Abstract
The present study was conducted to investigate the effects of dietary hydroxytyrosol (HT) on oxidative stress, inflammation and mitochondrial homeostasis in blunt snout bream (Megalobrama amblycephala). Fish were fed a low-fat diet (LFD, 5% lipid), a high-fat diet (HFD, 15% lipid), an LFD supplementing 200 mg/kg HT, or an HFD supplementing 200 mg/kg HT. After 10-week feeding, significant reduction of growth was observed in fish fed HFD, compared with other groups. HFD caused oxidative stress and more apoptosis of hepatocytes, while HT addition resulted in significant decrease of ROS and MDA contents, and the apoptotic hepatocytes. Moreover, the expression of genes involving inflammation of HFD group were elevated. Supplementing HT to HFD can attenuate this. All the activities of complexes of mitochondria in the HFD group were decreased compared with those in the LFD group, while supplementing HT to HFD significantly increased complex I-III activities. Furthermore, HFD downregulated the expressions of Atg5 and NRF-1 which induced the failure of mitophagy and biogenesis, while, supplementing HT to HFD reversed these expressions involving mitochondrial autophagy and biogenesis. In summary, adding HT to HFD relieved oxidative stress, apoptosis and inflammation, likely due to its regulation of mitochondrial homeostasis.
1. Introduction
Dietary lipid plays a pivotal role in nutrient homeostasis due to its function in energy supply, containing essential fatty acids and phospholipids for human and other vertebrate animals. Lipid is extensively used to improve energy level of fish diet, and has a protein-sparing effect to improve feed efficiency []. The use of high-lipid diets is booming as a common practice because of the increasing cost and decreasing supply of fish meal []. However, with dietary lipid increasing, there are many undesirable impacts emerging []. Recent studies using mammal models have indicated that fat accumulation can induce inflammation [,]. High-fat-diet-induced inflammation of fish is also the cause of appetite suppression, hypoimmunity and liver damage []. Moreover, accumulation of fat can increase the rate of lipid peroxidation and induce oxidative stress []. Therefore, in fish farming, one current issue is how to attenuate high-fat-induced inflammation and oxidative stress.
Evidence has implied that inflammation is the mitochondrial dysfunction-dependent event []. The impairment of mitochondrion function and autophagy induce NLRP3 inflammasome and NF-κB signaling activation, further activating the inflammatory cascade and macrophage recruitment [,]. The mitochondrial respiratory chain also plays a major role in cellular ROS formation. ROS are overproduced when mitochondria malfunction []. Thus, targeting mitochondrion is an effective strategy for preventing inflammation and oxidative stress.
Mitochondrial nutrients are considered as micronutrients maintaining mitochondrial homeostasis [], and sufficient mitochondrial nutrients in cells are effective in preventing mitochondrial dysfunction. Hydroxytyrosol (HT), a kind of polyphenol with small molecular weight, is regarded as the most efficient antioxidant in olives [,] and considered a mitochondrial nutrient []. HT enhances mitochondrial function via promoting mitochondrial complexes and decreasing free fatty acids in mammals []. Based on this, we investigated impacts of HT on anti-oxidative ability, apoptosis, the inflammatory response and mitochondrial homeostasis in blunt snout bream (Megalobrama amblycephala). This is an herbivorous fish and also a favorable fish for aquaculture with rapid growth, high flesh quality, and strong disease tolerance. In the past decades, the nutritional requirements of blunt snout bream have been determined. The optimal dietary protein content is approximately 30% and the optimal dietary lipid is 4–7% []. However, the blunt snout bream is more susceptible to high-fat-diet-induced metabolic disorders, due to its relatively low hepatosomatic index (liver weight/body weight) [,,,]. This study has implications for nutritional regulation of oxidative stress and chronic inflammation induced by a fat-enriched diet.
2. Materials and Method
2.1. Experimental Diet Preparation
A total of four experimental diets were made-up as presented in Table 1. According to our previous study, a diet containing 5% lipid was regarded as low-fat diet (LFD), and a diet with 15% lipid was named as high-fat diet (HFD) []. LFD with 200 mg/kg HT addition and HFD with 200 mg/kg HT addition were also prepared, referred to as LFD+HT and HFD+HT, respectively. The dose of HT was determined by our pilot test, in which 100–200 mg/kg HT supplementation had a lipid-lowering effect. All desiccative ingredients were fully crushed, weighed, and homogenized in oil. After that, water with 30% of the ingredients weight was included as binder. Then, hard pelleted feeds were produced through a laboratory pelletizer. After drying at 65 °C for eight hours, the diets were separated into sealed plastic bags and stored at −20 °C freezer. The crude protein and lipid contents of the experimental diets were determined according to our previous study [].

Table 1.
Formulation and proximate composition of the experimental diets (% dry matter).
2.2. Fish and Experimental Design
Experimental juveniles were purchased from a commercial hatchery in Guangzhou (China). The feeding trial was conducted in a RAS (recirculating aquaculture system). Before the feeding trial, fish were reared in 1000-L tanks and fed a commercial diet for 2 weeks. Then, 360 fish of similar size were randomly distributed into 12,200-L cylindrical tanks of fresh water at a stocking rate of 30 fish per tank. During the trial, the water temperature varied from 25 to 27 °C, dissolved oxygen fluctuated between 5.0 and 6.0 mg/L, and pH was maintained above 7.4. Fish were hand-fed to apparent satiation three times (08:00, 12:00, and 16:00 h) daily for ten weeks. Each diet was assigned in triplicate. During the feeding trial, no fish died, and no disease or other adverse events happened.
2.3. Sampling
After the 10-week feeding trial, fish were fasted for 24 h before sampling. After being euthanized by 100 mg/L MS-222 (Sigma, St. Louis, MO, USA), all the fish of each tank were weighed. Then, blood was drawn from ten randomly selected fish per tank from the caudal vessel using a 1-mL syringe, centrifuged (850× g, 10 min) at 4 °C, and the serum was separated and transferred to a −80 °C ultralow temperature freezer. After drawing blood, the liver was immediately removed (placed on ice), flash frozen in liquid nitrogen and then transferred to a −80 °C ultralow temperature freezer.
2.4. Biochemical Parameters
The activities of plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were assayed by commercial kits (Beijing BHKT Clinical Reagent, Beijing, China) according to our previous study []. The reactive oxygen species (ROS) level was detected in hepatic homogenates by the DCFH/DA method as in our previous study []. Moreover, superoxide dismutase (SOD) activity, malondialdehyde (MDA) and total antioxidant capacity (T-AOC) of liver samples were measured by commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
The liver samples were homogenized in pre-cooled buffer (0.25 m sucrose, 5 mm Tris–HCL and 1 mm EGTA, pH 7.4). Differential centrifugation was conducted to isolate mitochondria. After that, mitochondria were collected in hypotonic media (25 mm potassium phosphate, 5 mm MgCl2, pH 7.2) with repeated freezing and thawing three times to release enzymes. Then, the isolated mitochondria were used to detect mitochondrial complex I-V activities, according to the method descripted in a previous study [].
2.5. Apoptosis Determination
Liver samples were fixed in Bouin’s solution overnight and dehydrated in a graded ethanol series. Routine paraffin embedding and slicing to 5-μm thickness were conducted. Then, a TUNEL assay was applied to obverse apoptotic hepatocytes. The protocol is described in the manufacturer’s instructions (Nanjing Jian-Cheng Bioengineering Institute, Nanjing, China).
2.6. Gene Expression
Total RNA in liver samples was extracted using a FastPure Cell/Tissue Total RNA Isolation Kit (Vazyme Biotech Co., Ltd., Nanjing, China) according to the instruction provided by the manufacturer. Before the downstream experiment, RNA concentration and purity were measured by an NanoDrop spectrophotometer, and the quality was determined by the 1% agarose gel electrophoresis test. Genomic DNA was cleared by RNase-Free DNase. Complementary DNA (cDNA) was reverse-transcribed from 500 ng total RNA using a HiScript II 1st Strand cDNA Synthesis Kit (Vazyme Biotech Co., Ltd., Nanjing, China).
Quantitative Real-time PCR (qRT-PCR) was used to measure mRNA abundance using a SYBR® Green I fluorescence kit (Takara Co. Ltd., Kyoto, Japan). Primer characteristics of target genes and the reference genes used for qRT-PCR are shown in Table 2. Rpl13a was used as reference gene according to a previous study, and the reaction system, amplification process and mRNA abundance analysis method were conducted according to our previous study [].

Table 2.
Sequences of primers used for RT-PCR in this study.
2.7. Statistical Analysis
Data are presented as mean ± S.E. and were analyzed by one-way ANOVA using IBM SPSS Statistics 20. Duncan’s test was used for group comparison. The level of significance was set at p < 0.05.
3. Results
3.1. Growth and Blood Biochemistry
Growth performance data for blunt snout bream are presented in Table 3. The results showed fish fed HFD had obvious lower final body weight, weight gain and specific growth rate than in the other three groups, while, supplementary HT in the high-fat diet increased the values of the indexes. The feed efficiency ratio (FCR) of the HFD group was also obviously higher than in the other three groups, and HT administration decreased FCR. Feed intake showed no significant difference among all groups.

Table 3.
Growth performance of blunt snout bream fed with experimental diets.
The activities of AST and ALT in plasma were remarkably higher in the HFD group, which implied damage of the liver. Moreover, activities of these two enzymes in HFD+HT group were at the same level as the LFD group (Figure 1).
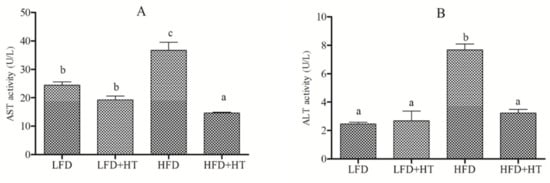
Figure 1.
Plasma AST (A) and ALT (B) activities of blunt snout bream fed with experimental diets. Bars with different letters are significantly different (p < 0.05).
3.2. Oxidative Status
A remarkable improvement of ROS level was observed in the liver of fish in the HFD group, and supplementation of HT in HFD significantly reduced hepatic ROS content (Figure 2). Likewise, the highest MDA content was found in the HFD group, and an intermediary value was observed in the HFD+HT group (Figure 2). HFD showed the lowest SOD and T-AOC activities among all groups, and HT addition increased SOD activity.
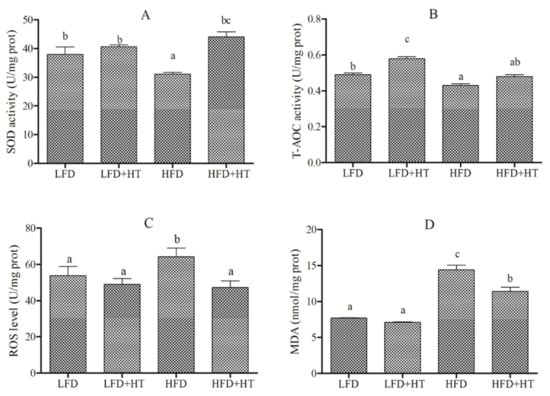
Figure 2.
(A) Activity of superoxide dismutase (SOD); (B) total antioxidant capacity (T-AOC); (C) concentrations of reactive oxygen species (ROS); (D) malondialdehyde (MDA) in liver of blunt snout bream fed with experimental diets. Bars with different letters are significantly different (p < 0.05).
3.3. Hepatocytes Apoptosis
The TUNEL method indicated DNA breakage of hepatocytes, which can be used to detect the level of apoptosis. Normal and apoptotic hepatocytes were stained amethyst and brown respectively (Figure 3). Apoptotic cells made up a small percentage of the total number of hepatocytes in the LFD group, whereas apoptotic cells constituted a high percentage of the hepatocytes in fish that were fed HFD. HT addition resulted in significant decrease of the apoptotic hepatocytes.
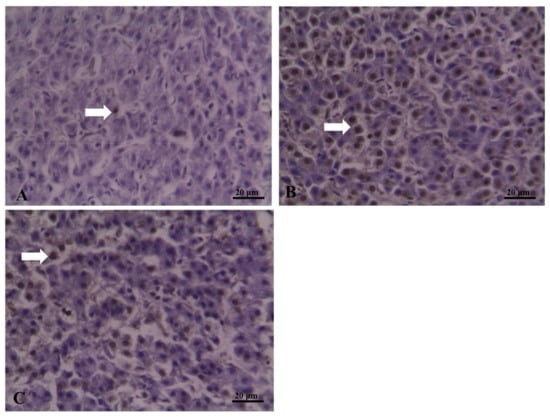
Figure 3.
Hepatocyte apoptosis in blunt Snout Bream: (A) normal cells (stained amethyst) and apoptotic cells (white arrow) from LFD group; (B) normal cells and apoptotic cells from HFD group; (C) normal cells and apoptotic cells from the HFD+HT group.
The expressions of apoptosis-related genes are listed in Figure 4. The results show that expressions of Bax, caspase 3 and caspase 9 genes were up-regulated in the HFD group, while HT supplementation significantly down-regulated the expressions to the levels of the LFD group. Conversely, the expression of Bcl-2 gene was down-regulated in HFD group, and an enhancement was observed by HT addition.
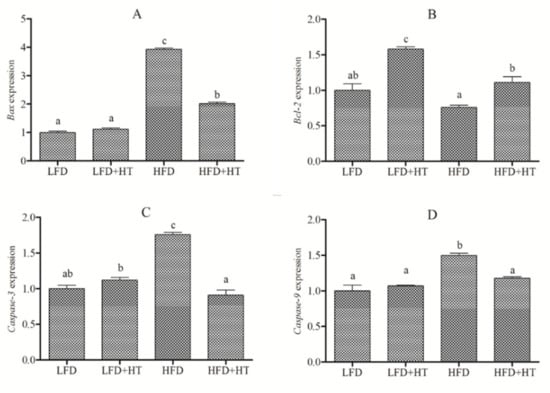
Figure 4.
Relative expression of apoptosis-related genes (A) Bax (B) Bcl-2 (C) Caspase-3 (D) Caspase-9 in the liver of blunt snout bream fed with experimental diets. Bars with different letters are significantly different (p < 0.05).
3.4. Inflammation
The expressions of inflammation-related genes are listed in Figure 5. Expression of IL-6, known as pro-inflammatory cytokines, was significantly up-regulated in the HFD group. CD68, considered as a macrophage marker and its gene expression was activated in the liver of fish fed HFD. CCR2 has a key role in the process of macrophage recruitment, and its gene expression was higher in HFD-fed fish. The p38 mitogen-activated protein kinase (p38MAPK) signaling pathway plays a key role in the production of pro-inflammatory cytokines, and its gene expression was higher in group fed with HFD. Moreover, the addition of HT down-regulated the expressions of IL-6, CD68, p38MAPK and CCR2.
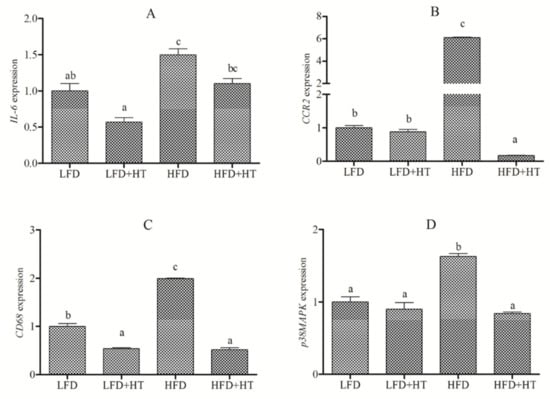
Figure 5.
Relative expression of inflammation-related genes (A) IL-6 (B) CCR2 (C) CD68 (D) p38MAPK in the liver of blunt snout bream fed with experimental diets. Bars with different letters are significantly different (p < 0.05).
3.5. Mitochondrial Function
Hepatic mitochondrial complex I-V activities are showed in Table 3. All the activities of complexes (I-V) in fish fed with HFD were lower than fish fed with LFD. The addition of HT significantly increased the activities of complex I-III.
The expressions of genes involving mitochondrial remodeling are presented in Table 4. PINK1, Mul1 and Atg5 play key roles in mitophagy, and all the expressions were down-regulated by HFD, while the addition of HT significantly increased the expressions of Mul1 and Atg5. NRF-1 and TFAM play key roles in mitochondrial biogenesis, and all the expressions were down-regulated by HFD. Mfn2 is one of the key points in the fusion of mitochondria, and all the expressions were down-regulated by HFD; the addition of HT significantly increased its expression. Fis1 plays a key role in fission of mitochondria, and its expression was down-regulated by HFD. The addition of HT significantly increased its expression. The regulatory factors of mitochondrial remodeling including PGC-1α, PGC-1β, FoxO and SIRT-1 were all down-regulated by HFD, while the addition of HT reverted these expressions to the levels of LFD (Table 5).

Table 4.
Activities of mitochondrial complexes in the liver of blunt snout bream fed with experimental diets.

Table 5.
Mitochondrial remodeling in the liver of blunt snout bream fed with experimental diets.
4. Discussion
In mammals, lipotoxicity is the main contributor of various diseases associated with excess fat accumulation, such as fatty liver, obesity and diabetes [,]. Excessive triglyceride deposition in liver is a biomarker of fatty liver. In some fish species, liver is a major site of lipid storage, and a large part of dietary energy is retained as liver lipid stores []. During autumn, extensive lipid infiltration of the hepatic parenchyma occurs depending on the species, e.g., for cod Gadus morhua L. []. This is not regarded as a pathological phenomenon. For most fish species, especially in freshwater farmed fish, if liver lipid content reaches a certain level, a severe metabolism syndrome emerges [,,]. In present study, oxidative stress, apoptosis and inflammation appeared in fish fed HFD due to lipid deposition, which is considered pathological.
Previous studies have indicated fat accumulation can increase fat peroxidation rate and induce oxidative stress []. Our results showed obvious increases of ROS and MDA levels in the HFD group, which implies oxidative stress. Although tissues have antioxidant defense systems to mitigate oxidative stress, including antioxidase and nonenzymatic antioxidant system, excessive ROS accumulation still can cause oxidative stress [,]. Mitochondria are the major source of ROS production due to oxidative phosphorylation []. Thus, oxidative stress mostly originates in the cell’s mitochondria. Actually, our previous study showed that blunt snout bream fed HFD have many damaged mitochondria with impairment of cristae and matrices [,]. Moreover, this study presented a decrease of the activities of complexes of the mitochondrial respiratory chain. ROS is primarily produced by complex I-III located at mitochondrial inner membrane and lower activities of complexes contribute to ROS overload []. Generally speaking, the excessive intake of fat destroys mitochondrial functions and causes oxidative stress. Supplementation of HT can reduce the contents of ROS and MDA in the liver and attenuate oxidative stress. Although HT is often considered a non-enzymatic antioxidant, it did not elevate total antioxidant capacity (T-AOC) in this study. Thus, the effects of HT in attenuating oxidative stress are mainly by promoting mitochondrial function with less ROS.
Hepatocyte apoptosis often appears in fatty liver, and is frequently considered as a hepatic steatosis biomarker in human []. In the present study, TUNEL positive cells of HFD fed fish were more prevalent than in other fish. Apoptotic pathways are involved in many extrinsic and intrinsic pathways associated with cellular stress []. There is widespread agreement that mitochondria are central to apoptosis []. In the present study, apoptosis of hepatocytes was also related to intrinsic mitochondrial-pathways characterized by mitochondrial damage with the release of cytochrome c, and activation of caspase 9 and, subsequently, caspase 3. Based on our previous study, cytochrome c is released from mitochondria into the cytosol when fish are fed HFD, with damage of mitochondrial permeability []. Release of cytochrome c from mitochondria is considered the primary event for apoptosis. HT supplementation significantly down-regulated the expressions of caspase 3 and 9 to the levels of the LFD group. Thus, we presume that the anti-apoptotic action of HT is based on the protection of mitochondrial integrity. Among proapoptotic members, Bax is perhaps the best-studied protein and is essential for mitochondrion-mediated apoptosis []. Functional analysis showed that BAX could promote the release of cytochrome c from mitochondria [,]. Conversely, Bcl-2 plays an anti-apoptosis role by ameliorating cytochrome c release, and decrease of the Bax/Bcl-2 ratio alleviates the amount of apoptosis [,]. In this study, the Bax expression level was decreased, and Bcl-2 was increased in fish fed the HT-supplemented diet.
Inflammatory cytokines are closely associated with the development of metabolic diseases [,,]. Inflammation is the key and early contributing factor for the occurrence of many chronic diseases []. A recent study in zebrafish (Danio rerio) indicated that long-term HFD feeding induced the increase of inflammatory markers []. Moreover, the HFD-related inflammation response inhibits the appetite of blunt snout bream, and is closely related to lipid abnormality and exacerbation of growth performance []. IL-6, a pro-inflammatory cytokine, has been proved to be a marker of inflammation []. In this study, its expression in the HFD group was significantly up-regulated. Furthermore, the recruitment of macrophages to the liver is caused by pro-inflammatory cytokines, which is an important process in inflammation-induced liver damage []. CCR2 and CD68 act as key points in the recruitment of macrophages [,]. Our t results showed that these two gene expressions were higher in fish fed HFD than in those fed LFD. These data generally indicate that macrophage recruitment is associated with inflammation in fish. Interestingly, the mitochondrion is a signaling platform for macrophage activation and functions, and is the master regulator of macrophages []. Therefore, in this study, the effects of HT on preventing inflammation of fish fed high-fat diets may also be related to mitochondrion-targeting. Likewise, the role of mitochondrial dysfunction is widely discussed in the context of various chronic inflammatory disorders [].
As a highly dynamic cell organelle, the mitochondrion is involved in many cellular processes, including ATP formation, apoptosis, and signal transduction, among others []. Hence, it is not surprising that mitochondrial dysfunction is implicated in several diseases, and it has been implicated in many diet-induced fatty liver diseases []. In response to extrinsic/intrinsic stimuli, mitochondria undergo reproduction (biogenesis) and degradation (mitophagy), as well as morphological dynamics (via fusion and fission), which maintains the homeostasis of mitochondria [].
Mitophagy is a selective autophagy and triggered when there are more damaged mitochondria in cells [] and can resist various stressors such as oxidative stress []. The dysfunction of mitophagy induces the retention of dysfunctional mitochondria resulting in oxidative stress, apoptosis and inflammation []. In this study, all autophagic and mitophagic markers, such as PINK1, Parkin, Mul1 and Atg5, were decreased in the HFD group, together with the inhibition of FoxO. Thus, we can conclude that the intake of HFD inhibits the activation of the mitophagy pathway, inducing the accumulation of damaged mitochondria.
Besides clearance of damaged mitochondria, maintenance of mitochondrial homeostasis also requires generation of fresh and healthy mitochondria by biogenesis. There is complex crosstalk between mitophagy and biogenesis, and their balance is very important in cellular homeostasis [], and some studies have even indicated that mitophagy is required for mitochondrial biogenesis []. In this study, as with mitophagy, the key factors of mitochondria biogenesis, NRF-1 and TFAM were down-regulated in the HFD group together with inhibition of PGC-1. Hence, the down-regulation of biogenesis would induce fewer fresh mitochondria, while, the supplementation of HT can revert these two processes to control level. Therefore, HT is involved in the regulation of mitochondrial homeostasis.
In addition, mitochondria constantly undergo fission and fusion to repair damaged components []. Mitochondrial fusion is mainly regulated by Mfn1 and 2, and fission is mediated by an out-membrane protein Drp1 and Fis1 []. Fission and fusion also play critical roles in maintaining functional mitochondria when cells face metabolic or environmental stresses. Fusion mitigates stress via mixing partially damaged mitochondria as a complementation method. Fission is needed to create new mitochondria and essential for providing dividing cells with adequate mitochondria []. It has been reported that fission is required for mitophagy as it was prevented in a negative mutant of Drp1 []. In all, failure of these processes leads to cell death and metabolic disorders. In this study, the mRNA levels of all fusion and fission proteins, such as Mfn1 and 2, Drp1 and Fis1, were decreased in the HFD group. Thus, we can conclude that the intake of HFD also inhibited the pathways of fusion and fission, while the supplementation of HT reverted these two processes to control level. Therefore, HT can regulate mitochondrial morphology to combat cellular stress via fusion and fission.
5. Conclusions
In conclusion, supplementing HT to HFD attenuated oxidative stress, apoptosis, inflammation and mitochondrial dysfunction in the liver. HT maintained mitochondrial homeostasis by activating mitophagy and biogenesis.
Author Contributions
Conceptualization, K.L. and Y.D.; data curation, Y.D., T.X., M.Y. and K.L.; formal analysis, Y.D.; methodology, T.X., M.Y., L.W., K.S. and C.Z.; supervision, K.L.; writing—original draft, Y.D.; writing—review & editing, K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by National Nature Science Foundation of China (31602171) and Nature Science Foundation of Fujian Province (2017J05056).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Basel and approved by Recommendations of Animal Research Institute Committee guidelines, Jimei University, China (Approval number: 2011-58).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hillestad, M.; Johnsen, F. High-energy/low-protein diets for Atlantic salmon: Effects on growth, nutrient retention and slaughter quality. Aquaculture 1994, 124, 109–116. [Google Scholar] [CrossRef]
- Du, Z.Y.; Clouet, P.; Zheng, W.H.; Degrace, P.; Liu, Y.J. Biochemical hepatic alterations and body lipid composition in the herbivorous grass carp (Ctenopharyngodon idella) fed high-fat diets. Br. J. Nutr. 2006, 95, 905–915. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.; Xu, W.; Li, J.; Li, X.; Huang, G.; Liu, W. Alterations of liver histology and blood biochemistry in blunt snout bream Megalobrama amblycephala fed high-fat diets. Fish. Sci. 2013, 79, 661–671. [Google Scholar] [CrossRef]
- Oka, T.; Nishimura, Y.; Zang, L.; Hirano, M.; Tanaka, T. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol. 2010, 10, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, P.; Wong, K.I.; Sun, X.; Reilly, S.M.; Uhm, M.; Liao, Z.; Skorobogatko, Y.; Saltiel, A.R. TBK1 at the Crossroads of Inflammation and Energy Homeostasis in Adipose Tissue. Cell 2018, 172, 731–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Y.; Cao, X.; Zhang, D.; Li, X.; Liu, W.; Jiang, G. Chronic inflammation is a key to inducing liver injury in blunt snout bream (Megalobrama amblycephala) fed with high-fat diet. Dev. Comp. Immunol. 2019, 97, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Wang, L.; Zhang, D.; Liu, W.; Xu, W. Berberine attenuates oxidative stress and hepatocytes apoptosis via protecting mitochondria in blunt snout bream Megalobrama amblycephala fed high-fat diets. Fish Physiol. Biochem. 2017, 43, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. The macrophage: Past, present and future. Eur. J. Immunol. 2007, 37, S9–S17. [Google Scholar] [CrossRef]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Eto, K.; Yamashita, H.; Ohsugi, M.; Otsu, M.; Hara, K.; Ueki, K.; Sugiura, S.; et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009, 15, 914–920. [Google Scholar] [CrossRef]
- Shadel, G.S.; Horvath, T.L. Mitochondrial ROS Signaling in Organismal Homeostasis. Cell 2015, 163, 560–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Shen, W.; Zhao, B.; Wang, Y.; Wertz, K.; Weber, P.; Zhang, P. Targeting mitochondrial biogenesis for preventing and treating insulin resistance in diabetes and obesity: Hope from natural mitochondrial nutrients. Adv. Drug Deliver. Rev. 2009, 61, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Manna, C.; Ragione, F.D.; Cucciolla, V.; Borriello, A.; Zappia, V. Biological Effects of Hydroxytyrosol, a Polyphenol from Olive Oil Endowed with Antioxidant Activity. Adv. Exp. Med. Biol. 1999, 472, 115. [Google Scholar] [PubMed]
- Roberto, F.; Patrizia, R.; Angelo, D.B.; Raffaela, F.; Maurizio, S.; Francesco, M.G.; Guido, M. Oxidative DNA Damage Is Prevented by Extracts of Olive Oil, Hydroxytyrosol, and Other Olive Phenolic Compounds in Human Blood Mononuclear Cells and HL60 Cells. J. Nutr. 2008, 138, 1411–1416. [Google Scholar]
- Liu, Z.; Wang, N.; Ma, Y.; Wen, D. Hydroxytyrosol Improves Obesity and Insulin Resistance by Modulating Gut Microbiota in High-Fat Diet-Induced Obese Mice. Front. Microbiol. 2019, 10, 390. [Google Scholar] [CrossRef]
- Hao, J.; Shen, W.; Yu, G.; Jia, H.; Li, X.; Feng, Z.; Ying, W.; Weber, P.; Wertz, K.; Sharman, E. Hydroxytyrosol promotes mitochondrial biogenesis and mitochondrial function in 3T3-L1 adipocytes. J. Nutr. Biochem. 2010, 21, 634–644. [Google Scholar] [CrossRef]
- Li, X.; Liu, W.; Jiang, Y.; Zhu, H.; Ge, X. Effects of dietary protein and lipid levels in protical diets on growth performance and body composition of blunt snout bream (Megalobrama amblycephala) fingerlings. Aquaculture 2010, 303, 65–70. [Google Scholar] [CrossRef]
- Li, J.; Zhang, D.; Xu, W.; Jiang, G.; Zhang, C.; Li, X.; Liu, W. Effects of dietary choline supplementation on growth performance and hepatic lipid transport in blunt snout bream (Megalobrama amblycephala) fed high-fat diets. Aquaculture 2014, 434, 340–347. [Google Scholar] [CrossRef]
- Cai, L.; Wang, L.; Song, K.; Lu, K.; Zhang, C.; Rahimnejad, S. Evaluation of protein requirement of spotted seabass (Lateolabrax maculatus) under two temperatures, and the liver transcriptome response to thermal stress. Aquaculture 2020, 516, 734615. [Google Scholar] [CrossRef]
- Dong, Y.; Li, L.; Espe, M.; Lu, K.; Rahimnejad, S. Hydroxytyrosol Attenuates Hepatic Fat Accumulation via Activating Mitochondrial Biogenesis and Autophagy through the AMPK Pathway. J. Agric. Food Chem. 2020, 68, 9377–9386. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, J.; Zhou, Z. In vitro effect of manganese chloride exposure on reactive oxygen species generation and respiratory chain complexes activities of mitochondria isolated from rat brain. Toxicol. Vitr. 2004, 18, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Luo, W.; Liu, H.; Zeng, C.; Liu, X.; Yi, S.; Wang, W. Transcriptome analysisand SSR/SNP markers information of the blunt snout bream (Megalobrama amblycephala). PLoS ONE 2012, 7, e42637. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Rahimnejad, S.; Lu, K.; Wang, L.; Liu, W. Effects of berberine on growth, liver histology, and expression of lipid-related genes in blunt snout bream (Megalobrama amblycephala) fed high-fat diets. Fish Physiol. Biochem. 2019, 45, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Chavez, J.A.; Summers, S.A. Lipid oversupply, selective insulin resistance, and lipotoxicity: Molecular mechanisms. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2010, 1801, 252–265. [Google Scholar] [CrossRef] [Green Version]
- Brookheart, R.T.; Michel, C.I.; Schaffer, J.E. As a Matter of Fat. Cell Metab. 2009, 10, 9–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrington, D.A.; Davidson, B.K.; Winemiller, K.O.; Layman, C.A. Influence of life history and seasonal hydrology on lipid storage in three neotropical fish species. J. Fish Biol. 2006, 68, 1347–1361. Available online: http://www.blackwell-synergy.com (accessed on 9 May 2006). [CrossRef]
- Grant, S.M.; Arown, J.A.; Boyce, D.L. Enlarged fatty livers of small juvenile cod: A comparison of laboratory-cultured and wild juveniles. J. Fish Biol. 1998, 52, 1105–1114. [Google Scholar] [CrossRef]
- Du, Z.Y.; Clouet, P.; Huang, L.M.; Degrace, P.; Zheng, W.H.; He, J.G.; Tian, L.X.; Liu, Y.J. Utilization of different dietary lipid sources at high level in herbivorous grass carp (Ctenopharyngodon idella): Mechanism related to hepatic fatty acid oxidation. Aquacult. Nutr. 2008, 14, 77–92. [Google Scholar] [CrossRef]
- Lu, K.L.; Xu, W.N.; Liu, W.B.; Wang, L.N.; Zhang, C.N.; Li, X.F. Association of Mitochondrial Dysfunction with Oxidative Stress and Immune Suppression in Blunt Snout Bream Megalobrama amblycephala Fed a High-Fat Diet. J. Aquat. Anim. Health 2014, 26, 100–112. [Google Scholar] [CrossRef]
- Zou, X.; Feng, Z.; Li, Y.; Wang, Y.; Wertz, K.; Weber, P.; Fu, Y.; Liu, J. Stimulation of GSH synthesis to prevent oxidative stress-induced apoptosis by hydroxytyrosol in human retinal pigment epithelial cells: Activation of Nrf2 and JNK-p62/SQSTM1 pathways. J. Nutr. Biochem. 2012, 23, 994–1006. [Google Scholar] [CrossRef]
- Vial, G.; Dubouchaud, H.; Couturier, K.; Cottet-Rousselle, C.; Taleux, N.; Athias, A.; Galinier, A.; Casteilla, L.; Leverve, X.M. Effects of a high-fat diet on energy metabolism and ROS production in rat liver. J. Hepatol. 2010, 54, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Ravera, S.; Bartolucci, M.; Cuccarolo, P.; Litamè, E.; Illarcio, M.; Calzia, D.; Degan, P.; Morelli, A.; Panfoli, I. Oxidative stress in myelin sheath: The other face of the extramitochondrial oxidative phosphorylation ability. Free Radic. Res. 2015, 49, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Bottje, W.; Iqbal, M.; Tang, Z.X.; Cawthon, D.; Okimoto, R.; Wing, T.; Cooper, M. Association of Mitochondrial Function with Feed Efficiency within a Single Genetic Line of Male Broilers. Poultry Sci. 2002, 81, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Wieckowska, A.; Zein, N.N.; Yerian, L.M.; Lopez, A.R.; Mccullough, A.J.; Feldstein, A.E. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology 2006, 44, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Zamzami, N.; Kroemer, G. The mitochondrion in apoptosis: How Pandora’s box opens. Nat. Rev. Mol. Cell Biol. 2001, 2, 67–71. [Google Scholar] [CrossRef]
- Chiu, S.M.; Xue, L.Y.; Usuda, J.; Azizuddin, K.; Oleinick, N.L. Bax is essential for mitochondrion-mediated apoptosis but not for cell death caused by photodynamic therapy. Brit. J. Cancer 2003, 89, 1590–1597. [Google Scholar] [CrossRef] [Green Version]
- Mcarthur, K.; Whitehead, L.W.; Heddleston, J.M.; Li, L.; Padman, B.S.; Oorschot, V.; Geoghegan, N.D.; Chappaz, S.; Davidson, S.; San, C.H.; et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 2018, 359, eaao6047. [Google Scholar] [CrossRef] [Green Version]
- Xie, S.; Lin, Y.; Wu, T.; Tian, L.; Liang, J.; Tan, B. Dietary lipid levels affected growth performance, lipid accumulation, inflammatory response and apoptosis of japanese seabass (Lateolabrax japonicus). Aquacult. Nutr. 2021, 27, 807–816. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, L.; Lu, T.; Zhang, Y.; Sui, X.; Li, Y.; Huang, X.; He, L.; Cai, J.; Zhou, C.; et al. MSCs ameliorate hepatocellular apoptosis mediated by PINK1-dependent mitophagy in liver ischemia/reperfusion injury through AMPKα activation. Cell Death Dis. 2020, 11, 256. [Google Scholar] [CrossRef]
- Ahonen, T.M.; Saltevo, J.T.; Kautiainen, H.J.; Kumpusalo, E.A.; Vanhala, M.J. The association of adiponectin and low-grade inflammation with the course of metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 285–291. [Google Scholar] [CrossRef]
- Sun, X.; Feng, R.; Li, Y.; Lin, S.; Zhang, W.; Li, Y.; Sun, C.; Li, S. Histidine supplementation alleviates inflammation in the adipose tissue of high-fat diet-induced obese rats via the NF-κB- and PPARγ-involved pathways. Br. J. Nutr. 2014, 112, 477–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Jin, Q.; Yao, Q.; Zhou, Y.; Zou, Y.; Li, Z.; Zhang, S.; Tu, C. Placental Growth Factor Contributes to Liver Inflammation, Angiogenesis, Fibrosis in Mice by Promoting Hepatic Macrophage Recruitment and Activation. Front. Immunol. 2017, 8, 801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mcgrath-Morrow, S.A.; Ndeh, R.; Collaco, J.M.; Poupore, A.K.; Dikeman, D.; Zhong, Q.; Singer, B.D.; D’Alessio, F.; Scott, A. The innate immune response to lower respiratory tract E. Coli infection and the role of the CCL2-CCR2 axis in neonatal mice. Cytokine 2017, 97, 108–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miura, K.; Yang, L.; van Rooijen, N.; Ohnishi, H.; Seki, E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am. J. Physiol.-Gastr. Liv. 2012, 302, G1310–G1321. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Ho, P. Mitochondria: A master regulator in macrophage and T cell immunity. Mitochondrion 2018, 41, 45–50. [Google Scholar] [CrossRef]
- Dela Cruz, C.S.; Kang, M. Mitochondrial dysfunction and damage associated molecular patterns (DAMPs) in chronic inflammatory diseases. Mitochondrion 2018, 41, 37–44. [Google Scholar] [CrossRef]
- Fouret, G.; Gaillet, S.; Lecomte, J.; Bonafos, B.; Djohan, F.; Barea, B.; Badia, E.; Coudray, C.; Feillet-Coudray, C. 20-Week follow-up of hepatic steatosis installation and liver mitochondrial structure and activity and their interrelation in rats fed a high-fat-high-fructose diet. Br. J. Nutr. 2018, 119, 368–380. [Google Scholar] [CrossRef] [Green Version]
- Kang, C.; Ji, L.L. PGC-1α overexpression via local transfection attenuates mitophagy pathway in muscle disuse atrophy. Free Radic. Biol. Med. 2016, 93, 32–40. [Google Scholar] [CrossRef]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Balancing mitochondrial biogenesis and mitophagy to maintain energy metabolism homeostasis. Cell Death Differ. 2015, 22, 1399–1401. [Google Scholar] [CrossRef] [Green Version]
- Yun, J.; Finkel, T. Mitohormesis. Cell Metab. 2014, 19, 757–766. [Google Scholar] [CrossRef] [Green Version]
- Palikaras, K.; Tavernarakis, N. Mitochondrial homeostasis: The interplay between mitophagy and mitochondrial biogenesis. Exp. Gerontol. 2014, 56, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Sin, J.; Andres, A.M.; Taylor, D.J.; Weston, T.; Hiraumi, Y.; Stotland, A.; Kim, B.J.; Huang, C.; Doran, K.S.; Gottlieb, R.A. Mitophagy is required for mitochondrial biogenesis and myogenic differentiation of C2C12 myoblasts. Autophagy 2016, 12, 369–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, D.C. Fusion and fission: Interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 2012, 46, 265–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grumati, P.; Coletto, L.; Sabatelli, P.; Cescon, M.; Angelin, A.; Bertaggia, E.; Blaauw, B.; Urciuolo, A.; Tiepolo, T.; Merlini, L.; et al. Autophagy is defective in collagen VI muscular dystrophies, and its reactivation rescues myofiber degeneration. Nat. Med. 2010, 16, 1313–1320. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).