Somatic Condition and Reproductive Potential as a Tandem in European Sardine: An Analysis with an Environmental Perspective in the Northern Adriatic (Gulf of Trieste)
Abstract
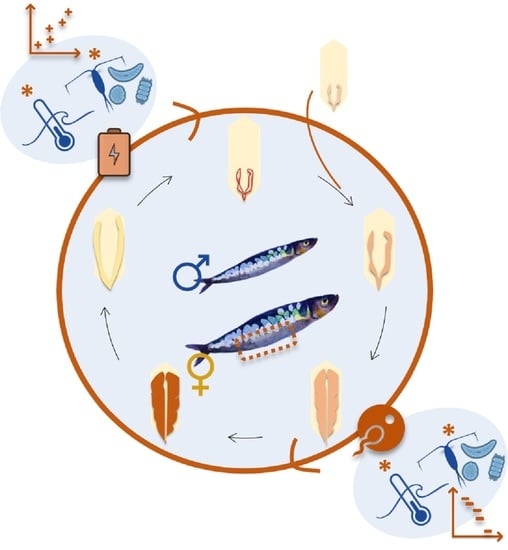
1. Introduction
2. Materials and Methods
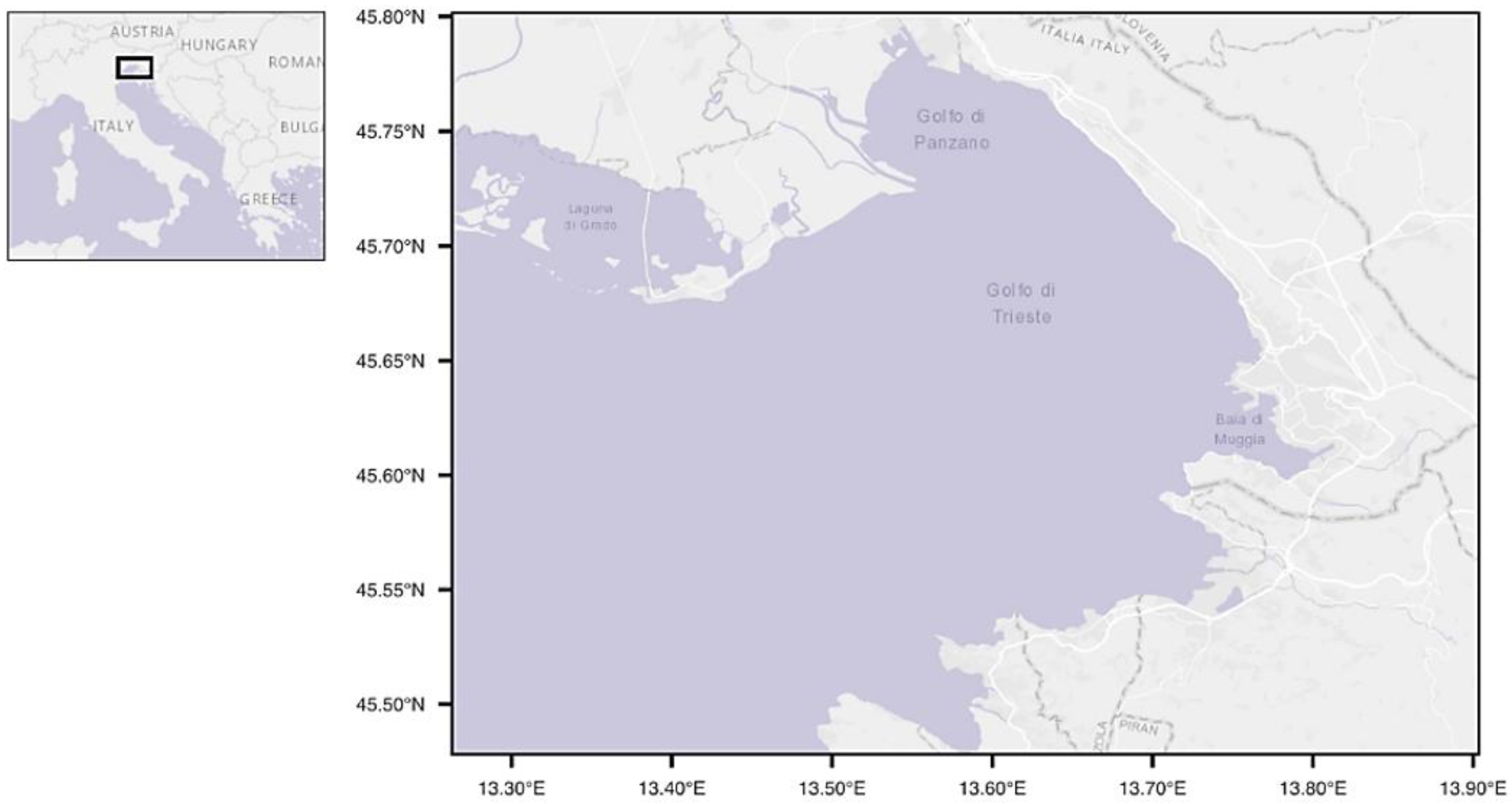
2.1. Sampling Collection
2.2. Somatic Condition Evaluation
2.3. Reproduction Analysis
2.4. Statistical Analysis
3. Results
3.1. Somatic and Reproductive Condition Analyses. Correlation with Environmental Parameters
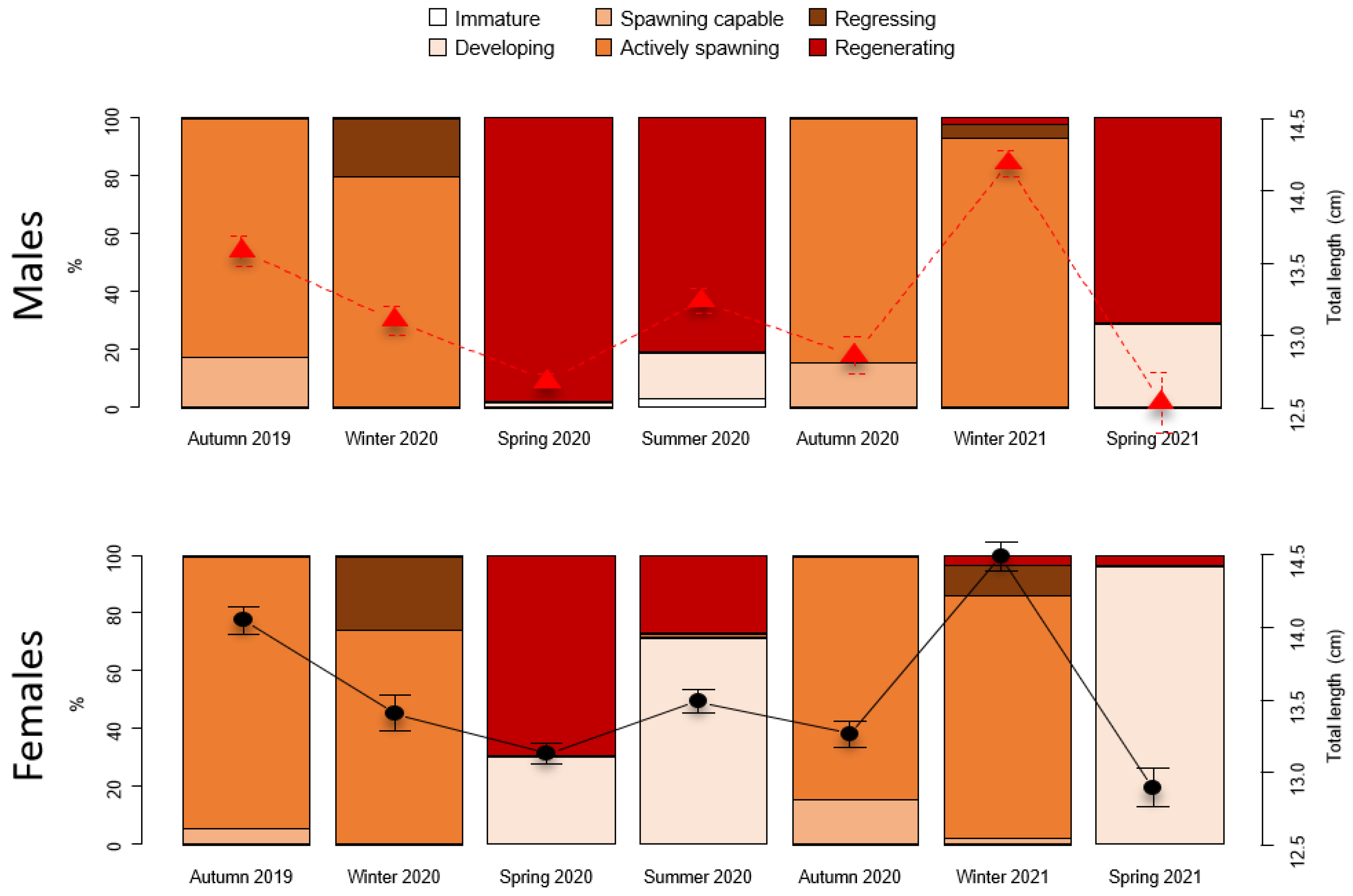
3.2. Sex Ratio and Reproductive Cycle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marteinsdottir, G.; Begg, G.A. Essential relationships incorporating the influence of age, size and condition on variables required for estimation of reproductive potential in Atlantic cod Gadus Morhua. Mar. Ecol. Prog. Ser. 2002, 235, 235–256. [Google Scholar] [CrossRef]
- Ganias, K.; Somarakis, S.; Nunes, C. Chapter 3 Reproductive Potential. In Biology and Ecology of Sardines and Anchovies; Ganias, K., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 79–121. [Google Scholar]
- Alonso-Fernández, A. Bioenergetics approach to fish reproductive potential: Case of Trisopterus luscus (Teleostei) on the Galician Shelf (NW Iberian Peninsula). Ph.D. Thesis, University of Vigo, Vigo, Spain, 2011. [Google Scholar]
- Albo-Puigserver, M.; Muñoz, A.; Navarro, J.; Coll, M.; Pethybridge, H.; Sánchez, S.; Palomera, I. Ecological energetics of forage fish from the Mediterranean Sea: Seasonal dynamics and interspecific differences. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 140, 74–82. [Google Scholar] [CrossRef]
- Albo-Puigserver, M.; Sánchez, S.; Coll, M.; Bernal, M.; Sáez-Liante, R.; Navarro, J.; Palomera, I. Year-round energy dynamics of sardine and anchovy in the north-western Mediterranean Sea. Mar. Environ. Res. 2020, 159, 105021. [Google Scholar] [CrossRef] [PubMed]
- Saraux, C.; Van Beveren, E.; Brosset, P.; Queiros, Q.; Bourdeix, J.H.; Dutto, G.; Gasset, E.; Jac, C.; Bonhommeau, S.; Fromentin, J.M. Small pelagic fish dynamics: A review of mechanisms in the Gulf of Lions. Deep Sea Res. Part II Top. Stud. Oceanogr. 2019, 159, 52–61. [Google Scholar] [CrossRef]
- Domínguez-Petit, R.; Saborido-Rey, F. New bioenergetic perspective of European hake (Merluccius merluccius L.) reproductive ecology. Fish. Res. 2010, 104, 83–88. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, S.K.; Kim, S.S.; Choi, M.S. Environmental factors affecting anchovy reproductive potential in the southern coastal waters of Korea. Anim. Cells Syst. 2013, 17, 133–140. [Google Scholar] [CrossRef][Green Version]
- Zwolinski, J.; Stratoudakis, Y.; Sares, E. Intra-annual variation in the batch fecundity of sardine off Portugal. J. Fish Biol. 2001, 58, 1633–1645. [Google Scholar] [CrossRef]
- Ganias, K.; Somarakis, S.; Machias, A.; Theodorou, A. Pattern of oocyte development and batch fecundity in the Mediterranean sardine. Fish. Res. 2004, 67, 13–23. [Google Scholar] [CrossRef]
- Tsikliras, A.C.; Koutrakis, E.T. Growth and reproduction of European sardine, Sardina pilchardus (Pisces: Clupeidae), in northeastern Mediterranean. Cah. Biol. Mar. 2013, 54, 365–374. [Google Scholar]
- Basilone, G.; Ferreri, R.; Aronica, S.; Mazzola, S.; Bonanno, A.; Gargano, A.; Pulizzi, M.; Fontana, I.; Giacalone, G.; Calandrino, P.; et al. Reproduction and Sexual Maturity of European Sardine (Sardina pilchardus) in the Central Mediterranean Sea. Front. Mar. Sci. 2021, 999. [Google Scholar] [CrossRef]
- Nunes, C.; Silva, A.; Soares, E.; Ganias, K. The use of hepatic and somatic indices and histological information to characterize the reproductive dynamics of Atlantic sardine Sardina pilchardus from the Portuguese coast. Mar. Coast. Fish. 2011, 3, 127–144. [Google Scholar] [CrossRef]
- Machias, A.; Tsimenides, N. Biological factors affecting the swimbladder volume of sardine (Sardina pilchardus). Mar. Biol. 1995, 123, 859–867. [Google Scholar] [CrossRef]
- Parrish, R.H.; Serra, R.; Grant, W.S. The monotypic sardines, Sardina and Sardinops: Their taxonomy, distribution, stock structure, and zoogeography. Can. J. Fish. Aquat. Sci. 1989, 46, 2019–2036. [Google Scholar] [CrossRef]
- Cury, P.; Bakun, A.; Crawford, R.J.M.; Jarre, A.; Quinones, R.A.; Shannon, L.J.; Verheye, H.M. Small pelagics in upwelling systems: Patterns of interaction and structural changes in “wasp-waist” ecosystems. ICES J. Mar. Sci. 2000, 57, 603–618. [Google Scholar] [CrossRef]
- Van Beveren, E.; Bonhommeau, S.; Fromentin, J.M.; Bigot, J.L.; Bourdeix, J.H.; Brosset, P.; Roos, D.; Saraux, C. Rapid changes in growth, condition, size and age of small pelagic fish in the Mediterranean. Mar. Biol. 2014, 161, 1809–1822. [Google Scholar] [CrossRef]
- Šimat, V.; Hamed, I.; Petričević, S.; Bogdanović, T. Seasonal changes in free amino acid and fatty acid compositions of sardines, Sardina Pilchardus (Walbaum, 1792): Implications for nutrition. Foods 2020, 9, 867. [Google Scholar] [CrossRef]
- Lleonart, J.; Maynou, F. Fish stock assessments in the Mediterranean: State of the art. Sci. Mar. 2003, 67, 37–49. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action; FAO: Rome, Italy, 2020; pp. 1–244. [Google Scholar]
- Morello, E.B.; Arneri, E. Anchovy and sardine in the Adriatic Sea—An ecological review. Oceanogr. Mar. Biol. Annu. Rev. 2009, 47, 221–268. [Google Scholar]
- Pacetti, D.; Balzano, M.; Colella, S.; Santojanni, A.; Frega, N.G. Effect of spawning on furan fatty acid profile of edible muscle and organ tissues from sardine (Sardina pilchardus) and anchovy (Engraulis encrasicolus). J. Agric. Food Chem. 2013, 61, 3969–3977. [Google Scholar] [CrossRef]
- Carpi, P.; Scarcella, G.; Cardinale, M. The saga of the management of fisheries in the Adriatic Sea: History, flaws, difficulties, and successes toward the application of the common fisheries policy in the Mediterranean. Front. Mar. Sci. 2017, 4, 423. [Google Scholar] [CrossRef]
- Brosset, P.; Fromentin, J.M.; Van Beveren, E.; Lloret, J.; Marques, V.; Basilone, G.; Bonanno, A.; Carpi, P.; Donato, F.; Čikeš-Keč, V.; et al. Spatio-temporal patterns and environmental controls of small pelagic fish body condition from contrasted Mediterranean areas. Prog. Oceanogr. 2017, 151, 149–162. [Google Scholar] [CrossRef]
- Popescu, I. Multiannual Plan for Small Pelagic Fish Stocks in the Adriatic Sea; EPRS European Parliamentary Research Service: Brussels, Belgium, 2018; pp. 1–8. [Google Scholar]
- Bertrand, M.; Brosset, P.; Soudant, P.; Lebigre, C. Spatial and ontogenetic variations in sardine feeding conditions in the Bay of Biscay through fatty acid composition. Mar. Environ. Res. 2022, 173, 105514. [Google Scholar] [CrossRef] [PubMed]
- Bandarra, N.M.; Batista, I.; Nunes, M.L.; Empis, J.M.; Christie, W.W. Seasonal changes in lipid composition of sardine (Sardina pilchardus). J. Food Sci. 1997, 62, 40–42. [Google Scholar] [CrossRef]
- Somarakis, S.; Ganias, K.; Tserpes, G.; Koutsikopoulos, C. Ovarian allometry and the use of the gonosomatic index: A case study in the Mediterranean sardine, Sardina pilchardus. Mar. Biol. 2004, 146, 181–189. [Google Scholar] [CrossRef]
- Volkoff, H.; London, S. Nutrition and reproduction in fish. In Encyclopedia of Reproduction, 2nd ed.; Skinner, M.K., Ed.; Academic Press: Oxford, UK, 2018; Volume 9, pp. 743–748. [Google Scholar]
- Vargas-Yáñez, M.; Giráldez, A.; Torres, P.; González, M.; García-Martínez, M.D.C.; Moya, F. Variability of oceanographic and meteorological conditions in the northern Alboran Sea at seasonal, inter-annual and long-term time scales and their influence on sardine (Sardina pilchardus Walbaum 1792) landings. Fish. Oceanogr. 2020, 29, 367–380. [Google Scholar] [CrossRef]
- Catalán, I.A.; Olivar, M.P.; Palomera, I.; Berdalet, E. Link between environmental anomalies, growth and condition of pilchard Sardina pilchardus larvae in the northwestern Mediterranean. Mar. Ecol. Prog. Ser. 2006, 307, 219–231. [Google Scholar] [CrossRef]
- Oliver, E.C.; Donat, M.G.; Burrows, M.T.; Moore, P.J.; Smale, D.A.; Alexander, L.V.; Benthuysen, J.A.; Feng, M.; Sen-Gupta, A.; Hobday, A.J.; et al. Longer and more frequent marine heatwaves over the past century. Nat. Commun. 2018, 9, 1324. [Google Scholar] [CrossRef] [PubMed]
- Viaroli, P.; Nizzoli, D.; Pinardi, M.; Soana, E.; Bartoli, M. Eutrophication of the Mediterranean Sea: A watershed—Cascading aquatic filter approach. Rend. Lincei 2015, 26, 13–23. [Google Scholar] [CrossRef]
- Brown, C.J.; Fulton, E.A.; Hobday, A.J.; Matear, R.J.; Possingham, H.P.; Bulman, C.; Christensen, V.; Forrest, R.E.; Gehrke, P.C.; Gribble, N.A.; et al. Effects of climate-driven primary production change on marine food webs: Implications for fisheries and conservation. Glob. Change Biol. 2010, 16, 1194–1212. [Google Scholar] [CrossRef]
- Guisande, C.; Vergara, A.R.; Riveiro, I.; Cabanas, J.M. Climate change and abundance of the Atlantic-Iberian sardine (Sardina pilchardus). Fish. Oceanogr. 2004, 13, 91–101. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Albouy, C.; Ben Rais Lasram, F.; Cheung, W.W.; Christensen, V.; Karpouzi, V.S.; Guilhaumon, F.; Mouillot, D.; Paleczny, M.; et al. The Mediterranean Sea under siege: Spatial overlap between marine biodiversity, cumulative threats and marine reserves. Glob. Ecol. Biogeogr. 2012, 21, 465–480. [Google Scholar] [CrossRef]
- Kogovšek, T.; Vodopivec, M.; Raicich, F.; Uye, S.I.; Malej, A. Comparative analysis of the ecosystems in the northern Adriatic Sea and the Inland Sea of Japan: Can anthropogenic pressures disclose jellyfish outbreaks? Sci. Total Environ. 2018, 626, 982–994. [Google Scholar] [CrossRef]
- Uitz, J.; Claustre, H.; Gentili, B.; Stramski, D. Phytoplankton class-specific primary production in the world’s oceans: Seasonal and interannual variability from satellite observations. Glob. Biogeochem. Cycles 2010, 24, GB3016. [Google Scholar] [CrossRef]
- Lejeusne, C.; Chevaldonné, P.; Pergent-Martini, C.; Boudouresque, C.F.; Pérez, T. Climate change effects on a miniature ocean: The highly diverse, highly impacted Mediterranean Sea. Trends Ecol. Evol. 2010, 25, 250–260. [Google Scholar] [CrossRef]
- Ben Rais Lasram, F.; Guilhaumon, F.; Albouy, C.; Somot, S.; Thuiller, W.; Mouillot, D. The Mediterranean Sea as a ‘cul-de-sac’for endemic fishes facing climate change. Glob. Change Biol. 2010, 16, 3233–3245. [Google Scholar] [CrossRef]
- Cheung, W.W.; Watson, R.; Pauly, D. Signature of ocean warming in global fisheries catch. Nature 2013, 497, 365–368. [Google Scholar] [CrossRef]
- Brosset, P.; Fromentin, J.M.; Ménard, F.; Pernet, F.; Bourdeix, J.H.; Bigot, J.L.; Van Beveren, E.; Pérez-Roda, M.A.; Choy, S.; Saraux, C. Measurement and analysis of small pelagic fish condition: A suitable method for rapid evaluation in the field. J. Exp. Mar. Biol. Ecol. 2015, 462, 90–97. [Google Scholar] [CrossRef]
- Zorica, B.; Keč, V.Č.; Vidjak, O.; Mladineo, I.; Balič, D.E. Feeding habits and helminth parasites of sardine (S. pilchardus) and anchovy (E. encrasicolus) in the Adriatic Sea. Mediterr. Mar. Sci. 2016, 17, 216–229. [Google Scholar] [CrossRef]
- Le Cren, E.D. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol. 1951, 20, 201–219. [Google Scholar] [CrossRef]
- Brosset, P.; Lloret, J.; Muñoz, M.; Fauvel, C.; Van Beveren, E.; Marques, V.; Fromentin, J.M.; Ménard, F.; Saraux, C. Body reserves mediate trade-offs between life-history traits: New insights from small pelagic fish reproduction. R. Soc. Open Sci. 2016, 3, 3160202. [Google Scholar] [CrossRef]
- Kent, M. Hand-held instrument for fat/water determination in whole fish. Food Control 1990, 1, 47–53. [Google Scholar] [CrossRef]
- Van Der Lingen, C.D.; Hutchings, L. Estimating the lipid content of pelagic fish in the southern Benguela by visual assessment of their mesenteric fat. Afr. J. Mar. Sci. 2005, 27, 45–53. [Google Scholar] [CrossRef]
- Brown-Peterson, N.J.; Wyanski, D.M.; Saborido-Rey, F.; Macewicz, B.J.; Lowerre-Barbieri, S.K. A standardized terminology for describing reproductive development in fishes. Mar. Coast. Fish. 2011, 3, 52–70. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: http://www.R-project.org/ (accessed on 21 February 2022).
- Zar, J.H. Biostatistical Analysis, 3rd ed.; Prentice-Hall: New Jersey, NJ, USA, 1996; pp. 1–662. [Google Scholar]
- NOAA/OAR/ESRL PSL, Boulder, Colorado, USA. Available online: https://psl.noaa.gov/data/gridded/data.noaa.oisst.v2.highres.html (accessed on 21 February 2022).
- NASA Goddard Space Flight Center, Ocean Ecology Laboratory, Ocean Biology Processing Group. Sea-Viewing Wide Field-of-View Sensor (SeaWiFS) Ocean Color Data; In 2018 Reprocessing; NASA OB.DAAC: Greenbelt, MD, USA. Available online: https://oceancolor.gsfc.nasa.gov/ (accessed on 21 February 2022).
- Druon, J.N.; Gascuel, D.; Gibin, M.; Zanzi, A.; Fromentin, J.M.; Colloca, F.; Hélaouët, P.; Coll, M.; Mannini, A.; Bluemel, J.K.; et al. Mesoscale productivity fronts and local fishing opportunities in the European Seas. Fish Fish. 2021, 22, 1227–1247. [Google Scholar] [CrossRef]
- Environmental Marine Information System. Available online: http://data.europa.eu/89h/bce67a6d-45ea-4fc1-a685-b775d5b31953 (accessed on 21 February 2022).
- Wright, P.J.; Trippel, E.A. Fishery-induced demographic changes in the timing of spawning: Consequences for reproductive success. Fish Fish. 2009, 10, 283–304. [Google Scholar] [CrossRef]
- Rosa, R.; González, L.; Broitman, B.R.; Garrido, S.; Santos, A.M.P.; Nunes, M.L. Bioenergetics of small pelagic fishes in upwelling systems: Relationship between fish condition, coastal ecosystem dynamics and fisheries. Mar. Ecol. Prog. Ser. 2010, 410, 205–218. [Google Scholar] [CrossRef]
- Conversi, A.; Peluso, T.; Fonda-Umani, S. Gulf of Trieste: A changing ecosystem. J. Geophys. Res. Oceans 2009, 114, C03S90. [Google Scholar] [CrossRef]
- Rizzi, J.; Torresan, S.; Critto, A.; Zabeo, A.; Brigolin, D.; Carniel, S.; Pastres, R.; Marcomini, A. Climate change impacts on marine water quality: The case study of the Northern Adriatic Sea. Mar. Pollut. Bull. 2016, 102, 271–282. [Google Scholar] [CrossRef]
- Lloret, J.; Palomera, I.; Salat, J.; Solé, I. Impact of freshwater input and wind on landings of anchovy (Engraulis encrasicolus) and sardine (Sardina pilchardus) in shelf waters surrounding the Ebre (Ebro) River delta (north-western Mediterranean). Fish. Oceanogr. 2004, 13, 102–110. [Google Scholar] [CrossRef]
- Caballero-Huertas, M.; Vargas-Yánez, M.; Frigola-Tepe, X.; Viñas, J.; Muñoz, M. Unravelling the drivers of variability in body condition and reproduction of the European sardine along the Atlantic-Mediterranean transition. Mar. Environ. Res. 2022. submitted. [Google Scholar]
- Mustać, B.; Sinovčić, G. Reproduction, length-weight relationship and condition of sardine, Sardina pilchardus (Walbaum, 1792), in the eastern Middle Adriatic Sea (Croatia). Period. Biol. 2010, 112, 133–138. [Google Scholar]
- Ganias, K. Linking sardine spawning dynamics to environmental variability. Estuar. Coast. Shelf Sci. 2009, 84, 402–408. [Google Scholar] [CrossRef]
- Ganias, K.; Somarakis, S.; Koutsikopoulos, C.; Machias, A. Factors affecting the spawning period of sardine in two highly oligotrophic Seas. Mar. Biol. 2007, 151, 1559–1569. [Google Scholar] [CrossRef]
- Mustać, B.; Sinovčić, G. Comparison of mesenteric and tissue fat content in relation to sexual cycle of the sardine, Sardina pilchardus (Walb., 1792), in the eastern Middle Adriatic fishery grounds (Croatia). J. Appl. Ichthyol. 2009, 25, 595–599. [Google Scholar] [CrossRef]
- Campanini, C.; Albo-Puigserver, M.; Gérez, S.; Lloret-Lloret, E.; Giménez, J.; Pennino, M.G.; Bellido, J.M.; Colmenero, A.I.; Coll, M. Energy content of anchovy and sardine using surrogate calorimetry methods. Mar. Environ. Res. 2021, 172, 105510. [Google Scholar] [CrossRef]
- Lloret, J.; Shulman, G.; Love, R.M. Condition and Health Indicators of Exploited Marine Fishes; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Bellido, J.M.; Brown, A.M.; Valavanis, V.D.; Giráldez, A.; Pierce, G.J.; Iglesias, M.; Palialexis, A. Identifying essential fish habitat for small pelagic species in Spanish Mediterranean waters. In Essential Fish Habitat Mapping in the Mediterranean; Valavanis, V.D., Ed.; Springer: Dordrecht, The Netherlands, 2008; Volume 203, pp. 171–184. [Google Scholar]
- Fernández-Corredor, E.; Albo-Puigserver, M.; Pennino, M.G.; Bellido, J.M.; Coll, M. Influence of environmental factors on different life stages of European anchovy (Engraulis encrasicolus) and European sardine (Sardina pilchardus) from the Mediterranean Sea: A literature review. Reg. Stud. Mar. 2021, 41, 101606. [Google Scholar] [CrossRef]
- Garrido, S.; Silva, A.; Marques, V.; Figueiredo, I.; Bryère, P.; Mangin, A.; Santos, A.M.P. Temperature and food-mediated variability of European Atlantic sardine recruitment. Prog. Oceanogr. 2017, 159, 267–275. [Google Scholar] [CrossRef]
- Riveiro, I.; Guisande, C.; Lloves, M.; Maneiro, I.; Cabanas, J.M. Importance of parental effects on larval survival in Sardina pilchardus. Mar. Ecol. Prog. Ser. 2000, 205, 249–258. [Google Scholar] [CrossRef]
- Grilli, F.; Accoroni, S.; Acri, F.; Bernardi Aubry, F.; Bergami, C.; Cabrini, M.; Campanelli, A.; Giani, M.; Guicciardi, S.; Marini, M. Seasonal and interannual trends of oceanographic parameters over 40 years in the northern Adriatic Sea in relation to nutrient loadings using the EMODnet chemistry data portal. Water 2020, 12, 2280. [Google Scholar] [CrossRef]
- Garrido, S.; Rosa, R.; Ben-Hamadou, R.; Cunha, M.E.; Chícharo, M.A.; van der Lingen, C.D. Spatio-temporal variability in fatty acid trophic biomarkers in stomach contents and muscle of Iberian sardine (Sardina pilchardus) and its relationship with spawning. Mar. Biol. 2008, 154, 1053–1065. [Google Scholar] [CrossRef]
- Garrido, S.; Rosa, R.; Ben-Hamadou, R.; Cunha, M.E.; Chícharo, M.A.; van der Lingen, C.D. Effect of maternal fat reserves on the fatty acid composition of sardine (Sardina pilchardus) oocytes. Comp. Biochem. Physiol. Biochem. Mol. Biol. 2007, 148, 398–409. [Google Scholar] [CrossRef]
- Abdelmoulah, A.; Hadj Ali Salem, M. Etude préliminaire sur les variations des lipides dans les différentes parties du corps de la sardine Sardina pilchardus (Walbaum, 1792) de la région de Bizerte. Bull. Inst. Natl. Sci. Tech. Oceanogr. Peche Salammbo 1981, 8, 53–58. [Google Scholar]
- McPherson, L.R.; Slotte, A.; Kvamme, C.; Meier, S.; Marshall, C.T. Inconsistencies in measurement of fish condition: A comparison of four indices of fat reserves for Atlantic herring (Clupea harengus). ICES J. Mar. Sci. 2011, 68, 52–60. [Google Scholar] [CrossRef]
- Krzeptowski, M. Biological characteristics of Sardine (Sardina pilchardus Walb.) off west Sahara. Acta Ichthyol. Piscat. 1983, 13, 13–38. [Google Scholar] [CrossRef]
- Garrido, S.; Ben-Hamadou, R.; Oliveira, P.B.; Cunha, M.E.; Chícharo, M.A.; van der Lingen, C.D. Diet and feeding intensity of sardine Sardina pilchardus: Correlation with satellite-derived chlorophyll data. Mar. Ecol. Prog. Ser. 2008, 354, 245–256. [Google Scholar] [CrossRef]
- Nikolioudakis, N.; Palomera, I.; Machias, A.; Somarakis, S. Diel feeding intensity and daily ration of the sardine Sardina pilchardus. Mar. Ecol. Prog. Ser. 2011, 437, 215–228. [Google Scholar] [CrossRef]
- Zorica, B.; Keč, V.C.; Vidjak, O.; Kraljevic, V.; Brzulja, G. Seasonal pattern of population dynamics, spawning activities, and diet composition of sardine (Sardina pilchardus Walbaum) in the eastern Adriatic Sea. Turk. J. Zool. 2017, 41, 892–900. [Google Scholar] [CrossRef]
- Zorica, B.; Keč, V.C.; Vrgoč, N.; Isajlović, I.; Piccinetti, C.; Mandić, M.; Marčeta, B.; Pešić, A. A review of reproduction biology and spawning/nursery grounds of the most important Adriatic commercial fish species in the last two decades. Acta Adriat. 2020, 61, 89–99. [Google Scholar] [CrossRef]
- Nejedli, S.; Petrinec, Z.; Kužir, S.; Srebočan, E. Annual oscillation of ovarian morphology in European pilchard (Sardina pilchardus Walbaum) in the Northern Adriatic Sea. Vet. Arh. 2004, 74, 97–106. [Google Scholar]
- Abderrazik, W.; Baali, A.; Schahrakane, Y.; Tazi, O. Study of reproduction of sardine, Sardina pilchardus in the North of Atlantic Moroccan area. Aquac. Aquar. Conserv. Legis. 2016, 9, 507–517. [Google Scholar]



| Variable/Index | Mean ± SD | Outcome | N | Test | Statistic | p | |
|---|---|---|---|---|---|---|---|
| Males | Females | ||||||
| LT (cm) | 13.17 ± 0.80 | 13.50 ± 0.90 | Males < Females | 704 | One-way analysis of means (not equal variances) | F = 27.04 | *** |
| WT (g) | 18.07 ± 3.40 | 19.98 ± 3.80 | Males < Females | 704 | Welch two sample t-test | t = 7.00 | *** |
| WE (g) | 16.01 ± 2.84 | 17.64 ± 3.23 | Males < Females | 704 | Welch two sample t-test | t = 7.11 | *** |
| Kn | 0.982 ± 0.113 | 1.023 ± 0.112 | Males < Females | 704 | Welch two sample t-test | t = 4.78 | *** |
| GSI | 1.906 ± 2.013 | 2.547 ± 2.268 | Males < Females | 704 | One-way analysis of means (not equal variances) | F = 15.54 | *** |
| HSI | 0.690 ± 0.447 | 0.785 ± 0.491 | Males < Females | 704 | Welch two sample t-test | t = 2.62 | * |
| Tissue fat content | 9.468 ± 4.333 | 10.353 ± 4.469 | Males < Females | 704 | Welch two sample t-test | t = 2.63 | * |
| Mesenteric fat | - | - | Males = Females | 704 | Wilcoxon rank sum test with continuity correction | W = 60366 | NS |
| % V | - | - | Males = Females | 704 | Pearson’s chi-squared test | χ² = 1.31 | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caballero-Huertas, M.; Frigola-Tepe, X.; Viñas, J.; Muñoz, M. Somatic Condition and Reproductive Potential as a Tandem in European Sardine: An Analysis with an Environmental Perspective in the Northern Adriatic (Gulf of Trieste). Fishes 2022, 7, 105. https://doi.org/10.3390/fishes7030105
Caballero-Huertas M, Frigola-Tepe X, Viñas J, Muñoz M. Somatic Condition and Reproductive Potential as a Tandem in European Sardine: An Analysis with an Environmental Perspective in the Northern Adriatic (Gulf of Trieste). Fishes. 2022; 7(3):105. https://doi.org/10.3390/fishes7030105
Chicago/Turabian StyleCaballero-Huertas, Marta, Xènia Frigola-Tepe, Jordi Viñas, and Marta Muñoz. 2022. "Somatic Condition and Reproductive Potential as a Tandem in European Sardine: An Analysis with an Environmental Perspective in the Northern Adriatic (Gulf of Trieste)" Fishes 7, no. 3: 105. https://doi.org/10.3390/fishes7030105
APA StyleCaballero-Huertas, M., Frigola-Tepe, X., Viñas, J., & Muñoz, M. (2022). Somatic Condition and Reproductive Potential as a Tandem in European Sardine: An Analysis with an Environmental Perspective in the Northern Adriatic (Gulf of Trieste). Fishes, 7(3), 105. https://doi.org/10.3390/fishes7030105