Abstract
Fishmeal is the most expensive feedstuff in the aquafeed and one of the most environmentally limiting factor of aquaculture development. Therefore, the search for alternative protein sources is a continuous process. The present feeding trial was conducted to evaluate the effects of replacing fishmeal with zooplankton biomass meal (ZBM) on the growth performance, nutrient utilization, intestine, and liver histological changes of grey mullet, Mugil cephalus (initial weight of 0.10 ± 0.01 g). Five isoproteic (35% crude protein) and isolipidic (8% crude lipid) diets were formulated as the control diet (Z0) and the other four diets (Z25, Z50, Z75, and Z100), where 25%, 50%, 75%, and 100% of fishmeal was replaced by ZBM, respectively. After 60 days of feeding, the final weight, weight gain, and daily growth index of the grey mullet fed the Z100 diet were higher than those fed the control diet (p < 0.05). In addition, the better values of feed conversion ratio, protein efficiency ratio and lipid efficiency ratio were recorded in the fish fed with the Z100 diet. Additionally, the intestinal villus length, crypts depth, and muscle thickness were significantly improved with ZBM inclusion (p < 0.05). Meanwhile, there were no histopathological changes observed on the liver when compared with the control group. From the economic point of view, dietary substitution of fishmeal by ZBM (Z100) reduced the cost of diet formulation by 18% and the price per kg weight gain by about 40%. Overall, according to the findings of this study, substituting fishmeal with ZBM up to 100% could improve growth performance, feed utilization, gut health status, and profit ability of rearing M. cephalus juveniles.
1. Introduction
The grey mullet, Mugil cephalus, is an economically valuable euryhaline and eurythermal species in many countries, contributing to large fisheries in estuarine and coastal areas [1,2,3]. Because of its strong tolerance to captivity, fast development, omnivorous feeding habits, and high market price, this fish species has been identified as a potential species for aquaculture diversification in the Mediterranean region, as well as in other parts of the world [2,4,5]. This reality necessitates the advancement of breeding technologies, as well as a suitable and cost-effective grow-out diet. However, the majority of commercial grey mullet aquaculture production also relies on wild-caught fry, which is less expensive but not sustainable [2,6,7]. Mullets are characterized as omnivorous, opportunistic feeders which thrive on any food that comes their way [8]. However, knowledge is scarce about how to formulate realistic feed for cultured mullets [9,10].
Microcrustaceans, Copepods, zooplankton, detritus, and benthic microalgae are the main food sources for grey mullet larvae [11]. This suggests that on-growing diets for this species could include high levels of fishmeal (FM) replacement with alternate protein sources [12,13,14,15]. This is also significant because the global supply of FM remains largely stable, resulting in high processing costs and a decline in its availability for large-scale use in high-quality fishmeal-based diets [16,17,18]. More research is required to find more cost-effective alternative protein-rich feed ingredients that are appropriate for omnivorous fish species [19,20].
Alternative protein sources may be the main factor that helps in lowering feed costs and increasing the industry’s economic viability. Zooplankton has been deemed “nutritionally superior live feed” for fish larvae and juveniles of cultivable species, demonstrating that it has a higher nutritional benefit than Artemia; therefore, it plays an important role in initial feeding for survival and growth [21,22,23]. Freshwater zooplankton biomass consists of three main species, Rotifera, Cladoceran, and Copepoda, which have been successfully used as live feed in the aquaculture industry [24,25,26,27]. According to biochemical research, zooplankton is high in proteins (45–52%), lipids (9–12%), essential amino acids, and essential fatty acids; it is a natural food source for many aquatic species and has high reproductive performance, as well as producing high biomass under the right culture conditions, enabling it to be used as an ingredient in the aqua-feed [28,29,30].
Zooplankton meal succeeded in replacing 100% of FM in the diet of Dicentrarchus labrax fingerlings without compromising growth performance and feed utilization [31]. The study of Sharahi, et al. [32] revealed that the replacement of FM with gammarus meal up to 20% significantly improved different growth performance criteria and feed utilization of Siberian sturgeon, Acipenser baerii. In addition, the use of Dahpnia magna meal as an FM alternative in the diet of grey mullet showed a quadratic regression trend in terms of growth and feed utilization, with the best replacement level at 75% of FM [33]. The previous studies showed that the total zooplankton meal could replace 100% of FM; however, using individual types of zooplankton (daphnia or gammarus) partially succeeded in replacing FM in the aquatic diet. Accordingly, the use of total zooplankton meal as feed ingredient to replace FM in the diet of grey mullet could be a promising alternative. Therefore, this study was conducted to evaluate the growth performance, feed utilization, carcass traits, histological status, and economic evaluation of grey mullet fed diets with different FM replacement levels with zooplankton biomass meal (ZBM).
2. Materials and Methods
The fish rearing, handling, and sampling procedures were approved by the institutional animal care and use committee of Alexandria University with approval No. AU:19/21/05/27/3/18.
2.1. Fish and Feeding Management
Grey mullet larvae were obtained from the Mediterranean Sea, Rosita Fry Collection Center, (RFCC), Al-Behira, Egypt. Before the growth trial, all fish were adapted to laboratory conditions for two weeks. The fish were fed 50% Artemia nauplii (15–20 nauplii mL−1) and 50% control diet for 7 days, then gradually weaned to the control diet during the second 7 days. After that, 1500 apparent healthy juveniles (initial body weight (BW) 0.10 ± 0.01 g and standard length (SL) 1.36 ± 0.05 cm) were randomly distributed into 15 glass aquariums (100 L). All aquaria were assigned to five groups, each group with three replicates (n = 100 fry per aquarium), and the fish were fed five times daily (9:00, 12:00, 15:00, 18:00, and 21:00) for 60 days. The daily feeding rate was approximately 15% of the fish body weight. The husbandry system was supplied with sand-filtered, chlorinated, and UV-disinfected water and about 70% of the water was exchanged daily.
Water quality conditions during the acclimation and experimental periods were as follows: temperature of around 17.6–20.7 °C; dissolved oxygen, in a range from 5.40 to 6.45 mg L−1; NH4+, 0.16–0.29 mg L−1; the photoperiod was 10L:14D (light: darkness).
2.2. Preparation of Diets and Zooplankton Biomass Meal (ZBM)
Zooplankton biomass was collected in May 2019 from El-Mahmoudia Canal (31°3007′ N and 29°4166′ E), using a standard plankton net (No. 25) of 55 μm mesh size with a diameter of 55 cm as described by Abo-Taleb, et al. [23], Manickam, et al. [34]. The collected freshwater zooplankton biomass consisted of 48.70% Rotifera, 46.10% Copepoda, 3.09% Cladocera, 1.21% Ostracoda and 0.91% Protozoa. The obtained biomass was cleaned, washed with distilled water, dried at 50 °C for 24 h, and finely ground using an electric mill. Approximate composition of ZBM and ingredients of the experiment diets are presented in Table 1. The control diet (Z0) was formulated with using FM as animal protein source (200 g/kg), then the FM was replaced by ZBM at the ratios of 25%, 50%, 75%, and 100% (Z25, Z50, Z75, and Z100), respectively, to form five iso-energetic and iso-nitrogenous experimental diets.

Table 1.
Approximate composition of the ingredients used in the experimental diet (dry matter, %).
All the ingredients were finely mashed and sieved through a 60-mesh sieve before being completely combined. To form a dough, water and oil were progressively added to the dry ingredients and the mixture was pelleted using a meat grinder. Finally, pellets were dried and crushed at 50 °C for 24 h, sieved to generate feed particles with an average size of 0.5 mm, and stored at −20 °C. The diet formulation and approximate composition are shown in Table 2.

Table 2.
Formulation and approximate composition of experimental diets (dry weight, %).
2.3. Growth Performance and Survival Determinations
Prior to the start of the growth trial, 50 fish were randomly collected to measure the initial weight and length. After 60-days, all the fish (after having fasted overnight) were taken from the aquarium with a dip net and gently anesthetized with 50 mg of clove oil l−1; then, their wet BW (g) and SL (cm) determined to the nearest 1.0 mg and 1.0 mm, respectively. Ten fish from each replicate per treatment were directly stored at −20 °C to analyze whole-body composition. In addition, 3 specimens per replicate were sacrificed with an overdose of anesthetic for assessing the histological changes in the liver and intestine. Survival rate (SR), weight gain (WG), daily growth index (DGI), thermal growth coefficient (TGC), length gain (LG), feed conversion ratio (FCR), protein efficiency ratio (PER), lipid efficiency ratio (LER), and condition factor (CF) were calculated using the following formulae:
Survival (%) = 100 × (final fish number/initial fish number)
WG (g) = final body weight − initial body weight
DGI (%) = (final body weight1∕3 − initial body weight1∕3)/days × 100
TGC= (final body weight1∕3 − initial body weight1∕3)/days × temperature °C
LG (cm) = (final body length − initial body length)
CF(g/cm3) = 100 × (live weight/final body length3)
FER (%) = 100 × (weight gain/total feed intake)
PER = weight gain/total protein intake (g)
LER = weight gain/total lipid intake (g)
2.4. Proximate Chemical Analysis
All chemical analyses of ingredients, experimental diets, and fish were examined by the standard methods of AOAC [35]. The whole fish that had been sampled before and after the experiment were ground and pooled. The moisture content of the samples was evaluated by drying them at 105 °C for 24 h. Before the chemical analysis, frozen body samples were freeze-dried. The ash content of feed and whole-body fish was determined using a muffle furnace at 550 °C for 5 h (Nabertherm B150, Bremen, Germany). Crude protein (N × 6.25) was determined using the Kjeldahl system method (VELP Scientifica, UDK 149, Usmate Velate, Italy). Crude lipid content was determined by petroleum ether extraction (40–60 °C) using a Soxhlet System (VELP Scientifica, SER 148, Usmate Velate, Italy).
2.5. Histological Examination of the Liver and Midgut
Hepatopancreas and midgut samples of six fish per treatment were selected randomly, then dissected and fixed in 10% buffered formalin solution for 24 h; then, they were dehydrated in ethanol, treated with xylene, embedded in paraffin wax, sectioned into 5 µm sections using a microtome, and stained with haematoxylin and eosin (H&E), according to conventional histological techniques [36]. The examination was carried out using an Olympus IX71 light microscope and a digital camera (C-4000 zoom) (Olympus Scientific Solutions Americas Inc., Waltham, MA, USA). Villus height, villus width, muscle thickness, and crypt depth were all measured in sections of the intestine according to Hamidian, et al. [37].
2.6. Economic Evaluation
An economic evaluation of the five experimented diets was calculated, including the cost of feed and the price of fish. The cost of experimental diets was calculated in Egyptian pounds (LE) according to international and local market prices, per kg, as follows: fishmeal, LE 40.00; zooplankton biomass meal, LE 20.00; soybean meal, LE 8.50; corn meal, LE 3.70; rice polishing, LE 4.00; fish oil, LE 27.00; vitamin and mineral premix, LE 30.00. As the zooplankton biomass meal was collected from nature, its cost was calculated based on the cost of collection, cleaning, drying, grinding, and sieving.
2.7. Statistical Analysis
The SPSS version 25.0 software was used to analyze the data using one-way analysis of variance, followed by Tukey’s honestly significant difference test. If there were significant differences between the groups, Tukey’s multiple comparison test was performed. Mean differences were considered significant at a p-value equal to or less than 0.05. All data are presented as mean ± standard error of the mean. In addition, to determine the fit regression model between increasing ZBM levels and different measurements, orthogonal quadratic contrasts were performed [38].
3. Results
3.1. Effects of ZBM on Growth Performance and Survival of Grey Mullet
The growth performance of grey mullet fed diets with different FM replacement levels by ZBM is presented in Table 3. The FBW, WG, LG, DGI, and TGC pronouncedly increased in fish fed on the ZBM-diets compared to the control (Z0). The highest significant values of growth performance were observed for fish fed Z100, compared with fish fed other diets (Z0, Z25, Z50 and Z75). A significantly lower condition factor (CF) was observed in fish fed Z75 in comparison with fish fed the control and Z50.

Table 3.
Survival rate and growth of grey mullet, Mugil cephalus, fed experimental diets for 60 days.
The increase in the FM replacement level with ZBM linearly increased survival and growth performance parameters. In addition, the WG of grey mullet was positively correlated with the percentage of FM replacement in the diet (R2 = 0.9848) according to the following equation: Y = 0.00468X + 1.526 (Figure 1).
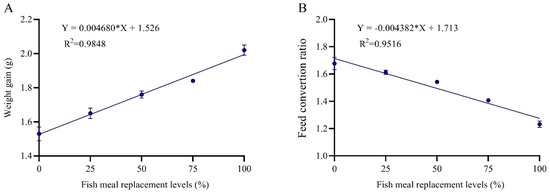
Figure 1.
The linear regression between weight gain (A) and feed conversion ratio (B), and the percentage of dietary fishmeal replacement level for grey mullet, Mugil cephalus, fed five experimental diets for 60 days.
3.2. Effects of ZBM on Feed Utilization of Grey Mullet
The effects of replacing FM with ZBM on feed and nutrient utilization parameters of grey mullet are presented in Table 4. The FER and PER were significantly influenced by different replacement levels. Fish fed on Z100 presented significantly higher PER and PPV and significantly lower FCR, compared with other investigated diets. A significant improvement was observed in PER values using replacement diets, as compared to the control diet (Table 4). There was a significant linear trend regarding the increasing replacement levels of FM with ZBM for all feed and nutrient utilization, whereas the FCR was positively correlated (R2 = 0.95) with FM replacement levels (Y = −0.004382*X + 1.713). However, the total lipid intake showed a significant quadratic trend with increasing ZBM inclusion and the maximum response was reached at level Z50 and decreased with the increasing of the inclusion levels.

Table 4.
Feed utilization of grey mullet, Mugil cephalus, fed experimental diets for 60 days.
3.3. Effects of ZBM on Whole-Body Composition of Grey Mullet
The effects of dietary FM replacement with ZBM on the approximate composition of grey mullet whole-body are presented in Table 5. In comparison with the other groups, the fish fed dietary Z100 had a higher crude protein content with a linear increasing trend with the increase in FM replacement with ZBM. The Z100 diet showed significant decrease in the lipid content compared to other groups. A significant decrease was observed in the ash content (inorganic matter) of the fish administrated with Z75 when compared with other groups.

Table 5.
The approximate composition (% on dry matter basis) of whole-body of grey mullet, Mugil cephalus, fed experimental diets for 60 days.
3.4. Effects of ZBM on Intestinal Histology and Histomorphometric Indices of Grey Mullet
According to Figure 2 and Figure 3, some of the intestinal morphological indexes, including villus width, villus height, crypt depth, and muscle thickness, were altered in grey mullet in response to the experimental diets. The inclusion of ZBM significantly increased villus length, width, and muscle thickness, compared with the low ZBM inclusion and the control group. The crypt depth increased in the fish administrated with Z100 followed by Z75 diet, while the lowest value was recorded in the control group.
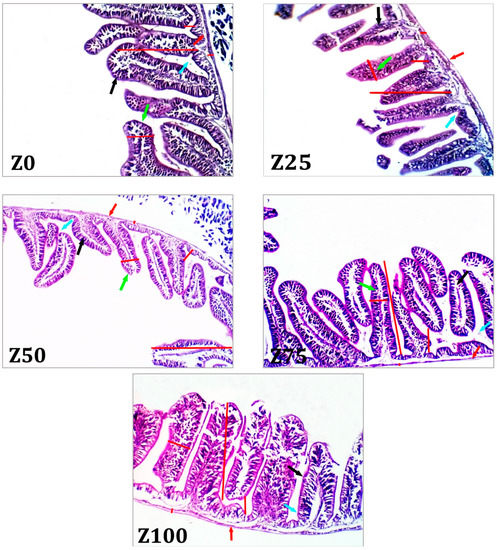
Figure 2.
Photomicrograph from intestine of the control (Z0) group showing normal histo-morphological structures of the villi and intestinal whale. The increase in ZBM levels show a comparatively increase in both villous length, crypts length and muscle thickness, with a characteristic increase in the number of goblet cells. Villous length (black arrow), crypt depth (blue arrow), villous width (green arrow), and muscle thickness (red arrow). Z0 = diet without zooplankton biomass meal (ZBM) inclusion; Z25, Z50, Z75, and Z100 have 25, 50, 75, and 100% fishmeal replaced with ZBM, respectively.

Figure 3.
Intestine histo-morphometrics indexes of grey mullet, Mugil cephalus, fed experimental diets for 60 days: (a) villus length; (b) villus width; (c) crypts length; (d) muscle thickness. Z0 = diet without zooplankton biomass meal (ZBM) inclusion; Z25, Z50, Z75, and Z100 have 25, 50, 75, and 100% fishmeal replaced with ZBM, respectively. The columns bearing different superscripts indicate significant differences as determined by Tukey’s test (p ≤ 0.05).
3.5. Effects of ZBM on Hepatic Histology of Grey Mullet
Hepatic histology is presented in Figure 4. The structure of the hepatocyte in Z0 recorded normally organized hepatic lobules with ill-district boundaries and radially arranged hepatic cords around a well-formed thin-walled central vein engorged with nucleated erythrocytes. Elongated and branched hepatic sinusoids were seen among the hepatic cords. The hepato-portal area and the hepato-pancreatic structures were ill-developed. In this study, no record of any pathological changes was observed in the other groups fed on Z25, Z50, Z75, and Z100, but there were hepatic blood vessels and hepatic sinusoids that appeared mildly dilated and increased numbers of circulating erythrocytes in groups fed on Z75 and Z100.

Figure 4.
Photomicrograph from liver of the control group showing normally organized hepatic lobules with ill district boundaries and radially arranged hepatic cords (black arrows) around a well-formed thin-walled central veins enfgorged with nucleated erythrocytes (green arrow). Hepatic sinusoids are seen among the hepatic cords (red arrows). There was no histopathological change in the other groups except Z75 and Z100; hepatic blood vessels and hepatic sinusoids in this group appeared moderately dilated, with increased numbers of circulating erythrocytes (green arrows). Z0 = diet without zooplankton biomass meal (ZBM) inclusion; Z25, Z50, Z75, and Z100 have 25, 50, 75, and 100% fishmeal replaced with ZBM, respectively.
3.6. Effects of ZBM on Economic Evaluation of Grey Mullet
The economic analysis of the experimented diets is presented in Table 6. The simple economic analysis of fish production in the present study showed that diet costs decreased with the increase in levels of ZBM in fish diets from 13.99 to 11.42 LE per kg diet.

Table 6.
Economic efficiency for production of 1 kg gain of grey mullet, Mugil cephalus, fed experimental diets for 60 days.
4. Discussion
The world production of zooplankton biomass represents hundreds of millions of tones. Only a small portion of this biomass is harvested and the remainder is not used in commercial products [17,39,40]. However, ZBM contains a high percentage of protein, reaching 45–52% crude protein on dry matter basis [41,42,43]. Therefore, the high protein content of ZBM indicates that it may be used as an alternative animal protein source in the diet of farmed fish [44], if we farm zooplankton. According to our knowledge, until now, there is no practical diet for grey mullet juveniles that contains ZBM as a total or partial replacement of FM.
The current study clearly confirmed that ZBM can totally replace FM in a practical diet for grey mullet juveniles. When compared to fish fed the FM-based diet, fish fed increasing levels of ZBM based-diets (Z25, Z50, Z75, and Z100) showed a trend toward better growth performance, feed consumption, carcass composition, and lower diet preparation expense (Z0). The results of the present study concluded that fish fed Z100 showed the highest WG, LG, DGI, FER, and survival, while achieved the lowest significant FCR value, compared with Z0 and other treatments. This data clearly confirms that 100% substitution of FM by ZBM is the ideal replacement of FM for grey mullet juveniles. The result was similar to the observation of Hongxia, et al. [45], who found that a 60% replacement level of FM by daphnia magna meal significantly increased the weight gain, specific growth rate, and protein efficiency of Pelteobagrus Fulvidraco fingerlings. Other studies have also found that replacing FM (control diet contained 35% FM) with zooplankton meal up to 100% significantly improved growth performance, PER, and FCR of seabass, Dicentrarchus labrax, fingerlings [31]. In addition, Dahpnia magna meal as an FM alternative in the diet (control diet contained 18% FM) of grey mullet larvae could replace up to 75% of FM, which recorded the best growth and feed utilization parameters [33]. The barramundi, Lates calcarifer, fingerlings fed diets containing 5–10% daphnia meal have high immune surveillance and disease resistance compared to the control group [46]. The study of Sharahi, et al. [32] revealed that the replacement of FM with gammarus meal up to 20% significantly improved different growth performance criteria and feed utilization of Siberian sturgeon, Acipenser baerii, juveniles.
In addition, Saravanan, et al. [47] found that, when feeding young yellow-tail damselfish, Neopomocentrus nemurus, larvae on copepod, they had better survival and growth performance. A previous study investigated the effect of a mixed copepod and rotifer diet on the survival and growth performance of first-feeding larvae of the southern flounder, Paralichthys lethostigma, larvae [48]. In addition, growth enhancement effect of zooplankton has been shown in several fish species, such as Atlantic cod, Gadus morhua, larvae [43,49] and rainbow trout, Oncorhynchus mykiss, juvenile [50].
For aquatic animals, zooplankton is a good source of protein, lipids, and carbohydrates [51]. In the present study, the results of whole-body composition indicated that feeding an increasing ZBM significantly increased crude protein and decreased lipid and ash content of grey mullet. The current findings are consistent with those of Aman, et al. [52], Manickam, et al. [53], who found a substantial increase in protein, lipid, and carbohydrate content in freshwater prawn, Macrobrachium rosenbergii, fed with wild mixed zooplankton. Previously, it was confirmed that Catla catla fry fed with different zooplankton, such as Copepoda, Thermocyclops decipiens, Crustacea, Moina micrura, and Daphnia spp., had higher protein, carbohydrate, and lipid content [20,54]. Abo-Taleb, et al. [33] reported that the use of Dahpnia magna meal replacing FM at a level of 75% significantly improved the protein and lipid contents of grey mullet. Carcass protein, lipid, and nutrient digestibility were improved in the juvenile of Australian freshwater crayfish, Cherax destructor, fed on different protein sources (meat, snail, soybean, yabby, and zooplankton) [55].
The study of the regression trend of the present results showed a significant linear regression among increasing levels of ZBM and different growth, feed utilization, and body composition criteria. Accordingly, ZBM could totally replace FM without negative impacts. In contrast, daphia meal as an FM replacer showed a quadratic regression trend and the best growth performance criteria were observed at 75% in the diet of grey mullet [33].
Since the GI tract is the primary site for feed digestion and nutrient absorption, its morphology can be used to assess intestinal health, feed consumption ability, and fish growth [56,57,58]. For normal intestinal functions, intestinal morphology must be healthy [59]. Some improvements in the structure of the intestine, such as deeper crypts, number of villi and shorter villi, can result in poor nutrient preoccupation and less function [60,61]. In the present study, the results showed that zooplankton meal improved the intestinal villi, muscle thickness, and number of goblet cells in the intestine of grey mullet. Similar results were also reported by Mona, et al. [62] for gilthead seabream, Sparus aurata, fry when fed marine rotifers and copepods. In addition, Dimitroglou, et al. [63] reported that the improvement of intestinal morphology by increasing villus length and density and increasing surface area of absorption boost the function of the intestine. In the present study, the morphological results indicate that dried zooplankton at 100% improved the gut development of grey mullet, which may also partially explain the rapid growth of fish fed this diet.
The liver of fish is a vital organ that oversees a variety of life functions, including protein, lipid, and carbohydrate metabolism, bile production, and detoxification. It also serves as a storage facility for a variety of compounds, mostly glycogen and lipids [64]. In the current study, when grey mullet was fed various levels of ZBM, no histopathological changes were seen, when compared with the control diet (Z0). These results are in agreement with [65], who showed that there was no effect, in control and fish oil, on liver functions.
Aside from the biological and nutritional assessment of dietary FM substitution, an economic study on alternate ingredient usage is critical to ensure the viability and economics of alternative schemes. The cost of diet preparation was reduced when FM was replaced with ZBM in this study, which is a clear reason for using this ingredient in commercial diets. The cost of weight gain by the diet containing 100% ZBM (9.46 L.E per kg gain) was less costly than the control diet by 40%, resulting in a higher economic efficiency ratio. Overall, this research study found that dietary FM could be replaced by ZBM up to 100% in grey mullet juvenile diets, with positive effects on growth performance and feed consumption, thus improving economic efficiency. In the same vein, the replacement of FM with Daphnia meal improved the ECR by 14.88% and 15.91% with 75 and 100% replacement levels, respectively [33]. One of the greatest impacts of replacement studies is the improvement of economic visibility of the diet, among other goals. Several alternatives have been used in partial replacement of FM and reduce feed costs, including high protein distillers dried grains [66,67], meat and bone meal [68], silkworm (Anaphe infracta) [69], insect meal [70], animal by-products [70], and whey protein [71]. Interestingly, the findings of the present study revealed the success of total replacement of FM with ZBM regarding the zootechnical performance and economic benefit by considering the natural collection of ZBM. This economic analysis could have differed if we had farmed the zooplankton with expectation of increasing their price. It is worth mentioning that the control diet in the current study contains high plant protein source (42% soyabean); therefore, other studies could be testing the total replacement of FM with ZBM at high animal protein diets.
5. Conclusions
The present study revealed that zooplankton meal (ZBM) could replace fishmeal (FM) up to 100% without compromising growth performance, feed utilization, and survival of grey mullet, Mugil cephalus. The relation between weight gain and increasing FM substitution levels follows linear regression with strong positive correlation. The whole-body crude protein was increased with the increase in ZBM levels in the diets. The histometric assays revealed an improvement of gut health with increasing ZBM levels. In addition, the total replacement of FM with ZBM improves the economic revenue of producing grey mullet, M. cephalus. Moreover, as the ZBM was collected from nature in the present study, its chemical composition could differ from season to season; therefore, it is recommended to determine the approximate composition for each collected batch before using it in the diet formulation to obtain an accurate diet formula. However, collecting zooplankton from nature is not a sustainable option; therefore, it could be suggested that farming zooplankton should be started massively. Moreover, further studies need to be conducted to examine the total replacement of FM in different diets with high FM levels.
Author Contributions
Conceptualization, H.A.A.-T., M.A., M.A.E., M.M.M. and M.M.M.E.-f.; methodology, H.A.A.-T., M.A., M.A.E., M.M.M., O.F.A. and M.M.M.E.-f.; software, M.A., A.T.M. and O.F.A.; validation, M.A.E., M.M.M. and M.M.M.E.-f.; formal analysis, A.T.M., M.A., A.M.A. and A.E.S.; investigation, M.A., H.A.A.-T., M.M.M., O.F.A. and M.M.M.E.-f.; resources, H.A.A.-T., A.T.M., A.M.A., M.M.M., M.M.M.E.-f. and K.M.A.; data curation, A.T.M., M.M.M. and O.F.A.; writing—original draft preparation, M.A., A.T.M., A.E.S. and O.F.A.; writing—review and editing, A.E.S. and A.T.M.; visualization, M.A.E., M.M.M., O.F.A., K.M.A. and A.E.S.; project administration, H.A.A.-T. and A.M.A.; funding acquisition, H.A.A.-T., A.T.M. and K.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
All the results in the current research are some of the outputs of a project funded from the budget of the Minister of Scientific Research office (a project of national strategy program for genetic engineering and biotechnology, phase III). Research project titled Innovation of new non-conventional methods for the use of plankton and their extracts as alternative natural sources for animal protein in fish diets and the medical enhancements (Project ID: 44 H/2019). Additionally, the authors would like to thank Taif University Researchers (Supporting Project number: TURSP-2020/267), Taif University, Taif, Saudi Arabia for supporting the publication of this work.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the institutional animal care and use committee of Alexandria University with the approval No. AU:19/21/05/27/3/18.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the authors upon reasonable request.
Acknowledgments
The researchers extend their sincere thanks to the Egyptian Ministry of Higher Education and Scientific Research for its continuous support. Additionally, the authors gratefully acknowledge Taif University Researchers (Supporting Project number: TURSP-2020/267), Taif University, Taif, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saleh, M. Cultured Aquatic Species Information Programme: Mugil Cephalus; FAO Fisheries Aquaculture Department: Rome, Italy, 2006. [Google Scholar]
- Whitfield, A.; Panfili, J.; Durand, J.-D. A global review of the cosmopolitan flathead mullet Mugil cephalus Linnaeus 1758 (Teleostei: Mugilidae), with emphasis on the biology, genetics, ecology and fisheries aspects of this apparent species complex. Rev. Fish Biol. Fish. 2012, 22, 641–681. [Google Scholar] [CrossRef]
- El-Hawarry, W.N. Effect of Nursery Age (Stunting) and Density on Polyculture Performance of Mullet Species; Mugil Capito and Mugil Cephalus. Alex. J. Vet. Sci. 2018, 57, 87–94. [Google Scholar]
- Crosetti, D. Culture of Grey Mullets: Current State of Grey Mullet Fisheries and Culture; CRC Press: Boca Raton, FL, USA, 2016; Volume 23, pp. 398–450. [Google Scholar]
- Abou-Gabal, A.A.; Abbas, E.M.; Ali, H.M.M.; El-Baramawi, N.; Khaled, A.A.; El Deeb, S.I. Molecular Identification of Grey Mullet species in the Mediterranean Sea of Egypt. Egypt. J. Aquat. Biol. Fish. 2018, 22, 125–139. [Google Scholar] [CrossRef]
- Biswas, G.; De, D.; Thirunavukkarasu, A.; Natarajan, M.; Sundaray, J.; Kailasam, M.; Kumar, P.; Ghoshal, T.; Ponniah, A.; Sarkar, A. Effects of stocking density, feeding, fertilization and combined fertilization-feeding on the performances of striped grey mullet (Mugil cephalus L.) fingerlings in brackishwater pond rearing systems. Aquaculture 2012, 338, 284–292. [Google Scholar] [CrossRef]
- El-Dahhar, A.; Salama, M.; Moustafa, Y.; Elmorshedy, E. Effect of using equal mixture of seaweeds and marine algae in striped mullet (Mugil cephalus) larval diets on growth performance and feed utilization. Arab. Aquacult. Soc. J. 2014, 9, 145–158. [Google Scholar]
- Parrino, V.; Cappello, T.; Costa, G.; Cannavà, C.; Sanfilippo, M.; Fazio, F.; Fasulo, S. Comparative study of haematology of two teleost fish (Mugil cephalus and Carassius auratus) from different environments and feeding habits. Eur. Zool. J. 2018, 85, 193–199. [Google Scholar] [CrossRef]
- Wassef, E.; El Masry, M.; Mikhail, F. Growth enhancement and muscle structure of striped mullet, Mugil cephalus L., fingerlings by feeding algal meal-based diets. Aquacult. Res. 2001, 32, 315–322. [Google Scholar] [CrossRef]
- Ghoshal, T.; Biswas, G.; Mukherjee, S.; Kumar, S.; Anand, P.; Raja, R.; Vijayan, K. Evaluation of Growth Performance in Mugil cephalus Juveniles Fed Diets Incorporated with Fermented Plant Feedstuffs Replacing Fishmeal or Diets Supplemented with Fish Gut Bacteria. Int. J. Food Process. Technol. 2018, 9, 728. [Google Scholar]
- Gisbert, E.; Mozanzadeh, M.T.; Kotzamanis, Y.; Estévez, A. Weaning wild flathead grey mullet (Mugil cephalus) fry with diets with different levels of fish meal substitution. Aquaculture 2016, 462, 92–100. [Google Scholar] [CrossRef]
- Jana, S.N.; Sudesh; Garg, S.K.; Sabhlok, V.P.; Bhatnagar, A. Nutritive evaluation of lysine-and methionine-supplemented raw vs heat-processed soybean to replace fishmeal as a dietary protein source for Grey Mullet, Mugil cephalus, and Milkfish, Chanos chanos. J. Appl. Aquac. 2012, 24, 69–80. [Google Scholar] [CrossRef]
- Kokou, F.; Sarropoulou, E.; Cotou, E.; Kentouri, M.; Alexis, M.; Rigos, G. Effects of graded dietary levels of soy protein concentrate supplemented with methionine and phosphate on the immune and antioxidant responses of gilthead sea bream (Sparus aurata L.). Fish Shellfish Immunol. 2017, 64, 111–121. [Google Scholar] [CrossRef]
- Kumar, V.; Lee, S.; Cleveland, B.M.; Romano, N.; Lalgudi, R.S.; Benito, M.R.; McGraw, B.; Hardy, R.W. Comparative evaluation of processed soybean meal (Enzo Meal TM) vs. regular soybean meal as a fishmeal replacement in diets of rainbow trout (Oncorhynchus mykiss): Effects on growth performance and growth-related genes. Aquaculture 2020, 516, 734652. [Google Scholar] [CrossRef]
- Li, S.; Dai, M.; Qiu, H.; Chen, N. Effects of fishmeal replacement with composite mixture of shrimp hydrolysate and plant proteins on growth performance, feed utilization, and target of rapamycin pathway in largemouth bass, Micropterus salmoides. Aquaculture 2021, 533, 736185. [Google Scholar] [CrossRef]
- Bell, J.G.; Waagbø, R. Safe and Nutritious Aquaculture Produce: Benefits and Risks of Alternative Sustainable Aquafeeds. In Aquaculture in The Ecosystem; Holmer, M., Black, K., Duarte, C.M., Marbà, N., Karakassis, I., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 185–225. [Google Scholar] [CrossRef]
- Huntington, T.; Hasan, M.R. Fish as Feed Inputs for Aquaculture–Practices, Sustainability and Implications: A Global Synthesis; FAO Fisheries & Aquaculture Technical Paper; FAO: Rome, Italy, 2009; pp. 1–61. [Google Scholar]
- Li, X.; Zheng, S.; Cheng, K.; Ma, X.; Wu, G. Use of alternative protein sources for fishmeal replacement in the diet of largemouth bass (Micropterus salmoides). Part II: Effects of supplementation with methionine or taurine on growth, feed utilization, and health. Amino Acids 2021, 53, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Kader, M.A.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Bulbul, M. Supplemental effects of some crude ingredients in improving nutritive values of low fishmeal diets for red sea bream, Pagrus major. Aquaculture 2010, 308, 136–144. [Google Scholar] [CrossRef]
- Kadhar, A.; Kumar, A.; Ali, J.; John, A. Studies on the survival and growth of fry of Catla catla (Hamilton, 1922) using live feed. J. Mar. Biol. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Rajkumar, M.; Santhanam, P.; Perumal, P. Laboratory culture of calanoid copepod, Acartia clausi Giesbrecht. Appl. Fish. Aquacult. 2004, 4, 5–8. [Google Scholar]
- Ashour, M.; Abo-Taleb, H.; Abou-Mahmoud, M.; El-Feky, M.M. Effect of the integration between plankton natural productivity and environmental assessment of irrigation water, El-Mahmoudia Canal, on aquaculture potential of Oreochromis niloticus. Turk. J. Fish. Aquat. Sci. 2018, 18, 1163–1175. [Google Scholar] [CrossRef]
- Abo-Taleb, H.; Ashour, M.; El-Shafei, A.; Alataway, A.; Maaty, M.M. Biodiversity of Calanoida Copepoda in Different Habitats of the North-Western Red Sea (Hurghada Shelf). Water 2020, 12, 656. [Google Scholar] [CrossRef]
- Evjemo, J.O.; Reitan, K.I.; Olsen, Y. Copepods as live food organisms in the larval rearing of halibut larvae (Hippoglossus hippoglossus L.) with special emphasis on the nutritional value. Aquacult. Int. 2003, 227, 191–210. [Google Scholar] [CrossRef]
- Fuentes, L.; Sánchez, F.J.; Lago, M.J.; Iglesias, J.; Pazos, G.; Linares, F. Growth and survival of Octopus vulgaris (Cuvier 1797) paralarvae fed on three Artemia-based diets complemented with frozen fish flakes, crushed zooplankton and marine microalgae. Sci. Mar. 2011, 75, 771–777. [Google Scholar] [CrossRef][Green Version]
- El-Gamal, M.M.; Othman, S.I.; Abdel-Rahim, M.M.; Mansour, A.T.; Alsaqufi, A.S.; El Atafy, M.M.; Mona, M.H.; Allam, A.A. Palaemon and artemia supplemented diet enhances sea bass, Dicentrarchus labrax, broodstock reproductive performance and egg quality. Aquacult. Rep. 2020, 16, 100290. [Google Scholar] [CrossRef]
- Magouz, F.I.; Essa, M.A.; El-Shafei, A.; Mansour, A.T.; Mahmoud, S.m.; Ashour, M. Effect of extended feeding with live copepods, Oithona nana, and Artemia franciscana on the growth performance, intestine histology, and economic viability of european seabass (Dicentrarchus labrax) postlarvae. Fresenius Environ. Bull. 2021, 30, 7106–7116. [Google Scholar]
- Conceição, L.E.C.; Yúfera, M.; Makridis, P.; Morais, S.; Dinis, M.T. Live feeds for early stages of fish rearing. Aquacult. Res. 2010, 41, 613–640. [Google Scholar] [CrossRef]
- Guerra-García, J.M.; Hachero-Cruzado, I.; González-Romero, P.; Jiménez-Prada, P.; Cassell, C.; Ros, M. Towards integrated multi-trophic aquaculture: Lessons from caprellids (Crustacea: Amphipoda). PLoS ONE 2016, 11, e0154776. [Google Scholar] [CrossRef]
- Abo-Taleb, H.; Zeina, A.; Ashour, M.; Mabrouk, M.M.; Sallam, E.A.; El-feky, M.M. Isolation and cultivation of the freshwater amphipod Gammarus pulex (Linnaeus, 1758), with an evaluation of its chemical and nutritional content. Egypt. J. Aquat. Biol. Fish. 2020, 24, 69–82. [Google Scholar] [CrossRef]
- Hassan, E.S.; Azab, M.A.; Abo-Taleb, A.H.; El-Feky, M.M. Effect of replacing fish meal in the fish diet by zooplankton meal on growth performance of Dicentrarchus labrax (Linnaeus, 1758). Egypt. J. Aquat. Biol. Fish. 2020, 24, 267–280. [Google Scholar] [CrossRef]
- Sharahi, A.R.; Falahatkar, B.; Efatpanah, I. Effect of fish meal replacement with Gammarus meal on growth and body composition of juvenile Siberian sturgeon, Acipenser baerii (Brandt, 1869). J. Aquat. Ecol. 2016, 6, 102–113. [Google Scholar]
- Abo-Taleb, H.A.; Ashour, M.; Elokaby, M.A.; Mabrouk, M.M.; El-feky, M.M.; Abdelzaher, O.F.; Gaber, A.; Alsanie, W.F.; Mansour, A.T. Effect of a New Feed Daphnia magna (Straus, 1820), as a Fish Meal Substitute on Growth, Feed Utilization, Histological Status, and Economic Revenue of Grey Mullet, Mugil cephalus (Linnaeus 1758). Sustainability 2021, 13, 7093. [Google Scholar] [CrossRef]
- Manickam, N.; Santhanam, P.; Bhavan, P.S.; Santhanam, P.; Begum, A.; Perumal, P. Techniques in the collection, preservation and morphological identification of freshwater zooplankton. In Basic and Applied Zooplankton Biology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 139–195. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis; Association of Official Analytical Chemists International: Washington, DC, USA, 2005. [Google Scholar]
- Bancroft, J.D. Histochemical Techniques, 2nd ed.; Butterworth-Heinemann: London, UK, 2013. [Google Scholar]
- Hamidian, G.; Zirak, K.; Sheikhzadeh, N.; Khani Oushani, A.; Shabanzadeh, S.; Divband, B. Intestinal histology and stereology in rainbow trout (Oncorhynchus mykiss) administrated with nanochitosan/zeolite and chitosan/zeolite composites. Aquacult. Res. 2018, 49, 1803–1815. [Google Scholar] [CrossRef]
- Wei, Y.; Shen, H.; Xu, W.; Pan, Y.; Chen, J.; Zhang, W.; Mai, K. Replacement of dietary fishmeal by Antarctic krill meal on growth performance, intestinal morphology, body composition and organoleptic quality of large yellow croaker Larimichthys crocea. Aquaculture 2019, 512, 734281. [Google Scholar] [CrossRef]
- Hewitt, R.P.; Watkins, J.L.; Naganobu, M.; Tshernyshkov, P.; Brierley, A.S.; Demer, D.A.; Kasatkina, S.; Takao, Y.; Goss, C.; Malyshko, A. Setting a precautionary catch limit for Antarctic krill. Oceanography 2002, 15, 26–33. [Google Scholar] [CrossRef]
- Naylor, R.L.; Hardy, R.W.; Bureau, D.P.; Chiu, A.; Elliott, M.; Farrell, A.P.; Forster, I.; Gatlin, D.M.; Goldburg, R.J.; Hua, K. Feeding aquaculture in an era of finite resources. Proc. Natl. Acad. Sci. USA 2009, 106, 15103–15110. [Google Scholar] [CrossRef]
- Holm, J.C.; Torrissen, K.R. Growth depression and acclimatization of protease activity in Atlantic salmon first-feeding fry responding to a diet supplemented with zooplankton. Aquacult. Int. 1987, 65, 171–174. [Google Scholar] [CrossRef]
- Massimo Perrone, F.; Della Croce, N.; Dell’anno, A.J.C.; Ecology. Biochemical composition and trophic strategies of the amphipod Eurythenes gryllus at hadal depths (Atacama Trench, South Pacific). Chem. Ecol. 2003, 19, 441–449. [Google Scholar] [CrossRef]
- Opstad, I.; Suontama, J.; Langmyhr, E.; Olsen, R.E. Growth, survival, and development of Atlantic cod (Gadus morhua L.) weaned onto diets containing various sources of marine protein. Int. J. Mar. Sci. 2006, 63, 320–325. [Google Scholar] [CrossRef]
- Baeza-Rojano, E.; Hachero-Cruzado, I.; Guerra-García, J.M. Nutritional analysis of freshwater and marine amphipods from the Strait of Gibraltar and potential aquaculture applications. J. Sea Res. 2014, 85, 29–36. [Google Scholar] [CrossRef]
- Hongxia, Z.; Yang, W.; Xuan, W.; Xiangqian, Z.; Guang, Y.; Jianchao, Z.; Guoxia, Z.; Dongqing, B. Effects of daphnia magna meal replacing fish meal on growth, biochemical indexes of Pelteobagrus fulvidraco and water quality indexes. Feed Ind. 2019, 2, 212–221. [Google Scholar]
- Chiu, S.-T.; Shiu, Y.-L.; Wu, T.-M.; Lin, Y.-S.; Liu, C.-H. Improvement in non-specific immunity and disease resistance of barramundi, Lates calcarifer (Bloch), by diets containing Daphnia similis meal. Fish Shellfish Immunol. 2015, 44, 172–179. [Google Scholar] [CrossRef]
- Saravanan, R.; Vijayanand, P.; Rajagopal, S. Copepod nauplii-a suitable feed for the hatchlings of yellow tail damsel (Neopomocentrus nemurus). J. Adv. Appl. Sci. Res. 2010, 1, 197–204. [Google Scholar]
- Wilcox, J.A.; Tracy, P.L.; Marcus, N.H. Improving live feeds: Effect of a mixed diet of copepod nauplii (Acartia tonsa) and rotifers on the survival and growth of first-feeding larvae of the southern flounder, Paralichthys lethostigma. J. World Aquacult. Soc. 2006, 37, 113–120. [Google Scholar] [CrossRef]
- Karlsen, Ø.; van der Meeren, T.; Rønnestad, I.; Mangor-Jensen, A.; Galloway, T.F.; Kjørsvik, E.; Hamre, K. Copepods enhance nutritional status, growth and development in Atlantic cod (Gadus morhua L.) larvae—can we identify the underlying factors? PeerJ 2015, 3, e902. [Google Scholar] [CrossRef] [PubMed]
- Azimi, A.; Hosseini, S.; Sudagar, M.; Aslanparviz, H. Effect of replacement of Caspian Sea gammarus meal by partial kilka fish meal on growth performance, feed conversion ratio and survival of juveniles of rainbow trout (Oncorhynchus mykiss). Iran. Fish. Sci. J. 2011, 20, 63–74. [Google Scholar]
- Manickam, N.; Bhavan, P.S.; Santhanam, P. Evaluation of nutritional profiles of wild mixed zooplankton in Sulur and Ukkadam Lakes of Coimbatore, South India. Turk. J. Fish. Aquat. Sci. 2017, 17, 509–517. [Google Scholar] [CrossRef]
- Aman, S.; Altaff, K. Biochemical profile of Heliodiaptomus viduus, Sinodiaptomus (Rhinediaptomus) indicus, and Mesocyclops aspericornis and their dietary evaluation for postlarvae of Macrobrachium rosenbergii. Zool. Stud. 2004, 43, 267–275. [Google Scholar]
- Manickam, N.; Bhavan, P.S.; Santhanam, P.; Muralisankar, T. Influence of wild mixed zooplankton on growth and muscle biochemical composition of the freshwater prawn Macrobrachium rosenbergii post larvae. Aquaculture 2020, 522, 735110. [Google Scholar] [CrossRef]
- Das, S.K.; Tiwari, V.; Venkateshwarlu, G.; Reddy, A.; Parhi, J.; Sharma, P.; Chettri, J. Growth, survival and fatty acid composition of Macrobrachium rosenbergii (de Man, 1879) post larvae fed HUFA-enriched Moina micrura. Aquaculture 2007, 269, 464–475. [Google Scholar] [CrossRef]
- Jones, P.L.; De Silva, S.S.; Mitchell, B.D. The effect of dietary protein source on growth and carcass composition in juvenile Australian freshwater crayfish. Aquacult. Int. 1996, 4, 361–376. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, H.; Yan, J.; Wang, R.; Liu, L. Effect of yeast polysaccharide on some hematologic parameter and gut morphology in channel catfish (Ictalurus punctatus). Fish Physiol. Biochem. 2012, 38, 1441–1447. [Google Scholar] [CrossRef]
- Sallam, A.E.; Almisherfi, H.M.; El-Feky, M.M.; Abdel-Ghany, H.M.; Salem, M.E.S. Feeding marbled spinefoot rabbitfish (Siganus rivulatus) juveniles with β-mannanase enzyme: An effective tool to enhance growth and immunity and induce low salinity tolerance. Aquacult. Nutr. 2020, 26, 1884–1894. [Google Scholar] [CrossRef]
- Abbas, E.M.; Ali, F.S.; Desouky, M.G.; Ashour, M.; El-Shafei, A.; Maaty, M.M.; Sharawy, Z.Z. Novel comprehensive molecular and ecological study introducing coastal mud shrimp (Solenocera crassicornis) recorded at the Gulf of suez, Egypt. J. Mar. Sci. Eng. 2021, 9, 9–22. [Google Scholar]
- Gao, Y.; Han, F.; Huang, X.; Rong, Y.; Yi, H.; Wang, Y. Changes in gut microbial populations, intestinal morphology, expression of tight junction proteins, and cytokine production between two pig breeds after challenge with Escherichia coli K88: A comparative study. J. Anim. Sci. 2013, 91, 5614–5625. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Becerril, M.; Angulo, C.; Estrada, N.; Murillo, Y.; Ascencio-Valle, F. Dietary administration of microalgae alone or supplemented with Lactobacillus sakei affects immune response and intestinal morphology of Pacific red snapper (Lutjanus peru). Fish Shellfish Immunol. 2014, 40, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-Z.; Li, D.; Chen, W.-J.; Ban, S.-N.; Liu, T.; Wen, H.; Jiang, M. Effects of dietary host-associated Lactococcus lactis on growth performance, disease resistance, intestinal morphology and intestinal microbiota of mandarin fish (Siniperca chuatsi). Aquaculture 2021, 540, 736702. [Google Scholar] [CrossRef]
- Mona, M.H.; Rizk, E.-S.T.; El-feky, M.; Elawany, M.E. Effect of nutritional quality of rotifers and copepods on sea bream (Sparus aurata) fry fish productivity. Egypt. J. Exp. Biol. 2019, 15, 135–142. [Google Scholar] [CrossRef]
- Dimitroglou, A.; Merrifield, D.; Moate, R.; Davies, S.; Spring, P.; Sweetman, J.; Bradley, G. Dietary mannan oligosaccharide supplementation modulates intestinal microbial ecology and improves gut morphology of rainbow trout, Oncorhynchus mykiss (Walbaum). J. Anim. Sci. 2009, 87, 3226–3234. [Google Scholar] [CrossRef]
- El-Bakary, N.; El-Gammal, H.L. Comparative histological, histochemical and ultrastructural studies on the liver of flathead grey mullet (Mugil cephalus) and sea bream (Sparus aurata). Glob. Vet. 2010, 4, 548–553. [Google Scholar]
- Parker, H.M.; Cohn, J.S.; O’Connor, H.T.; Garg, M.L.; Caterson, I.D.; George, J.; Johnson, N.A. Effect of fish oil supplementation on hepatic and visceral fat in overweight men: A randomized controlled trial. Nutrients 2019, 11, 475. [Google Scholar] [CrossRef]
- Allam, B.W.; Khalil, H.S.; Mansour, A.T.; Srour, T.M.; Omar, E.A.; Nour, A.A.M. Impact of substitution of fish meal by high protein distillers dried grains on growth performance, plasma protein and economic benefit of striped catfish (Pangasianodon hypophthalmus). Aquaculture 2020, 517, 734–792. [Google Scholar] [CrossRef]
- Goda, A.A.S.; Srour, T.M.; Omar, E.; Mansour, A.T.; Baromh, M.Z.; Mohamed, S.A.; El-Haroun, E.; Davies, S.J. Appraisal of a high protein distiller’s dried grain (DDG) in diets for European sea bass, Dicentrarchus labrax, fingerlings on growth performance, haematological status and related gut histology. Aquacult. Nutr. 2019, 25, 808–816. [Google Scholar] [CrossRef]
- Moutinho, S.; Martínez-Llorens, S.; Tomás-Vidal, A.; Jover-Cerdá, M.; Oliva-Teles, A.; Peres, H. Meat and bone meal as partial replacement for fish meal in diets for gilthead seabream (Sparus aurata) juveniles: Growth, feed efficiency, amino acid utilization, and economic efficiency. Aquaculture 2017, 468, 271–277. [Google Scholar] [CrossRef]
- Ijaiya, A.; Eko, E. Effect of replacing dietary fish meal with silkworm (Anaphe infracta) caterpillar meal on growth, digestibility and economics of production of starter broiler chickens. Pak. J. Nutr. 2009, 8, 845–849. [Google Scholar] [CrossRef]
- Chia, S.Y.; Tanga, C.M.; Osuga, I.M.; Alaru, A.O.; Mwangi, D.M.; Githinji, M.; Subramanian, S.; Fiaboe, K.K.; Ekesi, S.; van Loon, J.J. Effect of dietary replacement of fishmeal by insect meal on growth performance, blood profiles and economics of growing pigs in Kenya. Animals 2019, 9, 705. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.A.; Osman, A.; Al-Gabri, N.A.; Elsayed, S.A.; El-Rahman, A.; Ghada, I.; Elabbasy, M.T.; Ahmed, S.A.; Ibrahim, R.E. The effect of dietary replacement of fish meal with whey protein concentrate on the growth performance, fish health, and immune status of Nile Tilapia fingerlings, Oreochromis niloticus. Animals 2019, 9, 1003. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).