Abstract
Diseases of crucian carp (Carassius auratus) are closely related to intestinal parameters. Enterococcus faecalis has strong colonization ability in the intestinal tract, and produces natural antibiotics, bacteriocin, and other bacteriostatic substances, which can effectively inhibit some pathogenic bacteria and improve the intestinal microenvironment. This study aimed to assess the effects of E. faecalis YFI-G720 which was isolated from the intestinal of crucian carp on the growth, immunity, intestinal health, and disease resistance of crucian carp. Fish (48.16 ± 0.55 g) were fed four diets, commercial diet or diet containing E. faecalis at 105 CFU/g (EF1), 106 CFU/g (EF2), or 107 CFU/g (EF3) for 28 days. The results showed that supplementation of E. faecalis significantly improved the weight gain ratio (WGR) and the specific growth rate (SGR) compared with control group (p < 0.05). Intestinal mucosal epithelial cells in EF2 were intact and normal, but there was obvious vacuolation in CG. Compared with CG, serum C3 and IgM in EF2 were significantly increased at the end of the experiment (p < 0.05), and serum alkaline phosphatase was significantly higher in all experimental groups (p < 0.05). Among studied immune-related genes, expression was detected by qPCR, C3, IgM, and IL-1βwere upregulated in all experimental groups to varying degrees from 14 days, with highest expression in EF2 at 28 days. Intestinal microbiota structure analyzed through high-throughput sequencing, and the results showed that the relative abundance of Aeromonas and Acinetobacter decreased while Cetobacterium increased in all experimental groups, with the greatest changes in EF2. Challenge tests showed that fish fed E. faecalis were more resistant to Aeromonas veronii (p < 0.05). In conclusion, dietary E. faecalis YFI-G720 at 106 CFU/g can improve the health status, immune parameters, intestinal microbiota composition, and disease resistance of crucian carp.
1. Introduction
Crucian carp (Carassius auratus), an omnivorous and bottom-feeding freshwater fish, is one of the main aquaculture species in China [1,2]. In 2020, total production of crucian carp in China was 2.75 million tons, which constituted about 8.9% of the total freshwater fish aquaculture production [3]. Crucian carp meat contains 13% protein, 11% fat, and plenty of minerals, hence it is appreciated by consumers [4]. However, with the increasing development and intensification of aquaculture, diseases caused by pathogens affecting this species are gradually increasing, such as Aeromonas veronii, Aeromonas hydrophila, and Cyprinid herpesvirus 2 (CyHV-2) [5,6,7]. These pathogens mainly spread to other tissues and organs after infecting the intestinal tract. Therefore, the occurrence of diseases is closely related to intestinal health, especially the microbial composition of the intestinal [8,9]. The (species) diversity and stability of the intestinal microbial are important factors affecting host health [10,11]. Therefore, for the crucian carp industry, it is important to maintaining a balance of intestinal microbiota via good health management.
Probiotics have been proven to promote intestinal health, improve feed digestibility and absorption rate, and enhance disease resistance by regulating the intestinal microbiota [12,13]. Enterococcus faecalis, a facultative anaerobic Gram-positive bacterium belonging to the Enterococcaceae within Lactobacillus and Enterococcus, is one of the major intestinal microbiota components in humans and animals [14,15,16]. E. faecalis has strong tolerance to the environment and colonization ability in the intestinal tract that can help to form a barrier of lactic acid bacteria, preventing the invasion of foreign pathogens and viruses [17]. Additionally, its secondary metabolites—including organic acids, hydrogen peroxide, extracellular polysaccharides, and other substances—can inhibit the growth and reproduction of the various pathogenic bacteria [16,18]. E. faecalis can also facilitate the digestion and absorption of feed, enhance the activity of macrophages, and promote the immune responses of the host [19,20]. Studies have shown that dietary supplementation with 107–109 CFU/g E. faecalis can include the growth, immunity, and disease resistance of rainbow trout (Oncorhynchus mykiss Walbaum), Javanese carp (Puntius gonionotus Bleeker 1850), and mud crab (Scylla paramamosain) [19,20,21]. Thus, E. faecalis provides an important relevance for the development of probiotics in aquatic products.
A number of studies have verified the beneficial effects of probiotic bacteria such as Bacillus and Lactobacillus on crucian carp [22,23]. However, data regarding the effects of E. faecalis on the growth, immunity, and intestinal health of crucian carp are limited. E. faecalis YFI-G720 was isolated from the intestinal tract of crucian carp, and preliminary experiments showed that it could improve the intestinal microbiota of crucian carp. Therefore, the purpose of this study was to examine the effects of E. faecalis YFI-G720 supplementation of diet on growth performance, immune capacity, intestinal morphology, and the intestinal microbiota of crucian carp, as well as providing a reference for the application of probiotics in the crucian carp industry.
2. Materials and Methods
2.1. Experimental Diets
Experimental diets comprised puffed pellet feed which supplied by Tongwei Co., Ltd. (Chengdu, China). E. faecalis YFI-G720 stored at the China Center for type Culture Collection (CCTCC), Wuhan University, under preservation number CCTCC M2021312. E. faecalis was cultured overnight on brain heart infusion plates (BHI, Qingdao biological technology co., Ltd., Qingdao, China) at 28 °C and resuspended in sterilized phosphate-buffered saline (PBS). Colony-forming units (CFU) per mL of E. faecalis culture was determined by the plate dilution counting method. The E. faecalis cells were resuspended and sprayed on the commercial basal feed at 105 CFU/g, 106 CFU/g, and 107 CFU/g of diet [18], followed by thorough mixing. Feeds were vacuum dried at 30 °C overnight, stored in individual airtight containers at 4 °C, and produced every 3 days to maintain the cell count of E. faecalis.
2.2. Laboratory Fish and Rearing
The experiment was carried out in the Fish Disease Laboratory of the Yangtze River Fisheries Research Institute. Experimental crucian carp were obtained from a local farm in Wuhan, Hubei, China, and we randomly sampled for parasite observation, bacterial isolation, and CyHV-2 detection, and the results showed negative. Then crucian carp were acclimated in 300 L freshwater aquaculture tanks for 15 days. Next, 600 fish with a mean initial body weight of 48.16 ± 0.55 g were randomly assigned into four groups (three tanks per group, 50 fish per tank). The control group was fed with commercial basal diet, while EF1, EF2, and EF3 groups were fed E. faecalis diet supplemented at 105 CFU/g, 106 CFU/g, and 107 CFU/g, respectively. Fish were fed twice daily at 08:30 morning and 17:00 afternoon a day with the feeding rate of 3–5% of body weight. A quarter of the tank water was replaced every day. During the trial (4 weeks), the tank water temperature was maintained at 23–25 °C, pH 7.5–8.0, dissolved oxygen > 7.0 mg/L and flow rate > 4 mL/s. All experimental procedures were conducted according to the guidelines of the appropriate Animal Experimental Ethical Inspection of Laboratory Animal Center, Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences.
2.3. Growth Performance
Body weight of fish was measured at the beginning and the end of the experiment. Growth parameters were calculated according to the following formulae to measure the growth performance of crucian carp: WGR (weight gain ratio, %) = 100 × (final average body weight − initial average body weight)/initial body weight; SGR (specific growth rate, %/day) = 100 × [ln (final average body weight) − ln (initial average body weight)]/days; CF (condition factor, %) = 100 × final body weight/[body length (cm)]3; Survival rate (%) = 100 × final number of survived fish/initial number of fish.
2.4. Sample Collection
After 7, 14, 21, and 28 days of starting feed with special diet, three crucian carp from each tank were randomly selected, 500 μL blood was collected from the caudal vein of each fish with a 1 mL syringe and placed in plastic Eppendorf tubes containing anticoagulant solution (heparin). The tubes were kept at 4 °C overnight, centrifuged at 4000× g for 10 min, and the resulting serum was stored at −80 °C for serum biochemical index analysis. Meanwhile, spleen tissue was placed in a RNase-free centrifuge tubes containing 200 μL TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and stored at −80 °C for immunity gene analysis.
At the end of the experiment, three crucian carp from each tank were randomly collected and aseptically sacrificed in an ice bath. The anterior intestines were fixed in neutral 4% paraformaldehyde for analysis of intestinal morphology. Intestinal tissue was collected, frozen rapidly in liquid nitrogen, and stored at −80 °C for intestinal microbiota analyses.
2.5. Serum Biochemical Analysis
Complement 3 (C3), immunoglobulin M (IgM), triglyceride (TG), total cholesterol (TCHO), alkaline phosphatase (AKP), and aspartate amino transferase (AST) in serum were determined by Olympus600 automatic biochemical analyzer (Olympus, Tokyo, Japan) using a commercial kit (Guangzhou Dibao Medical Products Co., Ltd., Guangzhou, China).
2.6. Real-Time PCR Analysis of Immune-Related Genes
Immunity gene expression levels in spleen tissue of crucian carp were examined by quantitative fluorescence real-time PCR (qPCR) method. Total RNA was isolated using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and the residual trace of DNA was removed using Recombinant DNase I (RNase-free, Takara, Dalian, China). Complementary DNA (cDNA) was synthesized using a cDNA Synthesis SuperMix Kit (TransGen Biotech, Beijing, China). All qPCR experiments were performed using a SYBR Green Premix Ex Taq Kit (Takara, Taejin, Japan) by Rotor-Gene 6000 Real-time PCR System (Qiagen, Dusseldorf, Germany). Thermal cycling included denaturation at 95 °C for 5 min, followed by 30 cycles at 95 °C for 20 s, 56 °C for 20 s, 72 °C for 20 s, and 72 °C for 20 s. The primers for qPCR are shown in Table 1. The IgM gene sequence was found on NCBI (MK272741.1), and the primer was designed by primer 5. All experiments were repeated at least three times, and relative expression levels were calculated using the 2−ΔΔCt method with the β-actin gene used as an internal control gene for cDNA normalization.

Table 1.
Sequences of primer pairs used in real-time PCR.
2.7. Histopathology Analysis
Anterior intestine tissues were fixed in 4% paraformaldehyde for 24 h, dehydrated in a sequential ethanol series of alcohol (50–95%) and embedded in paraffin. Tissue blocks were sectioned (5 μm thick) and stained with hematoxylin and eosin (H&E). The intestinal structure was assessed and photographed under an Olympus BX53 microscope (Olympus, Tokyo, Japan).
2.8. Genomic DNA Extraction and 16S rRNA Gene Sequencing
Bacterial genomic DNA was extracted using a Bacterial DNA Kit (Omega Biotek, Winooski, VT, USA) following manufacturer’s instructions. PCR amplification of the bacterial V4–V5 region of the 16S rRNA gene was performed in a 50 μL reaction. The reaction contained 25 μL of Hot Start Taq 2 × Master Mix (New England BioLabs Inc., Ipswich, MA, USA), 2 μL of template DNA, 2 μL of each primer (338F, ACTCCTACGGGAGGCAGCA; 806R, GGACTACHVGGGTWTCTAAT [26]), and deionized water. Thermal cycling comprised an initial denaturation at 95 °C for 2 min, followed by 30 cycles at 95 °C for 15 s, 55 °C for 30 s, and 72 °C for 30 s, and a final extension at 72 °C for 5 min. After checking the quality by 1% agarose gel electrophoresis, samples were sequenced on an Illumina MiSeq PE300 high-throughput sequencing platform. The final effective data were obtained using the UCHIME v4.2 software [27]. Reads were clustered into operational taxonomical units (OTUs) at 97% identity [27]. The abundances of the corresponding OTUs in each group were calculated at the phylum and genus levels. Chao 1 (Bacterial richness index) and Shannon (Bacterial diversity index) alpha diversity indices were analyzed using QIIME (Version 1.7.0) [28].
2.9. Challenge Test
After 28 days of feeding trial, a challenge test was performed in each group with A. veronii which isolated from intestines of diseased crucian carp and preserved in our laboratory. A. veronii was inoculated into BHI medium and cultured overnight at 37 °C and settled by centrifugation then resuspended and adjusted to sterile phosphate-buffered saline (PBS). Thirty crucian carp were randomly selected from each group and challenged with 0.1 mL of A. veronii bacterial suspension (3.6 × 107 CFU/mL) by intraperitoneal injection. Mortality was recorded every 24 h for 10 days. The presence of A. veronii as the only etiological agent was confirmed by swabbing the intestinal tissues onto BHI plates and subsequent bacteria identification.
2.10. Statistical Analyses
Statistical analysis was performed using SPSS 19.0 (IBM Corp., Armonk, NY, USA). Differences between groups were detected using one-way analysis of variance (ANOVA) and tested by least significant difference (LSD) test. The results are presented as the mean ± standard deviation (SD). Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Growth Performance
After the feeding trial lasting 28 days, the survival rates of the four crucian carp groups were >95% (Table 2), and there was no significant difference between the groups. Compared with the CG, the fish in EF1 and EF2 groups had a significantly higher final weight, WGR and SCR (p < 0.05). Furthermore, SGR of fish in EF3 was significantly higher than in CG group (p < 0.05), but there was no difference in final weight or WGR between CG, EF1, and EF2 groups (p > 0.05). CF was unaffected by dietary treatment in all groups.

Table 2.
Growth performance of crucian carp fed with different experimental diets for 28 days.
3.2. Serum Biochemical Parameters
Dietary E. faecalis supplementation had a significant effect on some serum biochemical indices of crucian carp (Figure 1). C3 and IgM in the EF2 group increased with the progression of feeding trial and reached maximum values at 2.1 and 10.4 g/L at the end of 28 days of experiment, respectively, which were significantly higher than those in CG and EF1 groups (p < 0.05). AKP in the EF2 group was significantly higher than that in CG and EF3 groups at 21 days (p < 0.05), and it was significantly higher than in the other three groups at 28 days (p < 0.05). There were no significant differences in TG, TCHO, and AST among all groups (p > 0.05).
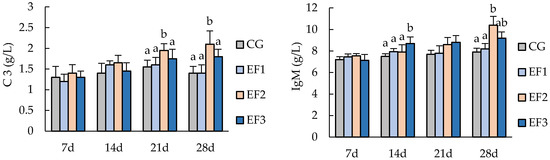
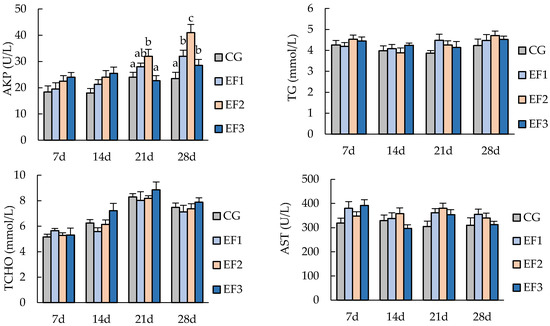
Figure 1.
Serum biochemical parameters of crucian carp. Each value represents mean ± SD (n = 3), and bars with different letters indicate significantly differences by LSD test for each time point (p < 0.05). C3, complement 3; IgM, immunoglobulin M; TG, triglyceride; TCHO, total cholesterol; AKP, alkaline phosphatase; AST, aspartate aminotransferase; CG, control group; EF1, E. faecalis at 105 CFU/g diet; EF2, E. faecalis at 106 CFU/g diet; EF3, E. faecalis at 107 CFU/g diet.
3.3. Changes in Immunity-Related Genes Expressions
Expression levels of immunity-related genes from the spleen tissue of crucian carp were measured on day 7, 14, 21, and 28, including the genes encoding IL-1β, C3, and IgM (Figure 2). After 21 days of experiment, the relative expression of IL-1β in the EF2 group was significantly upregulated compared with CG and EF1 groups (p < 0.05), and those in EF1 and EF2 groups were significantly upregulated compared with CG group and EF3 group at 28 days (p < 0.05). Expression of C3 in the EF2 group was upregulated at 7 days, and was significantly higher from 14 days than those in other groups (p < 0.05). Meanwhile, C3 expression in EF1 was significantly upregulated compared with CG at 21 days and 28 days (p < 0.05). Expression of IgM in EF3 group was significantly higher than in CG group from 14 days (p < 0.05), and that in EF2 was significantly upregulated from 21 days compared with CG and EF1 groups (p < 0.05), with expression peaking at 28 days.
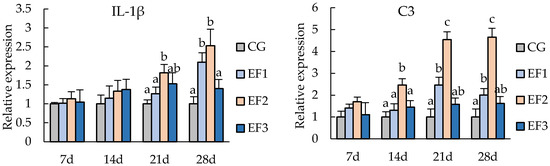
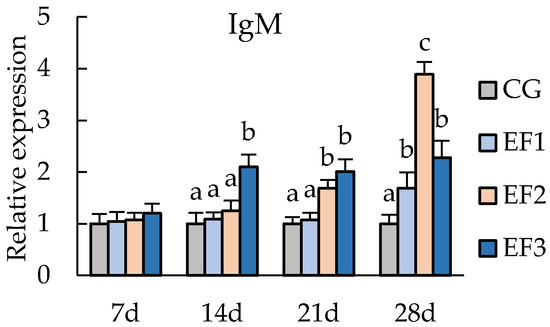
Figure 2.
Relative mRNA expression levels of immunity-related genes in crucian carp spleen tissue. Each value represents mean ± SD (n = 3), and bars with different letters indicate significantly differences by LSD test for each time point (p < 0.05). CG, control group; EF1, E. faecalis at 105 CFU/g diet; EF2, E. faecalis at 106 CFU/g diet; EF3, E. faecalis at 107 CFU/g diet.
3.4. Changes in Intestinal Morphology
After 28 days of feeding, crucian carp intestines from each fed with E faecalis supplemented diet had intact, orderly, and tight mucosal layers, submucosal layers, muscular layers, and outer membranes (Figure 3). Epithelial cells in the mucosal layer of intestinal tissue in CG group were disordered and vacuolated obviously. In the EF1 group, a few epithelial cells were ruptured or vacuolated at the top of the mucosal layer. Epithelial cells were intact and arranged normally in EF2 and EF3 groups.
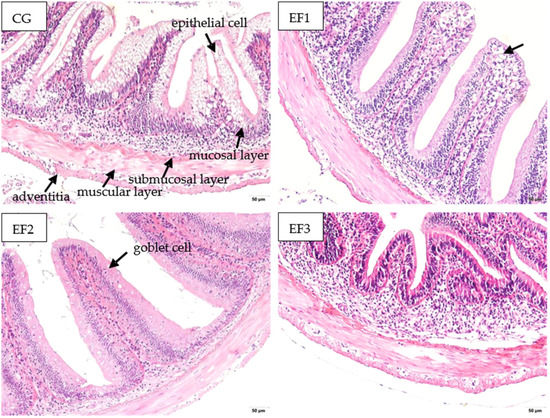
Figure 3.
Intestinal morphology analysis of crucian carp fed with different experimental diets for 28 days (200×). CG, control group; EF1, E. faecalis at 105 CFU/g diet; EF2, E. faecalis at 106 CFU/g diet; EF3, E. faecalis at 107 CFU/g diet.
3.5. Changes in the Intestinal Microbiota
We sequenced the 16S rRNA gene amplicons of the intestinal microbiota in the intestine samples of experimental crucian carp, and raw data have been deposited in the NCBI. The average number of OTUs obtained from the different samples ranged from 812 to 1037 (Table 3). The results showed no differences between Chao 1 indexe and OTUs in GC, EF1, and EF3 groups, but these parameters were significantly lower than in EF2 group (p < 0.05). There was no significant difference of Shannon index among all four groups (p > 0.05).

Table 3.
OTUs classification and alpha diversity index of microbial communities in the intestine samples.
To further explore the composition of the microbiota community richness in each group, the abundance in the intestinal microbiota of the top eight bacteria was calculated at the phylum and genus levels. At the phylum level, Proteobacteria, Firmicutes, Actinobacteria, and Bacteroidetes were the primary intestinal microbiota in all groups (Figure 4). Compared with CG group, the relative abundance of Proteobacteria was decreased in EF1, EF2, and EF3 groups, while the relative abundance of Actinobacteria was increased. At the genus level, the primary intestinal microbiota in all groups were Aeromonas, Magnetospirillum, Acinetobacter, and Cetobacterium (Figure 4). The relative abundance of Aeromonas and Acinetobacter was decreased in EF1, EF2, and EF3 groups after 28 days of feeding compared with CG group, while Cetobacterium was increased, and EF2 group exhibited the largest differences.
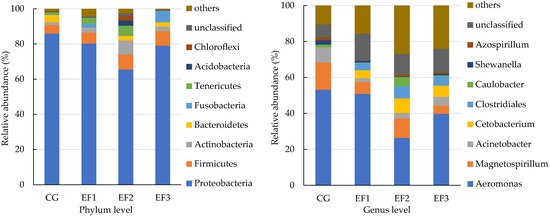
Figure 4.
Main relative abundance of the intestinal microbiota in crucian carp at the phylum and genus levels. CG, control group; EF1, E. faecalis at 105 CFU/g diet; EF2, E. faecalis at 106 CFU/g diet; EF3, E. faecalis at 107 CFU/g diet.
3.6. Challenge Test
The cumulative survival rates of crucian carp challenged with A. veronii for 10-day are shown in Figure 5. At the end of the 10 days challenge test, the cumulative survival rates were 24.44%, 56.67%, 67.78%, and 61.11% in the CG, EF1, EF2, and EF3 groups, respectively. Crucian carp in treatment groups showed a significantly higher survival rate than those in CG group after 28 days of feeding.
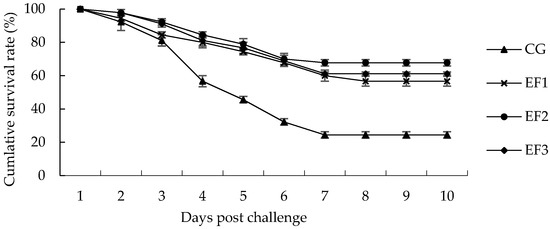
Figure 5.
Cumulative survival rate following challenge with A. veronii for 10 days. Each value represents the mean ± SD (n = 3). CG, control group; EF1, E. faecalis at 105 CFU/g diet; EF2, E. faecalis at 106 CFU/g diet; EF3, E. faecalis at 107 CFU/g diet.
4. Discussion
4.1. Growth Performance
Dietary supplementation of probiotics in aquaculture offers an ecofriendly prophylactic measure [29]. Their applications are increasing due to their positive impact on the growth and health of aquatic animals [30]. E. faecalis as a kind of lactic acid bacterium possessing growth-promoting potential [31]. Previous studies had demonstrated that E. faecalis had a positive impact on the growth of tilapia (Oreochromis niloticus) [18], rainbow trout (Oncorhynchus mykiss) [19], juvenile sea cucumber (Apostichopus japonicus selenka) [32], and other aquatic animals [33]. In the present study, following dietary supplementation with E. faecalis at 105–107 CFU/g, crucian carp exhibited significantly increased growth after 28 days, consistent with the results of these previous studies.
4.2. Serum Biochemical Parameters
Serum biochemical parameters are the main indices reflecting the health condition and physiological state of fish [34]. Serum parameters related to immunity and antioxidant capacity were measured in this study to confirm the positive effects of the E. faecalis on the health status of fish. TG, TCHO, and AST showed no significant differences after dietary intake of E. faecalis, in line with similar studies on crucian carp dietary supplementation with Bacillus subtilis [35]. C3, the most abundant complement protein in serum, is a bridge between natural and adaptive immunity, and plays an important role in immune defenses, regulation, and pathology [36]. AKP is a lysosomal enzyme involved in macrophage activation, and a promising antimicrobial agent [37]. In this study, C3 and AKP increased with progressing of feeding trial for diets supplemented with E. faecalis. Similarly, previous studies showed that dietary E. faecalis at 106 CFU/g can improve the serum C3 levels and AKP activity in both tilapia and blunt snout bream [18,38]. IgM is the first antibody produced by the body following stimulation by antigen, and it has strong cytotoxic and cytolytic activity [39]. Previous studies revealed higher serum IgM levels in crucian carp fed with Lactobacillus plantarum or Lactobacillus casei diets [23,40]. In the present study, dietary E. faecalis at 106–107 CFU/g may increase serum IgM at different times, similar to the results of these previous studies.
4.3. Immune Related Gene Expressions
Expression of immune-related genes in spleen also promotes the immune responses in fish. Specific immunity that mediate the production of cytokines and antibodies play an important role in the immune responses [24]. IL-1β plays a central role in the regulation of immune and inflammatory responses to infections by activating lymphocytes or inducing the release of other cytokines [41,42]. IgM, the main agent in humoral immunity, neutralizes antigens and activates the complement cascade [39]. C3 complement plays an antibacterial role by stimulating cell phagocytosis mediated by the complement activation pathway [43]. Heat-killed E. faecalis can induce cell-mediated immunity and enhanced IL12 and IFNgrel2 expression in crucian carp [14]. In addition, E. faecalis can upregulate the gene expression levels of TNF-α and IL-8 in tilapia [18]. In the present study, crucian carp fed with E. faecalis showed significantly increased expression of IL-1β, C3, and IgM genes in the spleen, consistent with the serum immune indices. This might be related to exopolysaccharides (EPSs), which can promote the expression of immune genes in aquatic animals, since E. faecalis is a lactic acid bacterium that synthesizes EPSs [44]. However, the timing at which IL-1β, C3, and IgM gene expression is first upregulated is inconsistent. This might be related to different pathways of immune gene expression and/or different mechanisms of genes involved in immune regulation [45].
4.4. Intestinal Morphology
In aquatic animals, the intestine is the main organ for food digestion and nutrient absorption [46]. As the largest immune organ in the body, the intestinal tract plays an important role in reducing the invasion of pathogenic bacteria [47]. Studies have shown that dietary ingredients may have an effect on fish intestines, for example, dietary supplementation with B. cereus or B. subtilis can improve the fold height and microvillus height of the intestines in crucian carp [22,35]. In addition, dietary E. faecalis can enhance the intestinal health and nutrient absorption in piglets by increasing the villus height [48]. In the present study, epithelial cells were intact and arranged normally without ruptured or vacuolation after feeding with E. faecalis at 106–107 CFU/g diet. This might be because E. faecalis forms a lactic acid barrier on the intestinal mucosa that resists the invasion of pathogenic bacteria and maintains the health of the intestinal tissue [17].
4.5. Changes in the Intestinal Microbiota
The intestinal microbiota plays important roles in intestinal physiology due to its critical impacts on metabolism and immune function [49,50]. Normally, the intestinal microbiota maintains a dynamic balance and stability in the intestinal environment, which can help the body to resist the invasion of pathogenic bacteria and enhance host immunity [51,52]. Diet plays a major role in altering the intestinal microbiota and metabolism [53]. In the present study, the richness of the intestinal microbial community of crucian carp increased after feeding them an appropriate amount of E. faecalis. From the perspective of phylum classification, Proteobacteria, Firmicutes, and Actinobacteria were the dominant phyla in all groups. A previous study reported slightly different results [54], possibly due to differences in environmental conditions or feeding habits [55]. Proteobacteria, the largest bacteria phylum, includes many pathogenic bacteria such as Escherichia coli, Salmonella, and Vibrio cholerae, and it has been proposed as a potential signature of dysbiosis and disease risk [56]. Firmicutes are considered beneficial bacteria in the intestines, exerting a positive influence on growth performance, immunity, digestion, and disease resistance in aquatic animals [57]. Actinobacteria can produce secondary metabolites, including extracellular enzymes and potent antibiotics [58]. Our results showed that the abundance of Firmicutes and Actinobacteria was increased after feeding E. faecalis for 28 days, but Proteobacteria was decreased compared with the CG group. It is assumed that E. faecalis might improve intestinal health by increasing the proliferation of Firmicutes and Actinobacteria, thereby inhibiting the growth of Proteobacteria.
At the genus level, our results showed that Aeromonas, Magnetospirillum, Acinetobacter, and Cetobacterium were the dominant genera in all groups. Aeromonas and Acinetobacter are associated with bacterial fish diseases, and they are also important opportunistic pathogens in crucian carp [5,59]. Cetobacterium can be abundant in various freshwater fish species, and it can produce large quantities of vitamin B-12 [60]. In the present study, compared with the control group, the abundance of the Cetobacterium was increased in EF1, EF2, and EF3 groups, while Aeromonas and Acinetobacter were decreased. This might be because E. faecalis produces bacteriocins and other bacteriostatic substances that inhibit the growth of pathogenic bacteria [61]. However, in this study, Enterococcus did not become the dominant genus. Similarly, the corresponding bacteria were not found in the intestinal of grass carp fed with Bacillus subtilis (108 CFU/g) for 4 weeks, but the microbiota structure changed [62]. This may be due to the short feeding time, low feeding amount, and other reasons that cannot occupy the intestinal niche, but through the production of some metabolites to regulate the intestinal microbiota structure.
4.6. Disease Resistance
A. veronii is regarded as one of the major pathogens, causing hemorrhagic septicemia, ulcerations, and ascites in crucian carp [63]. A. veronii exhibits significant virulence toward crucian carp, with an LD50 value of 1.31 × 107 CFU/mL [5]. In the present study, after a 10-day challenge with 3.6 × 107 CFU/mL A. veronii, the cumulative survival rate was 24.44% in the control group. Meanwhile, fish in the three E. faecalis groups all showed significantly higher cumulative survival rates, with the EF2 group showing the highest rate (67.78%). Similar results were observed in crucian carp fed diets containing Bacillus velezensis and Lactobacillus casei supplemented-diets [23,63]. This might be because E. faecalis can increase the abundance of intestinal probiotics and stimulate cellular and humoral immune functions, including complement C3 and IgM, to protect hosts against pathogen infection.
5. Conclusions
In this study, the addition of E. faecalis YFI-G720 to the diet positively influenced the growth performance, immune response, the intestinal microbiota structures, and resistance to pathogens in crucian carp. Long-term intake of fresh E. faecalis YFI-G720 at 106 CFU/g achieved a more pronounced effect. Therefore, E. faecalis YFI-G720 can be used as a potential probiotic in crucian carp farming. However, the reason that E. faecalis YFI-G720 improves the immunity of crucian carp is still unclear, and we will investigate the metabolites and functions of E. faecalis YFI-G720 to provide a reference for the efficient use.
Author Contributions
Y.Z., Y.X. and Y.L. conceived and designed the study, and performed the data collection, analysis, statistical analysis, writing of the manuscript, and conducted the software and literature review. Y.X. and Z.X. conducted animal management and sample collections. M.X., Y.L. and Z.X. performed the microbial analysis, immunity analysis, and literature review. Y.X., Y.Z., Y.F. and L.Z. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Key Research Development Program of China (2019YFD0900105), the National Natural Science Foundation of China (31802346), the Key Project of Scientific & Technological Innovation of Hubei Province (2018ABA101), and the Earmarked Fund for China Agriculture Research System (CARS-45-16).
Institutional Review Board Statement
In the present study, all experimental procedures were conducted according to guidelines of the appropriate Animal Experimental Ethical Inspection of Laboratory Animal Center, Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences (ID Number: YFI2021-zhouyong-02).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank Guangdong Yuequn Ocean Biological Research Development Co., Ltd. for their support during the preration of this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tang, M.; Xu, X.; Li, S.; Wu, T.; Yan, Y.; Gu, W. The metabolic responses of crucian carp blood to Cyprinid herpesvirus 2 infection. Aquaculture 2019, 498, 72–82. [Google Scholar] [CrossRef]
- Yang, F.; Yang, F.; Wang, G.; Kong, T.; Liu, B. Pharmacokinetics of florfenicol and its metabolite florfenicol amine in crucian carp (Carassius auratus) at three temperatures after single oral administration. Aquaculture 2019, 503, 446–451. [Google Scholar] [CrossRef]
- Fisheries and Fisheries Administration Bureau of Ministry of Agriculture and Industry. China Fisheries Yearbook; China Agriculture Press: Beijing, China, 2021; p. 25.
- Wu, S.J. Dietary Astragalus membranaceus polysaccharide ameliorates the growth performance and innate immunity of juvenile crucian carp (Carassius auratus). Int. J. Biol. Macromol. 2020, 149, 877–881. [Google Scholar] [CrossRef]
- Chen, F.; Sun, J.F.; Han, Z.R.; Yang, X.J.; Xian, J.A.; Lv, A.J.; Hu, X.C.; Shi, H.Y. Isolation, Identification and Characteristics of Aeromonas veronii From Diseased Crucian Carp (Carassius auratus gibelio). Front. Microbiol. 2019, 10, 2742. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yang, Y.; Liu, J.; Awan, F.; Lu, C.; Liu, Y. Inhibition of Aeromonas hydrophilainduced intestinal inflammation and mucosal barrier function damage in crucian carp by oral administration of Lactococcus lactis. Fish Shellfish Immunol. 2018, 83, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zeng, L.B.; Zhang, H.; Zhou, Z.; Ma, J.; Fan, Y.D. Cyprinid herpesvirus 2 infection emerged in cultured gibel carp, Carassius auratus gibelio in China. Vet. Microbiol. 2013, 166, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Rawls, J.F. Intestinal microbiota composition in fishes is influenced by host ecology and environment. Mol. Ecol. 2012, 21, 3100–3102. [Google Scholar] [CrossRef]
- She, R.; Li, T.T.; Luo, D.; Li, J.B.; Yin, L.Y.; Li, H.; Liu, Y.M.; Li, X.Z.; Yan, Q.G. Changes in the Intestinal Microbiota of Gibel Carp (Carassius gibelio) Associated with Cyprinid herpesvirus 2 (CyHV-2) Infection. Curr. Microbiol. 2017, 74, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Rawls, J.F.; Samuel, B.S.; Gordon, J.I. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc. Natl. Acad. Sci. USA 2004, 101, 4596–4601. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.K. Probiotics and immunity: A fish perspective. Fish Shellfish Immunol. 2010, 29, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Peera, H.; James, V. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Ther. Adv. Gastroenter 2013, 6, 39–51. [Google Scholar]
- Seyed, H.H.; Einar, R.; Alireza, S.M.; Maria, Á.E. Probiotic, prebiotic and synbiotic supplements in sturgeon aquaculture: A review. Rev. Aquacult. 2016, 8, 89–102. [Google Scholar]
- Matsuura, Y.; Takasaki, M.; Miyazawa, R.; Nakanishi, T. Stimulatory effects of heat-killed Enterococcus faecalis on cell-mediated immunity in fish. Dev. Comp. Immunol. 2017, 74, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nueno-Palop, C.; Narbad, A. Probiotic assessment of Enterococcus faecalis CP58 isolated from human gut. Int. J. Food Microbiol. 2011, 145, 390–394. [Google Scholar] [CrossRef]
- Sayyed, K.A.; Einar, R.; Fatimah, M.Y.; Hassan, M.D.; Aini, I. Properties of Enterococcus faecalis, a new probiotic bacterium isolated from the intestine of snakehead fish (Channa striatus Bloch). Afr. J. Microbiol. Res. 2014, 8, 2215–2222. [Google Scholar] [CrossRef][Green Version]
- Castro, M.S.; Molina, M.A.; Azpiroz, M.B.; Díaz, A.M.; Ponzio, R.; Sparo, M.D.; Manghi, M.A.; Canellada, A.M. Probiotic activity of Enterococcus faecalis CECT7121: Effects on mucosal immunity and intestinal epithelial cells. J. Appl. Microbiol 2016, 121, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Q.; Luo, L.; Zhou, Y.; Ling, H.Y.; Yang, Q.H.; Qi, D.S. Dietary administration of Enterococcus faecalis affects the growth, disease resistance and immune function of tilapia (Oreochromis niloticus). Aquacult. Rep. 2020, 18, 100440. [Google Scholar] [CrossRef]
- Uriel, R.E.; Shuichi, S.; Yutaka, H.; Hiroshi, F.; John, S. Effects of Inactivated Enterococcus faecalis and Mannan Oligosaccharide and Their Combination on Growth, Immunity, and Disease Protection in Rainbow Trout. N. Am. J. Aquacult. 2013, 75, 416–428. [Google Scholar]
- Sayyed, K.A.; Einar, R.; Fatimah, M.Y.; Hassan, H.D. Dietary supplement of Enterococcus faecalis on digestive enzyme activities, short-chain fatty acid production, immune system response and disease resistance of Javanese carp (Puntius gonionotus, Bleeker 1850). Aquacult. Nutr. 2017, 23, 331–338. [Google Scholar]
- Yang, Q.H.; Lü, Y.L.; Zhang, G.; Gong, Y.; Li, Z.Z.; Tran, N.T.; He, Y.Y.; Zhu, C.H.; Lu, Y.S.; Zhang, Y.L.; et al. Lactic acid bacteria, Enterococcus faecalis Y17 and Pediococcus pentosaceus G11, improved growth performance, and immunity of mud crab (Scylla paramamosain). Fish Shellfish Immunol. 2019, 93, 135–143. [Google Scholar] [CrossRef]
- Yang, G.; Cao, H.Z.; Jiang, W.H.; Hu, B.Q.; Jian, S.Q.; Wen, C.G.; Kajbaf, K.; Kumar, V.; Tao, Z.Y.; Peng, M. Dietary supplementation of Bacillus cereus as probiotics in Pengze crucian carp (Carassius auratus var. Pengze): Effects on growth performance, fillet quality, serum biochemical parameters and intestinal histology. Aquac. Res. 2019, 50, 2207–2217. [Google Scholar] [CrossRef]
- Kong, Y.D.; Li, M.; Tian, J.X.; Zhao, L.H.; Kang, Y.H.; Zhang, L.; Wang, G.Q.; Shan, X.F. Effects of recombinant Lactobacillus casei on growth performance, immune response and disease resistance in crucian carp, Carassius auratus. Fish Shellfish Immunol. 2020, 99, 73–85. [Google Scholar] [CrossRef]
- He, M.s.; Liu, G.y.; Liu, Y.h.; Yang, K.C.; Qi, X.Z.; Huang, A.G.; Liu, T.Q.; Wang, G.X.; Wang, E.L. Effects of geniposide as immunostimulant on the innate immune response and disease resistance in crucian carp. Aquaculture 2020, 529, 735713. [Google Scholar] [CrossRef]
- Fan, Y.D.; Zhang, X.P.; Zhou, Y.; Jiang, N.; Liu, W.Z.; Zeng, L.B. Molecular cloning of Gibel carp (Carassius auratus gibelio) complement component C3 and its expression profile after Cyprinid herpesvirus 2 infection. J. Vet. Med. Sci. 2020, 82, 47–55. [Google Scholar] [CrossRef]
- Tang, J.; Wang, W.; Jiang, Y.; Chu, W.H. Diazinon exposure produces histological damage, oxidative stress, immune disorders and gut microbiota dysbiosis in crucian carp (Carassius auratus gibelio). Environ. Pollut. 2021, 269, 116–129. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Grice, A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; Green, E.D.; et al. Topographical and temporal diversity of the human skin microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Nakhro, K.; Chowdhury, S.; Kamilya, D. Effects of potential probiotic Bacillus amyloliquefaciens FPTB16 on systemic and cutaneous mucosal immune responses and disease resistance of catla (Catla catla). Fish Shellfish Immunol. 2013, 35, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Arun, C.; Rahul, S. Probiotics in aquaculture: A promising emerging alternative approach. Symbiosis 2019, 77, 99–113. [Google Scholar]
- Wang, Y.B.; Tian, Z.Q.; Yao, J.T.; Li, W.F. Effect of probiotics, Enteroccus faecium, on tilapia (Oreochromis niloticus) growth performance and immune response. Aquaculture 2008, 277, 203–207. [Google Scholar] [CrossRef]
- Li, C.; Ren, Y.C.; Jiang, S.H.; Zhou, S.; Zhao, J.S.; Wang, R.J.; Li, Y.M. Effects of dietary supplementation of four strains of lactic acid bacteria on growth, immune-related response and genes expression of the juvenile sea cucumber Apostichopus japonicus Selenka. Fish Shellfish Immunol. 2018, 74, 69–75. [Google Scholar] [CrossRef]
- Safari, O.; Paolucci, M. Effect of in vitro selected synbiotics (galactooligosaccharide and mannanoligosaccharide with or without Enterococcus faecalis) on growth performance, immune responses and intestinal microbiota of juvenile narrow clawed crayfish, Astacus leptodactylus leptodactylus Eschscholtz, 1823. Aquacult. Nutr. 2018, 24, 247–259. [Google Scholar]
- Hossain, M.S.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Sony, N.M.; Ono, S.; Fujieda, T. Comparison of the effects of inosine and inosine monophosphate on growth, immune response, stress resistance and gut morphology of juvenile red sea bream, Pagrus major. Aquaculture 2016, 458, 64–74. [Google Scholar] [CrossRef]
- Cao, H.Z.; Yu, R.H.; Zhang, Y.Y.; Hu, B.Q.; Jian, S.Q.; Wen, C.G.; Kimia, K.; Vikas, K.; Gang, Y. Effects of dietary supplementation with β-glucan and Bacillus subtilis on growth, fillet quality, immune capacity, and antioxidant status of Pengze crucian carp (Carassius auratus var. Pengze). Aquaculture 2019, 508, 106–112. [Google Scholar] [CrossRef]
- Akhter, N.; Wu, B.; Memon, A.M.; Mohsin, M. Probiotics and prebiotics associated with aquaculture: A review. Fish Shellfish Immunol. 2015, 45, 733–741. [Google Scholar] [CrossRef] [PubMed]
- María, Á.E. An overview of the immunological defenses in fish skin. Int. Sch. Res. Not. 2012, 2012, 853470. [Google Scholar] [CrossRef]
- Zhong, X.; Liu, M.; Xu, C.; Liu, W.; Abasubong, K.; Li, X. Dietary supplementation of Streptococcus faecalis benefits the feed utilization, antioxidant capability, innate immunity, and disease resistance of blunt snout bream (Megalobrama amblycephala). Fish Physiol. Biochem. 2019, 45, 643–656. [Google Scholar] [CrossRef]
- Jinhwan, P.; Wooju, K.; Wi-Sik, K.; Hyun-Do, J.; Suhee, H. Cloning and expressional analysis of secretory and membrane-bound IgM in rock bream (Oplegnathus fasciatus) under megalocytivirus infection and vaccination. Fish Shellfish Immunol. 2019, 87, 275–285. [Google Scholar]
- Giri, S.S.; Sukumaran, V.; Oviya, M. Potential probiotic Lactobacillus plantarum VSG3 improves the growth, immunity, and disease resistance of tropical freshwater fish, Labeo rohita. Fish Shellfish Immunol. 2013, 34, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Austin, B. Innate immune responses in rainbow trout (Oncorhynchus mykiss, Walbaum) induced by probiotics. Fish Shellfish Immunol 2006, 21, 513–524. [Google Scholar] [CrossRef]
- Low, C.; Wadsworth, S.; Burrells, C.; Secombes, C. Expression of immune genes in turbot (Scophthalmus maximus) fed a nucleotide-supplemented diet. Aquaculture 2003, 221, 23–40. [Google Scholar] [CrossRef]
- Meng, F.X.; Sun, Y.N.; Liu, X.Z.; Wang, J.X.; Xu, T.J.; Wang, R.X. Analysis of C3 suggests three periods of positive selection events and different evolutionary patterns between fish and mammals. PLoS ONE 2012, 7, e37489. [Google Scholar] [CrossRef] [PubMed]
- Laiño, J.; Villena, J.; Kanmani, P.; Kitazawa, H. Immunoregulatory effects triggered by lactic acid bacteria exopolysaccharides: New insights into molecular interactions with host cells. Microorganisms 2016, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.J.; Li, Z.H.; Ou, M.F.; Wu, X.J.; Qiao, X.L.; Wei, W.; Liu, Y.; Ye, J.M.; Wang, W.N. Hypoimmunity and intestinal bacterial imbalance are closely associated with blue body syndrome in cultured Penaeus vannamei. Aquaculture 2020, 522, 735118. [Google Scholar] [CrossRef]
- Lee, S.; Katya, K.; Park, Y.; Won, S.; Seong, M.; Hamidoghli, A.; Bai, S.C. Comparative evaluation of dietary probiotics Bacillus subtilis WB60 and Lactobacillus plantarum KCTC3928 on the growth performance, immunological parameters, gut morphology and disease resistance in Japanese eel, Anguilla japonica. Fish Shellfish Immunol. 2017, 61, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, J.G.; Tattoli, I.; Girardin, S.E. The intestinal epithelial barrier: How to distinguish between the microbial flora and pathogens. Semin. Immunol. 2007, 19, 106–115. [Google Scholar] [CrossRef]
- Galeano, J.A.C.; Herrera, A.L.; Suescún, J.P. The probiotic Enterococcus faecium modifies the intestinal morphometric parameters in weaning piglets. Rev. Fac. Nac. Agron. Medellín 2016, 69, 7803–7811. [Google Scholar] [CrossRef]
- Gómez, G.D.; Balcázar, J.L. A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunol. Med. Microbiol. 2008, 52, 145–154. [Google Scholar] [CrossRef]
- Merrifield, D.L.; Dimitroglou, A.; Foey, A.; Davies, J.S.; Baker, T.M.R.; Bøgwald, J.; Castex, M.; Ringø, E. The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture 2010, 302, 1–18. [Google Scholar] [CrossRef]
- Blander, J.M.; Longman, R.S.; Iliev, I.D.; Sonnenberg, G.F.; Artis, D. Regulation of inflammation by microbiota interactions with the host. Nat. Immunol. 2017, 18, 851–860. [Google Scholar] [CrossRef]
- Emilie, C.; Yannick, G.; Kevin, M.; Bénédicte, L.; David, P.; Fabien, P.; Florian, N.; Denis, S. Bacterial community characterization of water and intestine of the shrimp Litopenaeus stylirostris in a biofloc system. BMC Microbiol. 2016, 16, 157. [Google Scholar]
- Brown, K.; DeCoffe, D.; Molcan, E.; Gibson, D.L. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients 2012, 4, 1095–1119. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.B.; Gatesoupe, F.J.; Li, T.T.; Wang, X.H.; Zhang, Q.Q.; Feng, D.Y.; Feng, Y.Q.; Chen, H.; Li, A.H. Significant improvement of intestinal microbiota of gibel carp (Carassius auratus gibelio) after traditional Chinese medicine feeding. J. Appl. Microbiol. 2018, 124, 829–841. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Miao, S.Y.; Zhao, C.Z.; Zhu, J.Y.; Hu, J.T.; Dong, X.J.; Sun, L.S. Dietary soybean meal affects intestinal homoeostasis by altering the microbiota, morphology and inflammatory cytokine gene expression in northern snakehead. Sci. Rep. 2018, 8, 113–122. [Google Scholar] [CrossRef]
- Ventura, M.; Canchaya, C.; Tauch, A.; Chandra, G.; Fitzgerald, G.F.; Chater, K.F.; Sinderen, D. Genomics of actinobacteria: Tracing the evolutionary history of an ancient phylum. Microbiol. Mol. Biol. Rev. 2007, 71, 495–548. [Google Scholar] [CrossRef]
- Lu, W.H.; Chen, H.; Zhou, Y.; Li, Y.J.; Wang, X.D.; Huag, C.G. Identification and drug sensitive test of the pathogen in Acinetobacter disease from hybrid crucian carp (Carassius auratus gibelio ♀ × Cyprinus carpio ♂). Fish. Sci. 2010, 29, 156–161. [Google Scholar]
- Tsuchiya, C.; Sakata, T.; Sugita, H. Novel ecological niche of Cetobacterium somerae, an anaerobic bacterium in the intestinal tracts of freshwater fish. Lett. Appl. Microbiol. 2008, 46, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Baños, A.; Ariza, J.J.; Nuñez, C.; Gil-Martínez, L.; García-López, J.D.; Martínez-Bueno, M.; Valdivia, E. Effects of Enterococcus faecalis UGRA10 and the enterocin AS-48 against the fish pathogen Lactococcus garvieae. Studies in vitro and in vivo. Food Microbiol. 2019, 77, 69–77. [Google Scholar] [CrossRef]
- Hao, K.; Wu, Z.Q.; Li, D.L.; Yu, X.B.; Wang, G.X.; Ling, F. Effects of Dietary Administration of Shewanella xiamenensis A-1, Aeromonas veronii A-7, and Bacillus subtilis, Single or Combined, on the Grass Carp (Ctenopharyngodon idella) Intestinal Microbiota. Probiotics Antimicro. 2017, 9, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.X.; Kang, Y.H.; Zhan, S.Z.; Ze, L.; Jin, S.N.; Chen, C.; Zhang, L.; Shen, J.Y.; Wang, C.F.; Wang, G.Q.; et al. Effect of Bacillus velezensis on Aeromonas veronii -Induced Intestinal Mucosal Barrier Function Damage and Inflammation in Crucian Carp (Carassius auratus). Front. Microbiol. 2019, 10, 2663. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).