Comparison of Metabolic Rates of Young of the Year Beluga (Huso huso), Sterlet (Acipenser ruthenus) and Bester Hybrid Reared in a Recirculating Aquaculture System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fishes
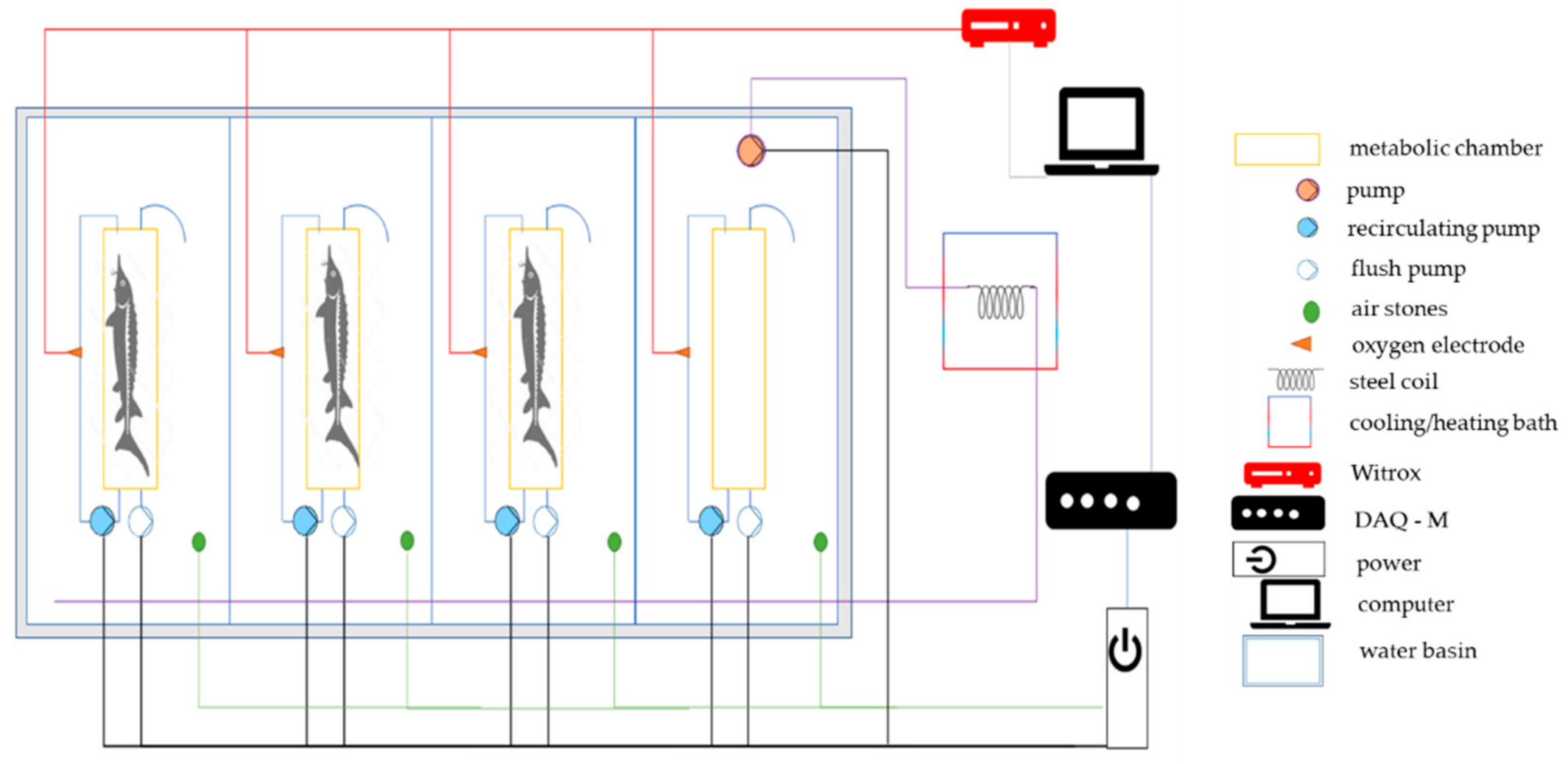
2.2. Respirometry Tests
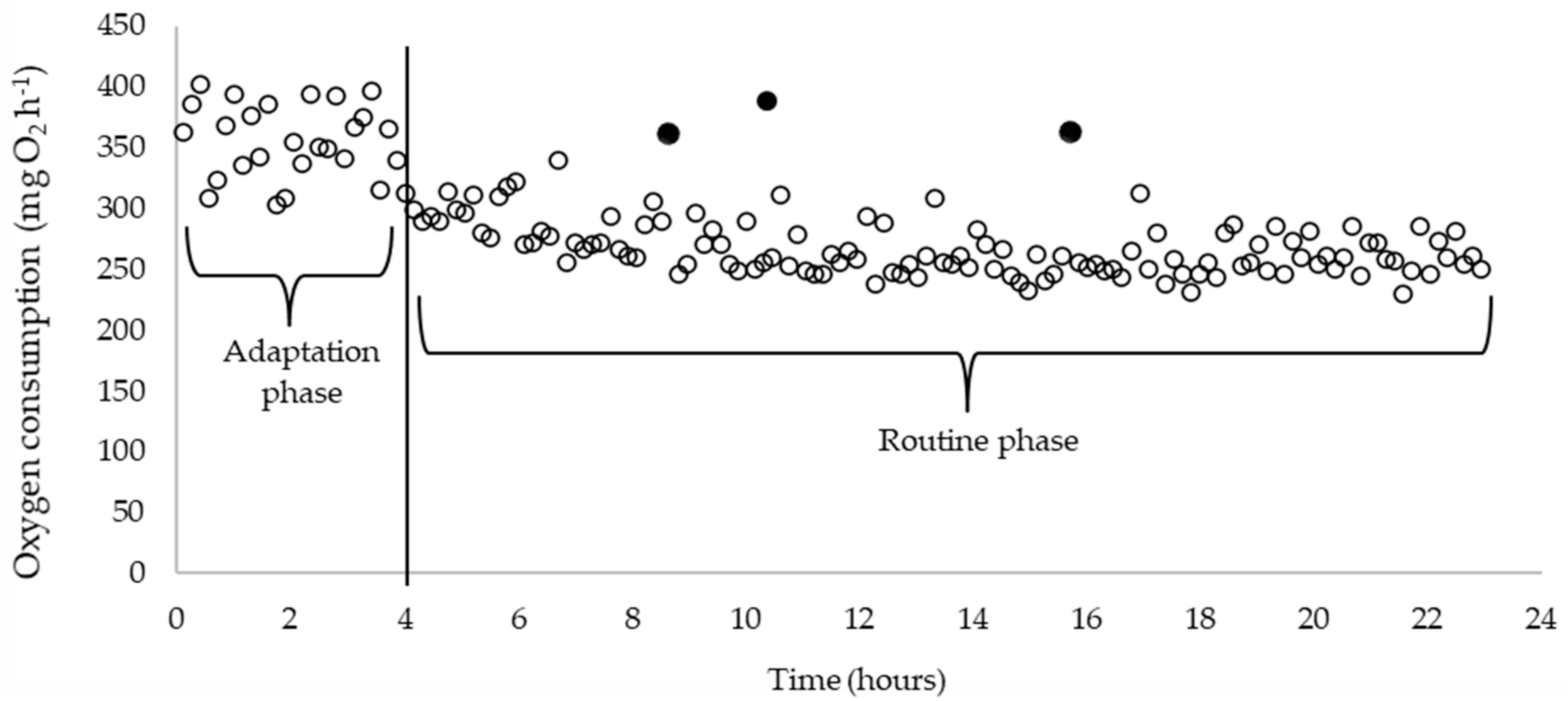
2.3. RMR and SMR Calculations
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pillay, T.V.R.; Kutty, M.N. Aquaculture: Principles and Practices, 2nd ed.; Blackwell Publishing: Hoboken, NJ, USA, 2005; pp. 7–41. [Google Scholar]
- Badiola, M.; Mendiola, D.; Bostock, J. Recirculating Aquaculture Systems (RAS) analysis: Main issues on management and future challenges. Aquac. Eng. 2012, 51, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Xiao, R.; Wei, Y.; An, D.; Li, D.; Ta, X.; Wu, Y.; Ren, Q. A review on the research status and development trend of equipment in water treatment processes of recirculating aquaculture systems. Rev. Aquac. 2019, 11, 863–895. [Google Scholar] [CrossRef]
- Cook, J.T.; McNiven, M.A.; Sutterlin, A.M. Metabolic rate of pre-smolt growth-enhanced transgenic Atlantic salmon (Salmo salar). Aquaculture 2000, 188, 33–45. [Google Scholar] [CrossRef]
- Chebanov, M.; Rosenthal, H.; Gessner, J.; van Anrooy, R.; Doukakis, P.; Pourkazemi, M.; Williot, P. Sturgeon hatchery practices and management for release—Guidelines. FAO Fish. Aquac. 2011, 110. [Google Scholar]
- Paschke, K.; Agüero, J.; Gebauer, P.; Díaz, F.; Mascaró, M.; López-Ripoll, E. Comparison of aerobic scope for metabolic activity in aquatic ectotherms with temperature related metabolic stimulation: A novel approach for aerobic power budget. Front. Physiol. 2018, 9, 1438. [Google Scholar] [CrossRef]
- Chabot, D.; Steffensen, J.F.; Farrell, A.P. The determination of standard metabolic rate in fishes. J. Fish Biol. 2016, 88, 81–121. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Reglero, P.; Ortega, A.; Folkvord, A.; Gándara, F.; de Rojas, A.H.; Moyano, M. First estimates of metabolic rate in Atlantic bluefin tuna larvae. J. Fish Biol. 2020, 97, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Burton, T.; Killen, S.S.; Armstrong, J.D.; Metcalfe, N.B. What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proc. R. Soc. B 2011, 278, 3465–3473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norin, T.; Clark, T.D. Measurement and relevance of maximum metabolic rate in fishes. J. Fish Biol. 2016, 88, 122–151. [Google Scholar] [CrossRef] [PubMed]
- Zupa, W.; Alfonso, S.; Gai, F.; Gasco, L.; Spedicato, M.T.; Lembo, G.; Carbonara, P. Calibrating Accelerometer Tags with Oxygen Consumption Rate of Rainbow Trout (Oncorhynchus mykiss) and Their Use in Aquaculture Facility: A Case Study. Animals 2021, 11, 1496. [Google Scholar] [CrossRef]
- Nelson, J. Oxygen consumption rate v. rate of energy utilization of fishes: A comparison and brief history of the two measurements. J. Fish Biol. 2016, 88, 10–25. [Google Scholar] [CrossRef] [Green Version]
- Gillooly, J.F.; Charnov, E.L.; West, G.B.; Savage, V.M.; Brown, J.H. Effects of size and temperature on developmental time. Nature 2002, 417, 70–73. [Google Scholar] [CrossRef]
- Helfman, G.S.; Collette, B.B.; Facey, D.E.; Bowen, B.W. The diversity of fishes. Biology, Evolution, and Ecology, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 57–165. [Google Scholar]
- Nagy, K.A.; Girard, I.A.; Brown, T.K. Energetics of free ranging mammals, reptiles, and birds. Annu. Rev. Nutr. 1999, 19, 247–277. [Google Scholar] [CrossRef]
- Lovegrove, B.G. The zoogeography of mammalian basal metabolic rate. Am. Nat. 2000, 156, 201–219. [Google Scholar] [CrossRef]
- Schaefer, J.; Walters, A. Metabolic cold adaptation and developmental plasticity in metabolic rates among species in the Fundulus notatus species complex. Funct. Ecol. 2010, 24, 1087–1094. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, J.B.; Schindler, D.E. Excess digestive capacity in predators reflects a life of feast and famine. Nature 2011, 476, 84–87. [Google Scholar] [CrossRef]
- Madenjian, C.P. Encyclopedia of Fish. Physiology: From Genome to Environment, 1st ed.; Academic Press: Oxford, UK, 2011; pp. 1675–1680. [Google Scholar]
- Canale, R.P.; Breck, J.E.; Shearer, K.D.; Neely, K.G. Validation of a bioenergetic model for juvenile salmonid hatchery production using growth data from independent laboratory feeding studies. Aquaculture 2013, 416–417, 228–237. [Google Scholar] [CrossRef]
- Hallajian, A.; Abdolhay, H.; Shadparvar, A.; Yarmohammadi, M.; Yazdanisadati, M. A factorial experiment for heritability estimation of the reproductive traits of the wild Persian sturgeon, Acipenser persicus. Iran. J. Fish. Sci. 2020, 19, 1954–1966. [Google Scholar] [CrossRef]
- Safabakhsh, M.; Mohseni, M.; Bahri, A.; Mohammadizadeh, F. Effect of dietary selenium on growth performance, survival rate and biochemical-blood profile of farmed juvenile Beluga (Huso huso). Iran. J. Fish. Sci. 2020, 19, 2077–2088. [Google Scholar] [CrossRef]
- Bronzi, P.; Rosenthal, H. Present and future sturgeon and caviar production and marketing: A global market overview. J. Appl. Ichthyol. 2014, 30, 1536–1546. [Google Scholar] [CrossRef]
- Ludwig, A.; Lippold, S.; Debus, L.; Reinartz, R. First evidence of hybridization between endangered Sterlets (Acipenser ruthenus) and exotic Siberian sturgeons (Acipenser baerii) in the Danube River. Biol. Invasions 2009, 11, 753–760. [Google Scholar] [CrossRef] [Green Version]
- Gisbert, E.; Cech, J.J.; Doroshov, S.I. Routine metabolism of larval green sturgeon (Acipenser medirostris Ayres). Fish Physiol. Biochem. 2001, 25, 195–200. [Google Scholar] [CrossRef]
- Kieffer, J.D.; Penny, F.M.; Papadopoulos, V. Temperature has a reduced effect on routine metabolic rates of juvenile shortnose sturgeon (Acipenser brevirostrum). Fish Physiol. Biochem. 2014, 40, 551–559. [Google Scholar] [CrossRef]
- Svendsen, J.C.; Genz, J.; Anderson, W.G.; Stol, J.A.; Watkinson, D.A.; Enders, E.C. Evidence of circadian rhythm, oxygen regulation capacity, metabolic repeatability and positive correlations between forced and spontaneous maximal metabolic rates in lake sturgeon Acipenser fulvescens. PLoS ONE 2014, 9, e94693. [Google Scholar] [CrossRef] [Green Version]
- Andrei, R.C.; Cristea, V.; Creţu, M.; Dediu, L.; Mogodan, A. The effect of temperature on the standard and routine metabolic rates of young of the year sterlet sturgeon (Acipenser ruthenus). Aquac. Aquar. Conserv. Legis. 2018, 11, 1467–1475. [Google Scholar]
- Stiller, K.T.; Vanselow, K.H.; Moran, D.; Riesen, G.; Koppe, W.; Dietz, C.; Schulz, C. The effect of diet, temperature and intermittent low oxygen on the metabolism of rainbow trout. Br. J. Nutr. 2007, 117, 784–795. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Kieffer, D.J. The effect of temperature on the resting and post-exercise metabolic rates and aerobic metabolic scope in shortnose sturgeon Acipenser brevirostrum. Fish Physiol. Biochem. 2017, 43, 1245–1252. [Google Scholar] [CrossRef]
- Mayfield, R.B.; Cech, J.J. Temperature Effects on Green Sturgeon Bioenergetics. Trans. Am. Fish. Soc. 2004, 133, 961–970. [Google Scholar] [CrossRef]
- Allen, P.J.; Cech, J.J. Age/size effects on juvenile green sturgeon, Acipenser medirostris, oxygen consumption, growth, and osmoregulation in saline environments. Environ. Biol. Fishes 2007, 79, 211–229. [Google Scholar] [CrossRef]
- McKenzie, D.; Cataldi, E.; Romano, P.; Owen, S.F.; Taylor, E.W.; Bronzi, P. Effects of acclimation to brackish water on the growth, respiratory metabolism, and swimming performance of young-of-the-year Adriatic sturgeon (Acipenser naccarii). Can. J. Fish. Aquat. Sci. 2001, 58, 1104–1112. [Google Scholar] [CrossRef]
- Secor, D.H.; Gunderson, T.E. Effects of hypoxia and temperature on survival, growth, and respiration of juvenile Atlantic sturgeon, Acipenser oxyrinchus. Fish. Bull. 1998, 96, 603–613. [Google Scholar]
- Rosewarne, P.J.; Wilson, J.M.; Svendsen, J.C. Measuring maximum and standard metabolic rates using intermittent-flow respirometry: A student laboratory investigation of aerobic metabolic scope and environmental hypoxia in aquatic breathers. J. Fish Biol. 2016, 88, 265–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svendsen, M.B.; Bushnell, S.P.G.; Steffensen, J.F. Design and setup of intermittent-flow respirometry system for aquatic organisms. J. Fish Biol. 2016, 88, 26–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahanbakhshi, A.; Shaluei, F.; Baghfalaki, M.; Ramazi, F.G.; Ahmadvand, S. Efficacy of 2-phenoxyethanol as an Anesthetic for Two Size of Persian Sturgeon, Acipenser persicus. J. Walailak. 2012, 9, 31–36. [Google Scholar]
- Svendsen, M.B.S.; Bushnell, P.G.; Christensen, E.A.F.; Steffensen, J.F. Sources of variation in oxygen consumption of aquatic animals demonstrated by simulated constant oxygen consumption and respirometers of different sizes. J. Fish Biol. 2016, 88, 51–64. [Google Scholar] [CrossRef]
- Svendsen, M.B.S.; Andersen, N.R.; Hansen, P.J.; Steffensen, J.F. Effects of Harmful Algal Blooms on Fish: Insights from Prymnesium parvum. Fishes 2018, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Rosewarne, P.J.; Svendsen, J.C.; Mortimer, R.J.G.; Dunn, A.M. Muddied waters: Suspended sediment impacts on gill structure and aerobic scope in an endangered native and an invasive freshwater crayfish. Hydrobiologia 2014, 722, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Urbina, M.A.; Glover, C.N. Relationship between fish size and metabolic rate in the oxy conforming inanga Galaxias maculatus reveals size-dependent strategies to withstand hypoxia. Physiol. Biochem. Zool. 2013, 86, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Seginer, I.; Mozes, N. A note on oxygen supply in RAS: The effect of water temperature. Aquac. Eng. 2012, 50, 46–54. [Google Scholar] [CrossRef]
- Summerfelt, S.T.; Vinci, B.J. Better management practices for recirculating aquaculture systems. In Environmental Best Management Practices for Aquaculture; Tucker, C.S., Hargreaves, J.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2008; Volume 297, pp. 389–426. [Google Scholar] [CrossRef]
- Burggren, W.W.; Randall, D.J. Oxygen uptake during hypoxic exposure in the sturgeon Acipenser transmontanus. Respir. Physiol. 1978, 34, 171–183. [Google Scholar] [CrossRef]
- Peake, S.J. Swimming and respiration. In Sturgeons and Paddlefish of North America; Le Breton, G.T.O., Beamish, F.W.H., McKinley, R.S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 147–166. [Google Scholar]
- Cai, L.; Johnson, D.; Fang, M.; Mandal, P.; Tu, Z.; Huang, Y. Effects of feeding, digestion and fasting on the respiration and swimming capability of juvenile Sterlet sturgeon (Acipenser ruthenus, Linnaeus 1758). Fish Physiol. Biochem. 2016, 43, 279–286. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, D.; Piraccini, G.; Papini, N.; Galli, C.; Bronzi, P.; Bolis, C.G.; Taylor, E.W. Oxygen consumption and ventilatory reflex responses are influenced by dietary lipids in sturgeon. Fish Physiol. Biochem. 1997, 16, 365–379. [Google Scholar] [CrossRef]
- Cai, L.; Johnson, D.; Mandal, P.; Gan, M.; Yuan, X.; Tu, Z.; Huang, Y. Integrating water flow, locomotor performance and respiration of Chinese sturgeon during multiple fatigue recovery cycles. PLoS ONE 2014, 9, e94345. [Google Scholar] [CrossRef]
- Cai, L.; Johnson, D.; Mandal, P.; Gan, M.; Yuan, X.; Tu, Z.; Huang, Y. Effect of exhaustive exercise on the swimming capability and metabolism of juvenile Siberian sturgeon. Trans. Am. Fish. Soc. 2015, 144, 532–538. [Google Scholar] [CrossRef]
- Cai, L.; Taupier, R.; Johnson, D.; Tu, Z.; Liu, G.; Huang, Y. Swimming capability and swimming behavior of juvenile Acipenser schrenckii. J. Exp. Zool. A Ecol. Integr. Physiol. 2013, 319, 149–155. [Google Scholar] [CrossRef]
- Patterson, J.T.; Mims, S.D.; Wright, R.A. Effects of body mass and temperature on routine metabolism of American paddlefish Polyodon spathula. J. Fish Biol. 2013, 82, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Steyermark, A.C. A high standard metabolic rate constrains juvenile growth. Zoology 2002, 105, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Biro, P.A.; Stamps, J.A. Do consistent individual differences in metabolic rate promote consistent individual differences in behavior? Trends Ecol. Evol. 2010, 25, 653–659. [Google Scholar] [CrossRef]
- Gesner, J.; Chebanov, M.; Freyhof, J. Huso huso. The IUCN Red List of Threatened Species 2010: E.T10269A3187455. Available online: https://dx.doi.org/10.2305/IUCN.UK.2010-1.RLTS.T10269A3187455.en (accessed on 13 June 2021).
- Furdean, S.; Lalescu, D.; Mihailov, S.A.; Grozea, A. Growth dynamic of the main morphological traits in a sterlet (Acipenser ruthenus) population reared into recirculating aquaculture system, from 2 to 6 months old. Res. J. Agric. Sci. 2017, 49, 128–134. [Google Scholar]
- Pakkasmaa, S.; Penttinen, O.P.; Piironen, J. Metabolic rate of Artic charr eggs depends on their parentage. J. Comp. Physiol. B 2006, 176, 387–391. [Google Scholar] [CrossRef]
- Brick, M.E.; Cech, J.J. Metabolic responses of juvenile striped bass to exercise and handling stress with various recovery environments. Trans. Am. Fish. Soc. 2002, 131, 855–864. [Google Scholar] [CrossRef]
- Enders, E.C.; Boisclair, D.; Boily, P.; Magnan, P. Effect of body mass and water temperature on the standard metabolic rate of juvenile yellow perch, Perca flavescens (Mitchill). Environ. Biol. Fishes 2006, 76, 399–407. [Google Scholar] [CrossRef]
- Tran-Duy, A.; Schrama, J.W.; Van Dam, A.A.; Verreth, J.A.J. Effects of oxygen concentration and body weight on maximum feed intake, growth and hematological parameters of Nile tilapia, Oreochromis niloticus. Aquaculture 2008, 275, 152–162. [Google Scholar] [CrossRef]
- Schmidt, N.K. Scaling: Why Is Animal Size So Important? Cambridge University Press: Cambridge, UK, 1984; pp. 7–32. [Google Scholar] [CrossRef]
- Mitz, S.V.; Newman, M.C. Allometric relationship between oxygen consumption and body weight of mosquitofish, Gambusia affinis. Environ. Biol. Fishes 1989, 24, 267–273. [Google Scholar] [CrossRef]
- Glazier, D.S. Beyond the ‘3/4-power law’: Variation in the intra- and interspecific scaling of metabolic rate in animals. Biol. Rev. Camb. Philos. Soc. 2005, 80, 611–662. [Google Scholar] [CrossRef]
- Labra, F.A.; Marquet, P.A.; Bozinovic, F. Scaling metabolic rate fluctuations. Proc. Natl. Acad. Sci. USA 2007, 104, 10900–10903. [Google Scholar] [CrossRef] [Green Version]
- Clarke, A.; Johnston, N.M. Scaling of metabolic rate with body mass and temperature in teleost fish. J. Anim. Ecol. 1999, 68, 893–905. [Google Scholar] [CrossRef]
- White, C.R.; Phillips, N.F.; Seymour, R.S. The scaling and temperature dependence of vertebrate metabolism. Biol. Lett. 2006, 2, 125–127. [Google Scholar] [CrossRef] [Green Version]
- Jobling, M. A study of some factors affecting rates of oxygen consumption of plaice, Pleuronectes platessa L. J. Fish Biol. 2006, 20, 501–516. [Google Scholar] [CrossRef]
- Rubalcaba, J.G.; Verberk, W.C.; Hendriks, A.J.; Saris, B.; Woods, H.A. Oxygen limitation may affect the temperature and size dependence of metabolism in aquatic ectotherms. Proc. Natl. Acad. Sci. USA 2020, 117, 31963–31968. [Google Scholar] [CrossRef]

| Species | Fish Weight (g) | Total Length (cm) | ||||
|---|---|---|---|---|---|---|
| Mean ± SE | Minimum | Maximum | Mean ± SE | Minimum | Maximum | |
| Acipenser ruthenus | 77.33 ± 5.36 | 54.00 | 99.00 | 28.2 ± 1.40 | 23.10 | 29.93 |
| Huso huso | 84.78 ± 4.42 | 67.00 | 107.00 | 30.2 ± 1.23 | 27.12 | 32.71 |
| Bester | 75.33 ± 2.50 | 65.00 | 89.00 | 27.9 ± 1.24 | 27.24 | 29.22 |
| Species | SMR | RMR | ||||
|---|---|---|---|---|---|---|
| Mean ± SE | Min | Max | Mean ± SE | Min | Max | |
| A. ruthenus | 21.04 ± 0.96 ** | 17.33 | 24.51 | 33.06 ± 1.17 ** | 29.63 | 38.52 |
| H. huso | 17.78 ± 0.60 * | 15.73 | 18.86 | 24.23 ± 1.32 * | 20.28 | 31.25 |
| Bester | 17.03 ± 0.38 * | 15.73 | 18.86 | 24.23 ± 1.32 * | 20.28 | 31.25 |
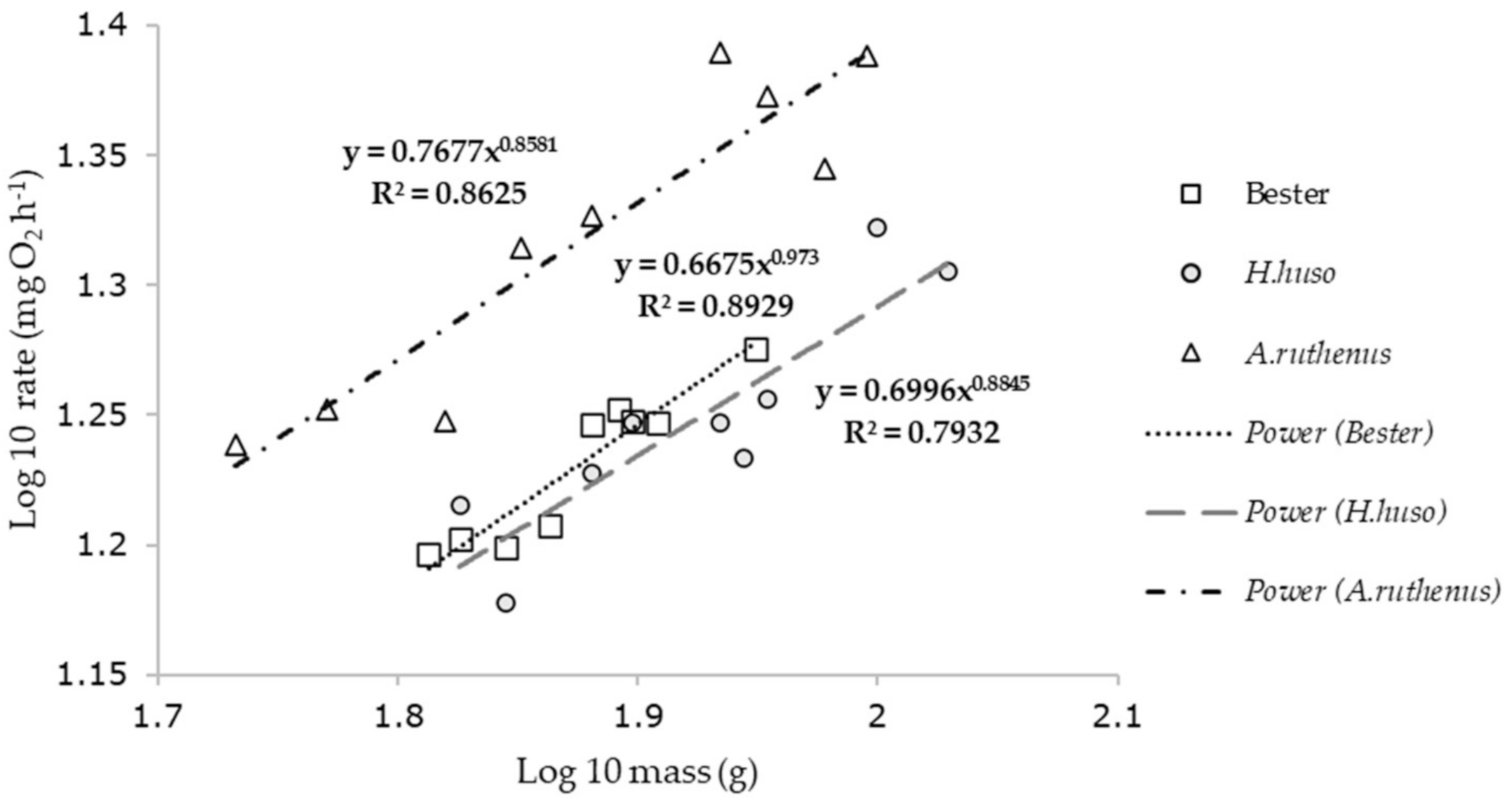
| Species | Regression Equations | ||
|---|---|---|---|
| y = oxygen uptake (mg h−1); x = mass (g) | r2 | n | |
| Acipenser ruthenus | y = 0.76x0.85 | 0.86 | 9 |
| H. huso | y = 0.69x0.88 | 0.78 | 9 |
| Bester | y = 0.66x0.9 | 0.89 | 9 |
| Species | Body Weight (g) | RMR (mg O2 kg−1 h−1) | Experimental Conditions | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| T (°C) | Rearing System | Fasting Period before Trial (hours) | Photoperiod (hours) | Acclimatization Period before Trial (hours) | ||||
| A. brevirostrum | 11 | 309 ± 33 | 25 | FTS | 24 | natural | 4 | [26] |
| 100 | 127 ± 8.8 | 15 | FTS | 24 | 12 L/12 D | 2 | [30] | |
| 100 | 199 ± 3 | 20 | FTS | 24 | 12 L/12 D | 12 | [30] | |
| 100 | 253 ± 18 | 25 | FTS | 24 | 12 L/12 D | 12 | [30] | |
| A. ruthenus | 14.58 ± 2.81 | approx. 320 | 20 | FTS | 48 | natural | 2 | [46] |
| A. oxyrinchus | 219.35 ± 2.2 | 217 ± 0.024 | 19.7 ± 0.05 | RAS | 12 | natural | - | [34] |
| approx. 69 | 200 to 300 | 19.26 | RAS | 12 | natural | - | [34] | |
| A. naccarii | 198 ± 0.015 | 110 ± 9 | 23 ± 1 | RAS | - | natural | 10 | [47] |
| 100 | 216 ± 25 | 23 ± 1 | RAS | 22 | natural | 22 | [33] | |
| A. sinensis | 8.38 ± 0.27 | 266.6 ± 30.94 | 19.3–20.8 | RAS | 8 | - | - | [48] |
| A. baeri | 14.5 ± 0.8 | 168.29 ± 2.29 | 20 ± 0.5 | RAS | 48 | natural | 1 | [49] |
| A. fulvescens | 30.51 ± 1.21 | 88.44 ± 3.54 | 17 ± 1 | FTS | 48 | 12 L/12 D | 20 | [27] |
| A. medirostris | 145.7 ± 3.8 | 63.36 ± 6.02 | 12 ± 0.01 | RAS | 72 | natural | - | [32] |
| 30.3 ± 17.5 | 270 | 24 | RAS | 24 | natural | 6 | [31] | |
| A. schrenckii | 32.7 ± 1.2 | 295.38 ± 10.42 | 20 ± 0.5 | RAS | 72 | 12 L/12 D | 2 | [50] |
| P. spathula | 500 ± 130 | 157.49 ± 22.54 | 21 ± 0.3 | P | 72 | natural | 1 | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crețu, M.; Guriencu, R.-C.; Dediu, L.; Stroe, M.-D. Comparison of Metabolic Rates of Young of the Year Beluga (Huso huso), Sterlet (Acipenser ruthenus) and Bester Hybrid Reared in a Recirculating Aquaculture System. Fishes 2021, 6, 46. https://doi.org/10.3390/fishes6040046
Crețu M, Guriencu R-C, Dediu L, Stroe M-D. Comparison of Metabolic Rates of Young of the Year Beluga (Huso huso), Sterlet (Acipenser ruthenus) and Bester Hybrid Reared in a Recirculating Aquaculture System. Fishes. 2021; 6(4):46. https://doi.org/10.3390/fishes6040046
Chicago/Turabian StyleCrețu, Mirela, Raluca-Cristina Guriencu, Lorena Dediu, and Maria-Desimira Stroe. 2021. "Comparison of Metabolic Rates of Young of the Year Beluga (Huso huso), Sterlet (Acipenser ruthenus) and Bester Hybrid Reared in a Recirculating Aquaculture System" Fishes 6, no. 4: 46. https://doi.org/10.3390/fishes6040046
APA StyleCrețu, M., Guriencu, R.-C., Dediu, L., & Stroe, M.-D. (2021). Comparison of Metabolic Rates of Young of the Year Beluga (Huso huso), Sterlet (Acipenser ruthenus) and Bester Hybrid Reared in a Recirculating Aquaculture System. Fishes, 6(4), 46. https://doi.org/10.3390/fishes6040046








