Shelf-Life Prediction of Glazed Large Yellow Croaker (Pseudosciaena crocea) during Frozen Storage Based on Arrhenius Model and Long-Short-Term Memory Neural Networks Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
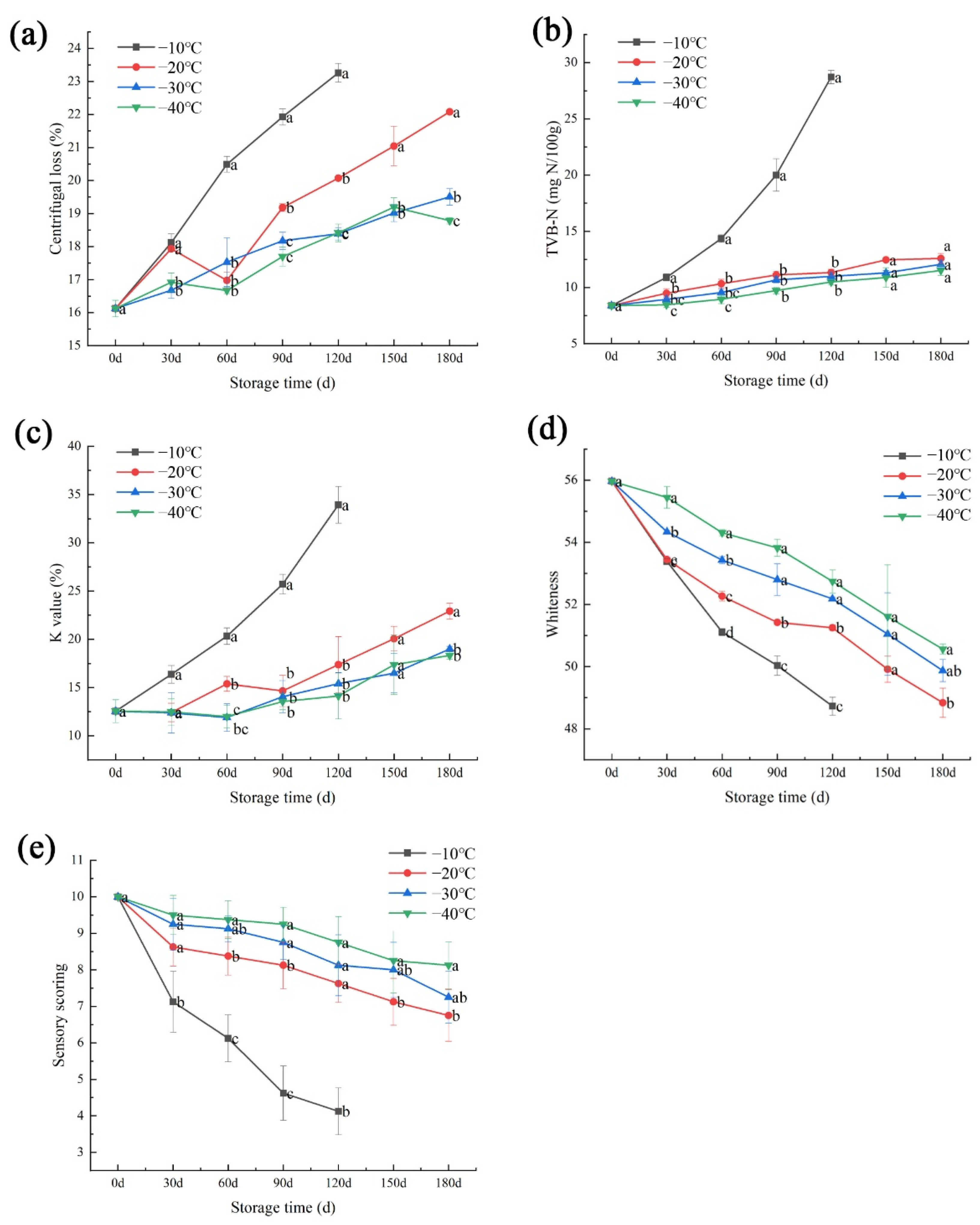
2.2. Centrifugal Loss
2.3. Total Volatile Base Nitrogen (TVB-N)
2.4. K Value
2.5. Color Measurement
2.6. Sensory Analysis
2.7. Shelf-Life Prediction
2.7.1. Arrhenius Model
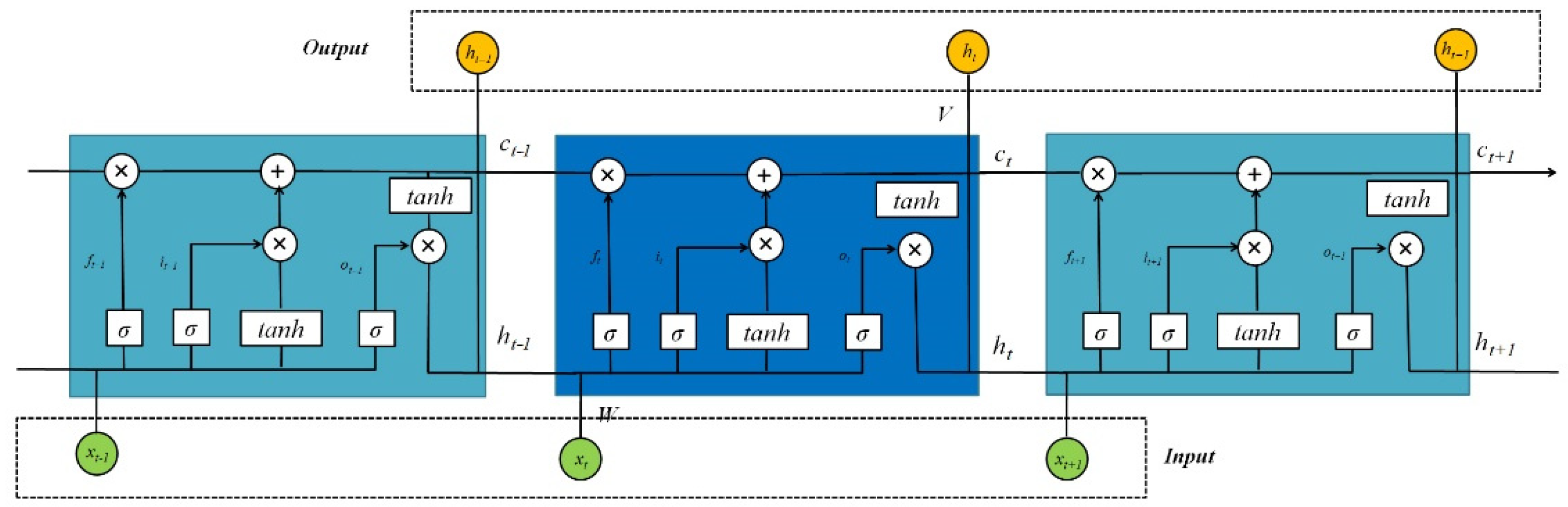
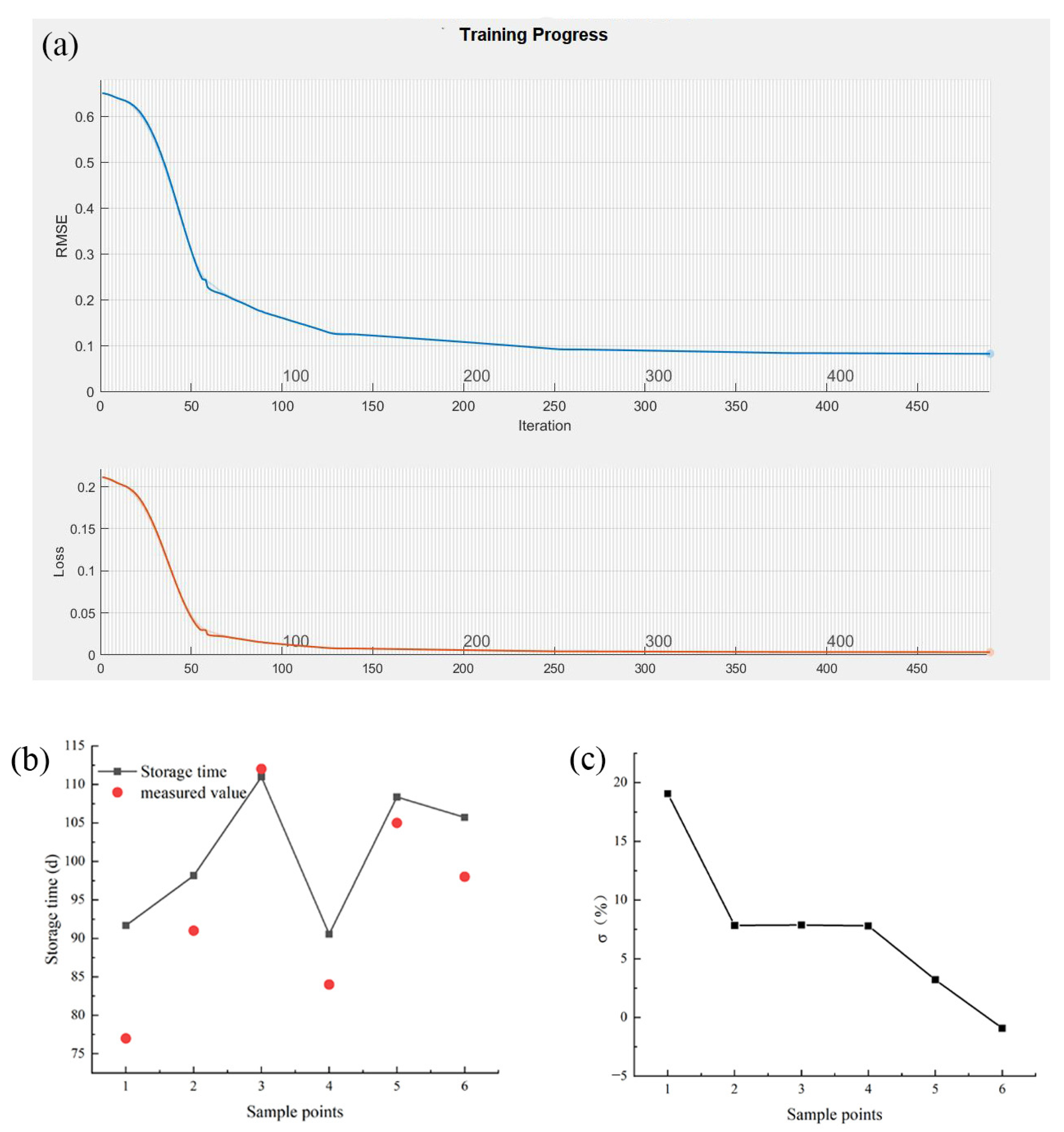
2.7.2. LSTM-NN Model
3. Results
4. Discussion
4.1. Arrhenius Model
4.1.1. Dynamical Analysis
4.1.2. Shelf-Life Modeling and Shelf-Life Forecasting
4.2. LSTM-NN Model
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- China Statistics Press. China Fishery Statistical Yearbook; China Agricultural Press: Beijing, China, 2020; pp. 22–23. [Google Scholar]
- Aponte, M.; Anastasio, A.; Marrone, R.; Mercogliano, R.; Peruzy, M.F.; Murru, N. Impact of gaseous ozone coupled to passive refrigeration system to maximize shelf-life and quality of four different fresh fish products. LWT 2018, 93, 412–419. [Google Scholar] [CrossRef]
- Cartagena, L.; Puértolas, E.; de Marañón, I.M. Application of high pressure processing after freezing (before frozen storage) or before thawing in frozen albacore tuna (Thunnus alalunga). Food Bioprocess Technol. 2020, 13, 1791–1800. [Google Scholar] [CrossRef]
- Truong, B.Q.; Buckow, R.; Nguyen, M.H.; Stathopoulos, C.E. High pressure processing of barramundi (Lates calcarifer) muscle before freezing: The effects on selected physicochemical properties during frozen storage. J. Food Eng. 2016, 169, 72–78. [Google Scholar] [CrossRef]
- Zhang, M.; Haili, N.; Chen, Q.; Xia, X.; Kong, B. Influence of ultrasound-assisted immersion freezing on the freezing rate and quality of porcine longissimus muscles. Meat Sci. 2018, 136, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tsironi, T.N.; Stoforos, N.G.; Taoukis, P.S. Quality and shelf-life modeling of frozen fish at constant and variable temperature conditions. Foods 2020, 9, 1893. [Google Scholar] [CrossRef] [PubMed]
- Trigo, M.; Rodríguez, A.; Dovale, G.; Pastén, A.; Vega-Gálvez, A.; Aubourg, S.P. The effect of glazing based on saponin-free quinoa (Chenopodium quinoa) extract on the lipid quality of frozen fatty fish. LWT 2018, 98, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.; Li, P.; Yu, W.; Wang, J.; Xie, J. Effects of glazing with preservatives on the quality changes of squid during frozen storage. Appl. Sci. 2019, 9, 3847. [Google Scholar] [CrossRef] [Green Version]
- Žoldoš, P.; Popelka, P.; Marcinčák, S.; Nagy, J.; Mesarčová, L.; Pipová, M.; Jevinová, P.; Nagyová, A.; Maľa, P. The effect of glaze on the quality of frozen stored Alaska pollack (Theragra chalcogramma) fillets under stable and unstable conditions. Acta Vet. Brno 2011, 80, 299–304. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Lei, Y.; Shen, H.; Hong, H.; Yu, X.; Zhu, B.; Luo, Y. Effect of glazing and rosemary (Rosmarinus officinalis) extract on preservation of mud shrimp (Solenocera melantho) during frozen storage. Food Chem. 2019, 272, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Mercogliano, R.; De Felice, A.; Cortesi, M.L.; Murru, N.; Marrone, R.; Anastasio, A. Biogenic amines profile in processed bluefin tuna (Thunnus thynnus) products. CyTA-J. Food 2013, 11, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Holman, B.W.B.; Kerry, J.P.; Hopkins, D.L. A review of patents for the smart packaging of meat and muscle-based food products. Recent Pat. Food. Nutr. Agric. 2018, 9, 3–13. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- English, M.M.; Scrosati, P.M.; Aquino, A.J.; McSweeney, M.B.; Gulam Razul, M.S. Novel carbohydrate blend enhances chemical and sensory properties of lobster (Homarus americanus) after one-year frozen storage. Food Res. Int. 2020, 137, 109697. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Wang, J.; Li, P.; Xie, J. Storage time prediction of glazed frozen squids during frozen storage at different temperatures based on neural network. Int. J. Food Prop. 2020, 23, 1663–1677. [Google Scholar] [CrossRef]
- Li, D.; Xie, H.; Liu, Z.; Li, A.; Li, J.; Liu, B.; Liu, X.; Zhou, D. Shelf life prediction and changes in lipid profiles of dried shrimp (Penaeus vannamei) during accelerated storage. Food Chem. 2019, 297, 124951. [Google Scholar] [CrossRef] [PubMed]
- Corzo, O.; Bracho, N.; Marjal, J. Color change kinetics of sardine sheets during vacuum pulse osmotic dehydration. J. Food Eng. 2006, 75, 21–26. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Y.; Shen, S.; Luo, Y.; Gao, L. Comparison of Arrhenius model and artificial neuronal network for the quality prediction of rainbow trout (Oncorhynchus mykiss) fillets during storage at different temperatures. LWT-Food Sci. Technol. 2015, 60, 142–147. [Google Scholar] [CrossRef]
- Mohammadi Lalabadi, H.; Sadeghi, M.; Mireei, S.A. Fish freshness categorization from eyes and gills color features using multi-class artificial neural network and support vector machines. Aquac. Eng. 2020, 90, 102076. [Google Scholar] [CrossRef]
- Marini, F. Artificial neural networks in foodstuff analyses: Trends and perspectives A review. Anal. Chim. Acta 2009, 635, 121–131. [Google Scholar] [CrossRef]
- Yu, L.; Qu, J.; Gao, F.; Tian, Y. A novel hierarchical algorithm for bearing fault diagnosis based on stacked LSTM. Shock Vib. 2019, 2019, 2756284. [Google Scholar] [CrossRef]
- Wang, H.; Kong, C.; Li, D.; Qin, N.; Fan, H.; Hong, H.; Luo, Y. Modeling quality changes in brined bream (Megalobrama amblycephala) fillets during storage: Comparison of the Arrhenius model, BP, and RBF neural network. Food Bioprocess Technol. 2015, 8, 2429–2443. [Google Scholar] [CrossRef]
- Cheng, G.; Wang, X.; He, Y. Remaining useful life and state of health prediction for lithium batteries based on empirical mode decomposition and a long and short memory neural network. Energy 2021, 232, 121022. [Google Scholar] [CrossRef]
- Han, Y.; Fan, C.; Xu, M.; Geng, Z.; Zhong, Y. Production capacity analysis and energy saving of complex chemical processes using LSTM based on attention mechanism. Appl. Therm. Eng. 2019, 160, 114072. [Google Scholar] [CrossRef]
- Conover, M.; Staples, M.; Si, D.; Sun, M.; Cao, R. AngularQA: Protein model quality assessment with LSTM networks. Comput. Math. Biophys. 2019, 7, 1–9. [Google Scholar] [CrossRef]
- Tan, M.; Ye, J.; Chu, Y.; Xie, J. The effects of ice crystal on water properties and protein stability of large yellow croaker (Pseudosciaena crocea). Int. J. Refrig. 2021, 130, 242–252. [Google Scholar] [CrossRef]
- Li, P.; Chen, Z.; Tan, M.; Mei, J.; Xie, J. Evaluation of weakly acidic electrolyzed water and modified atmosphere packaging on the shelf life and quality of farmed puffer fish (Takifugu obscurus) during cold storage. J. Food Saf. 2020, 40, e12773. [Google Scholar] [CrossRef]
- Yang, W.; Shi, W.; Zhou, S.; Qu, Y.; Wang, Z. Research on the changes of water-soluble flavor substances in grass carp during steaming. J. Food Biochem. 2019, 43, e12993. [Google Scholar] [CrossRef] [PubMed]
- Ozogul, Y.; Yuvka, İ.; Ucar, Y.; Durmus, M.; Kösker, A.R.; Öz, M.; Ozogul, F. Evaluation of effects of nanoemulsion based on herb essential oils (rosemary, laurel, thyme and sage) on sensory, chemical and microbiological quality of rainbow trout (Oncorhynchus mykiss) fillets during ice storage. LWT 2017, 75, 677–684. [Google Scholar] [CrossRef]
- Chaudhry, M.M.A.; Amodio, M.L.; Babellahi, F.; de Chiara, M.L.V.; Amigo Rubio, J.M.; Colelli, G. Hyperspectral imaging and multivariate accelerated shelf life testing (MASLT) approach for determining shelf life of rocket leaves. J. Food Eng. 2018, 238, 122–133. [Google Scholar] [CrossRef]
- Song, X.; Liu, Y.; Xue, L.; Wang, J.; Zhang, J.; Wang, J.; Jiang, L.; Cheng, Z. Time-series well performance prediction based on Long Short-Term Memory (LSTM) neural network model. J. Pet. Sci. Eng. 2020, 186, 106682. [Google Scholar] [CrossRef]
- Yin, X.; Luo, Y.; Fan, H.; Wu, H.; Feng, L. Effect of previous frozen storage on quality changes of grass carp (Ctenopharyngodon idellus) fillets during short-term chilled storage. Int. J. Food Sci. Technol. 2014, 49, 1449–1460. [Google Scholar] [CrossRef]
- Chen, H.-Z.; Zhang, M.; Bhandari, B.; Guo, Z. Evaluation of the freshness of fresh-cut green bell pepper (Capsicum annuum var. grossum) using electronic nose. LWT 2018, 87, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Qin, N.; Zhang, L.; Li, Q.; Prinyawiwatkul, W.; Luo, Y. Degradation of adenosine triphosphate, water loss and textural changes in frozen common carp (Cyprinus carpio) fillets during storage at different temperatures. Int. J. Refrig. 2019, 98, 294–301. [Google Scholar] [CrossRef]
- Ji, E.; Woong, H.; Bae, Y.; Hoon, S.; Se, J.; Hyun, H. Effect of tempering methods on quality changes of pork loin frozen by cryogenic immersion. Meat Sci. 2017, 124, 69–76. [Google Scholar] [CrossRef]
- Lau, M.; Tang, J.; Swanson, B. Kinetics of textural and color changes in green asparagus during thermal treatments. J. Food Eng. 2000, 45, 231–236. [Google Scholar] [CrossRef]
- Qing, X.; Niu, Y. Hourly day-ahead solar irradiance prediction using weather forecasts by LSTM. Energy 2018, 148, 461–468. [Google Scholar] [CrossRef]
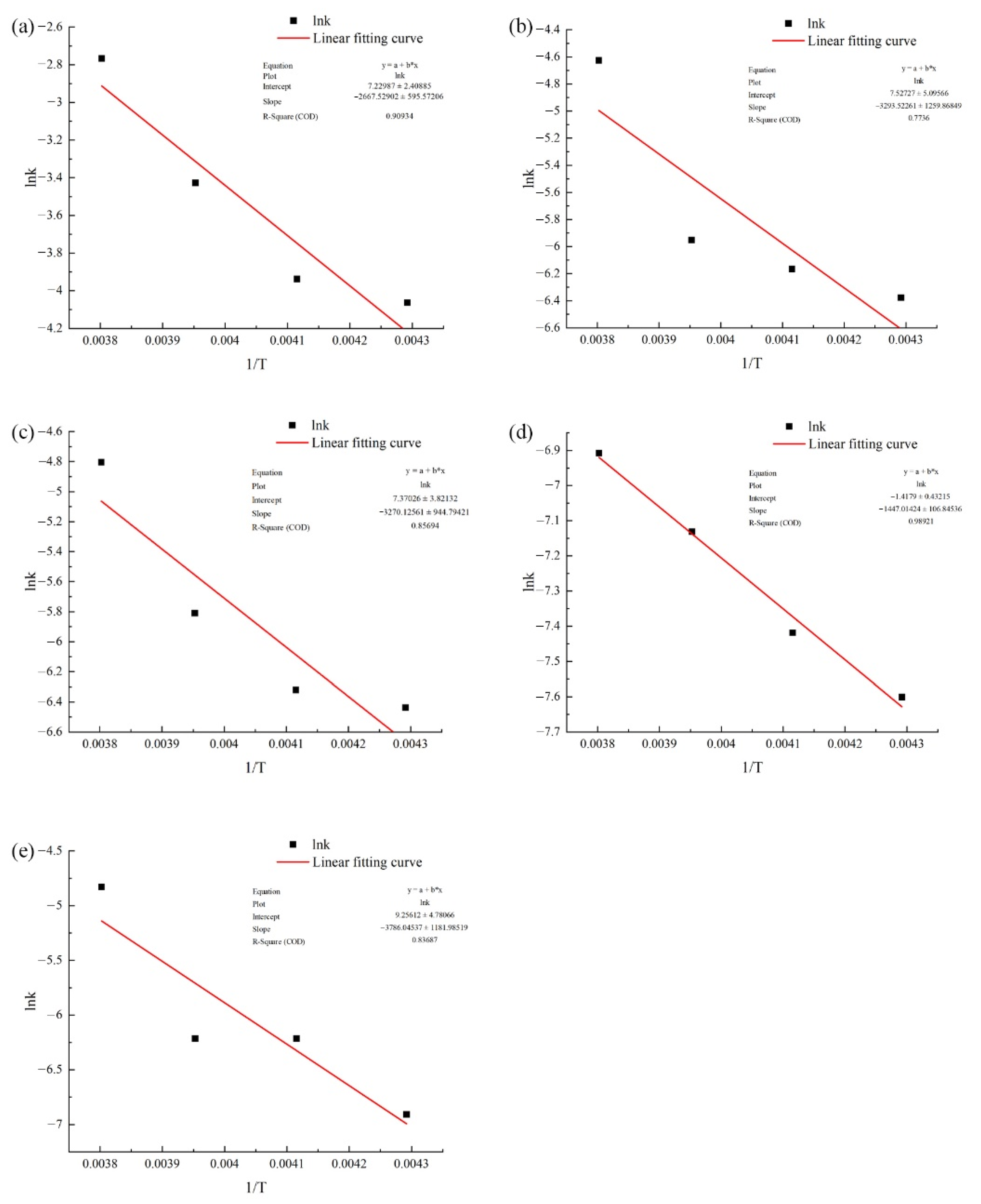
| Dynamics Model | Storage Temperature (°C) | Fitting Formula | Reaction Rate Constant | Determination Coefficient R2 | ∑R2 | Ea (kJ/mol) | k0 | |
|---|---|---|---|---|---|---|---|---|
| Centrifugal loss (%) | Zero-level dynamics model | −10 | y = 0.0629x + 16.13 | 0.0629 | 0.9863 | 3.8034 | 22.18 | e7.23 |
| −20 | y = 0.0325x + 16.13 | 0.0325 | 0.9316 | |||||
| −30 | y = 0.0195x + 16.13 | 0.0195 | 0.9843 | |||||
| −40 | y = 0.0172x + 16.13 | 0.0172 | 0.9012 | |||||
| First-level dynamics model | −10 | y = 16.13 exp(3.3 × 10−3 x) | 0.0033 | 0.9707 | 3.7822 | |||
| −20 | y = 16.13 exp(1.8 × 10−3 x) | 0.0018 | 0.9353 | |||||
| −30 | y = 16.13 exp(1.1 × 10−3 x) | 0.0011 | 0.9786 | |||||
| −40 | y = 16.13 exp(10−3 x) | 0.001 | 0.8976 | |||||
| TVB-N (mg N/100 g) | Zero-level dynamics model | −10 | y = 0.1451x + 8.39 | 0.1451 | 0.9404 | 3.8618 | ||
| −20 | y = 0.0258x + 8.39 | 0.0258 | 0.9677 | |||||
| −30 | y = 0.0208x + 8.39 | 0.0208 | 0.9775 | |||||
| −40 | y = 0.0165x + 8.39 | 0.0165 | 0.9762 | |||||
| First-level dynamics model | −10 | y = 8.39 exp(9.8 × 10−3 x) | 0.0098 | 0.9941 | 3.8885 | 27.38 | e7.53 | |
| −20 | y = 8.39 exp(2.6 × 10−3 x) | 0.0026 | 0.9462 | |||||
| −30 | y = 8.39 exp(2.1 × 10−3 x) | 0.0021 | 0.967 | |||||
| −40 | y = 8.39 exp(1.7 × 10−3 x) | 0.0017 | 0.9812 | |||||
| K value (%) | Zero-level dynamics model | −10 | y = 0.1604x + 12.56 | 0.1604 | 0.9706 | 3.5441 | ||
| −20 | y = 0.0479x + 12.56 | 0.0479 | 0.9127 | |||||
| −30 | y = 0.0266x + 12.56 | 0.0266 | 0.8558 | |||||
| −40 | y = 0.0244x + 12.56 | 0.0244 | 0.805 | |||||
| First-level dynamics model | −10 | y = 12.56 exp(8.2 × 10−3 x) | 0.0082 | 0.998 | 3.6562 | 27.19 | e7.37 | |
| −20 | y = 12.56 exp(3 × 10−3 x) | 0.003 | 0.942 | |||||
| −30 | y = 12.56 exp(1.8 × 10−3 x) | 0.0018 | 0.8829 | |||||
| −40 | y = 12.56 exp(1.6 × 10−3 x) | 0.0016 | 0.8333 | |||||
| Whiteness | Zero-level dynamics model | −10 | y = −0.0656x + 55.97 | 0.0656 | 0.9663 | 3.8751 | ||
| −20 | y = −0.0423x + 55.97 | 0.0423 | 0.939 | |||||
| −30 | y = −0.0339x + 55.97 | 0.0339 | 0.9813 | |||||
| −40 | y = −0.0284x + 55.97 | 0.0248 | 0.9885 | |||||
| First-level dynamics model | −10 | y = 55.97 exp(−10−3 x) | 0.001 | 0.9734 | 3.8859 | 12.03 | e−1.42 | |
| −20 | y = 55.97 exp(−8 × 10−4 x) | 0.0008 | 0.9446 | |||||
| −30 | y = 55.97 exp(−6 × 10−4 x) | 0.0006 | 0.9819 | |||||
| −40 | y = 55.97 exp(−5 × 10−4 x) | 0.0005 | 0.986 | |||||
| Sensory analysis | Zero-level dynamics model | −10 | y = −0.0558x + 10 | 0.0558 | 0.9278 | 3.799 | ||
| −20 | y = −0.0196x + 10 | 0.0196 | 0.9352 | |||||
| −30 | y = −0.0147x + 10 | 0.0147 | 0.9714 | |||||
| −40 | y = −0.0106x + 10 | 0.0106 | 0.9646 | |||||
| First-level dynamics model | −10 | y = 10 exp(−8 × 10−3 x) | 0.008 | 0.9765 | 3.8537 | 31.48 | e9.26 | |
| −20 | y = 10 exp(−2 × 10−3 x) | 0.002 | 0.9481 | |||||
| −30 | y = 10 exp(−2 × 10−3 x) | 0.002 | 0.9678 | |||||
| −40 | y = 10 exp(−10−3 x) | 0.001 | 0.9613 |
| Storage Temperature (°C) | Predicted Shelf-Life (d) | Measured Shelf-Life (d) | Relative Error (%) | |
|---|---|---|---|---|
| Centrifugal loss (%) | −5 | 88 | 98 | −9.56 |
| −10 | 107 | |||
| −20 | 160 | |||
| −30 | 246 | |||
| −40 | 394 | |||
| TVB-N (mg N/100 g) | −5 | 101 | 112 | −9.61 |
| −10 | 128 | |||
| −20 | 210 | |||
| −30 | 358 | |||
| −40 | 641 | |||
| K value (%) | −5 | 90 | 105 | −14.28 |
| −10 | 114 | |||
| −20 | 186 | |||
| −30 | 316 | |||
| −40 | 563 | |||
| Whiteness | −5 | 102 | 112 | −8.49 |
| −10 | 114 | |||
| −20 | 141 | |||
| −30 | 179 | |||
| −40 | 231 | |||
| Sensory analysis | −5 | 115 | 98 | 17.56 |
| −10 | 151 | |||
| −20 | 266 | |||
| −30 | 493 | |||
| −40 | 962 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, Y.; Tan, M.; Yi, Z.; Ding, Z.; Yang, D.; Xie, J. Shelf-Life Prediction of Glazed Large Yellow Croaker (Pseudosciaena crocea) during Frozen Storage Based on Arrhenius Model and Long-Short-Term Memory Neural Networks Model. Fishes 2021, 6, 39. https://doi.org/10.3390/fishes6030039
Chu Y, Tan M, Yi Z, Ding Z, Yang D, Xie J. Shelf-Life Prediction of Glazed Large Yellow Croaker (Pseudosciaena crocea) during Frozen Storage Based on Arrhenius Model and Long-Short-Term Memory Neural Networks Model. Fishes. 2021; 6(3):39. https://doi.org/10.3390/fishes6030039
Chicago/Turabian StyleChu, Yuanming, Mingtang Tan, Zhengkai Yi, Zhaoyang Ding, Dazhang Yang, and Jing Xie. 2021. "Shelf-Life Prediction of Glazed Large Yellow Croaker (Pseudosciaena crocea) during Frozen Storage Based on Arrhenius Model and Long-Short-Term Memory Neural Networks Model" Fishes 6, no. 3: 39. https://doi.org/10.3390/fishes6030039
APA StyleChu, Y., Tan, M., Yi, Z., Ding, Z., Yang, D., & Xie, J. (2021). Shelf-Life Prediction of Glazed Large Yellow Croaker (Pseudosciaena crocea) during Frozen Storage Based on Arrhenius Model and Long-Short-Term Memory Neural Networks Model. Fishes, 6(3), 39. https://doi.org/10.3390/fishes6030039








