Abstract
Brook trout populations in Prince Edward Island, Canada, have experienced over 50 pesticide-related fish kills since the 1960s. Life history evaluation of large sea-run brook trout recovered following two fish kill events was compared with a reference river using strontium:calcium otolith microchemistry. This study examined the dual hypotheses that anadromous brook trout are more likely to arise from sea-run mothers, and that freshwater entry timing makes them vulnerable to pesticide-induced fish kills. A total 89% of the fish exhibited an anadromous life history, and 77% of these were offspring of anadromous mothers, suggesting that anadromy is dominant in progeny of sea-run mothers. This study adds to our understanding of the maternal inheritance of anadromy in sea-run brook trout populations. Additionally, freshwater entry precedes the majority of fish kill events, illustrating that the overlap between migration and pesticide runoff contributes to the cumulative population risks to sea-run brook trout.
1. Introduction
The health of many aquatic ecosystems has been compromised by the cumulative impacts of human activities. Biodiversity in freshwater is declining faster than in terrestrial or marine environments [1]. While estuaries, which link fresh and marine waters, experience reduced biodiversity in systems affected by human development [2]. Diadromous fish species move between fresh and marine waters and factors that compromise the timing or restrict the spatial extent of movements pose a threat to migration [3,4,5]. Due to the multiple and cumulative threats to the habitat of diadromous fishes, many populations have been subject to pronounced declines and many species have experienced population extirpations within their historic range [6,7]. As human activities, including climate change, continue to infringe upon these environments, coherent conservation strategies are needed to protect diadromous species and the ecosystems they occupy [8,9].
Brook trout (Salvelinus fontinalis) are native to eastern North America and coastal populations are known to adopt a mixture of resident and sea-run life histories (i.e., facultative anadromy) [3,10]. Due to the spatial and temporal complexity of the habitat of anadromous brook trout, populations have been subject to declines across much of their native range, particularly south of the Bay of Fundy [11,12]. The sea-run life history strategy is thought to have an adaptive basis as anadromous mothers produce more eggs with enhanced fitness-related traits such as a shorter developmental time, faster juvenile growth rates, and higher survival [13]. Moreover, the progeny of sea-run females are the dominant proportion of young-of-year fish in areas where anadromous and resident spawners overlap [14]. Linking the contribution of anadromous parents to subsequent generations has primarily been explored in young-of-year offspring via stable-isotope analysis [14,15]. However, the marine isotopic signatures from anadromous mothers begin to change when juveniles commence exogenous feeding, making it difficult to detect the contribution of anadromous parents beyond early life stages [14,15]. Genetic differentiation has been used as a means of evaluating anadromous contributions [13] and sympatric sea-run and resident brook trout have been found to be genetically divergent, which might occur if sea-run progeny are themselves more likely to exhibit this phenotype [16]. In one brook trout study, Theriault et al. [17] used genetic methods to infer the heritability of the anadromous tactic as sea-run parentage was positively correlated with ultimate size at age 1. However, there are still knowledge gaps on the overall contribution of anadromous parents to subsequent generations and the perpetuation of the migratory life history.
Prince Edward Island (PEI), in eastern Canada, is well known for its anadromous brook trout populations, but the province also has a history of pesticide-related fish kills, where brook trout are the dominant species affected [18]. Since the 1960s, there have been at least 50 fish kills related to pesticide runoff. One study examining changes in the fish community after a fish kill found that brook trout suffered higher mortality than non-native rainbow trout (Oncorhynchus mykiss) and that the effects were strongest on young-of-year fish, but all sizes were affected [19]. Atlantic salmon (Salmo salar) were formerly widespread in the province, but overharvest and habitat deterioration have reduced their populations, making them less common in pesticide-related fish kills [18]. Overall, there is a lack of understanding of how these events influence the distribution, abundance and population demographics in local species assemblages.
Otoliths, or ear stones, incorporate certain environmental constituents in proportion to ambient concentration, and chemical analysis can be used to reconstruct chronological life history patterns [20]. In salmonid fishes, and some other species, elevated strontium concentrations are associated with marine occupation and prior to spawning, maternal signatures can be passed to eggs, and by examining otolith primordia of offspring, the maternal life history of offspring can be elucidated [21,22]. To date, opportunities to study the anadromous life history of large, sexually mature brook trout through otolith chemistry have been rare because otolith sampling is lethal and sacrificing large numbers of sexually mature fish would be ethically questionable given the significant reproductive contribution of these individuals to subsequent generations [13,23].
The overall aim of this study was to explore why brook trout are predisposed to the risk of pesticide-related fish kills and to use otolith microchemistry to explore the demographics of anadromous life history in coastal brook trout populations. Firstly, we examined how the timing of freshwater entry of anadromous brook trout relates to the timing of pesticide fish kills by analyzing 11 years of counting-fence data from an anadromous population on Montague River. It was predicted that freshwater entry would precede the occurrence of pesticide runoff events. Additionally, otolith microchemistry was used to establish the anadromous life history patterns of brook trout collected following fish kills in comparison to the signatures of samples taken from the Montague River counting fence. The prediction was that anadromous individuals would be more likely to arise from anadromous mothers, and that anadromous individuals would conduct repeated migrations once the anadromous life history strategy was adopted.
2. Materials and Methods
2.1. Study Sites
PEI has an area of 5560 km2 and is characterized by many short watersheds fed predominantly by groundwater. Temperatures in most rivers are suitable for salmonids, remaining below 18 °C throughout the year [24,25,26]. Following Holocene sea level rise, many estuaries are considered to be drowned river valleys, and these relatively large and productive estuaries are highly conducive for anadromous fishes [27,28]. Agricultural land use influences fish populations in the province as approximately 50% of the province is used for agricultural production [29,30]. As a consequence of the highly agricultural land-use sedimentation, nitrogen loading from fertilizers and pesticide runoff events are common phenomena in PEI waterways [31,32,33]. The present study took place in three PEI watersheds, Mill River (50.1 km2), Montague River (65.4 km2), and Trout River (51.9 km2) (Figure 1).
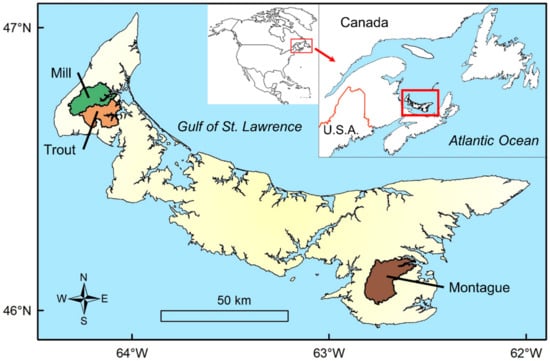
Figure 1.
Map showing the location of Prince Edward Island, Canada, and the three study watersheds.
2.2. Fish Community
The freshwater fish community on PEI is generally dominated by salmonids [29]. Brook trout are ubiquitous and the primary sportfish in the province, especially anadromous brook trout which have strong cultural and socioeconomic value [29]. Current land-use patterns, and historic activities play a dominant role in structuring current salmonid communities in PEI watersheds [26]. Atlantic salmon were widespread prior to human colonization but are now lost from over 60% of the watersheds the species once occupied and now populations are stronger in forested watersheds with abundant cobble substrate [34,35]. Non-native rainbow trout are present in approximately 30 rivers across the province. Rainbow trout tend to thrive in higher sloped reaches and in watersheds with higher agricultural land use, while brook trout juveniles tend to be dominant in headwater reaches [25]. Other common species in PEI, which have been recovered following pesticide fish kills include American eel (Anguilla rostrata), white perch (Morone americana) and several stickleback species (Gasterosteus spp.) [18]. Other diadromous species that are seasonally present in PEI waterways during spawning migrations including rainbow smelt (Osmerus mordax), blueback herring (Alosa aestivalis), and alewife (Alosa pseudoharengus) [18].
2.3. Timing of Brook Trout Movements and Fish Kills
Given the paucity of data on run timing for anadromous brook trout, a historic dataset from a fish trap at Knox’s Dam, Montague River was evaluated to establish the timing of freshwater return of anadromous brook trout. The fish trap was located in a technical concrete pool-and-weir-style fishway located at the head of tide. Fish moving upstream through the trap were considered to be moving from saltwater into freshwater. Historic data from the period 1982–1993 were provided by D. Cairns (Department of Fisheries and Oceans) and the counting fence was operated by the authors from 2011 to 2013. Fish were enumerated and individually measured (fork length, mm) and released upstream of the barrier. In total, 11 years of brook trout movement and abundance were evaluated including the total number of brook trout moving upstream each year, including 1982–1985, 1989–1990, 1991, 1993, and 2011–2013. Entry date was determined by finding the day of the year (Julian day) at which 50% of brook trout tend to be entering the river had arrived. Large female brook trout tend to be among the first to enter freshwater (3; S. Roloson, see results); therefore, the 50% cutoff includes the majority of the large breeding females. This date was used to compare the dates of fish kill events, in order to determine potential overlap between the timing of fish entry into freshwater and the incidence of pesticide runoff.
Data on fish kill timing were accessed from public records available online [18]. Of the 57 fish kills with information available from 1962 to 2019, 55 of these events list a specific date and two others list “summer” without a specific date. The Julian day of the fish kills with a specific date was used to enable comparisons of the ‘day of the year’ between subsequent years and with brook trout movement timing.
2.4. Fish Sampling and Otolith Microchemistry
Brook trout were opportunistically collected from two rivers following pesticide runoff events. The first fish kill sampled was on Trout River, which experienced a pesticide runoff event on 5 July 2012, and a subsample of 15 trout from 21.4 to 36.9 cm were collected on 6 July 2012 for otolith sampling. The Mill River fish kill occurred on 28 July 2013. Specimens were collected by local watershed officials who visually observed deceased trout on streambanks and on the streambed. Specimens were transported back to the lab for measuring fork length (mm) and weight (g) and extraction of saggital otoliths. Following removal, otoliths were rinsed with deionized water, left to dry for 24 h and stored in acid-washed 50 mL glass vials.
The third brook trout population was sampled from Montague River, where a counting fence captured fish as they ascended into freshwater from the estuary, so this river served as a reference as these fish were known to exhibit an anadromous life history. A subset of 31 fish from 15.6–38.1 cm were sampled for otolith microchemistry between 6 June and 29 June 2012. While fish at Montague River were sampled randomly during freshwater entry, samples at fish kills were opportunistically collected, and smaller fish could have been underrepresented due to the difficulty in finding and recovering smaller individuals.
Otoliths were processed at the Mass Spectroscopy Suite University of the Waikato in Hamilton, New Zealand. Prior to sampling, otoliths were stored in acid-washed glass vials and handled with non-metallic utensils to avoid trace metal contamination. Otoliths were mounted sulcus side up and sequentially polished with 1000, 2000, and 4000 grain wetted silicone carbide waterproof sandpaper until primordial nuclei were exposed. Decontamination procedures are outlined in Roloson et al. 2019 [36]. Once polished, otoliths were mounted on a single glass slide in batches [15,16,17,18,19,20] to allow for more efficient laser operation. Otoliths were analyzed with a Perkin Elmer Elan SCIEX DRCII laser ablation inductively coupled mass spectrometer (LAICP–MS) with a New Wave Research Nd:YAG 213 nm wavelength laser. To account for instrument drift, National Institute of Standards and Technology (NIST) 612 standard reference material was ablated prior, during and following sample ablation and for 1 min of every 15 min of laser operation time. The otolith core was identified using a transmitted light microscope and life history transects were oriented from the otolith primordia to the outer edge. The laser was set to a 20 µm beam diameter width at a frequency of 20 Hz and a travelling speed of 10 m·s–1. Brook trout otoliths were analyzed with the same protocols and run during the same sample runs as anadromous rainbow trout from PEI [36].
2.5. Statistical Analysis
Across all years, brook trout fresh water entry timing was summarized as the mean percent of total run entering freshwater each day. The mean daily proportion of entry was fitted using a Gaussian function: daily entry = a·exp(−(Julian Day−b)2/(2·c2), where a represents the maximum daily entry proportion, b the day on which the maximum occurred, and c the variance around the day. Fish kill date was represented as a cumulative proportion of fish kills for each Julian day. The median fish kill day was determined by fitting a two parameter logistic regression to the data of the form: cumulative proportion of fish kills = 100/(1 + 10(log10(Median Julian Day−Julian Day)·(Hillslope)), where hillslope is the shape parameter and median Julian day the day when half of the fish kills occurred. All summary statistics and curve fits were performed in Statistica v. 13.5.
Assessment of life history signatures was based upon previously established methods [37,38,39]. To assess natal core signatures, we took the mean otolith core Sr:Ca values from the first 25 sequential data points located in first 200 µm of the transect [37,40]. Rather than using a threshold to designate freshwater or marine core signatures, we used the univariate K-means clustering in the “factoextra” package in R, which uses an algorithm to separate core signatures into two groups based on differences in the data [37,40]. Differences in anadromous migratory history are much more explicit upon visual examination of line scans as it is common for migratory individuals to have Sr:Ca peaks reaching 4–7 mmol/mol. Therefore, to designate migratory life history, we followed Austin et al [38], who studied the closely related bull trout (Salvelinus confluentus). In that study, individuals with a Sr:Ca ratio that remained below 1 mmol/mol Sr:Ca were considered freshwater residents, and those with a ratio over 1 mmol/mol Sr:Ca were considered anadromous.
3. Results
3.1. Timing of Brook Trout Movement into Fresh Water
The timing of brook trout movements was determined from 11 years of abundance counts at Knox’s Dam (Montague River), which occurred intermittently between 1982 and 2013. Overall, the mean ± SD number of brook trout was 1268 ± 1020, with a high of 3069 brook trout counted in 1988 and a low of 193 in 1983. In the first time period, from 1982 to 1985, the mean annual brook trout count was 398 ± 234; between 1988 and 1993, the mean count was 2236 ± 515; and from 2011 to 2013, the mean count was 1138 ± 874. The timing of 50% entry spanned over a 43 day range from Julian Day 170 (18 June 1984) to Day 214 (2 August 1993). The median Julian Day of entry was 194 ± 13 SD. Peak freshwater entry is presented over the 11 year period, summarized in Figure 2, showing the daily run proportion entering freshwater each day. Figure 3 depicts the size distribution of brook trout entering freshwater when the authors operated the counting fence from 2011–2013 and it illustrates that larger individuals tend to be amongst the first to enter freshwater.
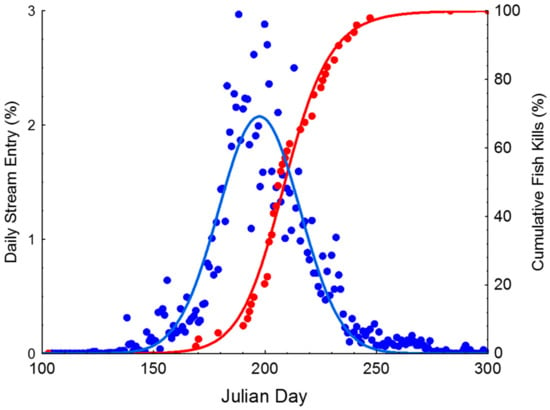
Figure 2.
Timing of freshwater entry based upon 11 years of abundance data from Montague River (blue) and the date of occurrence of pesticide fish kill events (red).
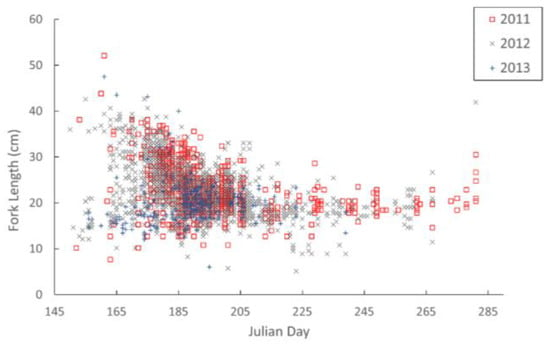
Figure 3.
Size vs. timing of brook trout (n = 853) travelling upstream through the counting fence at Knox’s Dam on Montague River in 2011.
3.2. Timing and Occurrence of Pesticide-Related Fish Kills
The timing of runoff events closely coincided with the timing of brook trout movements into freshwater. Mean ± SD Julian Day of all fish kill events was 212 ± 20. Fish kill dates ranged from Julian Day 169 (18 June 1977) to Day 283 (10 October 2017), with 29 occurring in July and 21 in August. The two rivers sampled following fish kills, Mill River and Trout River have numerous historic records of fish kills. Trout River has had six fish kills, which is the most of any watershed in the province, including fish kills in three consecutive years (2011, 2012, and 2013). In the 2011 event on Trout River (23 July 2011), 356 brook trout were collected with a mean ± SD size of 30.4 ± 9.5 cm. Following another runoff event on 5 July 2012, 1036 brook trout were collected with a mean size of 15 ± 6 cm SD. On 25 July 2013, 102 brook trout were collected with a mean ± SD size of 19.6 ± 8.6 cm. In the Mill River fish kill, which occurred on 27 July 2013, 71 fish were collected with a mean ± SD size of 33.8 ± 9.8 cm. Unfortunately, population surveys are not available following fish kills, which could help evaluate the percentage of the population that was affected by the runoff event. The extensive loss of brook trout following pesticide runoff events is reflected in publicly available summary reports for six recent fish kills, where brook trout were the dominant species found, constituting between 71% and 99% of fish collected following runoff events [18].
3.3. Otolith Chemistry and Anadromous Life History
From the microchemical evaluation of maternal phenotype a bimodal distribution of otolith cores was evident. Overall the K-means clustering algorithm estimated a cutoff value of 0.87 mmol/mol Sr:Ca, whereby individuals with core values above were designated as being from anadromous mothers, and those below were considered to be from freshwater mothers. Those identified to be descendants of anadromous mothers (n = 48), had a mean value of 1.26 mmol/mol Sr:Ca (±0.27 SD), and those from freshwater mothers (n = 16) had a mean Sr:Ca value of 0.47 mmol/mol (±0.23 SD), (Table 1).

Table 1.
Summary statistics and phenotypes of 64 brook trout sampled for otolith microchemistry where individual lengths were measured in fork length (FL, cm). Phenotypes are designated from Sr:Ca ratios as anadromous (An) or freshwater (Fw) for maternal life history (<200 µm) and migratory life history (>500 µm).
When lifetime migratory history patterns were considered, 89% of fish (57/64) exhibited an anadromous life history (Table 1). Of these, 77% (44/57) were from anadromous mothers and 23% (13/57) had freshwater mothers. Eleven percent (7/64) of individuals expressed a freshwater life history strategy, four of these arose from anadromous mothers and three from freshwater mothers.
The occurrence of repeated anadromous migrations was apparent at all sites as explicit annual Sr:Ca peaks were common (Figure 4). On Montague River, of the 31 individuals collected, 13 took one marine migration, 8 made two marine migrations and 4 made at least three. On Mill River, of the 18 individuals collected, 7 made two migrations, and another 7 showed evidence of three migrations; a single fish from the Mill river potentially made four marine migrations. On Trout River, of the 15 individuals sampled, 6 made a single migration, 5 made two migrations, and 3 made at least three migrations to the marine environment. At each site, there were individuals that exhibited non-typical Sr:Ca peaks suggestive of longer exposure to elevated salinity, or changing migratory patterns over the lifetime of the individual. On the Mill River, the representative non-typical migrant appeared to have multiple discontinuous migrations to higher-salinity environments, which may reflect different seasonal habitat use than typical individuals. Other atypical migrants showed increasing or decreasing Sr:Ca ratios over time, suggesting changes in migratory patterns throughout the individual’s lifetime.
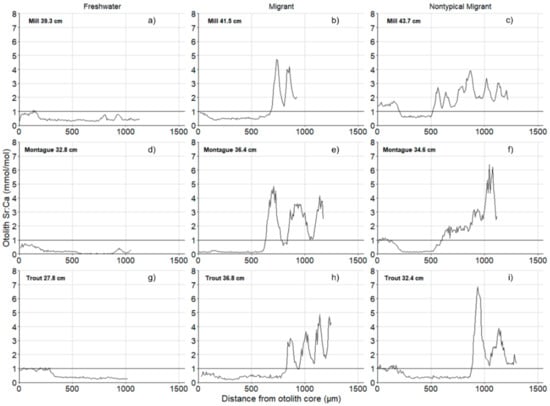
Figure 4.
Representative otolith microchemistry line scans for freshwater (a,d,g), migrant (b,e,h) and nontypical migrant (c,f,i) life histories from Mill, Montague and Trout River. Horizontal line shows threshold for determination of anadromous migratory strategy. Size of fish reported in fork length (cm).
4. Discussion
This study illustrates how freshwater entry for anadromous brook trout precedes the occurrence of fish kills and may explain why brook trout represent the most numerous species recovered from fish kills on PEI. Opportunistic life history evaluation via otolith microchemistry revealed the prevalence of anadromy in PEI brook trout populations killed by pesticides. Prior studies have attempted to trace the heritability and fitness benefits of an anadromous life history, but the connections have been tentative [14,15]. To date, previous studies have not examined the entire life history and parental contribution in anadromous brook trout. Given that the majority of individuals had anadromous mothers and exhibited an anadromous life history, this study highlights the significance of anadromy in coastal brook trout populations.
Otolith microchemistry has not been previously used to examine the inheritance of anadromy or the lifetime anadromous history in brook trout. Early attempts to demonstrate the maternal influence using stable isotopes were tenuous because those isotopic signatures do not persist long beyond exogenous feeding [14,15]. Otolith microchemistry showed that sea-run migration is often repeated throughout the individual’s lifespan and often arises from anadromous maternal origin, making otoliths a powerful tool for the study of anadromous life history [37,38,39]. These results using otolith microchemistry have the additional advantage of being able to show repeated patterns of anadromy over the life of the fish and many individuals undertook annual migrations to sea, once the strategy was adopted. Atypical migrant patterns could be explained by other studies that found anadromous brook trout leaving freshwater in the fall after spawning and overwintering under ice and in some cases returning to lower-salinity habitats during environmental events such as extreme cold [41,42]. Another similar study on PEI used otolith chemistry in conjunction with acoustic tracking to study non-native rainbow trout and showed that anadromous individuals regularly travelled between fresh and saltwater, possibly reflecting the presence of environmental constraints to habitat occupation [36].
The factors that influence the timing and demography of the freshwater return of anadromous brook trout are poorly understood despite decades of study. Previous studies, as well as the current study, illustrate a trend for large-bodied female brook trout to be the first individuals to return to freshwater; these females may dominate recruitment based upon the production of a greater number of eggs that tend to be larger and exhibit faster growth [17,43]. One reason that is speculated for freshwater return in brook trout, particularly those that return in early summer well in advance of fall spawning, is an obligate period of freshwater rearing for egg maturation [44,45]. Other drivers of entry into freshwater could be proximate environmental variables such as temperature, photoperiod, or dissolved oxygen that may differentially affect brook trout physiology based upon individual size. In their comprehensive review of anadromy in brook trout, Curry et al. [3] suggested that anadromous brook trout returned earlier in rivers where summer temperatures are high, and that these populations migrate to headwaters in order to find thermal refugia [46]. Thus, there may be river-specific attributes which affect the spatial distribution of sea-run brook trout that have returned to fresh water but it is unclear how this affects the potential susceptibility to pesticide runoff events.
While anadromy in brook trout can be seen to be maternally heritable, this does not necessarily imply clear genetic linkages. A study on brook trout microsatellite loci revealed that while there is differentiation between anadromous and resident populations, gene flow is likely mediated by freshwater resident males spawning with sea-run females, and so anadromy may not be a genetically mediated trait [17]. That study found, sea-run parentage was correlated with larger juvenile size at age 1, suggesting that size, over genetics, could be a determining factor in the decision to become anadromous. Larger females will have larger and more numerous eggs, which are more likely to produce large juveniles. Despite the strong maternal–progeny relationship for anadromy, small numbers of brook trout with freshwaters mothers were observed to become anadromous (and vice versa) in all watersheds studied. Given that the species commonly persists as a mixture of resident and anadromous forms, multiple strategies are likely important to mitigate catastrophic disturbance events such as fish kills. The long-term effects of these events are unknown and further study is warranted.
While pesticide-related fish kills have obvious negative impacts on fish populations, other attributes of agriculture may lead to increases in density. Curry et al. [15] found that the density of juvenile brook trout on PEI was not related to the contribution of anadromous parents at all, possibly because nitrate enrichment is driving the abundance of juveniles more than the contribution of anadromous mothers. This is supported by Gormley et al. [19], who found that juvenile brook trout density was positively correlated with the amount of land in potato agriculture in the watershed area. Previous studies have found a positive association between experimentally elevated nutrient levels, namely nitrate and juvenile salmonid growth [47]. On PEI, elevated nitrate concentrations are associated with increased invertebrate abundance of certain taxa [48], as well as increases in brook trout density [19], suggesting that juvenile brook trout are capable of capitalizing on the added resources associated with nutrient enrichment. Enhanced growth could thereby contribute to the continuance of anadromy provided density does not increase substantially, which would limit growth. Ultimately, there is a poor understanding of how nutrient enrichment, sedimentation, and pesticide-induced fish kills influence populations of brook trout in PEI rivers.
Pesticide-induced fish kills are always preceded by rainfall and a recent pesticide application. There are a number of interventions that can reduce this risk including increasing buffers along streams and reducing or eliminating the use of particular compounds that pose a high risk. Watershed models demonstrate that increases in buffer width from the current 15 m can achieve a significant reduction in sediment load to PEI streams [49]; however, the impact on pesticide runoff during rain events is less clear. A study specifically examining pesticide reduction by buffer strips on PEI showed that pesticides could be reduced further if buffers were increased to 30 m [50].
Regulating specific high-risk compounds can also reduce risk to fishes. Between 1977 and 2002, the insecticide azinphos-methyl was responsible for just over half of documented fish kills on PEI [51]. While azinphos-methyl was not banned in Canada, it is in the USA and much of the EU. PEI introduced restrictions on its use near waterways, and there has not been a fish kill attributed to azinphos-methyl since 2002. Measured residues of this compound have since declined across the region [33]. However, from 2002 onward, the fungicide chlorothalonil is the suspected toxic substance in at least 70% of fish kills [51]. As of the last available pesticide sales data for PEI between 2014 and 2016, chlorothalonil (125–140 tonnes) was second only to the fungicide mancozeb (303–330 tonnes) in terms of the quantity of active ingredient sold [52]. Thus, reductions in the use of chlorothalonil, which ranked the highest in a release-weighted risk assessment [53], would likely have the highest probability of protecting brook trout populations. The Canadian Pest Management Regulatory Authority has recently re-evaluated chlorothalonil and changed its regulations to allow a maximum of three applications per year, though the implications of this on chlorothalonil usage are still unclear [54].
5. Conclusions
This study demonstrates the risk to brook trout populations associated with timing of freshwater entry and the temporal overlap with pesticide runoff events. Additional anthropogenic risk factors, primarily associated with agriculture, are linked to sedimentation in streams [31,55,56], which can affect critical life cycle stages such as egg incubation. The general population status of brook trout on PEI is presumed to be healthy, but angler capture of brook trout on PEI was observed to have declined by 33% between 1973 and 1994 [57]. However, relatively little monitoring of these populations makes their status uncertain. The threats to brook trout in this region are consistent with observations elsewhere in their range that land-use change is as significant factor in the decline of the species [58]. Given the manner in which sea-run brook trout life history and anthropogenic stress coincide, enhanced monitoring of sea-run brook trout populations and exploration of alternative pest management strategies in agriculture are warranted.
Author Contributions
Conceptualization, S.D.R., methodology, S.D.R., B.J.H. and M.R.v.d.H.; data collection T.L.J., S.D.R. and K.M.K.; formal analysis, S.D.R., K.M.K., S.J.L. and M.R.v.d.H.; resources, B.J.H. and M.R.v.d.H.; writing—original draft preparation, S.D.R., K.M.K. and M.R.v.d.H.; writing—review and editing, S.D.R., K.M.K., S.J.L., B.J.H. and M.R.v.d.H.; supervision, M.R.v.d.H. funding acquisition, S.D.R. and M.R.v.d.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by an NSERC Industrial Postgraduate Scholarship to S.D.R., a University of Prince Edward Island Internal Research Grant and by the Prince Edward Island Wildlife Conservation Fund.
Institutional Review Board Statement
All fish were handled in accordance with approved University of Prince Edward Island animal care protocols. All fish collection activities complied with DFO Gulf Region License to Fish for Scientific Purposes.
Acknowledgments
We would like to thank all of those who assisted in the collection of field data especially M. Coffin and many other UPEI students as well as provincial government officials, R. MacFarlane and L. Jones. This manuscript is dedicated to the memory Daryl Guignion whose 40+ year career at UPEI had a profound influence on those involved in watershed ecology on PEI, including the authors of this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Su, G.; Logez, M.; Xu, J.; Tao, S.; Villéger, S.; Brosse, S. Human impacts on global freshwater fish biodiversity. Science 2021, 371, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Kume, M.; Terashima, Y.; Ye, F.; Kameyama, S.; Miya, M.; Yamashita, Y.; Kasai, A. Evaluation of fish biodiversity in estuaries using environmental DNA metabarcoding. PLoS ONE 2020, 15, e0231127. [Google Scholar] [CrossRef] [PubMed]
- Curry, A.A.; Bernatchez, L.; Whoriskey, F.; Audet, C. The origins and persistence of anadromy in brook charr. Rev. Fish Biol. Fish. 2010, 20, 557–570. [Google Scholar] [CrossRef]
- Goetz, F.A.; Beamer, E.; Connor, E.J.; Jeanes, E.; Kinsel, C.; Chamberlin, J.W.; Morello, C.; Quinn, T.P. The timing of anadromous bull trout migrations in estuarine and marine waters of Puget Sound, Washington. Environ. Biol. Fishes 2021, 104, 1073–1088. [Google Scholar] [CrossRef]
- Roloson, S.D.; Coffin, M.R.S.; Knysh, K.M.; van den Heuvel, M.R. Movement of non-native rainbow trout in an estuary with periodic summer hypoxia. Hydrobiologia 2021, 848, 4001–4016. [Google Scholar] [CrossRef]
- Limburg, K.E.; Waldman, J.R. Dramatic declines in north Atlantic diadromous fishes. Bioscience 2009, 59, 955–965. [Google Scholar] [CrossRef]
- Merg, M.L.; Dézerald, O.; Kreutzenberger, K.; Demski, S.; Reyjol, Y.; Usseglio-Polatera, P.; Belliard, J. Modeling diadromous fish loss from historical data: Identification of anthropogenic drivers and testing of mitigation scenarios. PLoS ONE 2020, 15, e0236575. [Google Scholar] [CrossRef]
- Charron, C.; St-Hilaire, A.; Ouarda, T.B.M.J.; van den Heuvel, M.R. Water temperature and hydrological modelling in the context of environmental flows and future climate change: Case study of the wilmot river (Canada). Water 2021, 13, 2101. [Google Scholar] [CrossRef]
- Verhelst, P.; Reubens, J.; Buysse, D.; Goethals, P.; Van Wichelen, J.; Moens, T. Toward a roadmap for diadromous fish conservation: The Big Five considerations. Front. Ecol. Environ. 2021, 19, 396–403. [Google Scholar] [CrossRef]
- MacCrimmon, H.; Campbell, S. World Distribution of Brook Trout, (Salvelinus fontinalis). J. Fish. Res. Board Can. 1969, 26, 1699–1725. [Google Scholar] [CrossRef]
- Eastern Brook Trout Joint Venture. Range-Wide Assessment of Brook Trout at the Catchment Scale: A Summary of Findings; Eastern Brook Trout Joint Venture. 2016. Available online: https://www.easternbrooktrout.org (accessed on 10 December 2021).
- Snook, E.L.; Letcher, B.H.; Dubreuil, T.L.; Zydlewski, J.; O’Donnell, M.J.; Whiteley, A.R.; Hurley, S.T.; Danylchuk, A.J. Movement patterns of Brook Trout in a restored coastal stream system in southern Massachusetts. Ecol. Freshw. Fish 2016, 25, 360–375. [Google Scholar] [CrossRef]
- Perry, G.M.L.; Audet, C.; Bernatchez, L. Maternal genetic effects on adaptive divergence between anadromous and resident brook charr during early life history. J. Evol. Biol. 2005, 18, 1348–1361. [Google Scholar] [CrossRef] [PubMed]
- Jardine, T.D.; Chernoff, E.; Curry, R.A. Maternal transfer of carbon and nitrogen to progeny of sea-run and resident brook trout (Salvelinus fontinalis). Can. J. Fish. Aquat. Sci. 2008, 65, 2201–2210. [Google Scholar] [CrossRef]
- Curry, R.A. Assessing the reproductive contributions of sympatric anadromous and freshwater-resident brook trout. J. Fish Biol. 2005, 66, 741–757. [Google Scholar] [CrossRef]
- Fraser, D.J.; Bernatchez, L. Allopatric origins of sympatric brook charr populations: Colonization history and admixture. Mol. Ecol. 1995, 14, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Thériault, V.; Garant, D.; Bernatchez, L.; Dodson, J.J. Heritability of life-history tactics and genetic correlation with body size in a natural population of brook charr (Salvelinus fontinalis). J. Evol. Biol. 2007, 20, 2266–2277. [Google Scholar] [CrossRef]
- PEI Department of Environment, Energy and Climate Action. Fish Kill Information and Statistics. 2020. Available online: www.princeedwardisland.ca/en/information/environment-water-and-climate-change/fish-kill-information-and-statistics (accessed on 21 December 2021).
- Gormley, K.L.; Teather, K.L.; Guignion, D.L. Changes in salmonid communities associated with pesticide runoff events. Ecotoxicology 2005, 14, 671–685. [Google Scholar] [CrossRef]
- Walther, B.D.; Limburg, K.E. The use of otolith chemistry to characterize diadromous migrations. J. Fish Biol. 2012, 81, 796–825. [Google Scholar] [CrossRef]
- Zimmerman, C.E.; Edwards, G.W.; Perry, K. Maternal Origin and Migratory History of Steelhead and Rainbow Trout Captured in Rivers of the Central Valley, California. Trans. Am. Fish. Soc. 2009, 138, 280–291. [Google Scholar] [CrossRef]
- Engstedt, O.; Engkvist, R.; Larsson, P. Elemental fingerprinting in otoliths reveals natal homing of anadromous Baltic Sea pike (Esox lucius L.). Ecol. Freshw. Fish. 2014, 23, 313–321. [Google Scholar] [CrossRef]
- Goodwin, J.C.A.; Andrew King, R.; Iwan Jones, J.; Ibbotson, A.; Stevens, J.R. A small number of anadromous females drive reproduction in a brown trout (Salmo trutta) population in an English chalk stream. Freshw. Biol. 2016, 61, 1075–1089. [Google Scholar] [CrossRef]
- Jiang, Y.; Somers, G. Modeling effects of nitrate from non-point sources on groundwater quality in an agricultural watershed in Prince Edward Island, Canada. Hydrogeol. J. 2009, 17, 707–724. [Google Scholar] [CrossRef]
- Knysh, K.M.; Giberson, D.J.; van den Heuvel, M.R. The influence of agricultural land-use on plant and macroinvertebrate communities in springs. Limnol. Oceanogr. 2016, 61, 518–530. [Google Scholar] [CrossRef]
- Roloson, S.D.; Knysh, K.M.; Coffin, M.R.S.; Gormley, K.L.; Pater, C.C.; van den Heuvel, M.R. Rainbow trout (Oncorhynchus mykiss) habitat overlap with wild Atlantic salmon (Salmo salar) and brook trout (Salvelinus fontinalis) in natural streams: Do habitat and landscape factors override competitive interactions? Can. J. Fish. Aquat. Sci. 2018, 75, 1949–1959. [Google Scholar] [CrossRef]
- van der Poll, H.W. Geology of Prince Edward Island; Report 83-1; Department of Energy and Forestry, Energy and Minerals Branch: Charlottetown, PE, Canada, 1983. [Google Scholar]
- Shaw, J. Geomorphic evidence of postglacial terrestrial environments on Atlantic Canadian Continental Shelves. Géographie Phys. Quat. 2005, 59, 141–154. [Google Scholar] [CrossRef]
- Guignion, D.; Dupuis, T.; Teather, K.; MacFarlane, R. Distribution and Abundance of Salmonids in Prince Edward Island Streams. Northeast. Nat. 2010, 17, 313–324. [Google Scholar] [CrossRef]
- PEI Department of Environment, Energy and Forestry. Resource Inventory. Corporate Land Use Inventory 2010; PEI Department of Environment, Energy and Forestry: Charlottetown, PE, Canada, 2010.
- Purcell, L.A.; Giberson, D.J. Effects of an azinphos-methyl runoff event on macroinvertebrates in the Wilmot River, Prince Edward Island, Canada. Can. Entomol. 2007, 139, 523–533. [Google Scholar] [CrossRef]
- Alberto, A.; St-Hilaire, A.; Courtenay, S.C.; van den Heuvel, M.R. Monitoring stream sediment loads in response to agriculture in Prince Edward Island, Canada. Environ. Monit. Assess. 2016, 188, 415. [Google Scholar] [CrossRef]
- Lalonde, B.; Garron, C. Temporal and Spatial Analysis of Surface Water Pesticide Occurrences in the Maritime Region of Canada. Arch. Environ. Contam. Toxicol. 2020, 79, 12–22. [Google Scholar] [CrossRef]
- Cairns, D.K.; MacFarlane, R.E.; Guignion, D.L.; Dupuis, T. The Status of Atlantic Salmon (Salmo salar) on Prince Edward Island (SFA 17) in 2011; DFO Canadian Science Advisory Secretariat Research Document 2012/090; Canadian Science Advisory Secretariat: Ottawa, ON, Canada, 2012. [Google Scholar]
- Cairns, D.K.; MacFarlane, R.E. The Status of Atlantic Salmon (Salmo salar) on Prince Edward Island (SFA 17) in 2013; DFO Canadian Science Advisory Secretariat Research Document 2015/019; Canadian Science Advisory Secretariat: Ottawa, ON, Canada, 2015. [Google Scholar]
- Roloson, S.D.; Landsman, S.J.; Tana, R.; Hicks, B.J.; Carr, J.W.; Whoriskey, F.; Van Den Heuvel, M.R. Otolith microchemistry and acoustic telemetry reveal anadromy in non-native rainbow trout (Oncorhynchus mykiss) in prince Edward Island, Canada. Can. J. Fish. Aquat. Sci. 2020, 77, 1117–1130. [Google Scholar] [CrossRef]
- Courter, I.I.; Child, D.B.; Hobbs, J.A.; Garrison, T.M.; Glessner, J.J.G.; Duery, S. Resident rainbow trout produce anadromous offspring in a large interior watershed. Can. J. Fish. Aquat. Sci. 2013, 70, 701–710. [Google Scholar] [CrossRef]
- Austin, C.S.; Bond, M.H.; Smith, J.M.; Lowery, E.D.; and Quinn, T.P. Otolith microchemistry reveals partial migration and life history variation in a facultatively anadromous, iteroparous salmonid, bull trout (Salvelinus confluentus). Environ. Biol. Fishes 2019, 102, 95–104. [Google Scholar] [CrossRef]
- Thibault, I.; Hedger, R.D.; Dodson, J.J.; Shiao, J.C.; Iizuka, Y.; and Tzeng, W.N. Anadromy and the dispersal of an invasive fish species (Oncorhynchus mykiss) in Eastern Quebec, as revealed by otolith microchemistry. Ecol. Freshw. Fish. 2010, 19, 348–360. [Google Scholar] [CrossRef]
- Liberoff, A.L.; Quiroga, A.P.; Riva-Rossi, C.M.; Miller, J.A.; Pascual, M.A. Influence of maternal habitat choice, environment and spatial distribution of juveniles on their propensity for anadromy in a partially anadromous population of rainbow trout (Oncorhynchus mykiss). Ecol. Freshw. Fish. 2014, 424–434. [Google Scholar] [CrossRef]
- Smith, M.W.; Saunders, J.W. Movements of Brook Trout, Salvelinus fontinalis (Mitchill), Between and Within Fresh and Salt Water. J. Fish. Res. Board Canada 1958, 15, 1403–1449. [Google Scholar] [CrossRef]
- Spares, A.D.; Dadswell, M.J.; MacMillan, J.; Madden, R.; O’Dor, R.K.; Stokesbury, M.J.W. To fast or feed: An alternative life history for anadromous brook trout Salvelinus fontinalis overwintering within a harbour. J. Fish Biol. 2014, 85, 621–644. [Google Scholar] [CrossRef]
- Castonguay, M.; Fitzgerald, J. Life history and movements of anadromous brook charr, Salvelinus fontinalis, in the St-Jean River, Gaspe, Quebec. Can. J. Zool. 1982, 60, 3084–3091. [Google Scholar] [CrossRef]
- Whoriskey, F.G.; Naiman, R.J.; Montgomery, W.L. Experimental sea ranching of brook trout Salvelinus fontinals. J. Fish. Biol. 1981, 19, 637–651. [Google Scholar] [CrossRef]
- McCormick, S.D.; Naiman, R.J. Hypoosmoregulation in an anadromous teleost: Influence of sex and maturation. J. Exp. Zool. 1985, 234, 193–198. [Google Scholar] [CrossRef]
- Curry, R.A.; Sparks, D.; van de Sande, J. Spatial and Temporal Movements of a Riverine Brook Trout Population. Trans. Am. Fish. Soc. 2002, 131, 551–560. [Google Scholar] [CrossRef]
- Johnston, N.T.; Perrin, C.J.; Slaney, P.A.; Ward, B.R. Increased juvenile salmonid growth by whole-river fertilization. Can. J. Fish. Aquat. Sci. 1990, 47, 862–872. [Google Scholar] [CrossRef]
- Purcell, L.A. The River Runs Through It: Evaluation of the Effects of Agricultural Land Use Practices on Macroinvertebrates in Prince Edward Island Streams Using Both New and Standard Methods. Master’s Thesis, University of Prince Edward Island, Charlottetown, PE, Canada, 2003. [Google Scholar]
- Sirabahenda, Z.; St-Hilaire, A.; Courtenay, S.C.; van den Heuvel, M.R. Assessment of the effective width of riparian buffer strips to reduce suspended sediment in an agricultural landscape using ANFIS and SWAT models. CATENA 2020, 195, 104762. [Google Scholar] [CrossRef]
- Dunn, A.M.; Julien, G.; Ernst, W.R.; Cook, A.; Doe, K.G.; Jackman, P.M. Evaluation of buffer zone effectiveness in mitigating the risks associated with agricultural runoff in Prince Edward Island. Sci. Total Environ. 2011, 409, 868–882. [Google Scholar] [CrossRef] [PubMed]
- PEI Department of Environment, Energy and Climate Change. Island Fish Kill from 1962–2019. 2019. Available online: https://www.princeedwardisland.ca/sites/default/files/publications/pei_fish_kills_summary_1962-2017.pdf (accessed on 20 December 2021).
- PEI Department of Environment, Energy and Climate Change. Prince Edward Island 2015–2016 Retail Pesticide Sales Report. 2020; 7p. Available online: https://www.princeedwardisland.ca/sites/default/files/publications/2015-2016_pesticide_sales_report_final_rfw.pdf (accessed on 19 November 2021).
- Dunn, A.M. A Relative Risk Ranking of Pesticides Used in Prince Edward Island; Environmental Protection Branch Report Series EPS-5-AR-04-03; Environment Canada: Dartmouth, NS, Canada, 2004; 48p. [Google Scholar]
- Health Canada Pest Management Regulatory Agency. Re-Evaluation Decision RVD2018-11 Chlorothalonil and Its Associated End-use Products for Agricultural and Turf Uses Final Decision. 2018. Available online: https://ipmcouncilcanada.org/wp-content/uploads/2018/07/RVD2018-11-chlorothalonil.pdf (accessed on 19 November 2021).
- Sirabahenda, Z.; St-Hilaire, A.; Courtenay, S.C.; Alberto, A.; van den Heuvel, M.R. A modelling approach for estimating suspended sediment concentrations for multiple rivers influenced by agriculture. Water 2017, 62, 2209–2221. [Google Scholar] [CrossRef]
- Alberto, A.; Courtenay, S.C.; St-Hilaire, A.; van den Heuvel, M.R. Factors influencing brook trout (Salvelinus fontinalis) egg survival and development in streams influenced by agriculture. J. Fish. Sci. 2017, 11, 9–20. [Google Scholar]
- Effort, Harvest, and Expenditures of Trout and Salmon Anglers on Prince Edward Island in 1994, from a Mail-Out Survey; Canadian Technical Report of Fisheries and Aquatic Sciences No. 2367; Cairns, D.K., Ed.; Fisheries and Oceans Canada (DFO): Charlottetown, PE, Canada, 1996; 45p. [Google Scholar]
- Stranko, S.A.; Hilderbrand, R.H.; Morgan, R.P.; Staley, M.W.; Becker, A.J.; Roseberry-Lincoln, A.; Perry, E.S.; Jacobson, P.T. Brook trout declines with land cover and temperature changes in Maryland. N. Am. J. Fish. Manag. 2008, 28, 1223–1232. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).