia illucens or poultry by-product meals as single or combined substitute ingredients to dietary plant proteins. Aquaculture 2021, 538, 736550. [Google Scholar] [CrossRef]
- Randazzo, B.; Zarantoniello, M.; Cardinaletti, G.; Cerri, R.; Giorgini, E.; Belloni, A.; Conto, M.; Tibaldi, E.; Olivotto, I. Hermetia illucens and poultry by-product meals as alternatives to plant protein sources in gilthead seabream (Sparus aurata) diet: A multidisciplinary study on fish gut status. Animals 2021, 11, 677. [Google Scholar] [CrossRef]
- Hua, K.; Bureau, D.P. Development of a model to estimate digestible lipid content of salmonid fish feeds. Aquaculture 2009, 286, 271–276. [Google Scholar] [CrossRef]
- Diaz, J.P.; Guyot, E.; Vigier, S.; Connes, R. First events in lipid absorption during post-embryonic development of the anterior intestine in gilt-head sea bream. J. Fish Biol. 1997, 51, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.A.; Garcia, M.I.; Riera, H.N.; Vélez, E.J.; Lutfi, E.; Fontanillas, R.; Gutiérrez, J.; Capilla, E.; Navarro, I. Effects of different dietary vegetable oils on growth and intestinal performance, lipid metabolism and flesh quality in gilthead sea bream. Aquaculture 2019, 519, 734881. [Google Scholar] [CrossRef]
- Zhang, C.N.; Wang, A.M.; Liu, W.B.; Yang, W.P.; Yu, Y.B.; Lv, L.L.; Huang, J.T.; Qi, Z.T. Effect of dietary lipid levels on fat deposition, lipid metabolize enzyme and antiocidantic activities of Chelon haematocheilus. J. Fish Sci. Chin. 2013, 20, 108–115. [Google Scholar] [CrossRef]
- Xu, J.H.; Qin, J.; Yan, B.L.; Zhu, M.; Luo, G. Effects of dietary lipid levels on growth performance, feed utilization and fatty acid composition of juvenile Japanese seabass (Lateolabrax japonicus) reared in seawater. Aquacult. Int. 2011, 19, 79–89. [Google Scholar] [CrossRef]
- Xu, Q.Y.; Xu, Z.C.; Wang, C.A.; Zhao, Z.G.; Luo, L. Effect of dietary lipid levels on liver free fatty acids, serum biochemical parameters and liver histological structure in mirror common carp at different temperatures. J. Northeast. Agric. Univ. 2012, 43, 118–126. [Google Scholar] [CrossRef]
- Miao, C.H. Pharmacology study of Penthorum chinense pursh extract in grass carp fatty liver disease. Sichuan Agric. Univ. 2012. Available online: https://scholar.google.com.hk/scholar?hl=zh-CN&as_sdt=0%2C5&q=Supplementation+with+probiotics+modifies+gut+flora+and+attenuates+liver+fat+accumulation+in+rat+nonalcoholic+fatty+liver+disease+model&btnG= (accessed on 4 April 2022).
- Lee, H.Y.; Park, J.H.; Seok, S.H.; Baek, M.W.; Kim, D.J.; Lee, K.E.; Paek, K.S.; Lee, Y.; Park, J.H. Human originated bacteria, Lactobacillus rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity effects in diet-induced obese mice. Biochim. Biophys. Acta. 2006, 1761, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Jing, H.; Li, Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J. Hepatol. 2008, 49, 821–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.Y.; Wan, Y.P.; Fang, Q.Y.; Lu, W.; Cai, W. Supplementation with probiotics modifies gut flora and attenuates liver fat accumulation in rat nonalcoholic fatty liver disease model. J. Clin. Biochem. Nutr. 2012, 50, 72–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapia-Paniagua, S.T.; Diaz-Rosales, P.; de la Banda, I.G.; Lobo, C.; Clavijo, E.; Balebona, M.C.; Moriñigo, M.A. Modulation of certain liver fatty acids in Solea senegalensis is influenced by the dietary administration of probiotic microorganisms. Aquaculture 2014, 424–425, 234–238. [Google Scholar] [CrossRef]
- Falcinelli, S.; Picchietti, S.; Rodiles, A.; Cossignani, L.; Merrifield, D.; Taddei, A.R.; Maradonna, F.; Olivotto, I.; Gioacchini, G.; Carnevali, O. Lactobacillus rhamnosus lowers zebrafish lipid content by changing gut microbiota and host transcription of genes involved in lipid metabolism. Sci. Rep. 2015, 5, 9336. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Luo, Y.E.; Zhang, Y.G.; Chen, X.X.; Wang, H.; Wu, Z.X. Effects of Bacillus subtilis on hepatic lipid metabolism and oxidative stress response in grass carp (Ctenopharyngodon idella) fed a high-fat diet. MLST 2020, 2, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Shen, B.Q.; Chen, J.M.; Guo, J.L.; Pan, Q.; Sun, L.H.; Ye, J.Y. Effect of adding Bacillus subtilis to diets on growth performance, digestive enzymes activity and body composition of fingerling black carp (Mylopharyngodon piceus). Acta Hydrobiol. Sin. 2013, 37, 48–53. [Google Scholar]
- Karimzadeh, S.; Amirkolaie, A.K.; Miandehy, S.P. The effects of different levels of beta plus on growth performance, microbial flora and blood parameters of Caspian trout, Salmo caspius (Kessler, 1877). IJAB 2014, 2, 292–298. [Google Scholar]
- Wu, Z.X.; Feng, X.; Xie, L.L.; Peng, X.Y.; Yuan, J.; Chen, X.X. Effect of probiotic Bacillus subtilis Ch9 for grass carp, Ctenopharyngodon idella (Valenciennes, 1844), on growth performance, digestive enzyme activities and intestinal microflora. J. Appl. Ichthyol. 2012, 28, 721–727. [Google Scholar] [CrossRef]
- Cheng, W.; Chiu, C.S.; Guu, Y.K.; Tsai, S.T.; Liu, C.H. Expression of recombinant phytase of Bacillus subtilis E20 in Escherichia coli HMS 174 and improving the growth performance of white shrimp, Litopenaeus vannamei, juveniles by using phytase-pretreated soybean meal-containing diet. Aquacul. Nutr. 2013, 19, 117–127. [Google Scholar] [CrossRef]
- Liu, C.H.; Wu, K.; Chu, T.W.; Meng, W.T. Dietary supplementation of probiotic, Bacillus subtilis E20, enhances the growth performance and disease resistance against Vibrio alginolyticus in parrot fish (Oplegnathus fasciatus). Aquacul. Int. 2018, 26, 63–74. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Soltan, M.A.; Jarmo, O.S.; Abdo, H.S. Combined effects of dietary malic acid and Bacillus subtilis on growth, gut microbiota and blood parameters of Nile tilapia (Oreochromis niloticus). Aquacul. Nutr. 2018, 24, 83–93. [Google Scholar] [CrossRef]
- Lei, K.; Li, Y.L.; Wang, Y.; Wen, J.; Wu, H.Z.; Yu, D.Y.; Li, W.F. Effect of dietary supplementation of Bacillus subtilis B10 on biochemical and molecular parameters in the serum and liver of high-fat diet-induced obese mice. J. Zhejiang Univ.-Sci. B. 2015, 16, 487–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.J.; Wei, F.; Yao, G.; Du, H.; Wang, M.; Liu, Z.; Li, Q.; An, L.; Tian, J.; Li, M.; et al. Drinking warm water improves growth performance and optimizes the gut microbiota in early postweaning rabbits during winter. Animals 2019, 9, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, C.; Bik, E.M.; DiGiulio, D.B.; Relman, D.A.; Brown, P.O. Development of the human infant intestinal microbiota. PLoS Biol. 2007, 5, e177. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.; Stephens, W.Z.; Adam, R.B.; Burns, A.R.; Stagaman, K.; David, L.A.; Bohannan, B.J.M.; Guillemin, K.; Rawls, J.F. Ontogenetic differences in dietary fat influence microbiota assembly in the zebrafish gut. mBio 2015, 6, e00687-15. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.Y.; Li, J.J.; Yu, Y.H.; Wang, J.; He, Z.; Van Nostrand, J.D.; Kempher, M.L.; Wu, L.; Wang, Y.; Liao, L.; et al. Environmental filtering decreases with fish development for the assembly of gut microbiota. Environ. Microbiol. 2016, 18, 4739–4754. [Google Scholar] [CrossRef]
- Stephens, W.Z.; Adam, R.B.; Stagaman, K.; Wong, S.; Rawls, J.F.; Guillemin, K.; Bohannan, B.J.M. The composition of the zebrafish intestinal microbial community varies across development. ISME J. 2016, 10, 644–654. [Google Scholar] [CrossRef] [Green Version]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).