Abstract
An assessment of the stock status and historical changes in abundance of Coilia mystus and C. nasus in the Yangtze River Estuary, China, was carried out based on field surveys conducted in 2019–2020 and published length-frequency (L/F) data from earlier periods. These two species’ current and past relative biomasses (B/BMSY) were estimated using a length-based Bayesian biomass estimation method (LBB). The LLB method also estimated their asymptotic lengths (Linf), current and optimum mean lengths at first capture (Lc; Lopt_c), and their ratios of natural and fishing mortality to growth (M/K; F/K). In response to increasing fishing pressure, both species’ maximum lengths declined, along with their B/BMSY ratio, which declined for C. mystus from 1.7 in 1982 to 0.47 in 2020 and for C. nasus from 1.7 in 2006 (or earlier) to 0.17 in 2020. These assessments show that both of the two Coilia species are overfished, with C. nasus impacted more severely than C. mystus. The prospect for the recovery of these two species is briefly discussed. This contribution will help toward the management of the population of these two Coilia species and provides a basis for evaluating the effect of the 10-year fishing ban in the Yangtze River.
1. Introduction
Due to their productivity and location, estuaries provide habitats and migratory routes to many species exploited by fisheries [1,2,3]. However, despite the immense ecological services they provide, estuaries are threatened habitats throughout the world, mainly by riverine pollution and human settlements [4]. Indeed, these traditional threats to estuaries and their resource are now intensified worldwide by ocean warming, deoxygenation, and acidification [5,6,7,8,9].
The Yangtze is the largest river in China and the third-largest river in the world. The Yangtze River Estuary (Figure 1) is the most important estuary in China and functions as a feeding and wintering ground for hundreds of species exploited by fisheries [10,11]. The depletion of fishery resources due to overfishing and environmental degradation in the Yangtze River Estuary has been relatively well documented in recent years [12,13,14,15,16].
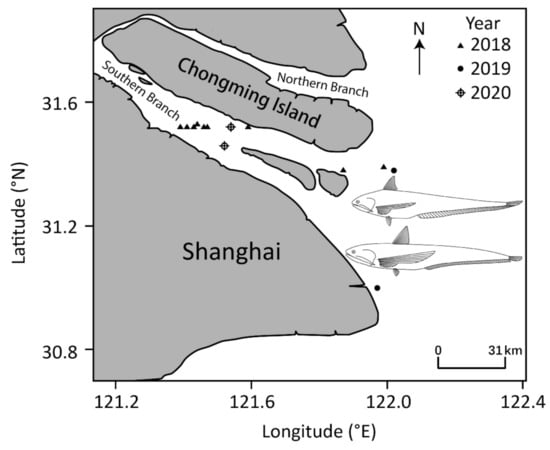
Figure 1.
Survey sites for C. mystus (2018–2020) and C. nasus (2019–2020) in the Yangtze River Estuary, China.
Osbeck’s grenadier anchovy (Coilia mystus) and Japanese grenadier anchovy (Coilia nasus) are small pelagic fish of the family Engraulidae. C. mystus is widely distributed in the coastal seas of countries in the Indo-Pacific, ranging from India in the West to China and Japan in the East [17], while C. nasus, also known as Japanese tapertail anchovy, has a more restricted distribution centered along the coastal seas of China, Japan, and South Korea in the Northwest Pacific (www.fishbase.org, accessed on 23 January 2022) [18].
These two species are commercially important [19,20], but in recent years, both declined in China and Japan, which is manifested in the decline of their catch and relative biomass (B/B0, i.e., current biomass relative to the estimated carrying capacity), in their catch consisting of younger individuals, and in a trend toward the miniaturization of body size [15,21,22,23,24]. Also, C. nasus is listed as an endangered species by the IUCN [25].
The Yangtze River and its estuary were the most important habitats for the two Coilia species, which supported two of the five major fisheries in the Yangtze River in the past decades. C. nasus was a fish sought-after by consumers in the Yangtze Basin, which resulted in a high market price for this species. C. nasus was mainly fished by drift net in Yangtze River Estuary. The catch of Shanghai in this area peaked in 1973 at 391 tonnes, then dropped to 49 tonnes by 1988 (Figure 2, right panel).
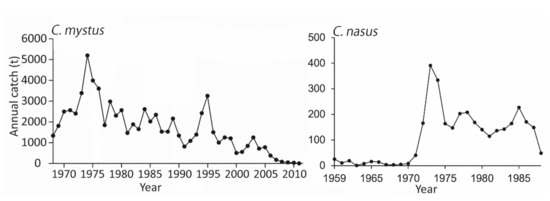
Figure 2.
Annual catch of two Coilia species in the Yangtze River Estuary (Left panel: catches of C. mystus by fishing season (April–July) from 1968 to 2011 [21,26,27,28,29,30]; right panel: catches of C. nasus of Shanghai in the Yangtze River Estuary from 1959 to 1988 [31]).
The main gears for C. mystus are gill nets and trap nets in the Yangtze River and its estuary. C. mystus accounted for almost half of China’s total domestic fishery catches before the 1960s [23,26]. Since the 1960s, the production of C. mystus has shown a fluctuating decrease in catch, which dropped from more than 5000 tonnes in 1973 to less than 20 tonnes in 2011 (Figure 2, left panel).
The stock of C. mystus in the Yangtze River can be clearly separated from the stocks in the Minjiang River and the Pearl River based on morphological, biochemical, and molecular criteria [32,33]. As for C. nasus, it is generally divided into three stocks according to ecotypes, i.e., landlocked stock, freshwater-resident stock, and anadromous stock [34,35]; the third stock is the focus of this study.
Scientific stock assessments of exploited fish populations are increasingly required in most countries to provide input for fisheries management [36,37]. A critical output of such assessments is the estimation of time series of mortality, from which the biomass of the exploited fish population can be determined and management or rehabilitation policies formulated.
In China, stock assessments have recently received a boost through the publications of robust methods for use in data-sparse situations, notably the CMSY and LBB approaches [38,39]. The latter approach—formally a length-based Bayesian biomass estimation method—has been successfully applied to over 60 populations of fish and invertebrates along the coast of China [40,41,42,43,44] and in other areas as well [45,46,47].
The LBB method applies a Bayesian Monte Carlo Markov Chain (MCMC) procedure to one (or several) length-frequency (L/F) sample(s) representative of an exploited population (over time) to estimate the population’s biomass (B) relative to its environmental carrying capacity (k), i.e., B/k, where k is roughly equivalent to unfished biomass (B0). Thus, we report here on B/B0.
Simultaneously, other key parameters are estimated by LBB from L/F samples. These include asymptotic length (Linf), length at 50% selectivity (Lc), and natural and fishing mortality relative to the K- parameter of the von Bertalanffy Growth Function, or VBGF (i.e., M/K and F/K). Since catch data, especially for recent years, is not available for C. mystus and C. nasus, we applied a length-based method to estimate their stock status. Thus, applying the LBB model to the two Coilia species presented above should help the management department to better understand their population and fisheries status, and provide a data basis for the evaluation of the effect of the 10-year fishing ban in the Yangtze River.
2. Materials and Methods
2.1. Data Sources
The L/F samples available for this analysis are presented in Tables S1 and S2 (see Supplementary Materials). The samples obtained from 2018 to 2020 originated from surveys of the South Branch of the Yangtze River Estuary in May, June, and July 2018, May 2019, and May 2020 by local fishers hired for the purpose and using set gillnets. Samples of more than 30 kg were split into subsamples, and individuals making up 10% of a sample or subsample were randomly selected and individually measured (standard and total lengths) and weighted. The collection and processing of the samples were carried out in accordance with the Specifications for oceanographic survey-Part 6: Marine biological survey (GB/T 12763.6-2007) [48].
The recent L/F that we obtained were then complemented with L/F data from the published literature to serve as a temporal contrast to the current data.
All the analyses in this paper were implemented using the R-code (version LBB_33. R), which can be downloaded from the website (http://oceanrep.geomar.de/id/eprint/44832/, accessed on 23 January 2022); their description and usage are documented in a User Guide [49]. The analyses and visualization were mainly based on the R package R2jags, Formula and ggplot2.
2.2. The LBB Method
The LBB method, as proposed by Froese et al. [39] assumes that the growth of fish is correctly described by the VBGF (see www.fishbase.org, accessed on 23 January 2022) [18,50,51], which has the form:
where Lt is the fish length at age t, Linf is the asymptotic length, i.e., the mean fish body length at an infinite age, K is a growth coefficient (here in year−1), and t0 is the theoretical age at zero length.
The LBB further assumes that the fraction of fish individuals that are retained by the fishing gear at length L, i.e., the selectivity of the fishing gear can be represented by
Also, it is assumed that the selectivity of the gear that produced the L/F samples is the same as that of the major gear in the commercial fishery, which was the case here.
From the length (Lstart) where S ≈ 1, when 100% of the fish coming in contact with the gear are retained, the numbers of length Li left in the population are given by
and
where NLi is the number of survivors at length Li, NLi-1 is their number at length Li-1, M and F are the instantaneous rates of natural and fishing mortality to which the population is exposed, and CLi is the number of individuals vulnerable to the gear, computed using the selection probabilities from Equation (2).
The maximum biomass of the unexploited population [52] is computed from
The optimum length at first capture Lc_opt that produces the maximum catch and biomass and leads to Lc_opt obtained from the equation
As shown by [39], relative yield-per-recruit (Y’/R) [53], can be re-expressed as
As CPUE is proportional to biomass, and fishing mortality is proportional to fishing effort, CPUE’/R could be obtained from Equation (6) by dividing it by F/M:
Then, the relative biomass (>Lc) when F = 0, i.e., before fisheries started, is given by
Thus, the relative biomass of exploited fishery can be obtained by
3. Results
L/F samples covering six periods were available for C. mystus (Figure 3) against three for C. nasus (Figure 4). As may be seen, the L/F data (left panels) were well fitted when the LBB model was applied to them (right panels).
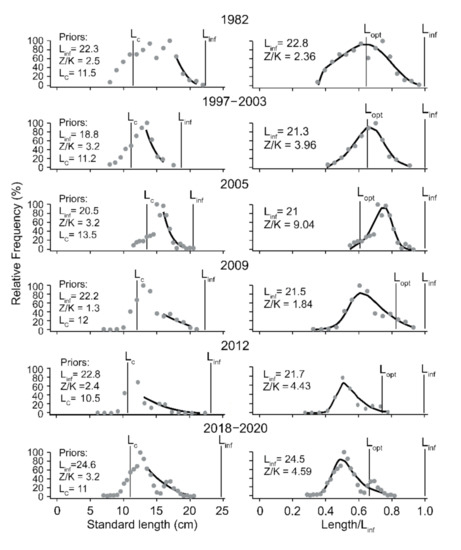
Figure 3.
LBB estimation of C. mystus from the Yangtze River Estuary. The panels on the left show the length-frequency (L/F) data from which priors are estimated for Lc, Linf, and Z/K. The panels on the right show the fit of the LBB master equation to the L/F data, which provides estimates of Z/K, M/K, F/K, Lc, and Linf, with Lopt computed from Linf and M/K.
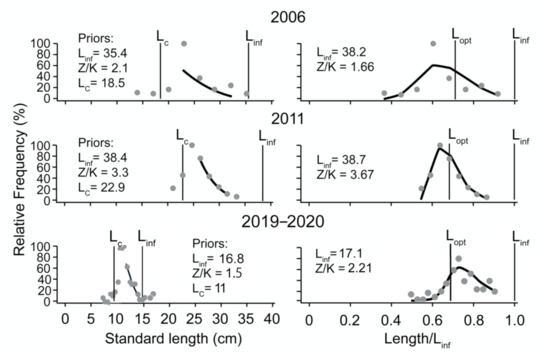
Figure 4.
LBB estimation of C. nasus in the Yangtze River Estuary. The panels on the left show the length-frequency (L/F) data from which priors are estimated for Lc, Linf and Z/K. The panels on the right show the fit of the LBB master equation to the L/F data, which provides estimates of Z/K, M/K, F/K, Lc, and Linf, with Lopt computed from Linf and M/K.
The estimated parameters, i.e., Linf, Lc_opt, F/K, Z/K, and F/M, are presented in Table 1. They show that the Linf estimates and the maximum length from each sample (Lmax) for both species have declined in recent years. This effect is particularly strong in C. nasus, whose Lmax of 350 mm in 2006 was reduced to 170 mm in 2019, while its Linf estimates went from 282 to171 mm in the same time span.

Table 1.
Parameters estimates and their 95% confidence limits (brackets) obtained via the LBB method applied to length-frequency samples covering different periods.
The length decline occurred with a sharp drop in relative biomass for both species (Table 2). For C. mystus, B/BMSY dropped from 1.7 in 1982 to 0.25 in 2012, then bounced back to 0.47 in 2020. The B/BMSY of C. nasus dropped from 1.70 in 2006 to 0.17 in 2020. This effect was caused by excessive fishing pressure, as expressed by the F/M ratio. For C. mystus, F/M increased from 0.44 in 1982, underwent some violent fluctuations, and stabilized at near 2 in recent years, and for C. nasus from 0.36 in 2006 to 1.62 in 2011, then declined to 0.55 along with fishing pressure reduced in 2020 (Table 2)

Table 2.
Estimated relative biomass (B/B0) and their 95% confidence limits (in brackets) as obtained by the LBB method.
According to the definitions in Table 3, the stock status for C. mystus in 1982 and C. nasus in 2006 was ‘healthy.’ However, after 2006, the status dropped to ‘overfished’ for both of the two fish species (Figure 5).

Table 3.
Definition of fish stock status, based on the estimation of B/BMSY in a given periods.
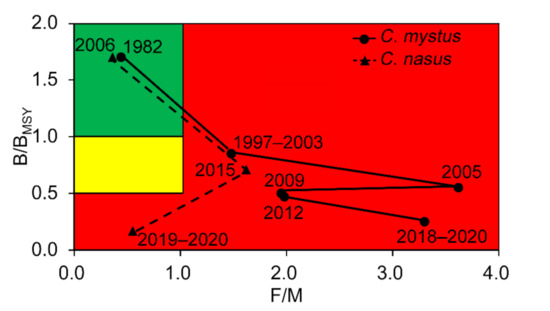
Figure 5.
Trajectories of the relative biomass (B/BMSY) and fishing pressure (F/M) for C. mystus and C. nasus in the Yangtze River Estuary, China. Red area: stocks that are being overfished and/or are outside of safe biological limits; yellow area: recovering stocks; green area: stocks subject to sustainable fishing pressure and/or a healthy stock biomass that can produce high yields close to MSY. Note that the location of 2006 data point for C. nasus is very questionable (see Discussion).
4. Discussion
A comparison of the results based on recent L/F samples suggests a worrying stock status for both Coilia species. This confirms the trend reported by other authors of worsening conditions for exploited fish populations in the Yangtze River Estuary, here illustrated by the much better state of the same fish population a few years or decades ago (Figure 5). When performing stock assessments, this illustrates the need to utilize data going as for back as possible, to avoid shifting baselines [54] and overly optimistic status assignments [55].
The B/BMSY values for C. mystus estimated here are consistent with other studies, based on different data and methods in the same period in the Yangtze River Estuary [16,56].
On the other hand, in the same period, the status of other stock of C. mystus in the Minjiang River Estuary (25°45′ N–26°30′ N and 119°30′ E–120°00′ E), another important estuary in southeastern China, was in much better shape [41,43]. It had a low F/M of 0.78 and a high B/BMSY of 1.3 in 2014 [41] and 1.9 in 2018 [43], having recovered from overfishing in the late 1980s and early 1990s [57]. This analysis illustrates that the fishing pressure in the Yangtze River Estuary far exceeds that in other parts of China’s coast, and that it is urgent to better manage the fishery resources of the Yangtze River Estuary.
What is comforting is that our data suggest that the status of C. mystus in recent years may have slightly recovered, possibly because of an early 2019 government ban on the issuance of special fishing licenses for C. mystus and C. nasus [58].
The available L/F data on C. nasus in the Yangtze River Estuary suggest that the stock had remained at a healthy level until 2006 and that its biomass declined only thereafter. However, the value of B/B0 for C. nasus in 2006 may well be an overestimate, because the L/F data used for its estimation was obtained during the spawning season [59], which led to an overrepresentation of adult fish in the sample.
This analysis is supported by the observation that 3~4-year-old individuals accounted for more than 80% of the spawning population in the 1970s, while it is reported to consist of 1~2-year-olds in more recent studies [60,61]. Similar truncations of population structure have been reported from other overexploited fish species [29,62].
In addition to fishing pressure, two other reasons have been attributed to the rapid depletion of the C. nasus population: (a) juveniles were caught as bycatch of the glass eel fishery [63], and (b) the construction of a dam led to a reduction of the spawning habitat and a shortening of the migration channel of C. nasus [64]. Indeed, similar causes have been attributed to the decline of C. nasus in the Ariake Sea, Japan [65].
The impact of the new dam on C. nasus was much more severe than on C. mystus because the former species migrates much further upstream than the latter [17,22]. With its biomass currently reduced to less than 20% of B0, C. nasus now probably suffers from depensation [66], which reduces recruitment when biomasses are low [39]. This situation was different even in the recent past, where the implementation of moderate control measures sufficed and led to a (slight) restoration of the stock [67].
Since the 1980s, China has adopted several management measures to control the fishing effort of the fisheries targeting Coilia spp., including limiting the number of fishing boats and nets, banning fishing in the spring, controlling the number of fishing licenses, and strengthening law enforcement (see Table S3 in Supplementary Materials). However, since the failure in the 1990s to ban the deep-water nets and glass eel nets that were extremely harmful to juveniles, fish stocks, especially for C. nasus, have been declining continuously. Furthermore, the 2003 dam project affected fish habitat downstream and altered the salinity and sediment supply to the estuary [61]. Thus, the dam added to the damage from legal and illegal overfishing.
Although we applaud the significant progress in the artificial propagation of C. nasus [68], we must point to the loss of genetic diversity that a massive reduction of the biomass as sea implies [69,70,71,72]. Since early 2020, much of the Yangtze River, including parts of the estuary, is officially declared protected by a ten-year fishing ban. This ban, implemented to recover the fishery resources of the Yangtze River, is the longest and strictest fishery ban in China’s history. We hope that its implementation is effective, as this would greatly improve the status of the fish of the Yangtze River and estuary.
5. Conclusions
Length-frequency data, when covering a longer time span and analyzed with the LBB method, can help straightforward inference on changes in the status of exploited fisheries resources. This is illustrated here in the case of two anchovy species, Coilia mystus and C. nasus, which are both overexploited by various fishing gears in the Yangtze Estuary. However, the life history of species must be considered when performing such assessments, as illustrated here by the fact that C. nasus, which performs longer upstream migrations than C. mystus, is, therefore, more affected by the construction of dams.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes7030095/s1, Table S1: Types and sources of the data available for this study, Table S2: Summary of the length data and sampling size for C. mystus and C. nasus in the Yangtze River Estuary used in this paper, Table S3: The main management measures and the fisheries events of C. mystus and C. nasus in the Yangtze River Estuary (1960s).
Author Contributions
L.Z.: Methodology, Formal analysis, Writing—Original draft; Z.L.: Data curation, Formal analysis; Y.H. and C.H.: Data curation, Investigation; S.T. and R.W.: Investigation, Supervision; D.P.: Methodology, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Science and Technology Commission of Shanghai Municipality (grant No. 22YF1416500), Tracking Evaluation of the Recovery Effect of Fishing-ban Resources in Key Waters of the Yangtze River (grant No. 17200292), Study on Aquatic index of biological integrity of the Yangtze River (grant No. 202003229) of Chinese Three Gorges Corporation.
Data Availability Statement
The data that support this study is derived from published papers and available in accompanying online Supplementary Materials.
Acknowledgments
D.P. thanks the Sea Around Us for support, itself funded by several philanthropic foundations, notably the Oak and Marisla Foundations. We also thank Elaine Chu for drafting our figures and the reviewers for their valuable and constructive comments on the manuscript.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Longhurst, A.R.; Pauly, D. Ecology of Tropical Oceans; Academic Press: Cambridge, MA, USA, 1987. [Google Scholar]
- Martinho, F.; Leitão, R.; Neto, J.; Cabral, H.; Marques, J.C.; Pardal, M.A. The use of nursery areas by juvenile fish in a temperate estuary, Portugal. Hydrobiologia 2007, 587, 281–290. [Google Scholar] [CrossRef]
- Martinho, F.; Leitão, R.; Neto, J.M.; Cabral, H.; Lagardère, F.; Pardal, M.A. Estuarine colonization, population structure and nursery functioning for 0-group sea bass (Dicentrarchus labrax), flounder (Platichthys flesus) and sole (Solea solea) in a mesotidal temperate estuary. J. Appl. Ichthyol. 2008, 24, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Elliott, M.; McLusky, D. The Need for Definitions in Understanding Estuaries. Estuar. Coast. Shelf Sci. 2002, 55, 815–827. [Google Scholar] [CrossRef] [Green Version]
- Houde, E.D.; Rutherford, E. Recent Trends in Estuarine Fisheries: Predictions of Fish Production and Yield. Estuaries 1993, 16, 161–176. [Google Scholar] [CrossRef]
- Roessig, J.M.; Woodley, C.M.; Cech, J.J.; Hansen, L.J. Effects of Global Climate Change on Marine and Estuarine Fishes and Fisheries. Rev. Fish Biol. Fish. 2004, 14, 251–275. [Google Scholar] [CrossRef]
- Gillanders, B.M.; Elsdon, T.S.; Halliday, I.A.; Jenkins, G.P.; Robins, J.B.; Valesini, F.J. Potential effects of climate change on Australian estuaries and fish utilising estuaries: A review. Mar. Freshw. Res. 2011, 62, 1115–1131. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.A.; Lenanton, R.C. Almost forgotten: Historical abundance of eel-tail catfish populations in south-western Australian estuaries and their decline due to habitat loss and historical overfishing. Reg. Stud. Mar. Sci. 2021, 41, 101605. [Google Scholar] [CrossRef]
- Cai, W.-J.; Hu, X.; Huang, W.-J.; Murrell, M.C.; Lehrter, J.C.; Lohrenz, S.E.; Chou, W.-C.; Zhai, W.-D.; Hollibaugh, J.T.; Wang, Y.; et al. Acidification of subsurface coastal waters enhanced by eutrophication. Nat. Geosci. 2011, 4, 766–770. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Huang, D.; Wu, Y.; Liang, J. Oxygen depletion off the Changjiang (Yangtze River) Estuary. Sci. China Ser. D Earth Sci. 2002, 45, 1137–1146. [Google Scholar] [CrossRef]
- Zhuang, P. Fisheries of the Yangtze Estuary, 1st ed.; China Agriculture Press: Beijing, China, 2006; 497p. [Google Scholar]
- Luo, M.B. Communities’ Response of Macrobenthos to Huge Engineering and the Ecological Restoration in Yangtze Estuarine, China. Ph.D. Thesis, East China Normal University, Shanghai, China, 2008. [Google Scholar]
- Yu, H.; Xian, W. The environment effect on fish assemblage structure in waters adjacent to the Changjiang (Yangtze) River estuary (1998–2001). Chin. J. Oceanol. Limnol. 2009, 27, 443–456. [Google Scholar] [CrossRef]
- Zhang, Z.; Mammola, S.; Xian, W.; Zhang, H. Modelling the potential impacts of climate change on the distribution of ichthyoplankton in the Yangtze Estuary, China. Divers. Distrib. 2020, 26, 126–137. [Google Scholar] [CrossRef] [Green Version]
- Zhai, L.; Liang, C.; Pauly, D. Assessments of 16 Exploited Fish Stocks in Chinese Waters Using the CMSY and BSM Methods. Front. Mar. Sci. 2020, 7, 483993. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, H.; Yang, G. Springtime spatial distributions of biogenic sulfur compounds in the Yangtze River Estuary and their responses to seawater acidification and dust. J. Geophys. Res. Biogeosci. 2021, 126, e2020JG006142. [Google Scholar] [CrossRef]
- Yu, X. Research on Biological Characteristics of Anadromous Spawning Coilia Mystus in Estuary of the Yangtze River. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2014. [Google Scholar]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication. Available online: www.fishbase.org (accessed on 23 January 2022).
- Cha, B.-Y.; Im, Y.-J.; Jo, H.-S.; Kwon, D.-H. A Fish Community Caught by a Stow Net in the Water off Hwaseong City, the West Sea, Korea. Korean J. Ichthyol. 2013, 25, 119–134. [Google Scholar]
- Choi, H.C.; Youn, S.H.; Huh, S.-H.; Park, J.M. Diet Composition and Feeding Habits of Two Engraulid Fish Larvae (Engraulis japonicus and Coilia nasus) in the Nakdong River Estuary, Korea. J. Coast. Res. 2018, 85, 346–350. [Google Scholar] [CrossRef]
- Zhang, G.X.; Hua, J.D. Changes in the resources of the Coilia mystus in the Yangtze River Estuary and estimation of its maximum sustainable yield. Fish. Sci. Technol. Inf. 1990, 5, 131–134. [Google Scholar] [CrossRef]
- Mao, C.Z.; Jiao, X.M.; Zhong, J.S.; Hua, W.H.; Zhang, X.Y.; Wu, J.X. Research progress on resource status and protection of Coilia nasus in Yangtze River Estuary. J. Huaihai Inst. Technol. 2015, 24, 78–83. [Google Scholar] [CrossRef]
- Zheng, Y. Evaluation of Coilia mystus of the Yangtze River Estuary. J. Anhui Agric. Sci. 2012, 40, 17140–17143. [Google Scholar]
- Itakura, H.; Yokouchi, K.; Kanazawa, T.; Matsumoto, M.; Matoba, T.; Wakiya, R.; Shirai, K.; Ishimatsu, A. Diverse downstream migration patterns of the anadromous Japanese grenadier anchovy Coilia nasus in the Chikugo River estuary and Ariake Sea, Japan. Reg. Stud. Mar. Sci. 2020, 39, 101436. [Google Scholar] [CrossRef]
- Hata, H. Coilia nasus (Corrected Version: 2019). The IUCN Red List of Threatened Species 2018, e.T98895427A143840780. 2018. Available online: https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T98895427A143840780.en (accessed on 1 September 2021).
- Wang, Y.H.; Ni, Y. On the fisheries resources and their exploitation of the Changjiang (Yangtze) River Estuary in Shanghai Region. J. Fish. China 1984, 8, 147–159. [Google Scholar]
- Shi, W.G.; Wang, B. Status quo of tapertail anchovy resource in the estuaries of the Yangtze River. Acta Hydrobiol. Sin. 2002, 26, 648–653. [Google Scholar]
- Liu, K.; Zhang, M.Y.; Xu, D.P.; Shi, W.G. Studies on resource change and MSY of Coilia mystus in the Yangtze River estuary. J. Shanghai Fish. Univ. 2004, 13, 298–303. [Google Scholar]
- Liu, Q.G.; Shen, J.Z.; Chen, M.K.; Tong, H.Y.; Li, J.L.; Chen, L.Q. Advances of the study on the miniaturization of natural economical fish resources. J. Shanghai Ocean. Univ. 2005, 14, 79–83. [Google Scholar]
- Liu, K.; Xu, D.P.; Duan, J.R.; Zhang, M.Y.; Fang, D.A.; Zhou, Y.F.; Shi, W.G. Fluctuation of biological characteristics and yield of Coilia mystus in fishing season after impoundment of the Three Gorges Dam in Yangtze Estuary. Resour. Environ. Yangtze Basin 2013, 22, 1282–1283. [Google Scholar]
- East China Sea Fisheries Research Institute. Fishes of Shanghai; Shanghai Science and Technology Press: Shanghai, China, 1992; 31p. [Google Scholar]
- Yuan, C.M.; Lin, J.B.; Qin, A.L.; Liu, R.H. On the classification of the anchovies, Coilia, from the lower Yangtze River and the Southeast Coast of China. J. Nanjing Univ. 1976, 2, 1–5. [Google Scholar]
- Yang, Q.; Zhao, F.; Song, C.; Zhang, T.; Miao, Z.B.; Zhuang, P. Analysis of morphological variations among four different geographic populations of Coilia mystus in the Yangtze River Estuary and its adjacent waters. Mar. Fish. 2019, 41, 294–303. [Google Scholar]
- Li, Y.; Xie, S.; Li, Z.; Gong, W.; He, W. Gonad development of an anadromous fish Coilia ectenes (Engraulidae) in lower reach of Yangtze River, China. Fish. Sci. 2007, 73, 1224–1230. [Google Scholar] [CrossRef]
- Ma, C.Y.; Cheng, Q.Q.; Zhang, Q.Y. Development of 12 polymorphic microsatellite markers in Coilia ectenes Jordan and Seale, 1905 (Clupeiformes: Engraulidae) and cross-species amplification in Coilia mystus (Linnaeus, 1758). Environ. Biol. Fishes 2011, 91, 243–249. [Google Scholar] [CrossRef]
- MSA. Magnuson-Stevens Fishery Conservation and Management Act, Public Law 94–265. As Amended by the Magnuson-Stevens Fishery Conservation and Management Reauthorization Act (P.L. 109-479). 2007. Available online: http://www.nmfs.noaa.gov/msa2005/docs/MSA_amended_msa%20_20070112_FINAL.pdf (accessed on 1 September 2021).
- CFP. Regulation (EU) No 1380/2013 of the European Parliament and of the Council of 11 December 2013 on the Common Fisheries Policy, amending Council Regulations (EC) No 1954/2003 and (EC) No 1224/2009 and repealing Council Regulations (EC) No 2371/2002 and (EC) No 639/2004 and Council Decision 2004/585/EC. Off. J. Eur. Union 2013, L354, 22–61. [Google Scholar]
- Froese, R.; Demirel, N.; Coro, G.; Kleisner, K.M.; Winker, H. Estimating fisheries reference points from catch and resilience. Fish Fish. 2017, 18, 506–526. [Google Scholar] [CrossRef] [Green Version]
- Froese, R.; Winker, H.; Coro, G.; Demirel, N.; Tsikliras, A.C.; Dimarchopoulou, D.; Scarcella, G.; Probst, W.N.; Dureuil, M.; Pauly, D. A new approach for estimating stock status from length frequency data. ICES J. Mar. Sci. 2018, 75, 2004–2015. [Google Scholar] [CrossRef]
- Liang, C.; Xian, W.; Liu, S.; Pauly, D. Assessments of 14 Exploited Fish and Invertebrate Stocks in Chinese Waters Using the LBB Method. Front. Mar. Sci. 2020, 7, 314. [Google Scholar] [CrossRef]
- Wang, L.; Lin, L.; Li, Y.; Xing, Y.; Kang, B. Sustainable Exploitation of Dominant Fishes in the Largest Estuary in Southeastern China. Water 2020, 12, 3390. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Liu, S.; Liang, C.; Zhang, H.; Xian, W. Stock Assessment Using LBB Method for Eight Fish Species from the Bohai and Yellow Seas. Front. Mar. Sci. 2020, 7, 164. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Ren, Q.-Q.; Liu, M.; Xu, Q.; Kang, B.; Jiang, X.-B. Fishery Stock Assessments in the Min River Estuary and Its Adjacent Waters in Southern China Using the Length-Based Bayesian Estimation (LBB) Method. Front. Mar. Sci. 2020, 7, 507. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Du, F.; Liu, M.; Bei, W.; Cai, Y.; Qiu, Y. Using LBB Tools to Assess Miter Squid Stock in the Northeastern South China Sea. Front. Mar. Sci. 2021, 7, 1192. [Google Scholar] [CrossRef]
- Kindong, R.; Gao, C.; Pandong, N.A.; Ma, Q.; Tian, S.; Wu, F.; Sarr, O. Stock Status Assessments of Five Small Pelagic Species in the Atlantic and Pacific Oceans Using the Length-Based Bayesian Estimation (LBB) Method. Front. Mar. Sci. 2020, 7, 592082. [Google Scholar] [CrossRef]
- Al-Mamun, M.A.; Liu, Q.; Chowdhury, S.R.; Uddin, M.S.; Nazrul, K.M.S.; Sultana, R. Stock Assessment for Seven Fish Species Using the LBB Method from the Northeastern Tip of the Bay of Bengal, Bangladesh. Sustainability 2021, 13, 1561. [Google Scholar] [CrossRef]
- Dimarchopoulou, D.; Mous, P.J.; Firmana, E.; Wibisono, E.; Coro, G.; Humphries, A.T. Exploring the status of the Indonesian deep demersal fishery using length-based stock assessments. Fish. Res. 2021, 243, 106089. [Google Scholar] [CrossRef]
- CNSMC (China National Standardization Management Committee). GB/T 12763.6-2007; Specifications for Oceanographic Survey—Part 6: MARINE Biological Survey. China National Standardization Management Committee: Beijing, China, 2007.
- Froese, R.; Winker, H.; Coro, G.; Demirel, N.; Tsikliras, A.C.; Dimarchopoulou, D.; Scarcella, G.; Probst, W.N.; Dureuil, M.; Pauly, D. A Simple User Guide for LBB (LBB_33a.R). 2019. Available online: http://oceanrep.geomar.de/44832/ (accessed on 1 September 2021).
- Von Bertalanffy, L. A quantitative theory of organic growth (inquiries on growth laws. II). Hum. Biol. 1938, 10, 181–213. [Google Scholar] [CrossRef]
- Pauly, D. Beyond our original horizons: The tropicalization of Beverton and Holt. Rev. Fish Biol. Fish. 1998, 8, 307–334. [Google Scholar] [CrossRef]
- Holt, S.J. The Evaluation of Fisheries Resources by the Dynamic Analysis Stocks, and Notes on the Time Factors Involved. In Proceedings of the Some Problems for Biological Fishery Surveys and Techniques for Their Solutions—A Symposium, Biarritz, France, 1–10 March 1956; International Commission for the Northwest Atlantic Fisheries: Halifax, NS, Canada Special Publication No.1. , 1958; pp. 77–95. [Google Scholar]
- Beverton, R.J.H.; Holt, S.J. Manual of Methods for Fish Stock Assessment, Part II. Tables Yield Funct. Fish. Tech. Pap. 1966, 38, 7–29. [Google Scholar]
- Pauly, D. Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol. Evol. 1995, 10, 430. [Google Scholar] [CrossRef]
- Schijns, R.; Pauly, D. Management implications of shifting baselines in fish stock assessments. Fish. Manag. Ecol. 2021, 29, 183–195. [Google Scholar] [CrossRef]
- Zhai, L.; Pauly, D. Yield-per-Recruit, Utility-per-Recruit, and Relative Biomass of 21 Exploited Fish Species in China’s Coastal Seas. Front. Mar. Sci. 2019, 6, 724. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, L.M.; Li, J.; Li, W.W.; Zhang, Y.Z. Resource assessment of Clupeiformes fishes in Fujian coastal waters. Mar. Fish. 2012, 34, 285–294. [Google Scholar]
- Fisheries Supervision and Administration Office of Yangtze River Basin. Announcement of the Ministry of Agriculture and Rural Affairs on Adjusting the Special Fishing Management System in the Yangtze River Basin. 2018. Available online: http://www.cjyzbgs.moa.gov.cn/tzgg/201904/t20190428_6220295.htm (accessed on 1 September 2021).
- Guan, W.B.; Chen, H.H.; He, W.H. Reproductive characteristics and condition status of Coilia mystus (Linnaeus) in the Changjiang River estuary. Prog. Fish. Sci. 2011, 32, 1–9. [Google Scholar]
- Yuan, C.F. The changes and causes in resources and population composition of Coilia nasus in the middle and lower reaches of the Yangtze River. Chin. J. Zool. 1988, 23, 12–14. [Google Scholar]
- Zhang, Y.M.; Xu, D.P.; Liu, K.; Shi, W.G. Studies on biological characteristics and change of resource of Coilia nasus Schlegel in the lower reaches of the Yangtze River. Resour. Environ. Yangtze Basin 2005, 14, 694–698. [Google Scholar]
- Esin, E.V.; Markevich, G.N.; Shkil, F.N. Rapid miniaturization of Salvelinus fish as an adaptation to the volcanic impact. Hydrobiologia 2020, 847, 2947–2962. [Google Scholar] [CrossRef]
- Ge, C.G.; Zhong, J.S.; Ge, K.K.; Li, A.D.; Liu, P.T.; Wang, M.X.; Yan, X. Analysis on the composition of by-catch in elver nets and the suggestions on the management of elver nets in Yangtze River estuary. J. Shanghai Ocean. Univ. 2013, 22, 391–397. [Google Scholar]
- Pan, B.Z.; Liu, X.Y. A review of water ecology problems and restoration in the Yangtze River Basin. J. Yangtze River Sci. Res. Inst. 2021, 38, 1–8. [Google Scholar] [CrossRef]
- Liu, H.B.; Jiang, T.; Yang, J. Current status and problems of estuarine tapertail anchovy (Coilia nasus) resource in the Ariake Sea of Japan. Fish. Inf. Strategy 2019, 34, 48–52. [Google Scholar] [CrossRef]
- Hutchings, J.A. Renaissance of a caveat: Allee effects in marine fish. ICES J. Mar. Sci. 2014, 71, 2152–2157. [Google Scholar] [CrossRef] [Green Version]
- Duan, J.R.; Zhang, H.Y.; Liu, K.; Xu, D.P.; Zhang, M.Y.; Shi, W.G. An Overview of Coilia ectenes in Jiangsu Section of the Yangtze River. Agric. Sci. Technol. 2012, 13, 1950–1954. [Google Scholar] [CrossRef]
- Xu, G.C.; Tang, X.; Zhang, C.X.; Gu, R.B.; Zheng, J.L.; Xu, P.; Le, G.W. First studies of embryonic and larval development of Coilia nasus (Engraulidae) under controlled conditions. Aquac. Res. 2011, 42, 593–601. [Google Scholar] [CrossRef]
- Smith, P.J.; Francis, R.; McVeagh, M. Loss of genetic diversity due to fishing pressure. Fish. Res. 1991, 10, 309–316. [Google Scholar] [CrossRef]
- Beardmore, J.A.; Mair, G.C.; Lewis, R.I. Biodiversity in aquatic systems in relation to aquaculture. Aquac. Res. 1997, 28, 829–839. [Google Scholar] [CrossRef]
- Diana, J.S. Aquaculture production and biodiversity conservation. Bioscience 2009, 59, 27–38. [Google Scholar] [CrossRef]
- Del Mar Ortega-Villaizan, M.; Noguchi, D.; Taniguchi, N. Minimization of genetic diversity loss of endangered fish species captive broodstocks by means of minimal kinship selective crossbreeding. Aquaculture 2011, 318, 239–243. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).