Abstract
French Polynesia is experiencing increasing coral bleaching events in shallow waters triggered by thermal anomalies and marine heatwaves linked to climate change, a trend that is replicated worldwide. As sea surface thermal anomalies are assumed to lessen with depth, mesophotic deep reefs have been hypothesized to act as refuges from anthropogenic and natural disturbances, the ‘deep reef refugia hypothesis’ (DRRH). However, evidence supporting the DRRH is either inconclusive or conflicting. We address this by investigating four assumptions of the DRRH focusing on the symbiotic association between anemones and anemonefish. First, we compare long-term temperature conditions between shallow (8 m) and mesophotic sites (50 m) on the island of Moorea from 2011–2020. Second, we compare the densities of the orange-fin anemonefish, Amphiprion chrysopterus between shallow and mesophotic (down to 60 m) reefs across three archipelagos in French Polynesia. Finally, we compare the percentage of anemone bleaching, as well as anemonefish reproduction, between shallow and mesophotic reefs. We found that the water column was well mixed in the cooler austral winter months with only a 0.19 °C difference in temperature between depths, but in the warmer summer months mixing was reduced resulting in a 0.71–1.03 °C temperature difference. However, during thermal anomalies, despite a time lag in warm surface waters reaching mesophotic reefs, there was ultimately a 1.0 °C increase in water temperature at both 8 and 50 m, pushing temperatures over bleaching thresholds at both depths. As such, anemone bleaching was observed in mesophotic reefs during these thermal anomalies, but was buffered compared to the percentage of bleaching in shallower waters, which was nearly five times greater. Our large-scale sampling across French Polynesia found orange-fin anemonefish, A. chrysopterus, in mesophotic zones in two high islands and one atoll across two archipelagos, extending its bathymetric limit to 60 m; however, orange-fin anemonefish densities were either similar to, or 25–92 times lower than in shallower zones. Three spawning events were observed at 50 m, which occurred at a similar frequency to spawning on shallower reefs at the same date. Our findings of thermal anomalies and bleaching in mesophotic reefs, coupled with mainly lower densities of anemonefish in mesophotic populations, suggest that mesophotic reefs show only a limited ability to provide refugia from anthropogenic and natural disturbances.
1. Introduction
Coral reefs are one the most threatened ecosystems worldwide and coral cover has declined over the last few decades due to a rapidly changing climate [1,2,3,4,5]. Sea surface temperature increases and anomalies, or marine heat waves (MHWs) have lethal consequences on coral reef benthic communities [6] and are the main cause of the worldwide decline in coral [7]. However, our knowledge focuses on shallow reef communities (2–15 m depth) and the increasing prevalence of mass mortalities at these depths [4,8]. While shallower ecosystems are increasingly threatened by global change and anthropogenic pressures, thermal stress and high light irradiance are assumed to attenuate with depth which may provide deeper reefs safe haven from environmental stressors occurring at the surface [9,10,11,12]. Mesophotic coral ecosystems (MCEs) are found from 30 m to the depth limit at which light is too low to sustain zooxanthellate photosynthetic coral growth [13], which ranges with location from 80 m [14], 100 m [15,16] to 150 m [17,18,19]. Coral reef habitats are usually contiguous from shallow reefs down a depth gradient [20], but MCEs may be less exposed to anthropogenic disturbances and other human impacts [21] than shallower and more coastal reefs [22]. As such, according to the ‘deep reef refuge hypothesis’ (DRRH) [9,23], MCEs have the potential to function as refugia for shallow reefs, aiding in their recovery. However, to date only one coral study has provided evidence for the DRRH [24] and despite considerable conservation interest and research, many questions remain unanswered [25].
The deep reef refuge hypothesis, specifically in response to thermal stress, rests on four main assumptions. First, that MCEs are less likely to experience temperature increases, anomalies, or MHWs that severely impact shallow reefs; however, few studies have compared water temperatures at both depths, and despite initial relief from anomalously warm temperatures, temperatures eventually increased even in MCEs [26,27]. Second, that MCEs are less susceptible to thermally induced bleaching compared to shallow reefs; however available data are often contradictory. Corals in some MCEs were afforded some protection from bleaching [11,28], but other MCEs were not immune to bleaching [18,29,30] (albeit to a lesser extent than at shallow reefs [26]) and others still may show greater susceptibility to thermal stress than their shallow counterparts [31]. Third, that there is overlapping species composition between shallow reefs and MCEs for some reef fishes and invertebrates [14,20,32,33]; but differences in fish abundance and community composition have also been found [13,34,35,36]. Fourth, that actively reproducing MCE populations act as a local recruitment source for shallow reefs; however, the data available are limited. While at least one species of fish, the three-spot damselfish, Chromis verater, and the corals Seriatopora hystrix and Montastraea cavernosa are genetically connected between MCEs and shallow reefs [37,38,39], some benthic sedentary organisms show limited vertical connectivity [13] and reduced reproduction at depth [40], but larval production may also be greater at depth compared to shallow reefs [41]. Evidence for the DRRH is therefore often contradictory and more data are needed from MCEs, especially for fishes.
Here, we address the potential for mesophotic areas to provide a thermal refuge for shallow water species by investigating the four assumptions of the DRRH in French Polynesia focusing on the symbiotic association between anemones and anemonefish. Anemonefish populations are known to mostly live in shallow waters between 1 m and 40 m deep [42]. In the first 10 m, anemonefish are directly and indirectly impacted by the cascading effects of temperature anomalies and MHWs which induce bleaching in their anemone hosts, severely impacting metabolic demand, reducing growth, causing chronic stress and reducing fecundity in anemonefish [43,44,45]. Other pomacentrids that also live associated with anemones for part of their life-cycle, such as the domino damselfish, Dascyllus trimaculatus [46], likely suffer the same impacts as anemonefish. Recently three field studies have shown the presence of anemonefish beyond a depth of 40 m, down to 60 m (Amphiprion bicinctus [47]; A. akindynos and A. perideraion [10]) and down to 70 m (A. clarkii [36]); therefore, mesophotic reefs may provide a refuge for this symbiotic association and may help trigger reef conservation research of mesophotic areas [48]. Here, we investigate the potential for the DRRH by determining: (1) long-term temperature conditions at shallow (8 m) and mesophotic (50 m) sites on the island of Moorea from 2011–2020, (2) the density of the orange-fin anemonefish, A. chrysopterus, in shallow and MCEs (down to 60 m) across three archipelagos in French Polynesia, as well as (3) the depth to which bleaching of their anemone hosts extends and (4) the depth to which anemonefish reproduction occurs.
2. Materials and Methods
2.1. Temperature Measurements at Shallow and Mesophotic Reefs
Moorea is a volcanic high island in the Society Islands (Figure 1A) measuring only 23 × 30 km, with a crater reaching 1212 m. The patch reefs in the lagoon reach depths of 20–30 m. The island is encircled by a barrier reef, whose sides slope down steeply to 30–40 m along a sandy bottom before continuing to depths of more than 60 m and the ocean around the island is more than 1500 m deep [49]. Sea temperatures were collected by the National Service of Observation CORAIL (National Center for Scientific Research INSU) in Moorea with HOBO data loggers (Onset Computer Corp., Pocasset, MA, US) installed on the reef outer slope on the north of Moorea at depths of 8 and 50 m. Mean hourly water temperatures were recorded from 1 October 2011 through 11 April 2020. Data are available on demand (http://observatoire.criobe.pf/wiki/tiki-index.php, accessed on 30 May 2021).
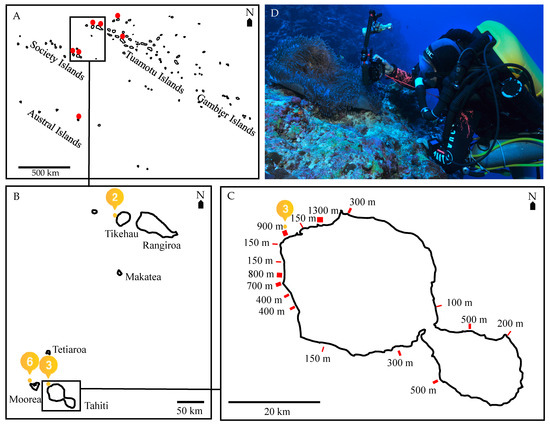
Figure 1.
Anemone and anemonefish surveying in French Polynesia. (A) Map of French Polynesia indicating the seven high islands/atolls that were surveyed from 2011–2021 (black triangles: no anemonefish found, red triangles: only shallow anemonefish found, blue triangles: presence of anemonefish at both shallow and mesophotic depths). (B) Map of the three high islands/atolls where orange-fin anemonefish, Amphiprion chrysopterus, were observed both in the shallow and mesophotic zone (>50 m depth): Moorea (17°28.870′ S, 149°53.990′ W) and Tahiti (17°32.487′ S, 149°37.568′ W) in the Society archipelago and Tikehau (15°1.057′ S, 148°17.184′ W) in the Tuamotu archipelago (blue triangles represent the location and the numbers represent the number of mesophotic anemonefish found). (C) Map of Tahiti indicating the 18 shallow and mesophotic transects and their lengths that were surveyed from 2011–2020. (D) A scuba diver (AH) using a rebreather and trimix gaz, photographing A. chrysopterus at 55 m in Tikehau (FZ).
2.2. Anemone and Anemonefish Surveys: Anemone Bleaching and Anemonefish Presence, Density Estimates and Reproduction at Shallow and Mesophotic Reefs
Anemone and anemonefish were surveyed along transects in seven high islands or atolls across three archipelagos in French Polynesia (Figure 1A): Tahiti and Moorea (Society Islands) (Figure 1B); Manihi, Rangiroa, Fakarava and Tikehau (Tuamotus) (Figure 1b); and Tubuai (Australes) from 2007–2021. Transect length and width varied with the topology of each site, mainly as a function of reef slope (e.g., Figure 1C). Surveys were carried out using open-circuit scuba diving at 8 m over a total distance of 3,389,250 m2 (Supplementary Materials Table S1). Mesophotic surveys (down to 70 m) were only possible using closed-circuit or “rebreather” mixed-gas breathing apparatus (rEvo™ rebreather, rEvo Rebreathers, Brussels, Belgium and Inspiration™ rebreather, Ambient Pressure Diving, Helston, Cornwall, UK) by AH, FZ and GS with each dive lasting on average 2–3 h (Figure 1D) over a total distance of 616,500 m2 (Supplementary Materials Table S1). Recently the use of closed-circuit apparatus by extensively trained divers enables exploration of mesophotic reefs, providing as much as 10–12 h of underwater autonomy [50,51,52,53].
In general, shallow to mesophotic reefs were surveyed along a depth gradient during every deep dive at each of the high islands/atolls described below (Supplementary Materials Table S1). In the Society Islands in Tahiti, 18 sites were monitored with transects ranging from 1,000–6,000 m in length and 50–100 m in width, resulting in a total surface area surveyed of 400,000 m2 for both shallow and mesophotic sites (Figure 1C); at Moorea, four mesophotic sites were monitored with transects ranging from 100–200 m in length and 10–100 m in width, resulting in a total surface area surveyed of 31,500 m2. At Moorea, shallow areas were extensively surveyed from 2007–2008 and less extensively thereafter, with over 700 transects of approx. 400 m in length and 10 m in width, resulting in a total surface area surveyed of 2,800,000 m2. In the Tuamotus: at Tikehau only 1 site was monitored in the mesophotic zone, along a 800 × 20 m transect, resulting in a total surface area surveyed of 16,000 m2 respectively; in the shallow zone, two transects were carried out ranging from 50–800 m in length and 5–20 m in width, resulting in a total surface area surveyed of 16,750 m2; at Rangiroa, only 1 mesophotic site was monitored along a 1000 × 50 m transect, resulting in a total surface area surveyed of 50,000 m2; in the shallow zone, two transects were carried out ranging from 100–1,000 m in length and 50 m in width, resulting in a total surface area surveyed of 55,000 m2. At Fakarava and Manihi, 2 sites were monitored from shallow to the mesophotic zone with two transects of 1000 × 50 and 100 × 50 m at each atoll, resulting in a total surface area surveyed of 55,000 m2 at both shallow and mesophotic depths. In the Australs, at Tubuai 3 mesophotic sites were monitored with transects ranging from 100 m in length and 20–50 m in width, resulting in a total surface area surveyed of 9000 m2, and 2 shallow sites were monitored with transects ranging from 100–500 m in length and 5–50 m in width, resulting in a total surface area surveyed of 7500 m2.
At Tahiti, over 440 shallow to deep dives were carried out spanning the whole year, with at least 25 dives in each month after summing dives over the entire time period from 2011–2021. In Moorea, 700 shallow sites were monitored mainly in 2007–2008 but afterwards as well and in addition, 6 mesophotic dives were carried out in total: 1 each in April and August 2019 and 1 each in February, June and July 2020 and 1 in February 2021. At Tikehau, 7 shallow to deep dives were carried out in total: 1 in December 2017, 1 in February 2018 and 5 in February 2021, 1 additional shallow transect was carried out in 2021. In Rangiroa, 20 shallow to deep dives were carried out in total: 6 in August 2018, 8 in November 2019 and 6 in December 2020, 1 additional shallow transect was carried out in 2020. In Fakarava, 14 shallow to deep dives were carried out in total; 5 in November 2018, 5 in March 2020 and 4 in July 2020. In Manihi, 4 shallow to deep dives were carried out in December 2019. In Tubuai, 3 shallow to deep dives were carried out in total: 1 in April 2019 and 2 in October 2019.
On finding anemonefish, photographs were taken to determine the number of individuals and their status (adult, juvenile, recruit). The anemones were also photographed to determine the number of anemones on the site and their health status (bleached or not) assessed by their colouration.
The presence of anemonefish eggs was determined by delicately lifting the tentacles of the anemone to reveal the rock beneath, which is non-invasive for the anemone [45]. When an egg clutch nest was identified, several close-up photographs were taken (Figure 1D) to determine the age of the eggs based on morphological development criteria (egg coloration, eye coloration, proportion of silver eyes in the nest; Supplementary Materials in [45]). Laying date was thus determined by subtracting the age of the eggs from the date the photograph was taken.
3. Results
3.1. Comparison of Temperature Measurements between Shallow and Mesophotic Reefs
Long-term temperature measurements on the outer reef of Moorea from 2011–2020 revealed that thermal regimes at mesophotic depths (50 m depth) were on average 0.536 ± 0.009 °C (mean ± SE) cooler than those in shallow water (8 m depth) (Figure 2).
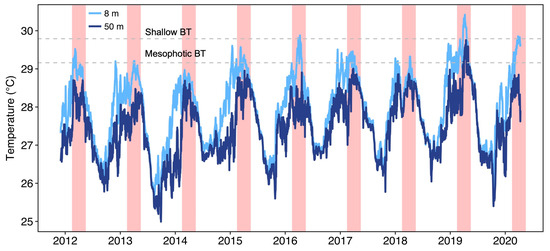
Figure 2.
Long-term benthic temperature recordings on the outer reef in Moorea, French Polynesia (17°28.870′ S, 149°53.990′ W) contrasted between shallow (8 m) and mesophotic (50 m) depths. Austral summer months (February, March and April) are indicated with red shading. BT = bleaching threshold temperature.
During the cooler austral winter months (May to September), the water column was well mixed with overlapping temperatures recorded between the shallow and mesophotic reefs (0.197 ± 0.005 °C difference between 8 and 50 m), with September being the coolest month at both depths (mean 26.63 and 26.43 °C respectively). With the approaching austral summer, mixing in the water column decreased slightly, but similar temperatures were still recorded between shallow and mesophotic reefs (0.492 ± 0.016 °C difference between 8 and 50 m in October and November respectively). At the beginning of the austral summer (December and January), water temperatures began to increase at 8 m (28.57 ± 0.019 °C), but the water column showed little mixing and water temperatures at 50 m were now 1.032 ± 0.023 °C cooler (27.36 ± 0.029 °C). Mixing in the water column increased for the hottest summer months (February, March and April) and the differences in temperature between 8 and 50 m decreased to 0.705 ± 0.015 °C, with peak temperatures occurring during March/April at both depths (28.79 ± 0.013 °C and 28.16 ± 0.017 °C at 8 and 50 m respectively outside of years with thermal anomalies). However, the warmest peak occurred on average around the 29 March at 8 m, with a 10-day lag before the warmest peak reached 50 m, around the 8 April. We established the bleaching threshold (BT; Figure 2), (1 °C above the mean of the month of the year that climatologically has the highest temperature [54]) from these March and April means respectively as 29.79 °C at 8 m and 29.16 °C at 50 m.
Long-term monitoring data of water temperatures from Moorea from 2011–2020 regressed linearly between 8 and 50 m (linear regression: F1, 2383 = 6145, p < 0.001, f(x) = 0.7583x + 6.2103, R2 = 0.72; Figure 3, grey solid line). Temperatures were similar between 8 and 50 m at the cooler end of the temperature scale, but as water temperature increases at 8 m the difference between the depths also increases, with temperatures at 8 m eventually reaching 1.0 °C higher than at 50 m (Figure 3) which corresponds to the months of December and January.
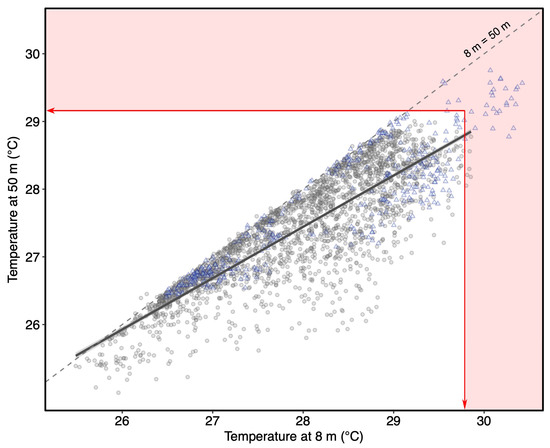
Figure 3.
Linear regression between water temperatures at 8 m and 50 m monitored from October 2011 to April 2020 (grey-filled circles; excluding the years with thermal anomalies 2016 and 2019) on the coral reef outer slope in Moorea. Temperature recordings from October 2018 to September 2019 (during the marine heatwave and severe coral bleaching event in 2019) are shown with a blue triangle (▲). The red zone represents when the bleaching thresholds at each depth (shallow BT: 29.79 °C; mesophotic BT: 29.16 °C) were exceeded.
The year 2019 was exceptionally warm in French Polynesia, and a severe coral bleaching event was observed in Moorea where 72% of pocilloporid coral colonies bleached at 10 m and mortality ranged from 11 % to 42 % four months after the warming event [55]. On 8 April 2019, water temperatures reached 30.42 °C at 8 m, 1.62 °C higher than the average in non-bleaching years (Figure 2 and blue triangles in Figure 3). However, despite water temperatures diverging with increasing temperature (Figure 3), water temperatures also increased by 1.56 °C at 50 m and reached 29.75 °C on the 15 April 2019 (Figure 2 and blue triangles in Figure 3). To date, these temperatures represent the highest temperatures ever recorded at both 8 m and 50 m in Moorea. The peak temperature at 50 m occurred 7 days later than at 8 m, a similar time lag as found during non-thermal stress years.
3.2. Comparison of Anemonefish Presence and Densities between Shallow and Mesophotic Reefs
Orange-fin anemonefish, Amphiprion chrysopterus, were found on shallow reefs (< 30 m) at five of the seven islands/atolls surveyed across three archipelagos in French Polynesia (Figure 1A; in red and blue), but only on both shallow and mesophotic reefs at three of the seven high islands/atolls surveyed (Figure 1A; in blue). Amphiprion chrysopterus were observed in shallow reefs at Tahiti and Moorea (Society Islands) and Rangiroa, Fakarava and Tikehau (Tuamotus), but were only observed in the mesophotic zone (below 40 m depth, between 48 and 60 m) at Tikehau, Moorea and Tahiti, belonging to two different archipelagos 300 km apart (Figure 1B). The host anemone, Heteractis magnifica, was the same species along the depth gradient, but in the mesophotic zone they were darker in colour and larger in size with a more flattened shape. These observations were made after over 500 mesophotic reef dives covering a total surface area of approximately 0.62 km2 (especially in Tahiti; Figure 1C) spanning ten years (January 2011–April 2021) with an additional 700 shallow dives spanning 15 years, covering a total surface area of approximately 3.39 km2 of shallow reefs across French Polynesia.
Anemonefish densities in shallow waters were estimated at 1.9 fish per 100,000 m2 in Tahiti, 1.79 fish per 100,000 m2 in Moorea, 1.16 fish per 100,000 m2 in Tikehau and 0.36 fish per 100,000 m2 in both Rangiroa and Fakarava. Despite being present in shallow waters, anemonefish were not found at mesophotic depths either in Rangiroa or in Fakarava. On the other hand, in Moorea, we located two groups of anemones (18 anemones in total) and six anemonefish. Firstly, we observed a group of 16 anemones, all H. magnifica, at a depth of 48 m containing two pairs of orange-fin anemonefish, A. chrysopterus, and all four fish were large adults (Figure 4A). The second group consisted of two anemones at a depth of 50 m, approx. 25 m away from first group, and hosted another adult anemonefish pair. In Tikehau at 55 m, we located a single H. magnifica anemone, containing an adult A. chrysopterus pair (Figure 4B). Finally, in Tahiti we found a single anemone and an adult pair at 60 m and another single adult in a single anemone at 55 m. Estimated anemonefish densities on mesophotic reefs, were 0 fish in Rangiroa, Fakarava, Manini and Tubuai; 0.0125 fish per 100,000 m2 in Tikehau; 0.075 fish per 100,000 m2 in Tahiti; and 1.90 fish per 100,000 m2 in Moorea. Estimated anemonefish densities were 25 and 92 times greater in shallow compared to mesophotic reefs in Tahiti and Tikehau respectively, but very similar in Moorea, although these estimations in Moorea might be biased due to all six anemonefish being found within 5 m of each other, in an anemone cluster coupled with the relatively small surveying effort at mesophotic depths. Even in shallow waters, precise density estimates are difficult as H. magnifica that host anemonefish in French Polynesia, are very scattered and vary between single isolated anemones in large areas to clusters of up to hundreds of anemones in a few tens of meters.

Figure 4.
Orange-fin anemonefish, Amphiprion chrysopterus, and their host, the magnificent sea anemone Heteractis magnifica, in the mesophotic zone. (A) One of the three pairs living in Moorea at a depth of 48–50 m, (B) one adult living at a depth of 55 m in Tikehau, fanning its eggs, (C) 6-day old eggs spawned at 50 m in Moorea on 18 June 2020 and (D) a bleached anemone at 50 m in Moorea, August 2019.
3.3. Comparison of Host Anemone Bleaching between Shallow and Mesophotic Reefs
Bleaching was monitored and quantified monthly in the shallow waters of Moorea from 2015–2021 (Supplementary Materials Table S2); however, bleaching was only monitored during the deep dives in the mesophotic zones. Nevertheless, at Moorea the mesophotic sites were visited four times. On one occasion, on 9 August 2019, six of the 16 anemones at 48 m were partially or completely bleached (Figure 4D), whereas the other two anemones at 50 m were healthy, i.e., 33% bleaching was observed at 50 m. The anemones had completely recovered their zooxanthellae and colour by February 2020 and were also 100% healthy when visited in June and again in July 2020. However, observed bleaching was higher in shallower waters for comparable dates. At the end of June 2019, 53% of anemones at the 52 sites monitored containing over 210 anemones in shallow reefs were bleached, vs. 33 % at 50 m. As per the mesophotic reefs, all shallow anemones had recovered and were healthy by February 2020, however, 26% of shallow water anemones were bleached in May and June 2020, compared to 0% bleaching at 48 to 50 m.
In Tahiti, no anemone bleaching was observed in the mesophotic zone on the 12 March 2016, and whilst bleaching was observed in the shallower zones in Tahiti it was not quantified, unlike in Moorea only 18 km away where 52% of the >100 anemones monitored were bleached in March 2016. On the 3 May 2021, no bleaching was seen either in shallow or mesophotic reefs in Tahiti or Moorea. Unfortunately, we do not have comparable measures of bleaching for shallow and mesophotic reefs in Tikehau, so a comparison cannot be carried out. Nevertheless, albeit based on our limited surveying at mesophotic reefs compared to our extensive monitoring on shallower reefs in Moorea, the average percentage of bleaching for comparable dates across two high islands on shallow and mesophotic reefs were 26% and 5.6%, respectively. The extent of anemone bleaching was nearly five times greater on shallow compared to mesophotic reefs.
3.4. Comparison of Reproductive Events between Shallow and Mesophotic Reefs
The four deep anemone territories in Moorea, Tahiti and Tikehau, were visited on a total of eight occasions (Moorea four times, and Tahiti and Tikehau twice) and we observed a total of three independent events of anemonefish reproduction in the mesophotic zone (Supplementary Materials Table S3). At Moorea, a nest was observed on 17 June 2020 at 48 m in the first large group of 16 anemones with eggs that had been laid 6 days prior on 11 June 2020 (Figure 4C; Supplementary Materials Figure S1). A nest was observed in the second group of two anemones at 50 m on the 29 July 2020 laid by the second anemonefish pair with eggs that had been laid 3 days prior on the 26 July 2020; Supplementary Materials Figure S2). Finally, in Tikehau we observed a nest on 17 February 2021 at 55 m with eggs that had been laid 6 days prior on 11 February 2021 (Figure 4B; Supplementary Materials Figure S3). Neither the number of eggs, egg development, nor parental care appeared any different from that observed for clutches laid at shallower depths. The developmental age of the eggs was determined from photographs (assuming similar developmental times to their shallower counterparts), enabling us to establish the precise date on which the eggs were laid. The two clutches in Moorea were laid during the first and last quarter moon, 8 days prior to and 6 days after the full moon respectively. The lunar spawning synchrony of the clutches laid in Moorea were compatible with the classical bi-monthly spawning pattern, the most common spawning pattern in anemonefish [56] and also in A. chrysopterus (DC, pers. comm). On the other hand, Tikehau’s spawning occurred during the new moon, 14 days prior to the full moon, could be consistent with the rarer strategy of three spawnings per lunar month.
In Moorea, we have monitored 772 spawnings from 74 breeding pairs on shallow reefs over three years. On the four occasions that the mesophotic reefs at Moorea were visited the % spawning frequency for the two pairs was 0%, 0%, 50% and 50% on 9 August 2019, 7 February 2020, 17 June 2020 and 30 July 2020, respectively. As a comparison, the spawning frequency for the 74 shallow breeding pairs (presence of eggs regardless of age, i.e., Days 1, 2, 3, 4, 5 and 6) were 17%, 35%, 25% and 11% on comparable dates. Similarly, on the two occasions that the mesophotic reefs at Tahiti were visited the percentage spawning frequency for the sole pair was 0% and 0% on 12 March 2016 and 3 May 2021. We determined that the spawning frequency for the 74 shallow breeding pairs in Moorea on the same dates were 6% and 14%. On the two occasions that the mesophotic reefs at Tikehau were visited the % spawning frequency for the sole pair was 0% and 100% on 25 February 2018 and 17 February 2021. We determined that the spawning frequency for the 74 shallow breeding pairs in Moorea on the same dates were 26 % and 9%. On average for all the dates combined, the spawning frequencies at mesophotic and shallow reefs for Tahiti, Moorea and Tikehau combined were highly similar at 25% and 19%, respectively.
4. Discussion
The long-term temperature monitoring data between shallow (8 m) and mesophotic reefs (50 m) revealed three interesting findings: a well-mixed water column in the cooler austral winter months, and less mixing in the hottest summer months; a seven-to-ten-day lag in high temperatures reaching mesophotic reefs from shallower waters during the summer; and a similar 0.95–1.10 °C increase in water temperature in the hottest summer months at both shallow and mesophotic reefs during mass bleaching event years. Correspondingly, we found that cnidarian mesophotic populations are not exempt from anthropogenic global warming, as bleaching in the magnificent sea anemone, Heteractis magnifica, was observed at a depth of 50 m as well as in shallower waters in Moorea during the thermal stress event of 2019. However, the bleaching frequency was five times lower on mesophotic reefs compared to shallower reefs. Our large-scale sampling across French Polynesia revealed that orange-fin anemonefish, Amphiprion chrysopterus, are found in their host anemones in the mesophotic zone down to 60 m; however, whilst their densities in MCEs were similar to those in shallower zones in Moorea, mesophotic densities were 25 and 92 times lower than in shallower zones in Tahiti and Tikehau respectively. Nevertheless, three successful spawning events were observed at these depths which corresponds to a similar spawning frequency found on shallow reefs.
Despite lower temperatures in mesophotic zones compared to those in shallow reefs, extreme temperatures in 2019 that induced a massive coral and anemone bleaching event in the shallow waters of Moorea lagoon [55] also resulted in extreme temperatures on mesophotic reefs and induced anemone bleaching at 50 m. The water temperatures in 2019 linearly regress in a similar manner to water temperatures in the other years (2011–2020), but temperatures in the hottest months of 2019 extend the regression line above the bleaching threshold at both depths (Figure 3). The strong positive temperature anomalies (>1 °C difference) during the warm season, exceeded the critical value at which anemones started to become bleached, both in the lagoon and at the deep sites. It remains to be determined if the bleaching threshold at mesophotic reefs is the same as at shallow reefs (29.79 °C) or is lower, i.e., 1 °C above the mean mesophotic temperature for April (29.16 °C), as anemones have adapted to the lower mesophotic temperatures. One other study has found that deeper corals show greater susceptibility to thermal stress than their shallow counterparts [31]. Temperature, rather than ocean acidification, has been demonstrated to be the key factor inducing anemone bleaching under 2100 warming scenario values [57]. As climate change continues and given the bleaching observed in Moorea at a depth of 50 m following a positive thermal anomaly, it is likely that bleaching events will become more severe and frequent, even in MCEs limiting the prospect that MCEs can be relied upon as a refuge for shallower reefs [18,26]. Nevertheless, the impacts of thermal stress resulted in five times less bleaching at 50 m than at shallower reefs. Such lower bleaching at depth agrees with other studies on corals [26], but nevertheless still highlights that mesophotic reefs are as at risk from climate change as their shallower counterparts, and as such should be given equivalent protection.
It is interesting to note that anemone bleaching does not occur at the same time in shallow and deep waters. The bleached status of anemones found at 50 m in August was relatively late in the year compared to shallower regions and may represent a delayed response of anemones in MCEs relative to shallow reefs as previously highlighted for corals [31]. The bleaching delay may be linked to the time lag taken for shallow warmer waters to reach mesophotic reefs that we highlighted from our long-term temperature monitoring data. Further studies are needed to understand the proximate mechanism driving this bleaching delay with depth. One other explanation could be the lack of food induced by the decrease in plankton abundance with elevated temperature [58]. Another major question is to understand why only a proportion of anemones bleach when it is likely that a high rate of clonality exists at each site.
The emergence of deep scientific diving techniques and especially closed-circuit diving [50,51,52,53] enabled us to describe, for the first time, the depth range of orange-fin anemonefish, A. chrysopterus, associated with magnificent sea anemones, H. magnifica, down to 60 m in several Polynesian islands and across two archipelagos. Our study agrees with and extends the bathymetric limit of A. chrysopterus from 0–40 m to 0–60 m [36]. Our study also indicates that both host sea anemones and anemonefishes can live across a large bathymetric range in different islands hundreds of miles away: anemonefish can live in mesophotic coral ecosystems (MCEs) both on high islands (Tahiti and Moorea) and in atolls (Tikehau). This species occurs in very distinct areas, from lagoonal areas (shallow, strong currents, turbid water) to the outer slope (from a few meters to 60 m deep, with little current and very clear waters).
Amphiprion chrysopterus, and many other anemonefish species, has become a preferred model organism to study marine dispersal dynamics [59,60]; however, the individuals at the deeper edge of the distribution are never included in parentage analysis studies, which would quantify their contribution to population replenishment and should be included in future dispersal studies. Furthermore, anemones have a large geographical distribution [61], and while anemones are known to photo-acclimate at depth by increasing the abundance of their symbiotic microalgae [62] there is still little knowledge regarding phenotypic adjustments [63] of anemonefish, or fishes in general, at depth, which would be interesting to study in the future. The host sea anemones may also have acclimated to depth in other ways as they were darker, larger and flatter, all changes likely linked to adaptations to cope with the lower access to light. In a similar manner to corals growing at depth, the flattened shape of anemones may be linked to the lower availability of light, a lower photosynthetic rate and lower growth [49] and the clade of symbiodinium may be the same at depth, as shown up to 172 m [64]. The change in anemone colour may be an adaptation of pigments to increase photosynthesis under low light conditions. In terms of their larger size, this may be an adaption to compensate for lower light levels at depth. Alternatively, gigantism of coral colonies has been attributed to the lower water currents at depth and may also thus explain the larger size of anemones at 50 m [49].
Our study also revealed that the densities of orange-fin anemonefish, A. chrysopterus, were either similar to (in Moorea) or 25–92 times (in Tahiti and Tikehau) less dense in mesophotic compared to shallow reefs. However, as the host anemone, H. magnifica, may occur in large clusters without another individual anemone for hundreds of meters, coupled with the fact that mesophotic surveys are more challenging technically and financially, our density estimates may have been biased. Nevertheless, population densities of the bicolor damselfish, Stegastes partitus, also decreased with depth [65] in agreement with our results.
The presence of three successful breeding events proves that anemonefish adults at depth were reproductively functional and perennial and showed similar, even a moderately higher, frequency of spawning than on shallower reefs. One of the spawning events appeared to follow the most common pattern of bi-monthly spawning events, approx. six days before the full moon. A second spawning event follows the pattern of tri-monthly spawning. Thus, depth does not appear to affect the sensitivity to lunar cycles for these individuals and the light of the full moon is known to penetrate clear oceanic waters to several hundred meters [66]. It is, therefore, not surprising that these mesophotic pairs may retain the benefits of synchronized reproduction to the lunar cycle similar to their shallow-water counterparts. Regular monitoring of these different pairs would be necessary in order to precisely determine the spawning pattern and synchronization between mesophotic and shallow water congeners. At least one species of fish, the three-spot damselfish, Chromis verater, is genetically connected between MCEs and shallow reefs [38] and it would be interesting to determine the connectivity of A. chrysopterus with shallower populations as well as assessing the genetic diversity of these mesophotic populations. In addition, parental environment can influence larval traits (Cortese et al. 2021 in review), therefore, the study of physiological and morphological characteristics of larvae from these deep anemonefish pairs would be interesting to elucidate any differences in larval traits that may be associated with depth.
5. Conclusions
Shallow water populations have been facing increasingly severe and frequent warming events in recent years [5] and mesophotic reefs, hypothesized to be less exposed to anthropogenic disturbances, are predicted to function as refugia for shallow reefs. The discovery of reproducing mesophotic anemonefish shows that these reefs have the potential to provide a refuge from anthropogenic disturbances and other human impacts. However, our observation of anemone bleaching in August 2019 at 50 m depth, albeit five times lower that at shallower reefs, suggests that even MCEs are affected by thermal stressor events and may not be the refuges from global warming as previously hoped. In a similar manner to corals that undergo bleaching at depth [26], bleaching in deep anemones may impact anemone survival. Furthermore, anemone bleaching is known to induce stress in resident fish, decreasing their fitness and reproduction [43,44,45]. Therefore, bleaching in mesophotic reefs jeopardises the ability of deep reefs to act as a refuge for anemonefish populations from global warming. Long-term monitoring is required to compare the duration of anemone bleaching between shallow and mesophotic areas to determine whether MCEs provide refuge for anemonefish in terms of bleaching duration. However, if the adverse effects of warming on shallow anemone hosts induce the same consequences in mesophotic anemones, it is plausible to wonder whether the reproductive success of mesophotic fish will be maintained at its current level and whether these individuals will be able to contribute to the maintenance of recruitment to shallow populations, limiting the potential for mesophotic reefs to act as refugia.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/fishes6030037/s1. Table S1. Clownfish transects. Table S2. Anemone bleaching. Table S3. Clownfish reproduction. Figure S1. Photograph of the nest at Moorea taken on 17th June 2020 at 48 m in the first large group of 16 anemones with eggs that had been laid 6 days prior on 11th June 2020. Figure S2. Photograph of the nest at Moorea in the second group of two anemones at 50 m taken on the 29th July 2020 laid by the second anemonefish pair with eggs that had been laid 3 days prior on the 26th July 2020. Figure S3. Photographs of the nest at Tikehau taken on 17th February 2021 at 55 m with eggs that had been laid 6 days prior on 11th February 2021.
Author Contributions
Conceptualization, A.H., S.C.M. and R.B.; diving and underwater pictures A.H., G.S. and F.Z.; data collection, A.H., G.S., F.Z., D.C., R.B. and S.C.M.; data analysis A.H., D.C. and S.C.M.; validation A.H., G.S., F.Z., D.C., R.B. and S.C.M.; writing—original draft, A.H., S.C.M. and R.B.; writing—review and editing, A.H., S.C.M., D.C., G.S. and R.B. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was provided by SNO CORAIL during visits to Tubuai and Tikehau, the Agence National de la Recherche (ANR-14-CE02-0005-01/Stay or Go) to Glenn Almany, S.C.M. and R.B., by the Haut-Commissariat de la République en Polynésie Française (HC/3041/DIE/BPT/) to S.C.M., Pacific Funds (BLEACH & ALAN) to S.C.M. and Millenium Nucleus for the Ecology and Conservation of Temperate Mesophotic Reef Ecosystem (NUTME) to R.B.
Institutional Review Board Statement
The study did not involve any animal testing or handling and was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of CNRS (CNRS 006725).
Data Availability Statement
The temperature data presented in this study are available on request from http://observatoire.criobe.pf/wiki/tiki-index.php (accessed on 19 August 2021). Data for anemonefish transects at each island are available in Supplementary Materials Table S1, for anemone bleaching in Supplementary Materials Table S2 and for anemonefish reproduction in Supplementary Materials Table S3 with photos from Moorea, Tahiti and Tikehau in Supplementary Materials Figures S1–S3 resp.
Acknowledgments
We would like to acknowledge the National Service of Observation of CRIOBE (SNO Corail) for the temperature data, Tikehau Diving Center and Tahiti Rebreather for deep diving facilities and Jules Schligler for providing data on anemone bleaching in 2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pandolfi, J.M.; Bradbury, R.H.; Sala, E.; Hughes, T.P.; Bjorndal, K.A.; Cooke, R.G.; McArdle, D.; McClenachan, L.; Newman, M.J.H.; Paredes, G.; et al. Global trajectories of the long-term decline of coral reef ecosystems. Science 2003, 301, 955–958. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral reefs under rapid climate change and ocean acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef]
- Spalding, M.D.; Brown, B.E. Warm-water coral reefs and climate change. Science 2015, 350, 769–771. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- Hughes, T.P.; Anderson, K.D.; Connolly, S.R.; Heron, S.F.; Kerry, J.T.; Lough, J.M.; Baird, A.H.; Baum, J.K.; Berumen, M.L.; Bridge, T.C.; et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 2018, 359, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Smale, D.A.; Wernberg, T.; Oliver, E.C.J.; Thomsen, M.; Harvey, B.P.; Straub, S.C.; Burrows, M.T.; Alexander, L.V.; Benthuysen, J.A.; Donat, M.G.; et al. Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat. Clim. Chang. 2019, 9, 306–312. [Google Scholar] [CrossRef]
- Hoey, A.S.; Howells, E.; Johansen, J.L.; Hobbs, J.-P.A.; Messmer, V.; McCowan, D.M.; Wilson, S.K.; Pratchett, M.S. Recent advances in understanding the effects of climate change on coral reefs. Diversity 2016, 8, 12. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Baird, A.H.; Connolly, S.R.; Dietzel, A.; Eakin, C.M.; Heron, S.F.; Hoey, A.S.; Hoogenboom, M.O.; Liu, G.; et al. Global warming transforms coral reef assemblages. Nature 2018, 556, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Glynn, P.W. Coral reef bleaching: Facts, hypotheses and implications. Glob. Chang. Biol. 1996, 2, 495–509. [Google Scholar] [CrossRef]
- Bridge, T.; Scott, A.; Steinberg, D. Abundance and diversity of anemonefishes and their host sea anemones at two mesophotic sites on the Great Barrier Reef, Australia. Coral Reefs 2012, 31, 1057–1062. [Google Scholar] [CrossRef]
- Muir, P.R.; Marshall, P.A.; Abdulla, A.; Aguirre, J.D. Species identity and depth predict bleaching severity in reef-building corals: Shall the deep inherit the reef? Proc. R. Soc. B Biol. Sci. 2017, 284, 20171551. [Google Scholar] [CrossRef]
- Slattery, M.; Lesser, M.P.; Brazeau, D.; Stokes, M.D.; Leichter, J.J. Connectivity and stability of mesophotic coral reefs. J. Exp. Mar. Bio. Ecol. 2011, 408, 32–41. [Google Scholar] [CrossRef]
- Kahng, S.E.; Copus, J.M.; Wagner, D. Recent advances in the ecology of mesophotic coral ecosystems (MCEs). Curr. Opin. Environ. Sustain. 2014, 7, 72–81. [Google Scholar] [CrossRef]
- Kahng, S.E.; Garcia-Sais, J.R.; Spalding, H.L.; Brokovich, E.; Wagner, D.; Weil, E.; Hinderstein, L.; Toonen, R.J. Community ecology of mesophotic coral reef ecosystems. Coral Reefs 2010, 29, 225–275. [Google Scholar] [CrossRef]
- Reed, J. Deepest Distribution of Atlantic Hermatypic Corals Discovered in the Bahamas. In Proceedings of the Fifth International Coral Reef Congress, French Polynesia, France, 27 May–1 June 1985; Antenne Museum-EPHE: Moorea, French Polynesia, France, 1985; pp. 249–254. [Google Scholar]
- Liddell, W.D.; Ohlhorst, S.L. Hard substrata community patterns, 1–120 m, North Jamaica. Palaios 1988, 3, 413. [Google Scholar] [CrossRef]
- Weiss, K.R. Can deep reefs rescue shallow ones? Science 2017, 355, 903. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.A.; Pinheiro, H.T.; Shepherd, B.; Papastamatiou, Y.P.; Luiz, O.J.; Pyle, R.L.; Bongaerts, P. Mesophotic coral ecosystems are threatened and ecologically distinct from shallow water reefs. Science 2018, 361, 281–284. [Google Scholar] [CrossRef]
- Hinderstein, L.M.; Marr, J.C.A.; Martinez, F.A.; Dowgiallo, M.J.; Puglise, K.A.; Pyle, R.L.; Zawada, D.G.; Appeldoorn, R. Theme section on “Mesophotic Coral Ecosystems: Characterization, Ecology, and Management. ” Coral Reefs 2010, 29, 247–251. [Google Scholar] [CrossRef]
- Lindfield, S.J.; Harvey, E.S.; Halford, A.R.; McIlwain, J.L. Mesophotic depths as refuge areas for fishery-targeted species on coral reefs. Coral Reefs 2016, 35, 125–137. [Google Scholar] [CrossRef]
- Andradi-Brown, D.A.; Beer, A.J.E.; Colin, L.; Hastuti; Head, C.E.I.; Hidayat, N.I.; Lindfield, S.J.; Mitchell, C.R.; Pada, D.N.; Piesinger, N.M.; et al. Highly diverse mesophotic reef fish communities in Raja Ampat, West Papua. Coral Reefs 2021, 40, 111–130. [Google Scholar] [CrossRef]
- Asher, J.; Williams, I.D.; Harvey, E.S. An assessment of mobile predator populations along shallow and mesophotic depth gradients in the Hawaiian archipelago. Sci. Rep. 2017, 7, 3905. [Google Scholar] [CrossRef] [PubMed]
- Bongaerts, P.; Ridgway, T.; Sampayo, E.M.; Hoegh-Guldberg, O. Assessing the “deep reef refugia” hypothesis: Focus on Caribbean reefs. Coral Reefs 2010, 29, 309–327. [Google Scholar] [CrossRef]
- Smith, T.B.; Glynn, P.W.; Maté, J.L.; Toth, L.T.; Gyory, J. A depth refugium from catastrophic coral bleaching prevents regional extinction. Ecology 2014, 95, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.A.; Andradi-Brown, D.A.; Gori, A.; Bongaerts, P.; Burdett, H.L.; Ferrier-Pagès, C.; Voolstra, C.R.; Weinstein, D.K.; Bridge, T.C.L.; Costantini, F.; et al. Key Questions for Research and Conservation of Mesophotic Coral Ecosystems and Temperate Mesophotic Ecosystems. In Mesophotic Coral Ecosystems; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Frade, P.R.; Bongaerts, P.; Englebert, N.; Rogers, A.; Gonzalez-Rivero, M.; Hoegh-Guldberg, O. Deep reefs of the Great Barrier Reef offer limited thermal refuge during mass coral bleaching. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Welch, D. Book Review: Climate Change as Social Drama: Global Warming in the Public Sphere. Sociol. Rev. 2016, 64, 387–390. [Google Scholar] [CrossRef]
- Laverick, J.H.; Rogers, A.D. Experimental evidence for reduced mortality of Agaricia lamarcki on a mesophotic reef. Mar. Environ. Res. 2018, 134, 37–43. [Google Scholar] [CrossRef]
- Nir, O.; Gruber, D.F.; Shemesh, E.; Glasser, E.; Tchernov, D. Seasonal mesophotic coral bleaching of Stylophora pistillata in the northern Red Sea. PLoS ONE 2014, 9, e84968. [Google Scholar] [CrossRef]
- Eyal, G.; Tamir, R.; Kramer, N.; Eyal-Shaham, L.; Loya, Y. The Red Sea: Israel. In Mesophotic Coral Ecosystems; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Smith, T.B.; Gyory, J.; Brandt, M.E.; Miller, W.J.; Jossart, J.; Nemeth, R.S. Caribbean mesophotic coral ecosystems are unlikely climate change refugia. Glob. Chang. Biol. 2016, 22, 2759–2765. [Google Scholar] [CrossRef]
- Brokovich, E.; Ayalon, I.; Einbinder, S.; Segev, N.; Shaked, Y.; Genin, A.; Kark, S.; Kiflawi, M. Grazing pressure on coral reefs decreases across a wide depth gradient in the Gulf of Aqaba, Red Sea. Mar. Ecol. Prog. Ser. 2010, 399, 69–80. [Google Scholar] [CrossRef]
- Malcolm, H.A.; Jordan, A.; Smith, S.D.A. Testing a depth-based habitat classification system against reef fish assemblage patterns in a subtropical marine park. Aquat. Conserv. Mar. Freshw. Ecosyst. 2011, 21, 173–185. [Google Scholar] [CrossRef]
- Feitoza, B.M.; Rosa, R.S.; Rocha, L.A. Ecology and zoogeography of deep-reef fishes in northeastern Brazil. Bull. Mar. Sci. 2005, 76, 725–742. [Google Scholar]
- Pearson, R.; Stevens, T. Distinct cross-shelf gradient in mesophotic reef fish assemblages in subtropical eastern Australia. Mar. Ecol. Prog. Ser. 2015, 532, 185–196. [Google Scholar] [CrossRef]
- Coleman, R.R.; Copus, J.M.; Coffey, D.M.; Whitton, R.K.; Bowen, B.W. Shifting reef fish assemblages along a depth gradient in Pohnpei, Micronesia. PeerJ 2018, 6, e4650. [Google Scholar] [CrossRef]
- Tenggardjaja, K.A.; Bowen, B.W.; Bernardi, G. Vertical and horizontal genetic connectivity in Chromis verater, an endemic damselfish found on shallow and mesophotic reefs in the Hawaiian archipelago and adjacent Johnston atoll. PLoS ONE 2014, 9, e115493. [Google Scholar] [CrossRef]
- Van Oppen, M.J.H.; Bongaerts, P.; Underwood, J.N.; Peplow, L.M.; Cooper, T.F. The role of deep reefs in shallow reef recovery: An assessment of vertical connectivity in a brooding coral from west and east Australia. Mol. Ecol. 2011, 20, 1647–1660. [Google Scholar] [CrossRef] [PubMed]
- Serrano, X.M.; Baums, I.B.; O’Reilly, K.; Smith, T.B.; Jones, R.J.; Shearer, T.L.; Nunes, F.L.D.; Baker, A.C. Geographic differences in vertical connectivity in the Caribbean coral Montastraea cavernosa despite high levels of horizontal connectivity at shallow depths. Mol. Ecol. 2014, 23, 4226–4240. [Google Scholar] [CrossRef] [PubMed]
- Shlesinger, T.; Grinblat, M.; Rapuano, H.; Amit, T.; Loya, Y. Can mesophotic reefs replenish shallow reefs? Reduced coral reproductive performance casts a doubt. Ecology 2018, 99, 421–437. [Google Scholar] [CrossRef]
- Holstein, D.M.; Paris, C.B.; Vaz, A.C.; Smith, T.B. Modeling vertical coral connectivity and mesophotic refugia. Coral Reefs 2016, 35, 23–37. [Google Scholar] [CrossRef]
- Mariscal, R.N.; Fautin, D.G.; Allen, G.R. Field Guide to Anemonefishes and Their Host Sea Anemones. Copeia 1993, 1993. [Google Scholar] [CrossRef]
- Norin, T.; Mills, S.C.; Crespel, A.; Cortese, D.; Killen, S.S.; Beldade, R. Anemone bleaching increases the metabolic demands of symbiont anemonefish. Proc. R. Soc. B Biol. Sci. 2018, 285, 20180282. [Google Scholar] [CrossRef]
- Beldade, R.; Blandin, A.; O’Donnell, R.; Mills, S.C. Cascading effects of thermally-induced anemone bleaching on associated anemonefish hormonal stress response and reproduction. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cortese, D.; Norin, T.; Beldade, R.; Crespel, A.; Killen, S.S.; Mills, S.C. Physiological and behavioural effects of anemone bleaching on symbiont anemonefish in the wild. Funct. Ecol. 2021, 35, 663–674. [Google Scholar] [CrossRef]
- Leray, M.; Beldade, R.; Holbrook, S.J.; Schmitt, R.J.; Planes, S.; Bernardi, G. Allopatric divergence and speciation in coral reef fish: The three-spot dascyllus, Dascyllus trimaculatus, species complex. Evolution 2010, 64, 1218–1230. [Google Scholar] [CrossRef]
- Brokovich, E.; Einbinder, S.; Shashar, N.; Kiflawi, M.; Kark, S. Descending to the twilight-zone: Changes in coral reef fish assemblages along a depth gradient down to 65 m. Mar. Ecol. Prog. Ser. 2008, 371, 253–262. [Google Scholar] [CrossRef]
- Steinberg, R.K.; van der Meer, M.H.; Pratchett, M.S.; van Herwerden, L.; Hobbs, J.-P.A. Keep your friends close and your anemones closer—Ecology of the endemic wideband anemonefish, Amphiprion latezonatus. Environ. Biol. Fishes 2020, 103, 1513–1526. [Google Scholar] [CrossRef]
- Kühlmann, D.H.H. Composition and ecology of deep-water coral associations. Helgoländer Meeresunters. 1983, 36, 183–204. [Google Scholar] [CrossRef]
- Pyle, R.L. Assessing undiscovered fish biodiversity on deep coral reefs using advanced self-contained diving technology. Mar. Technol. Soc. J. 2000, 34, 82–91. [Google Scholar] [CrossRef]
- Pyle, R.L. Exploring deep coral reefs: How much biodiversity are we missing? Glob. Biodivers. 1996, 6, 3–7. [Google Scholar]
- Sieber, A.; Pyle, R. A review of the use of closed-circuit rebreathers for scientific diving. Underw. Technol. 2010, 29, 73–78. [Google Scholar] [CrossRef]
- Pinheiro, H.T.; Mazzei, E.; Moura, R.L.; Amado-Filho, G.M.; Carvalho-Filho, A.; Braga, A.C.; Costa, P.A.S.; Ferreira, B.P.; Ferreira, C.E.L.; Floeter, S.R.; et al. Fish biodiversity of the Vitória-Trindade seamount chain, southwestern Atlantic: An updated database. PLoS ONE 2015, 10, e0118180. [Google Scholar] [CrossRef]
- Liu, G.; Strong, A.; Skirving, W.; Arzayus, F. Overview of NOAA Coral Reef Watch Program’s Near-Real Time Satellite Global Coral Bleaching Monitoring Activities. In Proceedings of the 10th International Coral Reef Symposium, Okinawa, Japan, 28 June–2 July 2003; Gurugram: Okinawa, Japan, 2006; Volume 1793, pp. 1783–1793. [Google Scholar]
- Burgess, S.C.; Johnston, E.C.; Wyatt, A.S.J.; Leichter, J.J.; Edmunds, P.J. Response diversity in corals: Hidden differences in bleaching mortality among cryptic Pocillopora species. Ecology 2021, 102, e03324. [Google Scholar] [CrossRef]
- Seymour, J.R.; Barbasch, T.A.; Buston, P.M. Lunar cycles of reproduction in the clown anemonefish Amphiprion percula: Individual-level strategies and population-level patterns. Mar. Ecol. Prog. Ser. 2018, 594, 193–201. [Google Scholar] [CrossRef]
- Pryor, S.H.; Andrews, L.; Kelaher, B.P.; Tagliafico, A.; Scott, A. Ocean temperature, but not acidification, causes sea anemone bleaching under a near-future climate scenario. Coral Reefs 2021, 40, 355–364. [Google Scholar] [CrossRef]
- Tada, K.; Sakai, K.; Nakano, Y.; Takemura, A.; Montani, S. Size-fractionated phytoplankton biomass in coral reef waters off Sesoko Island, Okinawa, Japan. J. Plankton Res. 2003, 25, 991–997. [Google Scholar] [CrossRef]
- Beldade, R.; Holbrook, S.J.; Schmitt, R.J.; Planes, S.; Malone, D.; Bernardi, G. Larger female fish contribute disproportionately more to self-replenishment. Proc. R. Soc. B Biol. Sci. 2012, 279, 2116–2121. [Google Scholar] [CrossRef] [PubMed]
- Beldade, R.; Holbrook, S.J.; Schmitt, R.J.; Planes, S.; Bernardi, G. Spatial patterns of self-recruitment of a coral reef fish in relation to island-scale retention mechanisms. Mol. Ecol. 2016, 25, 5203–5211. [Google Scholar] [CrossRef]
- Emms, M.A.; Saenz-Agudelo, P.; Giles, E.C.; Gatins, R.; Nanninga, G.B.; Scott, A.; Hobbs, J.P.A.; Frisch, A.J.; Mills, S.C.; Beldade, R.; et al. Comparative phylogeography of three host sea anemones in the Indo-Pacific. J. Biogeogr. 2020, 47, 487–500. [Google Scholar] [CrossRef]
- Dixon, A.K.; Needham, D.; Al-Horani, F.A.; Chadwick, N.E. Microhabitat use and photoacclimation in the clownfish sea anemone Entacmaea quadricolor. J. Mar. Biol. Assoc. UK 2014, 94, 473–480. [Google Scholar] [CrossRef]
- MacDonald, C.; Bridge, T.C.L.; Jones, G.P. Depth, bay position and habitat structure as determinants of coral reef fish distributions: Are deep reefs a potential refuge? Mar. Ecol. Prog. Ser. 2016, 561, 217–231. [Google Scholar] [CrossRef]
- Rouzé, H.; Galand, P.E.; Medina, M.; Bongaerts, P.; Pichon, M.; Pérez-Rosales, G.; Torda, G.; Moya, A.; Bardout, G.; Périé-Bardout, E.; et al. Symbiotic associations of the deepest recorded photosynthetic scleractinian coral (172 m depth). ISME J. 2021, 15, 1564–1568. [Google Scholar] [CrossRef]
- Goldstein, E.D.; D’Alessandro, E.K.; Sponaugle, S. Demographic and reproductive plasticity across the depth distribution of a coral reef fish. Sci. Rep. 2016, 6, 34077. [Google Scholar] [CrossRef] [PubMed]
- Favorite, F.; Laevastu, T.; Straty, R. Oceanography of the Northestern Pacific Ocean and Eastern Bering Sea and Relations to Various Living Marine Resources; National Marine Fisheries Service, Northwest and Alaska Fisheries Center: Washington, WA, USA, 1977. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).