Length–Weight Relationships and Growth Parameters of Common and Leafy Seadragons (Syngnathidae) from a Public Aquarium
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stiller, J.; Wilson, N.G.; Rouse, G.W. A spectacular new species of seadragon (Syngnathidae). R. Soc. Open Sci. 2015, 2, 140458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollom, R. Phyllopteryx taeniolatus. The IUCN Red List of Threatened Species 2017, e.T17177A67624517. Available online: https://www.iucnredlist.org/species/17177/67624517 (accessed on 10 December 2021).
- Pollom, R. Phycodurus eques. The IUCN Red List of Threatened Species 2017, e.T17096A67622420. Available online: https://www.iucnredlist.org/species/17096/67622420 (accessed on 11 December 2021).
- Aylesworth, L.; Pollom, R. Phyllopteryx dewysea (Errata Version Published in 2017). The IUCN Red List of Threatened Species 2016, e.T87568739A115514038. Available online: https://www.iucnredlist.org/species/87568739/115514038 (accessed on 10 December 2021).
- Stanton, L.M.; Foster, S.J.; Vincent, A.C.J. Identifying National Conservation Status, Legislation and Priorities for Syngnathid Fishes Globally; Fisheries Centre Research Reports 29(2); Institute for the Oceans and Fisheries, The University of British Columbia: Vancouver, BC, Canada, 2021. [Google Scholar] [CrossRef]
- Klanten, O.S.; Gaither, M.R.; Greaves, S.; Mills, K.; O’Keeffe, K.; Turnbull, J.; McKinnon, R.; Booth, D.J. Genomic and morphological evidence of distinct populations in the endemic common (weedy) seadragon Phyllopteryx taeniolatus (Syngnathidae) along the east coast of Australia. PLoS ONE 2020, 15, e0243446. [Google Scholar] [CrossRef] [PubMed]
- Kuiter, R.H. Note sur les soins parentaux, l’éclosion et l’élevage des dragons de mer (Syngnathidae). Rev. Fr. D’aquariol. 1988, 14, 113–120. [Google Scholar]
- Sanchez-Camara, J.; Booth, D.J.; Turon, X. Reproductive cycle and growth of Phyllopteryx taeniolatus. J. Fish Biol. 2005, 67, 133–148. [Google Scholar] [CrossRef]
- Sanchez-Camara, J.; Martin-Smith, K.; Booth, D.J.; Fritschi, J.; Turon, X. Demographics and vulnerability of a unique Australian fish, the weedy seadragon Phyllopteryx taeniolatus. Mar. Ecol. Prog. Ser. 2011, 422, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Forsgren, K.L.; Lowe, C.G. The life history of weedy seadragons, Phyllopteryx taeniolatus (Teleostei: Syngnathidae). Mar. Freshw. Res. 2016, 57, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Froese, R. Cube law, condition factor and weight–length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Hay, A.; Xian, W.; Bailly, N.; Liang, C.; Pauly, D. The why and how of determining length-weight relationships of fish from preserved museum specimens. J. Appl. Ichthyol. 2020, 36, 373–379. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 7th ed.; W.H. Freeman and Company: New York, NY, USA, 2017. [Google Scholar]
- Pauly, D. The gill-oxygen limitation theory (GOLT) and its critics. Sci. Adv. 2021, 7, eabc6050. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D. The relationships between gill surface area and growth performance in fish: A generalization of von Bertalanffy’s theory of growth. Ber. Dtsch. Wiss. Komm. Meeresforsch. 1981, 28, 251–282. [Google Scholar]
- Pauly, D. Tropical fishes: Patterns and propensities. J. Fish Biol. 1998, 53 (Suppl. A), 1–17. [Google Scholar]
- Pauly, D. Why do fish reach first maturity when they do? J. Fish Biol. 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Branstetter, S. Age and growth validation of newborn sharks held in laboratory aquaria, with comments on the life history of the Atlantic sharpnose shark, Rhizoprionodon terraenovae. Copeia 1987, 291–300. Available online: https://www.jstor.org/stable/1445764 (accessed on 1 January 2022). [CrossRef]
- Debelius, H.; Baensch, H.A.; Moosleitner, H. Marine Atlas: The Joint Aquarium Care of Invertebrates and Tropical Marine Fishes; Tetra Books: Blacksburg, VA, USA, 1994; p. 1200. [Google Scholar]
- Prein, M.; Kunzmann, A. Structural organization of the gills in pipefish (Teleostei, Syngnathidae). Zoomorphology 1987, 107, 161–168. [Google Scholar] [CrossRef]
- Pütter, A. Die Ernährung der Wassertiere und der Stoffhaushalt der Gewässer. Z. Allgeneine Physiol. 1909, 7, 148. [Google Scholar]
- Hughes, G.M.; Morgan, M. The Structure of Fish Gills in Relation to Their Respiratory Function. Biol. Rev. 1973, 48, 419–475. [Google Scholar] [CrossRef]

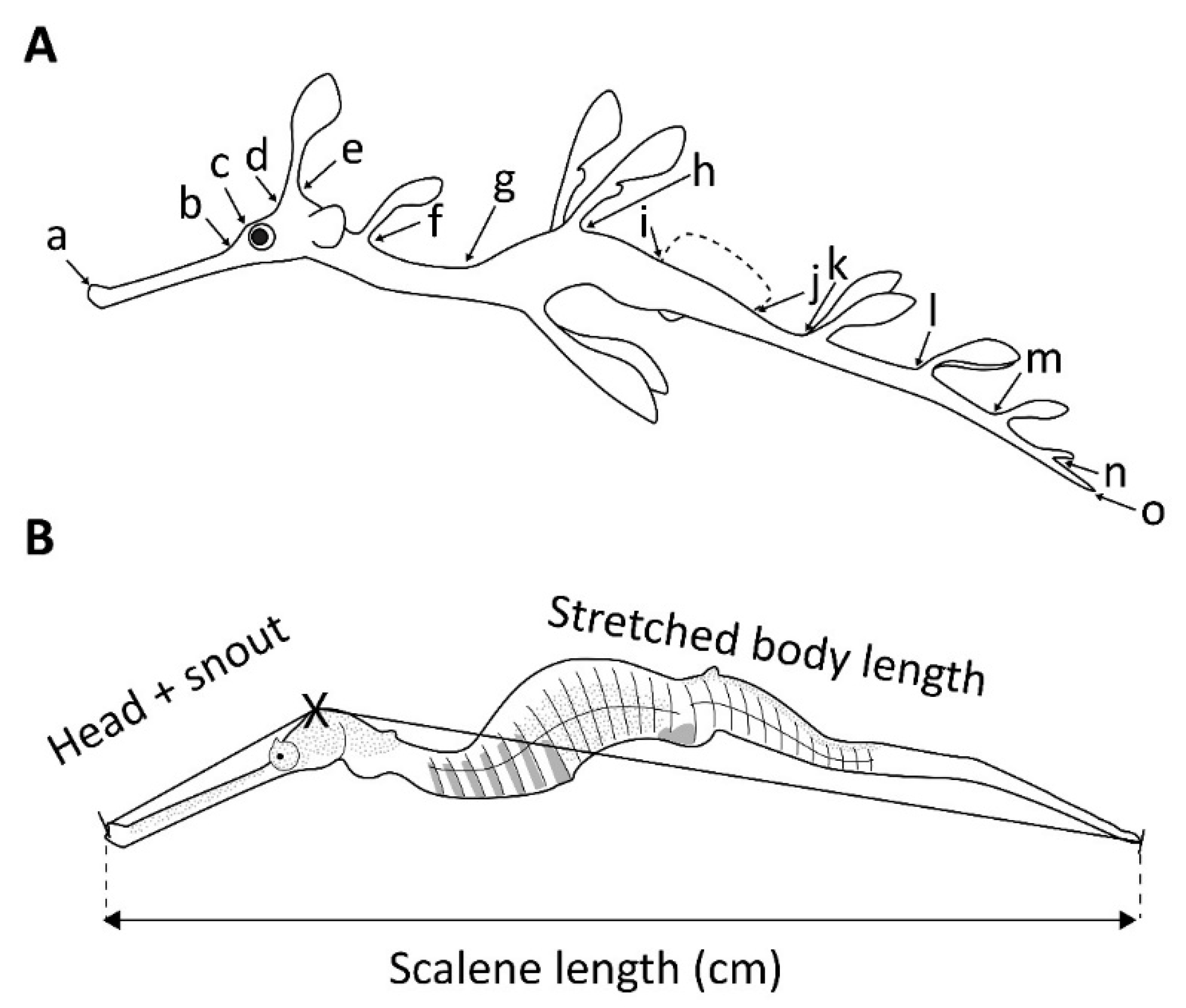
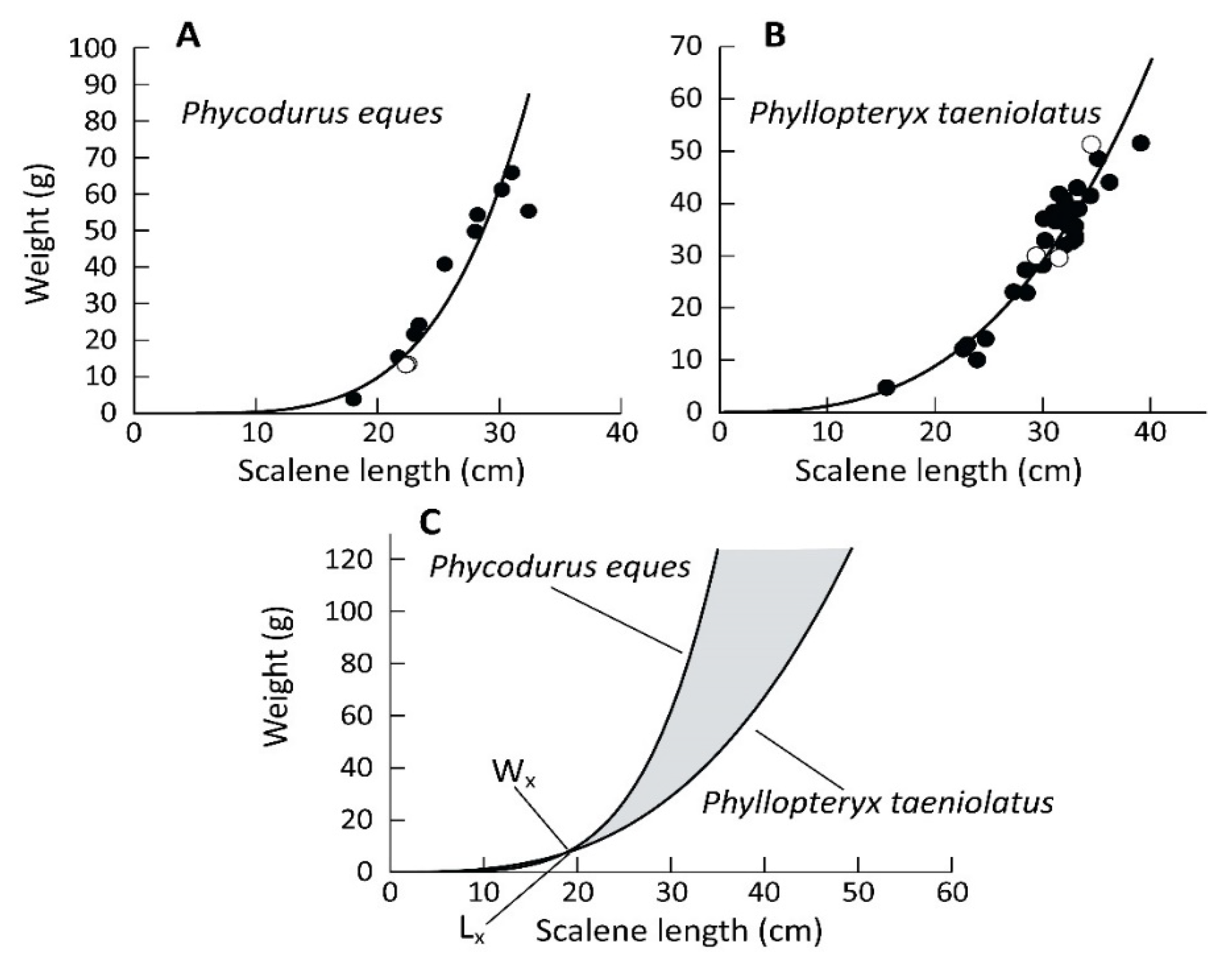

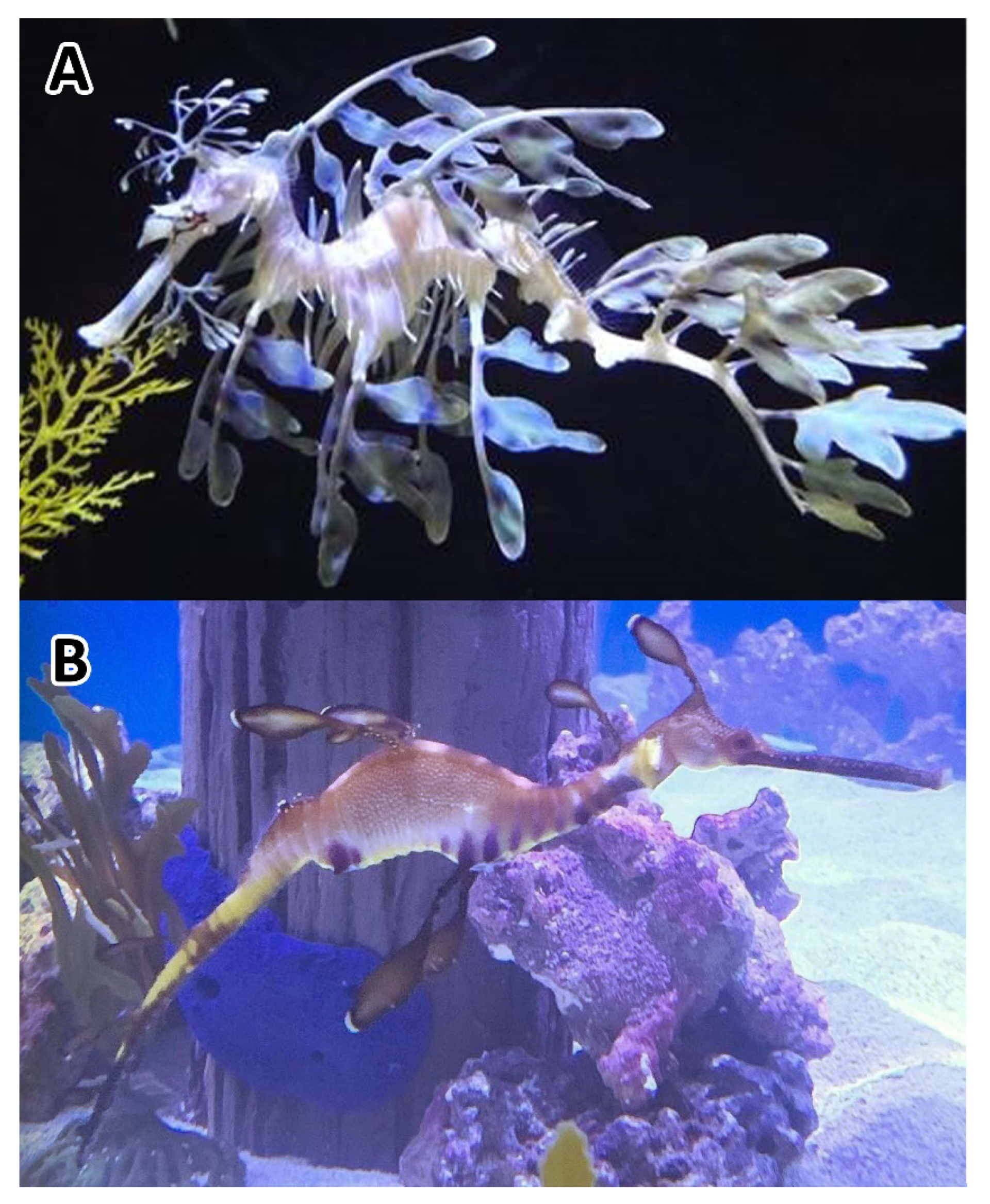
| Hatching Day | Death Day | Age (Days) | Sex | Length (mm) | Weight (g) |
|---|---|---|---|---|---|
| 1/9/2013 | 6/12/2013 | 96 | -- | 85 | 0.75 |
| 19/9/2013 | 25/11/2013 | 67 | -- | 91 | 0.66 |
| 19/9/2013 | 3/12/2013 | 75 | -- | 92 | 0.63 |
| 19/9/2013 | 1/12/2013 | 73 | -- | 92 | 0.70 |
| 19/9/2013 | 12/1/2013 | 73 | -- | 96 | 0.76 |
| 9/1/2013 | 8/12/2013 | 98 | -- | 98 | 0.77 |
| 21/3/2020 | 11/4/2020 | 228 | -- | 152 | 5.04 |
| 15/12/2009 | 7/4/2011 | 478 | ♀ | 155 | 4.69 |
| 1/8/2012 | 25/7/2013 | 358 | -- | 210 | 14.71 |
| -- | 20/1/2020 | -- | ♂ | 324 | 35.62 |
| 15/12/2018 | 12/1/2020 | 393 | ♂ | 226 | 12.05 |
| 15/12/2018 | 18/12/2019 | 368 | ♂ | 230 | 12.89 |
| 10/12/2018 | 19/2/2020 | 436 | ♀ | 239 | 10.00 |
| -- | 15/1/2020 | -- | ♂ | 351 | 48.56 |
| 10/12/2018 | 17/2/2020 | 434 | -- | 245 | -- |
| 1/8/2012 | 13/8/2013 | 377 | ♀ | 247 | 14.02 |
| 15/12/2009 | 18/5/2015 | 980 | ♀ | 273 1 | 23.04 1 |
| 15/12/2014 | 13/6/2017 | 911 | ♂ | 284 | 27.24 |
| 1/3/2016 | 30/1/2018 | 700 | ♀ | 285 | 22.84 |
| 15/12/2015 | 9/1/2019 | 1121 | ♀ | 294 | 29.91 2 |
| 15/12/2005 | 2/2/2015 | 3336 | ♀ | 299 | 28.17 |
| -- | 14/5/2016 | -- | ♂ | 327 | 32.63 |
| -- | 5/5/2016 | -- | ♀ | 330 | 35.65 |
| 15/12/2005 | 2/7/2014 | 3121 | ♀ | 300 | 28.16 |
| 15/12/2011 | 23/6/2015 | 1286 | ♀ | 301 | 37.05 |
| 15/12/2015 | 18/1/2020 | 1495 | ♀ | 302 | 32.90 |
| 15/12/2011 | 24/9/2020 | 3206 | ♀ | 310 | 38.25 |
| 15/12/2015 | 23/1/2020 | 1500 | ♀ | 312 | 36.55 |
| 15/12/2015 | 23/5/2020 | 1621 | ♀ | 315 | 41.77 |
| 15/12/2014 | 15/3/2016 | 456 | ♂ | 315 | 29.52 |
| 19/9/2013 | 19/1/2020 | 2313 | ♀ | 316 | 41.60 |
| -- | 2/2/2016 | -- | ♀ | 320 | 31.90 |
| 15/12/2011 | 12/8/2015 | 1336 | ♂ | 320 | 40.65 |
| 15/12/2012 | 73//2018 | 1908 | ♂ | 324 | 39.19 |
| 15/12/2012 | 21/8/2020 | 2806 | ♂ | 325 | 36.79 |
| 15/12/2011 | 20/2/2015 | 1163 | ♂ | 330 | 33.15 |
| 1/12/2011 | 28/12/2016 | 1854 | ♂ | 330 | 33.70 |
| 15/12/2014 | 18/1/2020 | 1860 | ♀ | 332 | 42.99 |
| 1/12/2011 | 25/4/2015 | 1241 | ♂ | 333 | 38.90 |
| 15/12/2011 | 19/1/2014 | 766 | -- | 338 | 27.68 |
| 15/12/2014 | 13/12/2020 | 2190 | ♂ | 344 | 41.45 |
| 15/12/2014 | 23/5/2020 | 1986 | ♀ | 345 | 51.27 |
| 15/12/2009 | 1/4/2015 | 1933 | ♂ | 362 | 44.00 |
| -- | 22/5/2011 | -- | -- | 266 | 17.00 |
| 15/12/2011 | 20/2/2015 | 1163 | ♂ | 391 | 51.54 |
| Hatching Day | Death Day | Age (Days) | Sex | Length (mm) | Weight (g) |
|---|---|---|---|---|---|
| 15/12/2011 | 22/3/2012 | 98 | -- | 109 | 2.80 |
| 13/12/2010 | 7/4/2011 | 115 | -- | 124 | 3.90 |
| 13/12/2010 | 2/5/2011 | 140 | -- | 160 | 3.43 |
| 15/12/2009 | 25/3/2011 | 465 | ♀ | 180 | 4.00 |
| 27/11/2017 | 31/3/2019 | 489 | ♀ | 217 | 15.44 |
| 27/11/2017 | 25/1/2019 | 424 | ♂ | 223 | 13.25 1 |
| 27/11/2017 | 14/2/2019 | 444 | ♂ | 225 | 13.59 2 |
| 15/12/2018 | 11/3/2020 | 452 | ♂ | 230 | 21.70 |
| 15/12/2018 | 2/4/2020 | 474 | ♀ | 234 | 24.32 |
| 15/12/2009 | 19/8/2011 | 612 | ♂ | 255 | 40.84 |
| 15/12/2011 | 19/6/2018 | 2378 | ♂ | 280 | 49.83 |
| 23/12/2012 | 19/6/2019 | 2369 | ♀ | 282 | 54.36 |
| 23/12/2012 | 25/9/2019 | 2467 | ♂ | 302 | 61.27 |
| 15/12/2011 | 14/5/2020 | 3073 | ♂ | 310 | 65.92 |
| 23/12/2012 | 23/3/2015 | 820 | ♀ | 324 | 55.38 |
| Item Number | Length Step (mm) | Phyllopteryx taeniolatus | Phycodurus eques | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | ||
| 1 | a–b | 49.6 | 50.6 | 55.6 | 35.6 | 37.5 | 15.5 | 40.1 | 33.5 |
| 2 | b–c | 9.4 | 10.3 | 11.9 | 8.0 | 7.3 | 3.9 | 10.4 | 9.5 |
| 3 | a–d | 9.4 | 10.3 | 11.2 | 7.7 | 13.0 | 9.4 | 12.7 | 11.1 |
| 4 | d–e | 9.4 | 11.9 | 14.4 | 7.5 | 12.0 | 5.7 | 12.7 | 13.7 |
| 5 | e–f | 14.2 | 14.7 | 18.1 | 12.0 | 15.8 | 4.8 | 17.6 | 15.5 |
| 6 | f–g | 23.5 | 31.9 | 31.8 | 21.0 | 22.6 | 14.0 | 25.4 | 26.0 |
| 7 | g–h | 47.0 | 51.8 | 56.6 | 33.7 | 28.6 | 6.8 | 30.0 | 29.3 |
| 8 | h–i | 32.3 | 39.2 | 50.8 | 25.8 | 31.3 | 10.3 | 33.6 | 31.1 |
| 9 | i–j | 35.4 | 39.9 | 41.5 | 29.7 | 46.0 | 23.5 | 51.9 | 46.2 |
| 10 | j–k | 16.0 | 19.5 | 20.3 | 15.4 | 30.2 | 9.2 | 28.6 | 23.6 |
| 11 | k–l | 30.6 | 30.8 | 37.4 | 27.5 | 30.7 | 12.3 | 29.8 | 25.7 |
| 12 | l–m | 25.7 | 30.0 | 34.7 | 26.4 | 20.2 | 9.4 | 19.7 | 23.3 |
| 13 | m–n | 31.3 | 21.1 | 22.8 | 17.5 | 27.9 | 6.0 | 14.4 | 18.0 |
| 14 | n–o | 11.0 | 19.9 | 26.3 | 15.3 | 7.7 | 7.4 | 18.1 | 18.3 |
| 15 | Sum | 345 | 377 | 433 | 282 | 331 | 132 | 345 | 325 |
| 16 | ScL | 320 | 360 | 392 | 260 | 260 | 115 | 275 | 265 |
| 17 | Ratio | 0.928 | 0.955 | 0.904 | 0.921 | 0.785 | 0.871 | 0.797 | 0.815 |
| Item (Definition; Units) | Phyllopteryx taeniolatus | Phycodurus eques |
|---|---|---|
| n (number of fishes used in LMR) | 31 | 10 |
| a (multiplicative term of LWR; cm and g) | 0.000139 | 0.0000135 |
| b (exponent of LWR) | 2.92 | 4.51 |
| r2 (coefficient of determination of linear LWR) | 0.930 | 0.912 |
| N (number of fishes used for the VBGF) | 40 | 15 |
| L∞ (asymptotic ‘scalene’ length, in cm) | 32.4 | 29.9 |
| W∞ (asymptotic weight, in g) | 36.3 | 60.4 |
| K (growth coefficient; year−1) | 1.09 | 0.93 |
| Item (Units) | Phyllopteryx taeniolatus1 | Phycodurus eques2 |
|---|---|---|
| Scalene length (cm) | 32.0 | 27.0 |
| Whole specimen weight (g) | 34.3 | 25.6 |
| Weight of appendages (g) | 0.04 | 1.44 |
| Weight of stalks (g) | 0.42 | 2.40 |
| Appendages and stalks in total weight (%) | 1.35 | 15.0 |
| Species | W∞ (g) | K (Year−1) | Ø | Source |
|---|---|---|---|---|
| Katsuwonus pelamis | 55,200 | 0.179 | 2.41 | Table 7 in Pauly (1981) [15] |
| Pollachius virens | 11,331 | 0.141 | 1.85 | |
| Labrus merula | 990 | 0.234 | 1.36 | |
| Blennius pholis | 102 | 0.746 | 1.21 | |
| Phycodurus eques | 60.4 | 0.930 | 1.16 | This study |
| 36.3 1 | 1.113 | 1.09 | Kuiter (1988) [7] and FishBase | |
| Phyllopteryx taeniolatus | 36.3 | 1.090 | 1.08 | This study |
| 29.9 2 | 2.20 | 1.32 | Forsgren and Lowe (2006) [11] | |
| 40.3 2 | 1.52 | 1.25 | Sanchez-Camara et al. (2011) [9] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauly, D.; Matsushige, L.; Malacane, J.; Hay, A.; Chu, E.; Warren, M. Length–Weight Relationships and Growth Parameters of Common and Leafy Seadragons (Syngnathidae) from a Public Aquarium. Fishes 2022, 7, 77. https://doi.org/10.3390/fishes7020077
Pauly D, Matsushige L, Malacane J, Hay A, Chu E, Warren M. Length–Weight Relationships and Growth Parameters of Common and Leafy Seadragons (Syngnathidae) from a Public Aquarium. Fishes. 2022; 7(2):77. https://doi.org/10.3390/fishes7020077
Chicago/Turabian StylePauly, Daniel, Leslee Matsushige, Janet Malacane, Amanda Hay, Elaine Chu, and Melanie Warren. 2022. "Length–Weight Relationships and Growth Parameters of Common and Leafy Seadragons (Syngnathidae) from a Public Aquarium" Fishes 7, no. 2: 77. https://doi.org/10.3390/fishes7020077
APA StylePauly, D., Matsushige, L., Malacane, J., Hay, A., Chu, E., & Warren, M. (2022). Length–Weight Relationships and Growth Parameters of Common and Leafy Seadragons (Syngnathidae) from a Public Aquarium. Fishes, 7(2), 77. https://doi.org/10.3390/fishes7020077








