Descriptions of Two New Species, Sillago muktijoddhai sp. nov. and Sillago mengjialensis sp. nov. (Perciformes: Sillaginidae) from the Bay of Bengal, Bangladesh
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Sillago muktijoddhai Gao and Saha sp. nov.
3.1.1. Holotype
3.1.2. Paratypes
3.1.3. Etymology
3.1.4. Diagnosis
3.1.5. Description
3.1.6. Color of Fresh Specimens
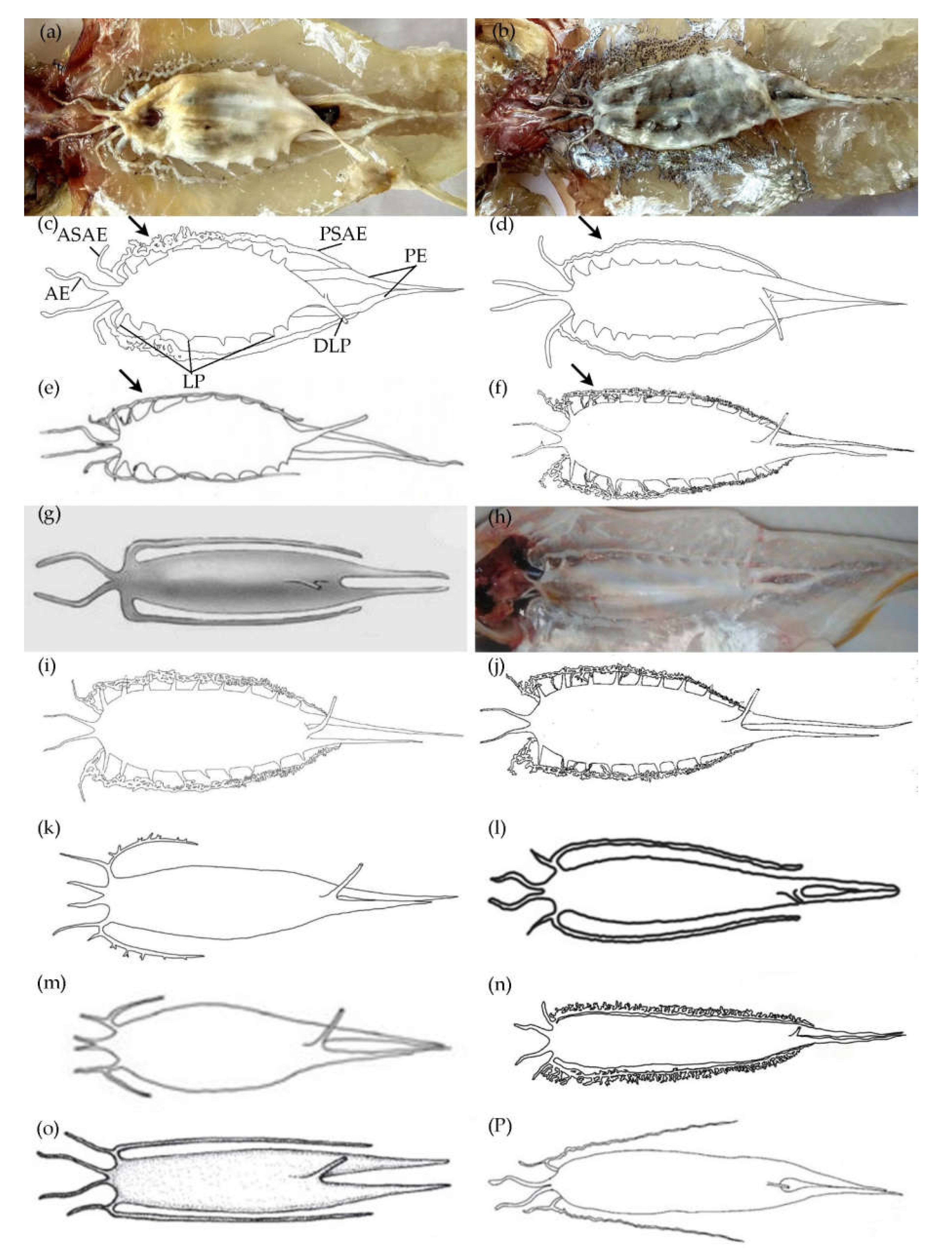
3.1.7. Swimbladder
3.1.8. Habitat
3.1.9. Distribution
3.2. Sillago mengjialensis Gao, Baki and Saha sp. nov.
3.2.1. Holotype
3.2.2. Paratypes
3.2.3. Etymology
3.2.4. Diagnosis
3.2.5. Description
3.2.6. Color of Fresh Specimens
3.2.7. Swimbladder
3.2.8. Habitat
3.2.9. Distribution
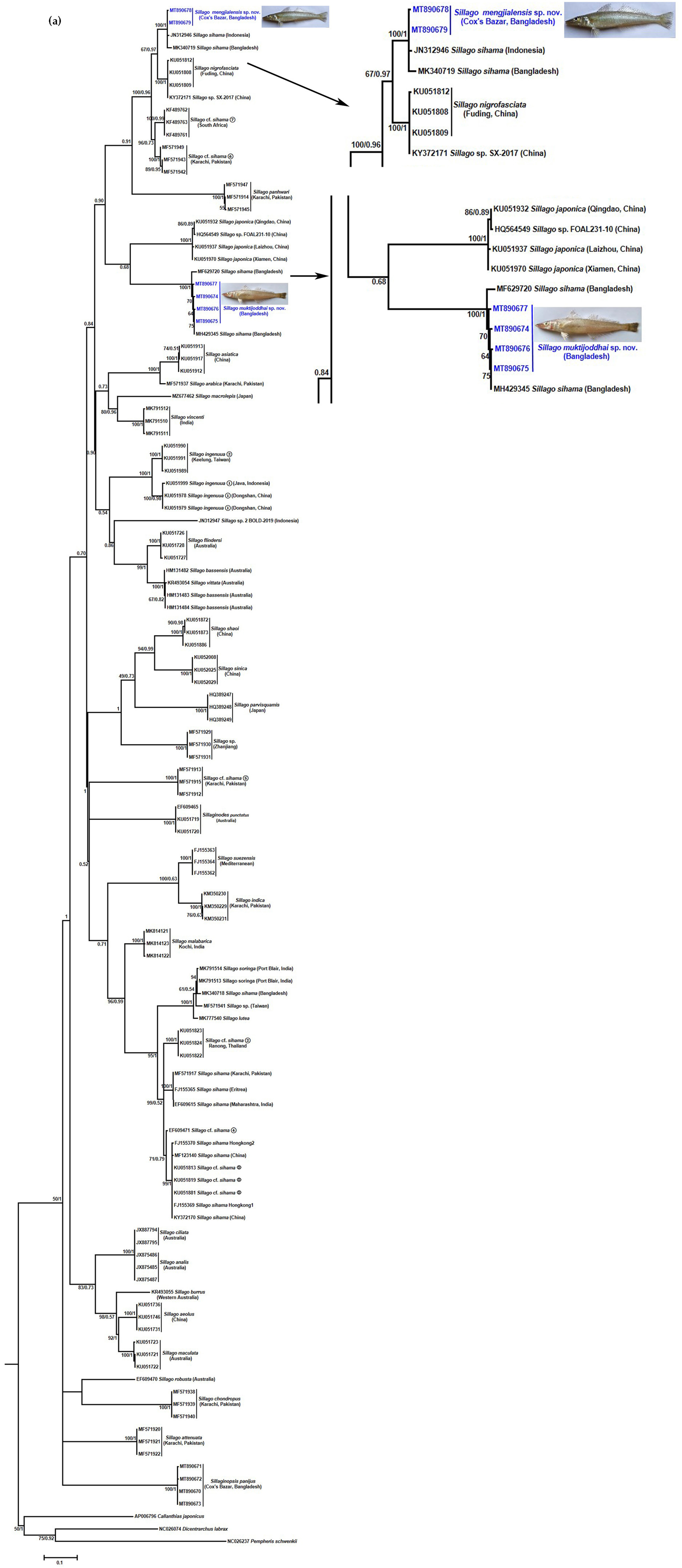
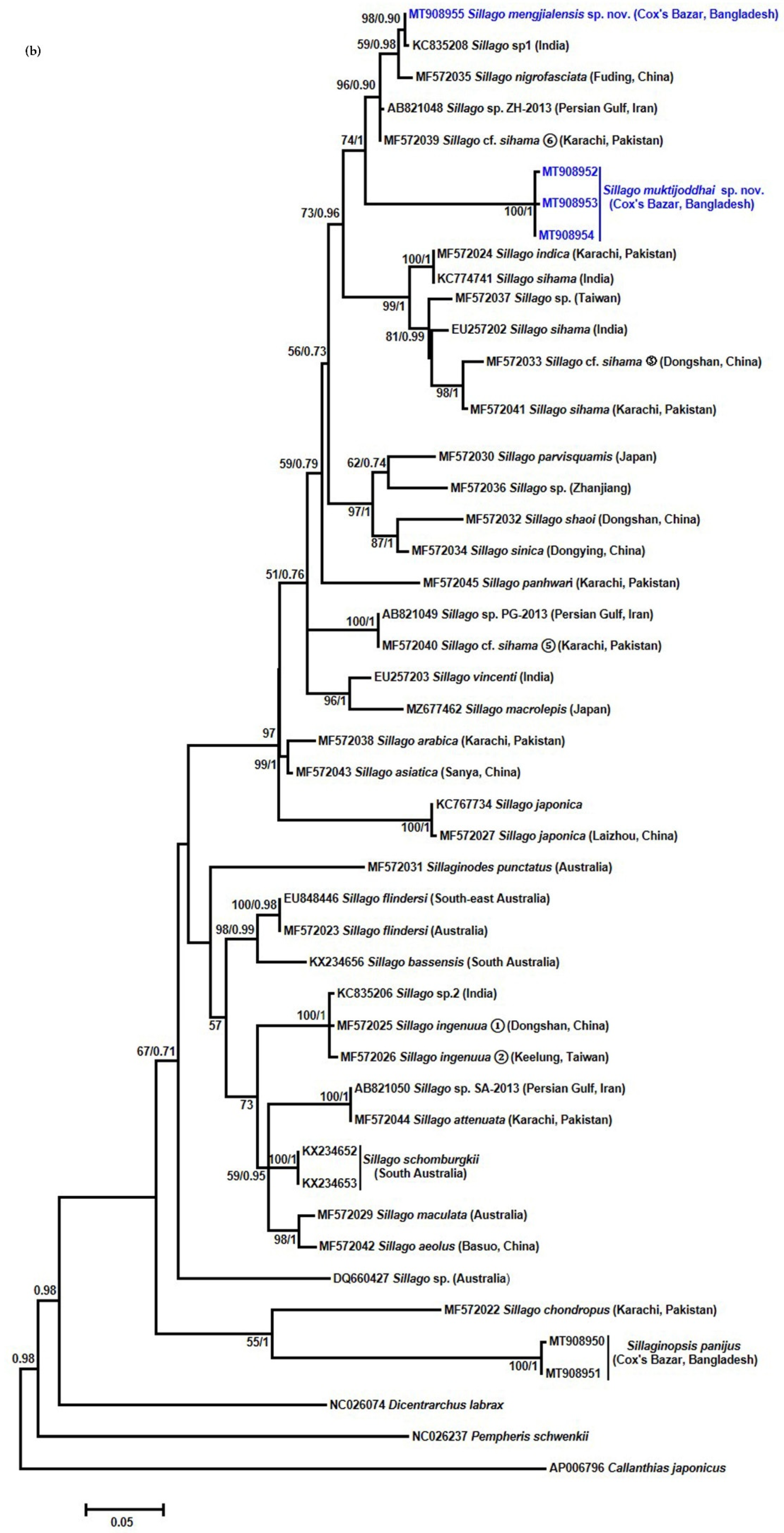
3.3. Molecular Analysis of the COI and 16S rRNA Gene
4. Discussion
- 1.
- Body with dark blotches or spots …………………………………………….................. 2
- -
- Body without dark blotches or spots ……...............………………………................. 3
- 2.
- Anal fin rays ≥ 23; vertebrae more than 34 ………………………….……... S. caudicula
- -
- Anal fin rays less than 23; vertebrae 34………………...…...……......... S. intermedius
- 3.
- HL/SL less than 33% ………………………………………………………………………. 4
- -
- HL/SL is 33% ……………………………………………............……….S. megacephalus
- 4.
- Second dorsal fin membrane with many rows of clear dusky spots ……...…..…….. 5
- -
- Second dorsal fin membrane without any row of dusky spots ……………………. 6
- 5.
- Second dorsal fin with 5 or 6 rows of dusky spots along with rays .… S. parvisquamis
- -
- Second dorsal fin with 3 or 4 rows of dusky spots along with rays …......... S. sinica
- 6.
- Anal fin membrane hyaline ………………………………………..…………………….. 7
- -
- Anal fin membrane usually with spots …………………………………………......... 8
- 7.
- Preopercle and most of the opercle without scales ……………..…………. S. suezensis
- -
- Preopercle and most of the opercle with scales ………….…......…………….......… 9
- 8.
- Gill rakers on the first arch 3–4/7–8; posterior sub extension of anterolateral extensions of swim bladder thin; roots of two posterior extensions not adjoining and two posterior extensions not adjoining …………….…….…………………………... S. indica
- -
- Gill rakers on the first arch 3–4/5–6; posterior sub extension of anterolateral extensions of swim bladder strong; roots of two posterior extensions not adjoining and two posterior extensions not adjoining ………………... S. shaoi
- -
- Gill rakers on the first arch 3–5/8–10; posterior sub extension of anterolateral extensions of swim bladder strong for about a half-length towards beginning, then the remainder is simple and thin; roots of two posterior extensions not adjoining and two posterior extensions not adjoining ………………………... S. muktijoddhai sp. nov.
- -
- Gill rakers on the first arch 2–4/5–8; posterior sub extension of anterolateral extensions of swim bladder strong; roots of two posterior extensions adjacent and two posterior extensions in close proximity ………..……… S. nigrofasciata
- -
- Gill rakers on the first arch 3–4/8–10; posterior sub extension of anterolateral extensions of swim bladder thin; roots of two posterior extensions adjoining and two posterior extensions in close proximity …..…. S. mengjialensis sp. nov.
- 9.
- Anterior extensions not joined at the origin; lacks an anterior sub-extension of the anterolateral extension; no lateral processes; two posterior tapering extensions are separated from each other ………….……………...……… S. sihama (Southern Red Sea)
- -
- Snout length 40.4–45.5% of head length; anterior extensions not joined at the origin; nine to ten lateral processes; the base of two posterior extensions adjoining and two posterior extensions adjoining …… S. cf. sihama ③ (China)
- -
- Snout length 39.4–46.8% of head length; anterior extensions not joined at the origin; eight to nine lateral processes; the base of two posterior extensions not adjoining and two posterior extensions not adjoining …………… S. malabarica
- -
- Snout length 39.5–46.5% of head length; anterior extensions joined at the origin; no lateral processes; two narrow posterior extensions separated from each other ….....................................................................................…... S. panhwari
- -
- Snout length 38.9–48.0% of head length; posterior sub extension of anterolateral extensions with some dwarf blind tubules, one-sided and outward and about one-third to half-length of the swim bladder; two dumpy posterior extensions separated from each other ……...…...……… S. parasihama
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKay, R.J. A Revision of the Fishes of the Family Sillaginidae; Memoirs of the Queensland Museum: South Brisbane, Australia, 1985; Volume 22, pp. 1–73. [Google Scholar]
- Johnson, G.D. Percomorph phylogeny: Progress and problems. Bull. Mar. Sci. 1993, 52, 3–28. [Google Scholar]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V.H. Fishes of the World, 5th ed.; John Wiley, Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Greenwood, P.H.; Rosen, D.E.; Weitzmann, S.H.; Myers, G.S. Phyletic studies of teleostean fishes, with a provisional classification of living forms. Bull. Am. Mus. Nat. Hist. 1966, 131, 339–455. [Google Scholar]
- McKay, R.J. An Annotated and Illustrated Catalogue of the Sillago, Smelt or Indo-Pacific Whiting Species Known to Date. FAO; Sillaginid fishes of the world (family Sillaginidae); Food and Agriculture Organisation of the United Nations: Rome, Italy, 1992; Species Catalogue Volume 14, pp. 1–87. [Google Scholar]
- Kaga, T.; Imamura, H.; Nakaya, K. A new sand whiting, Sillago (Sillago) caudicula, from Oman, the Indian Ocean (Perciformes: Sillaginidae). Ichthyol. Res. 2010, 57, 367–372. [Google Scholar] [CrossRef]
- Gao, T.X.; Ji, D.P.; Xiao, Y.S.; Xue, T.Q.; Yanagimoto, T.; Setoguma, T. Description and DNA barcoding of a new Sillago species, Sillago sinica (Perciformes: Sillaginidae) from coastal waters of China. Zool. Stud. 2011, 50, 254–263. [Google Scholar]
- Golani, D.; Fricke, R.; Tikochinski, Y. Sillago suezensis, a new whiting from the northern Red Sea, and status of Sillago erythraea Cuvier (Teleostei: Sillaginidae). J. Nat. Hist. 2014, 48, 413–428. [Google Scholar] [CrossRef]
- Xiao, J.G.; Song, N.; Han, Z.Q.; Gao, T.X. Description and DNA barcoding of a new Sillago species, Sillago shaoi (Perciformes: Sillaginidae), in the Taiwan Strait. Zool. Stud. 2016, 55, 1–18. [Google Scholar]
- Panhwar, S.K.; Farooq, N.; Qamar, N.; Shaikh, W.; Mairaj, M. A new Sillago species (family Sillaginidae) with descriptions of six sillaginids from the northern Arabian Sea. Mar. Biodivers. 2017, 48, 2225–2231. [Google Scholar] [CrossRef]
- Xiao, J.G.; Yu, Z.S.; Song, N.; Gao, T.X. Description of a new species, Sillago nigrofasciata sp. nov. (Perciformes, Sillaginidae) from the southern coast of China. Zookeys 2021, 1011, 85–100. [Google Scholar] [CrossRef]
- Divya, P.R.; Kumar, R.G.; Joy, L.; Shanis, C.P.R.; Basheer, V.S.; Lal, K.K. Re-Description of Sillago malabarica, Silver Whiting from Southern Indian Waters. Thalass. Int. J. Mar. Sci. 2021, 37, 627–640. [Google Scholar] [CrossRef]
- Yu, Z.S.; Guo, T.; Xiao, J.G.; Song, N.; Gao, T.X. Identification and DNA barcoding of a new Sillago species in Beihai and Zhanjiang, China, with a key to related species. J. Ocean. Univ. China. 2022; in press. [Google Scholar]
- Dutt, S.; Sujatha, K. On the seven species of fishes of the family Sillaginidae from Indian waters. Mahasagar—Bull. Natl. Inst. Oceanogr. 1980, 13, 371–375. [Google Scholar]
- McKay, R.J. The fishes of the family Sillaginidae from India with a description of a new species. J. Mar. Biol. Assoc. India 1980, 18, 375–385. [Google Scholar]
- Dutt, S.; Sujatha, K. A new record of sand whiting, Sillago intermedius (Wondratana, 1977) (Pisces: Sillaginidae) from Indian waters. Mahasagar—Bull. Natl. Inst. Oceanogr. 1984, 7, 187–188. [Google Scholar]
- McKay, R.J.; Dutt, S.; Sujatha, K. Sillago (Parasillago) indica. In A Revision of the Fishes of the Family Sillaginidae; McKay, R.J., Ed.; Memoirs of the Queensland Museum: South Brisbane, Australia, 1985; Volume 22, pp. 38–39. [Google Scholar]
- Dutt, S.; Sujatha, K. On the new species of Sillago Cuvier, 1817 (Teleostei: Sillaginidae) from India. Proc. Natl. Acad. Sci. India Sect. B 1982, 48, 611–614. [Google Scholar]
- Varadharajan, D.; Pushparaja, N.; Soundarapandian, P. Fish resources in Mallipattinam coast, southeast coast of India. Int. J. Pharm. Biol. Arch. 2012, 3, 871–876. [Google Scholar]
- Rahman, A.K.A. Freshwater Fishes of Bangladesh; Zoological Society of Bangladesh, Department of Zoology, University of Dhaka: Dhaka, Bangladesh, 1989; pp. 1–364. [Google Scholar]
- Rahman, A.K.A.; Kabir, S.M.H.; Ahmed, M.; Ahmed, A.T.A.; Ahmed, Z.U.; Begum, Z.N.T.; Hasan, M.A. Encyclopedia of Flora and Fauna of Bangladesh; Khondker, M., Ed.; Marine Fishes; Asiatic Society of Bangladesh: Dhaka, Bangladesh, 2009; Volume 24, pp. 189–190. [Google Scholar]
- Cheng, J.; Xiao, J.G.; Song, N.; Saha, S.; Qin, J.G.; Nomura, H.; Panhwar, S.K.; Farooq, N.; Shao, K.T.; Gao, T.X. Molecular phylogeny reveals cryptic diversity and swimbladder evolution of Sillaginidae fishes (Perciformes) across the Indo-West Pacific Ocean. Divers. Distrib. 2020, 27, 82–94. [Google Scholar] [CrossRef]
- Kuiter, R.H. Coastal Fishes of South-Eastern Australia; University of Hawaii Press: Honolulu, HI, USA, 1993; ISBN 1-86333-067-4. [Google Scholar]
- Tikochinski, Y.; Shainin, I.; Hyams, Y.; Motro, U.; Golani, D. Genetic evidence for an undescribed species previously considered as Sillago sihama from the northern Red Sea. Mar. Biol. Res. 2013, 9, 309–315. [Google Scholar] [CrossRef]
- Kaga, T. Phylogenetic systematics of the family Sillaginidae (Percomorpha: Order Perciformes). Zootaxa 2013, 3642, 1–105. [Google Scholar] [CrossRef]
- Shao, K.; Shen, S.; Chen, L. A newly recorded sandborer, Sillago (Sillaginopodys) chondropus Bleeker, with a synopsis of the fishes of family Sillaginidae of Taiwan. Bull. Inst. Zool. Acad. Sin. 1986, 25, 141–150. [Google Scholar]
- Kaga, T.; Ho, H.C. Redescription of Sillago (Parasillago) indica McKay, Dutt and Sujatha, 1985 (Perciformes: Sillaginidae), with a reassignment to the subgenus Sillago. Zootaxa 2012, 3513, 61–67. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D. DNA barcoding Australia’s fish species. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Ward, R.D.; Holmes, B.H. An analysis of nucleotide and amino acid variability in the barcode region of cytochrome c oxidase I (cox1) in fishes. Mol. Ecol. 2007, 7, 899–907. [Google Scholar] [CrossRef]
- Lakra, W.S.; Verma, M.S.; Goswami, M.; Lal, K.K.; Mohindra, V.; Punia, P.; Gopalakrishnan, A.; Singh, K.V.; Ward, R.D.; Hebert, P. DNA barcoding Indian marine fishes. Mol. Ecol. Resour. 2011, 11, 60–71. [Google Scholar] [CrossRef]
- Krück, N.C.; Tibbetts, I.R.; Ward, R.D.; Johnson, J.W.; Loh, W.K.W.; Ovenden, J.R. Multi-gene barcoding to discriminate sibling species within a morphologically difficult fish genus (Sillago). Fish. Res. 2013, 143, 39–46. [Google Scholar] [CrossRef]
- Xue, T.Q.; Du, N.; Gao, T.X. Phylogenetic relationships of four Sillaginidae species based on partial sequences of the COI and cytochrome b genes. J. Ocean. Univ. China 2010, 40, 91–98. [Google Scholar]
- Dias, P.J.; Wakefield, C.B.; Fairclough, D.V.; Jackson, G.; Travers, M.J.; Snow, M. Real-time PCR validation of visually identified snapper Chrysophrys auratus (Sparidae) eggs. J. Fish Biol. 2016, 88, 811–819. [Google Scholar] [CrossRef]
- Vinod, B.H.; Kumar, N.B.A.P.; Gireesh-babu, P.; Chaudhari, A. Genetic characterisation and molecular identification of two morphologically similar species of whiting, Sillago sihama (Forsskål, 1775) and Sillago vincenti McKay, 1980. Indian J. Fish. 2013, 60, 59–65. [Google Scholar]
- Hou, G.; Chen, W.T.; Lu, H.S.; Cheng, F.; Xie, S.G. Developing a DNA barcode library for perciform fishes in the South China Sea: Species identification, accuracy and cryptic diversity. Mol. Ecol. Resour. 2017, 18, 137–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Hubert, N.; Huang, Y.; Wang, X.; Gan, X.; Peng, Z.; He, S. DNA barcoding the ichthyofauna of the Yangtze River: Insights from the molecular inventory of a mega-diverse temperate fauna. Mol. Ecol. Resour. 2018, 19, 1278–1297. [Google Scholar] [CrossRef] [PubMed]
- Thu, P.T.; Huang, W.C.; Chou, T.K.; Van Quan, N.; Van Chien, P.; Li, F.; Liao, T.Y. DNA barcoding of coastal ray-finned fishes in Vietnam. PLoS ONE 2019, 14, e0222631. [Google Scholar] [CrossRef]
- Habib, K.A.; Kim, C.G.; Oh, J.; Neogi, A.K.; Lee, Y.H. Aquatic Biodiversity of Sundarbans, Bangladesh, 2nd ed.; Korea Institute of Ocean Science and Technology (KIOST): Busan, Korea, 2017; pp. 1–394. [Google Scholar]
- Ahmed, M.S.; Datta, S.K.; Saha, T.; Hossain, Z. Molecular characterization of marine and coastal fishes of Bangladesh through DNA barcodes. Ecol. Evol. 2021, 11, 3696–3709. [Google Scholar] [CrossRef]
- Xiao, J.G.; Song, N.; Gao, T.X.; McKay, R.J. Redescription and DNA barcoding of Sillago indica (Perciformes: Sillaginidae) from the coast of Pakistan. Pak. J. Zool. 2016, 48, 317–323. [Google Scholar]
- Hebert, P.D.N.; Stoeckle, M.Y.; Zemlak, T.S.; Francis, C.M. Identification of birds through DNA barcodes. PLoS Biol. 2004, 2, e312. [Google Scholar] [CrossRef] [Green Version]
- Ward, R.D.; Hanner, R.; Hebert, P.D.N. The campaign to DNA barcode all fishes, FISH-BOL. J. Fish Biol. 2009, 74, 329–356. [Google Scholar] [CrossRef]


| Meristics and Morphometric Measurements (mm) | S. muktijoddhai sp. nov. | S. mengjialensis sp. nov. | ||
|---|---|---|---|---|
| Holotype (FELOUC142377) | Paratypes (n = 123) | Holotype (FELOUC142378) | Paratypes (n = 86) | |
| Dorsal fins | XI, I+21 | X–XI, I+20–22 | XI, I+20 | XI, I+20–22 |
| Anal fin | II+22 | II+21–23 | II+22 | II+20–23 |
| Pectoral fin rays | 16 | 15–18 | 15 | 15–17 |
| Pelvic fin rays | I+5 | I+5 | I+5 | I+5 |
| Caudal fin rays | 17 | 16–17 | 17 | 16–17 |
| Scales in the lateral line | 69 | 68–72 | 68 | 66–72 |
| Scales above/below the lateral line | 5/12 | 4–6/10–13 | 5/11 | 4–5/10–12 |
| Gill rakers on first arch | 3 + 9 = 12 | 3–5 + 8–10= 11–14 | 3 + 9 = 12 | 3–4 + 8–10 = 11–13 |
| Vertebrae (AV+HV+CV) | – | 12–14 + 5–8 + 12–15 = 32–35 | – | 13–14 + 5–8 + 12–15 = 31–35 |
| Total weight (TW, g) | 6.9 | 2.28–41.59 | 8.2 | 2.1–16.9 |
| Total length (TL) | 107 | 61.36–191 | 113 | 63.1–142.54 |
| Standard length (SL) | 92 | 53.29–161 | 98 | 53.97–121.69 |
| Head length (HL) | 26 | 14.79–49 | 28 | 14.85–33.46 |
| Upper jaw length (UJL) | 5 | 3.24–8.39 | 5 | 2.50–7.73 |
| Lower jaw length (LJL) | 4 | 2.6–7.43 | 4 | 2.3–6.19 |
| Postorbital length (PL) | 10 | 4.95–21 | 10 | 5.71–13 |
| Snout length (slw) | 8 | 3.3–16 | 8 | 2.91–11.0 |
| Eye diameter (ED) | 5 | 3.69–9 | 6 | 3.35–9.17 |
| Interorbital width (IW) | 4 | 2.94–8 | 5 | 2.08–7.0 |
| Caudal peduncle depth (CPD) | 6.31 | 4.11–11 | 7.31 | 3.41–9.08 |
| Caudle peduncle length (CPL) | 8 | 4.05–17.77 | 10.73 | 3.28–12.84 |
| First dorsal fin base (D1L) | 18 | 9.83–34 | 18 | 10.16–25.93 |
| Second dorsal fin base (D2L) | 33 | 20.35–59 | 35 | 20.45–44.09 |
| Anal fin base (AL) | 34 | 21.68–59 | 36 | 19.37–44.12 |
| Pectoral fin length (ptl) | 15 | 7.71–26 | 15 | 7.9–18.04 |
| Pelvic fin length (pvl) | 14 | 7.18–25 | 14 | 6.85–19.46 |
| Body width (BW) | 10 | 5.13–21 | 10 | 6.23–14.88 |
| Body depth (BD) | 16 | 8.31–26 | 17 | 8.58–19.6 |
| As % of SL | ||||
| Body depth (BD) | 17.39 | 12.08–19.49 | 17.35 | 10.72–20.34 |
| Head length (HL) | 28.26 | 21.81–31.31 | 28.57 | 25.02–31.68 |
| Caudal peduncle length (CPL) | 8.70 | 6.66–13.13 | 10.95 | 5.11–13.25 |
| As % of HL | ||||
| Eye diameter (ED) | 19.23 | 16.28–29.54 | 21.43 | 16.67–29.87 |
| Interorbital width (IW) | 15.38 | 13.70–21.87 | 17.86 | 13.65–25.33 |
| Snout length (SLw) | 30.77 | 20.53–35.98 | 28.57 | 17.3–37.53 |
| Postorbital length (PL) | 38.46 | 32.14–46.51 | 35.71 | 29.62–44.59 |
| DCP/LCP | 78.88 | 56.05–94.1 | 68.13 | 54.52–94.97 |
| Species | Dorsal Fins | Anal Fin | Scales in Lateral Line | Scales above/ below Lateral Line | Gill Rakers First Arch | Vertebrae | Blotches | HL/SL (%) | SLw/HL (%) |
|---|---|---|---|---|---|---|---|---|---|
| S. muktijoddhai sp. nov. (n = 124) | X–XI,I,20–22 | II,21–23 | 68–72 | 4–6/10–13 | 3–5 + 8–10 = 11–14 | 12–14 + 5–8 + 12–15 = 32–35 | Absent | 21.8–31.3 | 20.5–35.98 |
| S. mengjialensis sp. nov. (n = 87) | XI,I,20–22 | II,20–23 | 66–72 | 4–5/10–12 | 3–4 + 8–10 = 11–13 | 13–14+5–8+12–15 = 31–35 | Absent | 25–31.7 | 17.3–37.5 |
| S. indica [36] (n = 72) | X–XI,I,20–22 | II,21–23 | 68–71 | 5–6/10–12 | 3–4 + 7–8 = 10–12 | 33–35 | Absent | 27.5–32.4 | 40.1–46.9 |
| S. sihama (Red Sea) [8] (n = 11) | XI,I,20–21 | II,21–23 | 70–74 | – | 4+9 | 14 + 2–8 + 12–18 = 34 | Absent | – | – |
| S. cf. sihama ③ (China) [9] | XI,I,20–23 | II,21–23 | 68–72 | 5–6/10–12 | 3 + 8–9 | 34 | Absent | 24.0–30.0 | – |
| S. shaoi [9] (n = 39) | XI,I,20–22 | II,21–22 | 70–73 | 5–6/10–12 | 3–4 + 5–6 | 35 | Absent | 26.1–31.0 | 41.8–50.2 |
| S. suezensis [8] (n = 92) | X–XII,I,19–22 | II,18–22 | 63–74 | – | 3–4 + 8–10 | 13 + 3 + 18 = 34 | Absent | 26.6–27.0 | – |
| S. sinica [7] (n = 53) | X–XI,I,20–22 | II,21–23 | 75–79 | 5–6/9–11 | 2–4 + 6–8 | 37–39 | Absent | 24.7–29.8 | 33.8–45.1 |
| S. panhwari [10] (n = 55) | X–XII,I,20–22 | II,18–23 | 69–84 | 3–4/7–10 | 3–4 + 7–9 | 33 | Absent | 27.9–35.0 | 39.5–46.5 |
| S. caudicula [6] (n = 4) | XI,I,22–23 | II,23–24 | 71 | 5/11 | 4+11 | 14–15 + 6 + 14–15 = 35–36 | Present | 29.0–30.1 | – |
| S. intermedius [1] | XI,I,21–22 | II,21–22 | 67–70 | 6–7/8–9 | – | 34 | Present | 30.0–31.0 | – |
| S. megacephalus [1] (n = 1) | XI,I,22 | II,23 | 70 | 5/10–11 | – | – | Absent | 33.0 | – |
| S. parvisquamis [1] | XII–XIII,I,20–22 | II,22–24 | 79–84 | 7/11–12 | 1–2 + 7–9 | 39–40 | Absent | 25.9–27.7 | – |
| S. nigrofasciata [11] (n = 108) | X–XII,I,20–22 | II,20–22 | 67–75 | 4–6/9–12 | 2–4/5–8 | 34–35 | Black stripe | 25.1–30.8 | – |
| S. malabarica [12] (n = 34) | XI–XII,I,21–24 | II,22–24 | 68–72 | 4–5/8–9.5 | 3–4 + 6–8 | 13 + 4 + 17 = 34 | Absent | 25–30.4 | 39.4–46.8 |
| S. parasihama [13] (n = 48) | XI–XII,I,18–21 | II,19–21 | 65–70 | 4–5/8–10 | 2–3 + 5–7 | 14 + 4–7 + 13–16 = 34 | Absent | 18.4–29.0 | 38.9–48.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, S.; Song, N.; Yu, Z.; Baki, M.A.; McKay, R.J.; Qin, J.; Gao, T. Descriptions of Two New Species, Sillago muktijoddhai sp. nov. and Sillago mengjialensis sp. nov. (Perciformes: Sillaginidae) from the Bay of Bengal, Bangladesh. Fishes 2022, 7, 93. https://doi.org/10.3390/fishes7030093
Saha S, Song N, Yu Z, Baki MA, McKay RJ, Qin J, Gao T. Descriptions of Two New Species, Sillago muktijoddhai sp. nov. and Sillago mengjialensis sp. nov. (Perciformes: Sillaginidae) from the Bay of Bengal, Bangladesh. Fishes. 2022; 7(3):93. https://doi.org/10.3390/fishes7030093
Chicago/Turabian StyleSaha, Shilpi, Na Song, Zhengsen Yu, Mohammad Abdul Baki, Roland J. McKay, Jianguang Qin, and Tianxiang Gao. 2022. "Descriptions of Two New Species, Sillago muktijoddhai sp. nov. and Sillago mengjialensis sp. nov. (Perciformes: Sillaginidae) from the Bay of Bengal, Bangladesh" Fishes 7, no. 3: 93. https://doi.org/10.3390/fishes7030093
APA StyleSaha, S., Song, N., Yu, Z., Baki, M. A., McKay, R. J., Qin, J., & Gao, T. (2022). Descriptions of Two New Species, Sillago muktijoddhai sp. nov. and Sillago mengjialensis sp. nov. (Perciformes: Sillaginidae) from the Bay of Bengal, Bangladesh. Fishes, 7(3), 93. https://doi.org/10.3390/fishes7030093








