Abstract
Chum salmon (Oncorhyncus keta) is a cold-water species reported to migrate within a wide range of habitats, including Korea, Japan, North America, and Russia, playing important roles in the river–sea nutrient cycle and food web. However, research on this species has not been widely performed in South Korea owing to its geographical location at the southern edge of migration. In this study, we analyzed the spatial distribution and morphological characteristics of chum salmon migrating to South Korea using the length–weight relationship. We also analyzed 3 years of catch, sex ratio, and individual information (total length (cm), weight (kg), n = 4400) from ten rivers (eight in the East coast and two on the South coast) with a total of 17 years of water quality and the distance they traveled (n = 50) using multivariate analysis. As a result, we discovered a trend of less migration in the southern part of South Korea for all individuals migrating to South Korea. Furthermore, the weight ratio of males/females was significantly different (p < 0.05). Based on the length–weight relationship analysis, the a and b values were between 0.0011 and 0.038 and 2.65 and 3.49, respectively. In the correlation analysis, catch is negatively correlated with distance traveled and temperature (p < 0.05), and positively correlated with pH, dissolved oxygen, distance, and female ratio (p < 0.05). This is possibly the result of differences in water quality during early life stages or the presence of an estuarine barrage at the mouth of the Nakdong River. This research may be used as fundamental distribution and morphological variations of chum salmon in South Korea.
1. Introduction
Salmonids comprise 11 genera, with 65–70 species worldwide [1]. The subfamily Salmonidae is composed of five to nine extant genera and approximately 30 species [2]. Among these, chum salmon (Oncorhynchus keta) is a cold-water fish distributed around the North Pacific Ocean including the Bering Sea, Alaska, and East Asia [3]. It is also understood that 95% of chum salmon biomass consists of oceanic organic matter, which enhances river–ocean nutrient circulation during decomposition following spawning [4]. Moreover, the behavior of returning from oceans to natal rivers to spawn is publicly well-known, rendering it aesthetically valuable [5]. For these reasons, the NPAFC (North Pacific Anadromous Fish Commission) has recognized the benefits of salmon for the ecosystem, and a significant amount of research on the lifecycles and resource management of Salmonidae has been conducted [6,7,8,9].
Chum salmon stock enhancement programs have been established by the North Pacific nations (Canada, Japan, Russia, South Korea, and so on) for representative migration-associated rivers and streams. These programs involve catching adult individuals for artificial fertilization and fry release [10,11], in order to obtain sustainable growth and breeding stock. By obtaining a relatively large number of chum salmon stock, studies on chum salmon in terms of the effect of spatial differences on morphological [12], genetic [13], and distribution differences [14] are globally documented. Species morphology and sex ratio, for instance, are an effective measure for identifying patterns and predictions for communities [15,16]; however, few studies of the spatial differences and morphology of chum salmon in South Korea have been conducted, owing to the limited migration of this species to South Korea, which is at the southern boundary of the distribution range in East Asia [17,18]. Additionally, research on the distribution and migration routes of chum salmon in South Korea has not been systematically performed; we hypothesize that those in Korea follow patterns similar to those of Japan and/or Russia [19]. Moreover, the return rate to South Korea has dropped significantly to nearly 1% owing to habitat fragmentation from dams and weir construction [20], high mortality at the juvenile stage [21], and a rise in ocean and river temperatures [22]. In addition, water quality deterioration is another important factor that reduces larval development and growth rates [23].
Therefore, the aims of the current research were to (1) identify distribution patterns of chum salmon migrating to South Korea; (2) localize regional morphological differences (body shape); (3) compare the sex ratio between chum salmon populations; and (4) quantify the relationship between water quality and the catch. In other words, we investigated the morphological differences between specimens from different regions in South Korea. This allows the determination of chum salmon distribution and morphological features affected by spatial differences in South Korea.
2. Materials and Methods
2.1. Study Area
This study was conducted in the south, east, and southeastern Korean peninsula region (Figure 1). In the ten rivers in the study, the eight uppermost rivers flow west to east, the second-lowest study station (St. 9, The Milyang River) flows to the southeast sea, and the lowermost study station (St. 10, the Seomjin River) flows to the southern sea of South Korea. These rivers are in temperate climate regions [24] with similar annual precipitation patterns, with over 60% of precipitation from June to September [25] due to the East Asian monsoon, and are driest in winter (November–January); consequently, hydrologic drought occurs from winter to early spring. Additionally, South Korea is an example of a tilted landform, where a chain of mountains is distributed along the eastern periphery of South Korea [26], making west-to-east rivers relatively short, small, and steep riverine systems. In addition, steep riverine systems on the eastern slope of South Korea often exhibit stream depletion, except in the wet season, while southward flowing rivers show a relatively low rate of stream depletion.
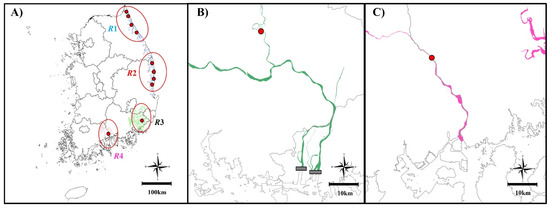
Figure 1.
Distribution of study sites across South Korea. (A) In R1 area, St. 1, St. 2, St. 3, and St. 4 exist from the uppermost part towards the lower regions. R2 comprises St. 5 to St. 8 (St. 5 at the top), R3 comprises St. 9, and R4 comprises St. 10 (St. 1—the Myeong-pa stream, St. 2—the Buk stream, St. 3—the Namdae stream, St. 4—the Yeongok stream St. 5—the Song stream, St. 6—the Wang-pi stream, St. 7—the Pyeonghae-Namdae stream St. 8—the Osip stream, St. 9—the Milyang River, and St. 10—the Seomjin River). (B) Expansion of the R3 region; Grey-rectangles are estuarine barrages existing in the mouth of the Nakdong River. (C) Expansion of the R4 region.
Currently, multi-purpose dams of different sizes exist in most South Korean rivers to prevent floods and droughts and to encourage eco-friendly public recreation [27]. In the study area, no artificial obstacles existed except for one on the Milyang River (St. 9). Furthermore, there is an estuarine barrage in the mouth of the Nakdong River, which was built in 1987 to satisfy human needs, such as salt intrusion prevention and a sustainable freshwater supply [28], and causing impoundment between the river and the sea [29]. The construction of the estuarine barrage has dramatic effects on fish communities [30], especially for migratory fishes [31]. Salmonid migration and behavior are closely related to environmental factors such as water velocity, topography, and water temperature [8,32,33], which possess the possibility of being affected by the estuarine barrage.
2.2. Data Collection
All chum salmon data were collected from national salmon hatchery institutions. Data for chum salmon migrating to the St. 1–St. 4 rivers were collected by the Aquatic Living Resources Center of the East Sea (Uljin, South Korea), affiliated with the Korea Fisheries Resources Agency (FIRA); data for those migrating to the St. 5–St. 8 rivers were collected by the Gyeongsangbuk-do Research Center for Freshwater Fishes, also located in Uljin. Data collected in St. 9 were from the Gyeongsangnam-do Research Center for Freshwater Fish, located in Milyang. In addition, the data collected in St. 10 were from the Seomjingang River Fish Museum (Jeonranam-do), affiliated with the Institute of Ocean and Fisheries Science. The methodology of catching adult chum salmon is to install a series of fixed shore nets (5 × 5 cm mesh size; length, 80 m; width, 1.5 m) on rivers to which the chum salmon migrate to obstruct their migration. The nets guide them to artificial fishways heading to the center, where their sex was identified and eggs were collected. Each center accumulates the annual catch from each river and records information for each adult individual, such as total length (cm, ±0.1 cm), weight (kg, ±0.01 kg), and male/female rate. Individual sex was identified by comparing the mouth parts; males have a longer and sharper upper jaw and teeth, and females have a shorter, smoother upper jaw and teeth.
To obtain water quality data, we used the water environment information system [34]. We collected monthly data of pH, dissolved oxygen (DO, mg/L), biological oxygen demand (BOD, mg/L), total nitrogen (TN, mg/L), total phosphorus (TP, mg/L), water temperature (°C), and conductivity (µS/cm). We utilized water quality data from 2014 to 2019 for R1 (n = 9), and 2004 to 2020 for R2 (n = 24) and R4 (n = 17). Water quality in R3 was excluded owing to insignificant and insufficient data.
2.3. Data Analysis
We hypothesized that chum salmon in South Korea will display significantly different mean values for total length (cm) and weight (kg) based on sex. To analyze the spatial morphology differences in accordance with regions and sexes, we used an independent t-test on mean total length (cm) and weight (kg) on different sexes using SPSS25. The dataset used in this analysis consists of St. 1, St. 2, and St. 3 in 2018; St. 5 in 2018–2020; and St. 9 and St. 10 in the year 2020.
We also performed calculations of length–weight relationships (LWRs) introduced by Froese [35] on chum salmon in South Korea. The formula for the LWR is as follows:
W = aLb,
(W: weight in grams; L: length in cm; a and b: parameters).
We used the natural logarithm (ln) on both sides and constructed a linear regression model using SigmaPlot 10.0. The transformed LWR formula is shown below:
lnW = ln a + b lnL,
(W: weight in grams; L: length in cm; a and b: parameters).
We then eliminated outliers until the coefficient of determination (r2) reached 0.95. Additionally, to analyze differences in sex ratio in accordance with the regions, a one-way analysis of variance (one-way ANOVA) was carried out using SPSS 25. We subdivided ten study stations into four regional groups: R1, R2, R3, and R4 (R1 = St. 1–4, R2 = St. 5–8, R3 = St. 9, and R4 = St. 10), and then utilized a one-way ANOVA for the data from the four regions. Statistical significance was set at p < 0.05. We excluded years for which accurate sex ratio data were not available and utilized sex ratio data only from five years (2014–2016, 2018, and 2019) for R1 and 17 years (2004–2020) for R2 and R4. R3 region is excluded owing to insignificant data (only the year 2020 is available).
To determine the correlation between chum salmon distribution and sex ratio and water quality, we performed a principal component analysis (PCA) using R version 4.0.5. We specifically collected monthly water quality data from September to November to match migration times to freshwater, and then averaged them to yield the annual data. Apart from the correlation between distribution, sex ratio, and water quality, we also used regional distances measured from the uppermost stream (St. 1) as another environmental parameter.
3. Results
3.1. Morphological Characteristic Differences
Among over 120,000 chum salmons that migrated to South Korea within seventeen years, we were able to calculate the mean total length and body weights of chum salmon in South Korea using individual 4400 chum salmons whose body information was recorded. The mean total length was 68.49 cm (±5.4, standard deviation) and 2.66 kg (±0.71). A total of 2070 males and 2330 females from six rivers (St. 1, St. 2, St. 3, St. 6, St. 9, and St. 10) were used to identify differences in total length and body mass. Females that migrated to South Korea had a mean total length of 68.42 cm (±4.7), while males were 68.55 cm (±5.9) on average, showing no significant differences (p > 0.05, Table 1). On the other hand, the weight for females was calculated to be 2.69 kg (±0.67) and 2.65 kg (±0.75) for males, which was significantly different related to the sex (p < 0.05, Table 1).

Table 1.
Mean length and mean weight of chum salmon (Oncorhynchus keta) surveyed in South Korea (* t-test, p < 0.05).
The LWRs of chum salmon during all study periods were calculated. For individuals migrating to South Korea, the a value was 0.0047 (±0.0009) and the b value was 3.129 (±0.023) (n = 2074). The a and b values differed based on the sex: 0.0038 (±0.00056) and 3.18 (±0.038), respectively, for females; 0.0052 (±0.0007) and 3.1 (±0.035), respectively, for males. Annual a and b values were calculated for the year 2018 from St. 1 (n = 18), St. 2 (n = 11), and St. 3 (n = 432). We also calculated the LWRs from St. 6 (n = 1384) in 2018, 2019, and 2020, as well as St. 9 (n = 49) and St. 10 (n = 386) in 2020. Specifically, specimens sampled at St. 1, St. 2, and St. 3 showed a values of 0.0011 (±0.004), 0.004 (±0.001), and 0.0013 (±0.0002), respectively. St. 6 showed an average a value of 0.009 ±0.001. In addition, St. 9 and St. 10 showed a values of 0.038 (±0.017) and 0.005 (±0.001), respectively (Figure 2). Chum salmon from St. 1, St. 2, and St. 3 showed b values of 3.45 (±0.05), 3.17 (±0.08), and 3.49 (±0.52), respectively. In addition, chum salmon from St. 6, St. 9, and St. 10 showed average b values of 2.97 (±0.03), 2.65 (±0.14), and 3.1 (±0.07), respectively (Figure 3).
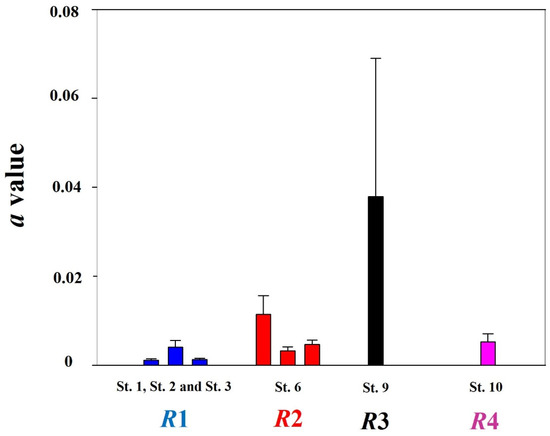
Figure 2.
Regional a values for chum salmon (Oncorhynchus keta) individuals that migrated to South Korea from 2018 to 2020. The a value showed the greatest value on the R3 region, while the R1 region showed the smallest value. For the a values, we calculated them using datasets in the year 2018 for R1 regions. Datasets from 2018 to 2020 were used to calculate the a values in R2, while datasets in 2020 were used for R3 and R4 regions (n = 422 for St. 1; 11 for St. 2; 18 for St. 3; 258 for St. 6 in 2018, 566 in 2019, and 883 in 2020. For St. 9 and St. 10, the ‘n’ is 43 and 291, respectively).
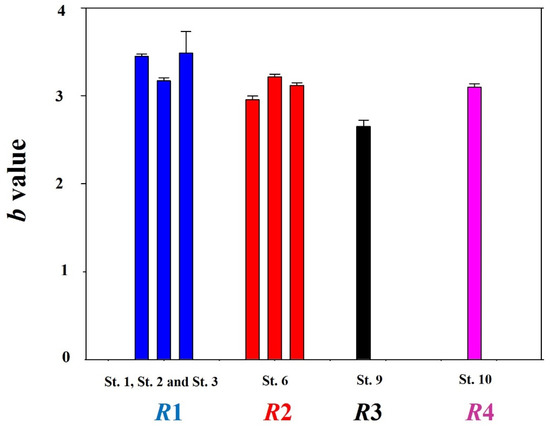
Figure 3.
Regional b values for chum salmon (Oncorhynchus keta) individuals that migrated to South Korea from 2018 to 2020. Unlike Figure 2, the R1 region showed the greatest b value among four regions, while R3 showed the smallest value (n = 422 for St. 1; 11 for St. 2; 18 for St. 3; 258 for St. 6 in 2018, 566 in 2019, and 883 in 2020. For St. 9 and St. 10, the ‘n’ is 43 and 291, respectively).
3.2. Regional Sex Ratio Differences
The sex ratio data from St. 1, St. 3, and St. 4 obtained over a period of five years were used. In addition, data obtained from St. 2 for four years were used. We grouped these four streams into the R1 region, as they were relatively close to each other. Data regarding the sex of chum salmon from St. 6 and St. 10 obtained over a 17-year period were used to calculate the sex ratio and were termed R2 and R4, respectively. For the R1 region, the proportion of females was 44.66% (±7.619), while the proportions of females in the other two regions (R2 and R4) were 41.95% (±4.53) and 39.79% (±7.585), respectively (Table A1, Table A2, Table A3 and Table A4).
We used a one-way ANOVA to examine the significance of the sex ratio in the three regions. St. 9 was not included because of insufficient data (n = 1). None of the regions showed significant differences in sex ratio (p > 0.05).
3.3. Correlation of Distribution with Water Quality
We first analyzed the regional water quality values and how they differ between the areas. The seven water quality parameters (temperature, conductivity, TP, TN, BOD, pH, and DO) are presented in Table A5. All parameters, except for TN, were significantly different in accordance with regional differences (Table A6). Specifically, for R1, pH and DO are significantly higher than other regions, while other values are mostly lower than other regions. To evaluate the correlations of chum salmon migration numbers with each parameter, all seven parameters were selected for PCA (Figure 4). In addition, the regional catch, female ratio, and regional distance were selected as inherent values of each individual. In the ordination, 39.9% of the variation was related to axis 1 (PC1), while axis 2 (PC2) explained 17.4% (total 57.3% of variance) in the biplot. Each vector indicates the direction and strength of each environmental variable relative to the overall interrelations. In Figure 4, distance, TP, and temperature have a relatively strong positive correlation to PC1, while BOD and TN show a relatively strong negative correlation to PC2. For vectors presented in the PCA, the catch was positively correlated with pH (p < 0.01), female ratio (p < 0.05), and DO (p < 0.05), while it was negatively correlated with temperature (p < 0.05) and distance (p < 0.01, Table 2). Each colored number (1–44) in the biplot represents different physiochemical properties, including water quality and female ratio. Physiochemical properties in R1 (1–9) seem to be assembled in the bottom-left side of the biplot, where relatively high positive correlations with catch, pH, DO, and female ratio and negative correlations with temperature, distance, conductivity, and TP can be observed. Physiochemical properties from R2 (10–26) are more or less gathered in the top-middle side of the biplot, showing relatively strong negative correlations with BOD and TN. However, the explanatory power of the R2 region using PC1 is relatively low. Though there is only one data from R3, physiochemical properties from R3 and R4 are relatively located together, showing positive correlations with temperature, distance, conductivity, and TP.
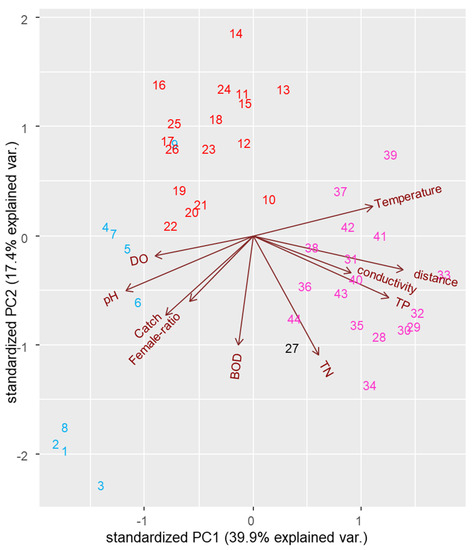
Figure 4.
Principal component analysis (PCA) of ten variables (catch, female ratio, dissolved oxygen (DO), pH, biological oxygen demand (BOD), total nitrogen (TN), total phosphorus (TP), conductivity, distance, and temperature). The parameterized stations (1–9: R1, 10–26: R2, 27: R3, 28–44: R4) were gathered in accordance with three regions (R1, R2, R4).

Table 2.
The Pearson correlation coefficient and significance (p) between the variables. Significance at * p < 0.05 and ** p < 0.01.
4. Discussion
The distribution of chum salmon in South Korea is principally concentrated on R1 (Table A1, Table A2, Table A3 and Table A4), and this seems to be due to the large number of offspring released in the R1 region [10]. Ruggerone and Irvine [36] calculated that approximately 70% of chum salmon individuals returning to Asia are from artificial hatchery production; therefore, the number of offspring produced by artificial fertilization could be the most crucial factor in the total catch. Furthermore, relatively high genetic diversity due to a large number of males/females could prevent the Allee effect in the R1 region.
Regional differences in the sex ratio of chum salmon individuals were not significant. It is likely that the insignificance is due to the relatively small study area in each region (R1–R4). Although there was no significant regional difference in the female ratio, we were able to see a positive correlation with the catch. Brykov et al. [37] documented that the female ratio in the Kamchatka River showed a negative correlation with spawner density, which differs from our results. For sex determination of sockeye salmon (O. nerka), the temperature can alter their sex in the early life history stages [38]. Thus, the same strategy could be applied to chum salmon, but no related data are available. Moreover, we suppose that other salmon variables such as migration age and early life stage diet could be potential factors in sex determination. So, there needs to be additional data collection at national salmon hatchery institutions for further research. No significant difference was found in the total length, yet we found a significant difference in their weights (p < 0.05). A significant difference in weight may be attributed to the presence of eggs in the female.
In the PCA, catch is positively correlated with DO and pH, while it is negatively correlated with temperature and distance (Figure 4, Table 2). In the early stages of the life history of salmon, a high DO [39,40] and a low temperature [41,42] is particularly important for water quality and have been well investigated in terms of early mortality based on past research. In addition, low pH results in the disappearance of fish populations as a result of water quality reducing site use or choice [43,44], as well as for chum salmon [45]. Therefore, R2, R3, and R4 regions may not be able to hold large numbers of the species owing to relatively polluted water quality parameters, such as low DO and a relatively small number of fry released. We also observed high conductivity in R4, and it is thought to be due to the water quality measuring station located in a brackish-zone [46], despite its rather inland location. Changes in water quality (often caused by global warming), such as DO or temperature, can possibly cause a more severe decrease in abundance in Korea than in other countries, as Korea lay on the southern edge of chum salmon’s migration. Specifically, for water temperature, we observed that the mean temperature in all regions was about 15–18 °C (Table A5). Chum salmons have an acute preference for temperatures of 7 °C to 11 °C [3], which is considerably lower than referred regions. As global warming proceeded [47], Japan has already experienced a decrease in the carrying capacity and loss of migration routes of chum salmons [48,49], which gives us the possibility that carrying capacity in South Korea is also, or more severely, decreased.
To the best of our knowledge, this study represents the assessment of spatial morphological characteristics of chum salmon in South Korea, including a study carried out by Myeong and Kim [50], in which LWR data were excluded. In addition, research on the morphology of chum salmon has focused on juvenile [51], fish scales [52], and otoliths [53,54] globally. Froese et al. [55] used a Bayesian approach for estimating LWRs in fish and subdivided the fish body shape into four types: eel-like, elongated, fusiform, and short and deep. Analysis revealed that fish with an elongated body shape exhibit an a value of 0.0018–0.00842 at the 95% confidence interval and an a value of 0.00514–0.0245 for fusiform fish. As individuals migrating to South Korea exhibited a values in the range of 0.0011–0.038, it is expected that those in South Korea have a more or less ‘blended’ body shape with elongated and fusiform structures. Fish are the most susceptible vertebrates to environmentally induced morphological variations, demonstrating greater variance within and between populations [56,57]. Thus, environmental variables such as prey composition in early life stages or genetic variation may alter the a value of chum salmon in South Korea. In addition, it is a global phenomenon that its weight is in a gradual decline [36], so it may affect the a value. Likewise, research on the b value of chum salmon has not been well documented. From the information presented in FishBase [58], it is shown that the b value of chum salmon varies from 2.93 to 3.25, which establishes the b values from St. 9 (2.65), St. 1 (3.45), and St. 3 (3.49) as outliers. The low b values for St. 9 could be attributed to the presence of the Nakdong estuary barrage. Many studies have observed that, in the presence of an estuary barrage and water impoundment, salmon migration is often delayed and obstructed [59], due to increased residence time [60], loss of direction [61], and susceptibility to disease [62]. Moreover, data were collected rather upstream (Figure 1) for St. 9 and St. 10. They cease foraging during their migration and have to rely on stored energy [63], so upstream migration in a river may have decreased the b value. Alternatively, the relatively high values for St. 1 and St. 3 (3.45 and 3.49, respectively) could be attributed to relatively short migration distances and higher water quality in early life history stages. In addition, the b value for St. 3 may be inaccurate owing to the lack of available data (n = 18). So far, not enough available data for Korean chum salmon’s length–weight relationship are presented, which implies demands for further research to compare the regional morphologies of South Korea.
Thus far, we have performed an analysis of data on the distribution and morphological characteristics of chum salmon in South Korea. They possess a large geographic distribution [64], while their body weight exhibits more or less fluctuating patterns [65], indicating that the long-term monitoring of migration to South Korea is vital. However, to fully discover migration patterns and morphological characteristics, further data regarding chum salmon migration to South Korea are required. In addition, along with the size, spawn age is also an important life-history trait [66]; thus, measuring the age of individuals of this species during migration would also be a focus for further research. Moreover, more detailed consideration of the geographical traits of South Korea and global warming is necessary, as released offspring often suffer high mortality due to increased sea temperatures [67], which would be fatal for offspring released to southern rivers (R3 and R4). In this point of view, though marine water quality parameters (SST, sea surface temperature and SSS, sea surface salinity, among others) play an important role in the catch [68], we only utilized inland water quality data, which indeed leaves a limitation to fully discover the correlation of water quality with the catch. The return rate of chum salmon in South Korea is thought to be around 1% [10], which is relatively low compared with that in Japan (~5%, [11]). To understand the mechanisms underlying the migration of its offspring, fish preserves for immature individuals, as well as the maintenance of good water quality, should be established first.
5. Conclusions
Chum salmon (O. keta) are found circumpolar in their distribution; however, the South Korean peninsula marks the southern edge of its migration range. Results show that the sex ratios showed little variation, while morphological characteristics did show significant variation relative to the location within the sampling area. Also, there was a strong relationship between water quality and developmental growth (the a value). As well, DO and pH showed a positive relationship to catch while temperature and distance had a negative relationship. The more polluted the water the lower the diversity and alterations to the temperature will also impact the species morphology and sex ratios. Results also show that the long-term monitoring of the Chum Salmon population in South Korea is important to determine how further changes in water quality and climate will affect the population within South Korea. These changes will require further study to determine the effects on the Chum Salmon population.
Author Contributions
Conceptualization, D.H. and H.J.; methodology, D.H., E.J. and H.J.; data curation D.-H.K., K.B.S. and H.-W.K.; validation, M.J.M.L. and H.-W.K.; resources, D.-H.K.; writing—original draft preparation, D.H., E.J. and J.-S.G.; writing—review and editing, D.H., G.-J.J. and K.B.S.; supervision, J.-S.G., K.B.S., D.-H.K., G.-J.J., M.J.M.L., H.-W.K. and H.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation of Korea, grant number NRF-2020R1C1C1009066.
Data Availability Statement
The datasets used during and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
The catch of chum salmon (Oncorhynchus keta) individuals and the number of females (female ratio) in the R1 region.
Table A1.
The catch of chum salmon (Oncorhynchus keta) individuals and the number of females (female ratio) in the R1 region.
| Region | St. Number | Year | Catch | The Number of Female | Female Ratio |
|---|---|---|---|---|---|
| R1 | St. 1 | 2014 | 2214 | 919 | 0.4151 |
| R1 | St. 1 | 2015 | 532 | 245 | 0.4605 |
| R1 | St. 1 | 2016 | 1564 | 831 | 0.5313 |
| R1 | St. 1 | 2018 | 1627 | 772 | 0.4745 |
| R1 | St. 1 | 2019 | 166 | 56 | 0.3373 |
| R1 | St. 2 | 2014 | 1638 | 718 | 0.4383 |
| R1 | St. 2 | 2016 | 1370 | 634 | 0.4628 |
| R1 | St. 2 | 2018 | 3430 | 1382 | 0.4029 |
| R1 | St. 2 | 2019 | 1057 | 364 | 0.3444 |
| R1 | St. 3 | 2014 | 31,833 | 15,261 | 0.4794 |
| R1 | St. 3 | 2015 | 18,151 | 9108 | 0.5018 |
| R1 | St. 3 | 2016 | 11,262 | 6342 | 0.5631 |
| R1 | St. 3 | 2018 | 6635 | 3010 | 0.4537 |
| R1 | St. 3 | 2019 | 2790 | 1208 | 0.433 |
| R1 | St. 4 | 2014 | 4011 | 2139 | 0.5333 |
| R1 | St. 4 | 2015 | 4249 | 2062 | 0.4853 |
| R1 | St. 4 | 2016 | 3462 | 1674 | 0.4835 |
| R1 | St. 4 | 2018 | 1971 | 876 | 0.4444 |
| R1 | St. 4 | 2019 | 457 | 110 | 0.2407 |

Table A2.
The catch of chum salmon (Oncorhynchus keta) individuals and the number of females (Female ratio) in the R2 region.
Table A2.
The catch of chum salmon (Oncorhynchus keta) individuals and the number of females (Female ratio) in the R2 region.
| Region | St. Number | Year | Catch | The Number of Female | Female Ratio |
|---|---|---|---|---|---|
| R2 | St. 6 | 2004 | 454 | 191 | 0.4229 |
| R2 | St. 6 | 2005 | 205 | 77 | 0.3756 |
| R2 | St. 6 | 2006 | 1221 | 534 | 0.4373 |
| R2 | St. 6 | 2007 | 1615 | 691 | 0.4279 |
| R2 | St. 6 | 2008 | 375 | 135 | 0.36 |
| R2 | St. 6 | 2009 | 706 | 266 | 0.3768 |
| R2 | St. 6 | 2010 | 1145 | 504 | 0.4402 |
| R2 | St. 6 | 2011 | 727 | 365 | 0.5021 |
| R2 | St. 6 | 2012 | 1286 | 541 | 0.4207 |
| R2 | St. 6 | 2013 | 1286 | 561 | 0.4362 |
| R2 | St. 6 | 2014 | 2091 | 881 | 0.4213 |
| R2 | St. 6 | 2015 | 1339 | 522 | 0.3898 |
| R2 | St. 6 | 2016 | 1077 | 488 | 0.4531 |
| R2 | St. 6 | 2017 | 1136 | 451 | 0.397 |
| R2 | St. 6 | 2018 | 442 | 145 | 0.3281 |
| R2 | St. 6 | 2019 | 881 | 391 | 0.4438 |
| R2 | St. 6 | 2020 | 1651 | 822 | 0.4979 |

Table A3.
The catch of chum salmon (Oncorhynchus keta) individuals and the number of females (Female ratio) in the R3 region.
Table A3.
The catch of chum salmon (Oncorhynchus keta) individuals and the number of females (Female ratio) in the R3 region.
| Region | St. Number | Year | Catch | The Number of Sex-Identified Individuals | Female Ratio |
|---|---|---|---|---|---|
| R3 | St. 9 | 2020 | 197 | 61/95 | 0.391 |

Table A4.
The catch of chum salmon (Oncorhynchus keta) individuals and the number of females (Female ratio) in the R4 region.
Table A4.
The catch of chum salmon (Oncorhynchus keta) individuals and the number of females (Female ratio) in the R4 region.
| Region | St. Number | Year | Catch | The Number of Female | Female Ratio |
|---|---|---|---|---|---|
| R4 | St. 10 | 2004 | 163 | 75 | 0.4601 |
| R4 | St. 10 | 2005 | 243 | 102 | 0.4198 |
| R4 | St. 10 | 2006 | 387 | 165 | 0.4264 |
| R4 | St. 10 | 2007 | 419 | 179 | 0.4272 |
| R4 | St. 10 | 2008 | 68 | 29 | 0.4265 |
| R4 | St. 10 | 2009 | 96 | 37 | 0.3854 |
| R4 | St. 10 | 2010 | 54 | 27 | 0.5 |
| R4 | St. 10 | 2011 | 59 | 30 | 0.5085 |
| R4 | St. 10 | 2012 | 79 | 36 | 0.4557 |
| R4 | St. 10 | 2013 | 162 | 57 | 0.3519 |
| R4 | St. 10 | 2014 | 188 | 61 | 0.3245 |
| R4 | St. 10 | 2015 | 208 | 48 | 0.2308 |
| R4 | St. 10 | 2016 | 124 | 36 | 0.2903 |
| R4 | St. 10 | 2017 | 265 | 88 | 0.3321 |
| R4 | St. 10 | 2018 | 704 | 239 | 0.3395 |
| R4 | St. 10 | 2019 | 692 | 316 | 0.4566 |
| R4 | St. 10 | 2020 | 569 | 244 | 0.4288 |

Table A5.
Water qualities measured in each region.
Table A5.
Water qualities measured in each region.
| Parameters | Regions | Average | Standard Deviation | Standard Error | n |
|---|---|---|---|---|---|
| pH | R1 | 8.95 | 0.46 | 0.15 | 9 |
| R2 | 7.83 | 0.36 | 0.07 | 24 | |
| R4 | 7.57 | 0.19 | 0.05 | 17 | |
| DO (mg/L) | R1 | 10.79 | 1.16 | 0.39 | 9 |
| R2 | 9.62 | 1.12 | 0.23 | 24 | |
| R4 | 9.28 | 1.19 | 0.29 | 17 | |
| BOD (mg/L) | R1 | 0.93 | 0.29 | 0.1 | 9 |
| R2 | 0.68 | 0.22 | 0.04 | 24 | |
| R4 | 0.75 | 0.19 | 0.05 | 17 | |
| TN (mg/L) | R1 | 1.25 | 0.36 | 0.12 | 9 |
| R2 | 1.3 | 0.96 | 0.196 | 24 | |
| R4 | 1.56 | 0.29 | 0.071 | 17 | |
| TP (mg/L) | R1 | 0.014 | 0.003 | 0.001 | 9 |
| R2 | 0.017 | 0.009 | 0.002 | 24 | |
| R4 | 0.043 | 0.012 | 0.003 | 17 | |
| Temperature (°C) | R1 | 15.64 | 1.78 | 0.595 | 9 |
| R2 | 17.77 | 1.87 | 0.38 | 24 | |
| R4 | 18.76 | 1.14 | 0.28 | 17 | |
| Conductivity (µS/cm) | R1 | 227.63 | 119.67 | 39.89 | 9 |
| R2 | 154.61 | 54.05 | 11.03 | 24 | |
| R4 | 4534.29 | 5326.83 | 1291.95 | 17 |

Table A6.
One-way ANOVA results in accordance with different regions.
Table A6.
One-way ANOVA results in accordance with different regions.
| Parameters | Source of Variance | Sum of Squares | df | Mean Square | F | Significance |
|---|---|---|---|---|---|---|
| pH | Between groups | 11.72 | 2 | 5.86 | 53.31 | <0.01 |
| Within group | 5.17 | 47 | 0.11 | |||
| Total | 16.89 | 49 | ||||
| DO (mg/L) | Between groups | 13.72 | 2 | 6.86 | 5.18 | <0.01 |
| Within group | 62.22 | 47 | 1.32 | |||
| Total | 75.95 | 49 | ||||
| BOD (mg/L) | Between groups | 0.40 | 2 | 0.20 | 3.98 | <0.05 |
| Within group | 2.37 | 47 | 0.05 | |||
| Total | 2.77 | 49 | ||||
| TN (mg/L) | Between groups | 0.85 | 2 | 0.42 | 0.85 | 0.44 |
| Within group | 23.55 | 47 | 0.50 | |||
| Total | 24.39 | 49 | ||||
| TP (mg/L) | Between groups | 0.008 | 2 | 0.004 | 47.13 | <0.01 |
| Within group | 0.004 | 47 | <0.001 | |||
| Total | 0.012 | 49 | ||||
| Temperature (°C) | Between groups | 56.14 | 2 | 28.07 | 10.44 | <0.01 |
| Within group | 126.40 | 47 | 2.69 | |||
| Total | 182.54 | 49 | ||||
| Conductivity (µS/cm) | Between groups | 2.13 × 109 | 2 | 1.07 × 109 | 11.04 | <0.01 |
| Within group | 4.54 × 109 | 47 | 9.66 × 107 | |||
| Total | 6.67 × 108 | 49 |
References
- Jobling, M.; Arnesen, A.M.; Benfey, T.; Carter, C.; Hardy, R.; Le Francois, N.R.; O’Keefe, R.; Koskela, J.; Lamarre, S.G. The salmonids (family: Salmonidae). In Finfish Aquaculture Diversification, 1st ed.; Le Francois, N., Jobling, M., Eds.; MPG Books Group: London, UK, 2010; p. 234. [Google Scholar]
- Norden, C.R. Comparative osteology of representative salmonid fishes, with particular reference to the grayling (Thymallus arcticus) and its phylogeny. J. Fish. Res. Board Can. 1961, 18, 679–791. [Google Scholar] [CrossRef]
- Groot, C.; Margolis, L. Pacific Salmon Life Histories, 1st ed.; UBCPress: Vancouver, BC, Canada, 1991; pp. 234–237. [Google Scholar]
- Bilby, R.; Beach, E.W.; Fransen, B.R.; Walter, J.K.; Bisson, P.A. Transfer of Nutrients from Spawning Salmon to Riparian Vegetation in Western Washington. Trans. Am. Fish. Soc. 2003, 132, 733–745. [Google Scholar] [CrossRef]
- Kumala, S.; Haapasaari, P.; Karjalaien, T.P.; Kuikka, S.; Pakarinen, T.; Romakkaniemi, A.; Vuorinen, P.J. Ecosystem services provided by Baltic salmon- a regional perspective to the socio-economic benefits associated with a keystone migratory species. In Socio-Economic Importance of Ecosystem Services in the Nordic Countries-Scoping Assessment in the Context of The Economics of Ecosystems and Biodiversity (TEEB); Kettunen, M., Koljonen, M.-L., Kallio-Nyberg, I., Förster, J., Eds.; Nordic Council of Ministers: Copenhagen, Denmark, 2012; pp. 266–276. [Google Scholar]
- Barton, L.H. Tanana River, Alaska, Fall Chum Salmon Radio Telemetry Study. In Fishery Research Bulletin; Gunstrom, G.K., Fried, S.M., Buklis, L.S., Schmidt, D.C., Eds.; Alaska Department of Fish and Game Devision of Commercial Fisheries: Juneau, Alaska, 1992; Volume 92-01, pp. 1–22. [Google Scholar]
- Seeb, L.W.; Crane, P.A.; Kondzela, C.M.; Wilmot, R.L.; Urawa, S.; Varnavskaya, N.V.; Seeb, J.E. Migration of pacific rim chum salmon on the high seas: Insight from genetic data. Environ. Biol. Fishes 2004, 69, 21–36. [Google Scholar] [CrossRef]
- Esteve, M. Observations of spawning behavior in Salmoninae: Salmo, Oncorhynchus and Salvelinus. Rev. Fish Biol. 2005, 15, 1–21. [Google Scholar] [CrossRef]
- Urawa, S.; Sato, S.; Crane, P.A.; Agler, B.; Josephson, R.; Azumaya, T. Stock-specific Ocean Distribution and Migration of Chum Salmon in the Bering Sea and North Pacific Ocean. NPAFC 2009, 5, 131–146. [Google Scholar]
- Lee, H.S.; Seong, K.B.; Lee, C.H. History and status of the chum salmon enhancement program in Korea. The Sea 2007, 12, 73–80. [Google Scholar]
- Miyakoshi, Y.; Nagato, M.; Kitada, S.; Kaeriyama, M. Historical and Current Hatchery Programs and Management of Chum Salmon in Hokkaido, Northern Japan. Rev. Fish. Sci. 2013, 21, 469–479. [Google Scholar] [CrossRef]
- Beacham, T.D.; Murray, C.B. Comparative developmental biology of chum salmon (Oncorhynchus keta) from the Fraser River, British Columbia. Can. J. Fish. Aquat. Sci 1986, 43, 252–262. [Google Scholar] [CrossRef]
- Kijima, A.; Fujio, Y. Relationship between average heterozygosity and river population size in chum salmon (Oncorhynchus keta). Bull. Japan. Soc. Sci. Fish 1984, 50, 603–608. [Google Scholar] [CrossRef]
- Okazaki, T. Distribution, migration and possible origins of genetically different populations of chum salmon Oncorhynchus keta along the eastern coast of northern Japan. Fish. Sci. 1986, 52, 983–994. [Google Scholar] [CrossRef][Green Version]
- Pease, A.A.; González, A.A.; Rodiles-Hernández, R.; Winemiller, K.O. Functional diversity and trait-environment relationships of stream fish assemblages in a large tropical catchment. Freshw. Biol. 2012, 57, 1060–1075. [Google Scholar] [CrossRef]
- Fryxell, D.C.; Arnett, H.A.; Apgar, T.M.; Kinnison, M.T.; Palkovacs, E.P. Sex ratio variation shapes the ecological effects of a globally introduced freshwater fish. Proc. Royal Soc. B 2015, 282, 20151970. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.E.; Kim, G.G.; Lee, Y.H. Genetic similarity-dissimilarity among Korea Chum salmons of each stream and their relationship with Japan salmons. The Sea 2007, 12, 94–101. [Google Scholar]
- Kwon, O.N.; Kim, J.K.; Yoon, M.G.; Kim, D.H.; Hong, K.E. Marine prey selectivity of released juvenile chum salmon (Oncorhynchus keta) during early marine migration in Korean waters. J. Kor. Soc. Fish. Mar. Edu. 2014, 26, 421–429. [Google Scholar]
- Seo, H.; Kim, S.; Seong, K.; Kang, S. Variability in scale growth rates of chum salmon (Oncorhynchus keta) in relation to climate changes in the late 1980s. Prog. Oceanogr. 2006, 68, 205–216. [Google Scholar] [CrossRef]
- Gleick, P.H. Making every drop count. Sci. Am. 2001, 284, 40–45. [Google Scholar] [CrossRef]
- Fukuwaka, M.; Suzuki, T. Early sea mortality of chum salmon juveniles in the open coastal waters of the Japan Sea. NPAFC 2001, 2, 7–8. [Google Scholar]
- Battin, J.; Wiley, M.W.; Ruckelshaus, M.H.; Palmer, R.N.; Korb, E.; Bartz, K.K.; Maki, H. Projected impacts of climate change on salmon habitat restoration. PNAS 2007, 104, 6720–6725. [Google Scholar] [CrossRef]
- Bams, R.A.; Lam, C.N.H. Influence of deteriorating water quality on growth and development of chum salmon (Oncorhynchus keta) larvae in a Japanese-style keeper channel. Can. J. Fish. Aquat. 1983, 40, 2098–2104. [Google Scholar] [CrossRef]
- Ko, E.J.; Do, Y.; Kim, H.; Song, H.S.; Wood, T.S.; Chon, T.S. Effective detection methods for Pectinatella magnifica Leidy 1851 colony distribution using statoblasts. Bio. Invasions 2021, 23, 981–987. [Google Scholar] [CrossRef]
- Jeong, K.S.; Kim, D.K.; Joo, G.J. Delayed influence of dam storage and discharge on the determination of seasonal proliferations of Microcystis aeruginosa and Stephanodiscus hantzschii in a regulated river system of the lower Nakdong River (South Korea). Water Res. 2007, 41, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H. The Geography of Korea. Soc. Stud. 1988, 79, 141–145. [Google Scholar] [CrossRef]
- Normile, D. Restoration or devastation? Science 2010, 327, 1568–1570. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.; Mukhopadhyay, M.; Mitra, P.; Bagchi, M.; Karamkar, H. Impact of Farakka barrage on the hydrology and fishery of Hoogly estuary. Estuaries 1996, 19, 710–722. [Google Scholar] [CrossRef]
- Tekile, A.; Kim, I.; Kim, J. Mini-review on river eutrophication and bottom improvement techniques, with special emphasis on the Nakdong River. J. Environ. Sci. 2015, 30, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.D.; Jang, M.H.; Jo, H.B.; Jeong, K.S.; Kim, G.Y.; Joo, G.J. Changes of fish assemblages after construction of an estuary barrage in the lower Nakdong River, South Korea. Limnology 2016, 17, 183–197. [Google Scholar] [CrossRef]
- Hooper, T.; Austen, M. Tidal barrages in the UK: Ecological and social impacts, potential mitigation, and tools to support barrage planning. Renew. Sust. Energ. Rev. 2013, 23, 289–298. [Google Scholar] [CrossRef]
- Ellis, D.V. Swimming speeds of sockeye and coho salmon on spawning migration. J. Fish. Res. Board Can. 1966, 23, 181–187. [Google Scholar] [CrossRef]
- Akita, M.T.; Makiguchi, H.; Nii, H.; Nakao, K.; Sandahl, J.F.; Ueda, H. Upstream migration of chum salmon through a restored segment of the Shibetsu River. Ecol. Freshw. 2006, 15, 125–130. [Google Scholar] [CrossRef]
- Water Environment Information System. Available online: http://water.nier.go.kr/ (accessed on 29 May 2021).
- Froese, R. Cube law, condition factor and weight-length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Ruggerone, G.T.; Irvine, J.R. Number and biomass of natural and hatchery-origin pink salmon, chum salmon, and sockeye salmon in the north Pacific Ocean, 1925–2015. Mar. Coast. Fish. 2018, 10, 152–168. [Google Scholar] [CrossRef]
- Brykov, V.A.; Kukhleysky, A.D.; Shevlyakov, E.A. Sex ratio control in pink salmon (Oncorhynchus gorbuscha) and chum salmon (O. keta) populations: The possible causes and mechanism of changes in the sex ratio. Russ. J. Genet. 2008, 44, 786–792. [Google Scholar] [CrossRef]
- Craig, J.K.; Foote, C.J.; Wood, C.C. Evidence of Temperature-Dependent Sex Determination in Sockeye Salmon (Oncorhynchus nerka). Can. J. Fish. Aquat. Sci. 1996, 53, 141–147. [Google Scholar] [CrossRef]
- Alderdice, D.F.; Wickett, W.P.; Brett, J.R. Some effects of temporary exposure to low dissolved oxygen levels on Pacific salmon eggs. J. Fish. Res. Board Can. 1958, 15, 229–250. [Google Scholar] [CrossRef]
- Fellman, J.B.; Hood, E.; Nagorski, S.; Hudson, J.; Pyare, S. Interactive physical and biotic factors control dissolved oxygen in salmon spawning streams in coastal Alaska. Aquat. Sci. 2019, 81, 1–11. [Google Scholar] [CrossRef]
- Beacham, T.D.; Murray, C.B. Effect of female size, egg size, and water temperature on development biology of chum salmon (Oncorhynchus keta) from the Nitinat River, British Columbia. Can. J. Fish. Aquat. Sci. 1985, 42, 1755–1765. [Google Scholar] [CrossRef]
- Zinichev, V.V.; Zotin, A.I. Selected temperature and optimums for development in prolarvae and larvae of chum salmon, Oncorhynchus keta. J. Ichthyol. 1987, 27, 141–144. [Google Scholar]
- Jensen, K.; Snekvik, C. Low pH levels wipe out salmon and trout populations in southernmost Norway. Ambio 1972, 1, 223–225. [Google Scholar]
- Rosseland, B.O.; Sevaldrub, I.; Svalastog, D.; Muniz, I.P. Studies on freshwater fish populations—effects of acidification on reproduction, population structure, growth and food selection. In Proceedings of the Ecological impact of acid precipitation, Sandefjord, Norway, 11 March 1980; Drabløs, D., Tollan, A., Eds.; SNSF-prosjektet, As-NLH. pp. 336–337. [Google Scholar]
- Rombough, P.J. Effects of low pH on eyed embryos and alevins of Pacific salmon. Can. J. Fish. Aquat. 1983, 40, 1575–1582. [Google Scholar] [CrossRef]
- Park, E.O.; Suh, H.L.; Soh, H.Y. Spatio-temporal distribution of Acartia (Copepoda: Calanoida) species along a salinity gradient in the Seomjin River estuary, South Korea. J. Nat. Hist. 2015, 79, 2799–2812. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Kaeriyama, M.; Seo, H.; Qin, Y. Effect of global warming on the life history and population dynamics of Japanese chum salmon. Fish. Sci. 2014, 80, 251–260. [Google Scholar] [CrossRef][Green Version]
- Abdul-Aziz, O.I.; Nathan, J.M.; Katherine, W.M. Potential climate change impacts on thermal habitats of Pacific salmon (Oncorhynchus keta spp.) in the North Pacific ocean and adjacent seas. Can. J. Fish. Aquat. Sci. 2011, 68, 1660–1680. [Google Scholar] [CrossRef]
- Myeong, J.G.; Kim, Y.U. Morphological Study of Oncorhynchus spp. In Korea-V. Comparison of Skeletal Characters of Chum Salmon, O. keta, Masu Salmon O. masou and Rainbow Trout O. mykiss. Korean J. Fish. Aquat. Sci. 1996, 29, 208–229. [Google Scholar]
- Kohan, M.L.; Mueter, F.J.; Orsi, J.A.; McPhee, M.V. Variation in size, condition, and abundance of juvenile chum salmon (Oncorhynchus keta) in relation to marine factors in Southeast Alaska. Deep Sea Res. Part II Top. Stud. Oceanogr. 2019, 165, 340–347. [Google Scholar] [CrossRef]
- Marr, J.C. Age, length and weight studies of three species of Columbia River salmon (Oncorhynchus keta, O. gorbuscha and O. kisutch); Department of Research Fish Commission of the State of Oregon: Salem, OR, USA, 1944; Volume 2, pp. 157–197. [Google Scholar]
- Liu, W.; Zhan, P.R.; Tang, F.J. Study on morphological development of fall chum salmon (Oncorhynchus keta) otoliths in Heilongjiang River. Acta Hydrobiol. Sin. 2010, 34, 1069–1076. [Google Scholar] [CrossRef]
- Toshihiko, S.; Kentaro, H.; Kei, S.; Kyuji, W.; Kengo, S.; Yukihiro, H.; Shigeto, K.; Tomoki, S.; Fumihisa, T.; Shupei, S. Stock composition of adult chum salmon Oncorhynchus keta caught in a setnet fishery estimated using genetic identification, scale patterns, and otolith thermal marking. Fish Sci. 2020, 86, 271–286. [Google Scholar]
- Froese, R.; Thorson, J.T.; Reyes, R.B., Jr. A Bayesian approach for estimating length weight relationships in fishes. J. Appl. Ichthyol. 2014, 30, 78–85. [Google Scholar] [CrossRef]
- Allendorf, F.W.; Ryman, N.; Utter, F. Genetics and Fishery Management: Past, Present, and Future, 1st ed.; University of Washington Press: Seattle, DC, USA, 1987; pp. 1–19. [Google Scholar]
- Wimberger, P.H. Plasticity of fish body shape, the effects of diet, development, family and age in two species of Geophagus (Pisces: Cichlidae). Biol. J. Linn. Soc. Lond. 1992, 45, 197–218. [Google Scholar] [CrossRef]
- FishBase. Available online: https://www.fishbase.se (accessed on 1 June 2021).
- Marthers, R.G.; De carlos, M.; Crowley, K.; Teangana, D.Ó. A review of the potential effect of Irish hydroelectric installations on Atlantic salmon (Salmo Sar L.) populations, with particular reference to the river Erne. Proc. R. Ir. Acad. 2002, 102B, 69–79. [Google Scholar] [CrossRef]
- Mee, D.M.; Kirkpatrick, A.J.; Stonehewer, R.O. Post impoundment fishery investigations on the Tawe Barrage, South Wales. In Barrages: Engineering Design & Environmental Impacts; Burt, N., Watts, J., Eds.; John Wiley & Sons Ltd: West Sussex, UK, 1996; pp. 395–408. [Google Scholar]
- Russell, I.C.; Moore, A.; Ives, S.; Kell, L.T.; Ives, M.J.; Stonehewer, R.O. The migratory behaviour of juvenile and adult salmonids in relation to an estuarine barrage. Hydrobiologia 1998, 371, 321–334. [Google Scholar] [CrossRef]
- Plumb, J.M.; Perry, R.W.; Adams, N.S.; Rondorf, D.W. The effect of river impoundment and hatchery rearing on the migration behavior of juvenile steelhead in the lower Snake River, Washington. N. Am. J. Fish. Manag. 2006, 26, 438–452. [Google Scholar] [CrossRef]
- Hasler, A.D.; Allan, T.S.; Ross, M.H. Olfactory imprinting and homing in Salmon: Recent experiments in which salmon have been artificially imprinted to a synthetic chemical verify the olfactory hypothesis for salmon homing. Am. Sci. 1978, 66, 347–355. [Google Scholar] [PubMed]
- Salo, E.O. Life history of chum salmon (Oncorhynchus keta). In Pacific Salmon Life Histories, 2nd ed; Groot, C., Margolis, L., Eds.; UBCPress: Vancouver, BC, Canada, 1991; Volume 1, pp. 231–310. [Google Scholar]
- Zavolokin, A.V.; Zavolokin, E.A.; Khokhlov, Y.N. Changes in size and growth of anadromous chum salmon (Oncorhynchus keta) from 1962-2007. NPAFC 2009, 5, 157–163. [Google Scholar]
- Roff, D.A. The Evolution of Life Histories: Theory and Analysis, 1st ed.; Chapman & Hall, Ed.; Springer: New York, NY, USA, 1993. [Google Scholar]
- Fukuwaka, M. Density-dependence of chum salmon in coastal waters of the Japan Sea. NPAFC 2000, 2, 75–81. [Google Scholar]
- Azumaya, T.; Ishida, Y. An evaluation of the potential influence of SST and currents on the oceanic migration of juvenile and immature chum salmon (Oncorhynchus keta) by a simulation model. Fish. Oceanogr. 2004, 13, 10–23. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).