Development of Carbon Dioxide Barriers to Deter Invasive Fishes: Insights and Lessons Learned from Bigheaded Carp
Abstract
1. Background
2. Bigheaded Carp
3. Chicago Area Waterway System
4. Carbon Dioxide in the Atmosphere
5. CO2 and Fish Physiology
6. CO2 and Fish Behavior
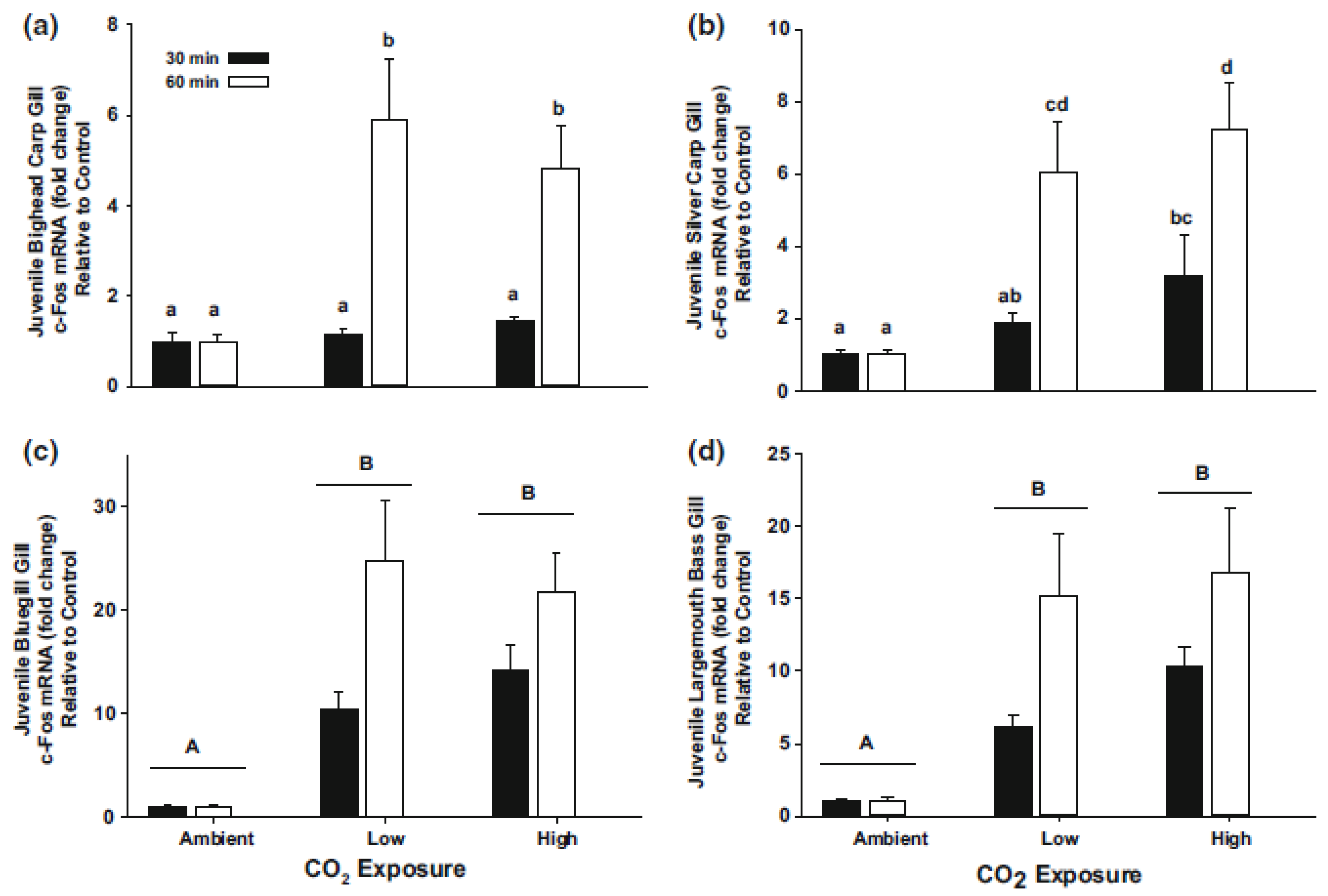
7. CO2 and Physiological Responses
8. CO2 as a Potential Fish Barrier
9. Questions from Avoidance Data
10. Factors Influencing the Avoidance of CO2
11. Factors Influencing CO2 Tolerance
12. Management Implications
13. Future Work
14. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Lodge, D.M.; Williams, S.; MacIsaac, H.J.; Hayes, K.R.; Leung, B.; Reichard, S.; Mack, R.N.; Moyle, P.B.; Smith, M.; Andow, D.A.; et al. Biological Invasions: Recommendations for US Policy and Management. Ecol. Appl. 2006, 16, 2035–2054. [Google Scholar] [CrossRef]
- Britton, J.R.; Davies, G.D.; Harrod, C. Trophic Interactions and Consequent Impacts of the Invasive Fish Pseudorasbora Parva in a Native Aquatic Foodweb: A Field Investigation in the UK. Boil. Invasions 2009, 12, 1533–1542. [Google Scholar] [CrossRef]
- Gozlan, R.E.; Britton, J.R.; Cowx, I.; Copp, G.H. Current Knowledge on Non-Native Freshwater Fish Introductions. J. Fish Boil. 2010, 76, 751–786. [Google Scholar] [CrossRef]
- Wilcove, D.S.; Rothstein, D.; Dubow, J.; Phillips, A.; Losos, E. Quantifying Threats to Imperiled Species in the United States. BioScience 1998, 48, 607–615. [Google Scholar] [CrossRef]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the Environmental and Economic Costs Associated with Alien-Invasive Species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Pejchar, L.; Mooney, H.A. Invasive Species, Ecosystem Services and Human Well-Being. Trends Ecol. Evol. 2009, 24, 497–504. [Google Scholar] [CrossRef]
- Strayer, D.L.; Dudgeon, D. Freshwater Biodiversity Conservation: Recent Progress and Future Challenges. J. N. Am. Benthol. Soc. 2010, 29, 344–358. [Google Scholar] [CrossRef]
- Wu, J. Landscape Sustainability Science: Ecosystem Services and Human Well-Being in Changing Landscapes. Landsc. Ecol. 2013, 28, 999–1023. [Google Scholar] [CrossRef]
- Jenkins, M. Prospects for Biodiversity. Science 2003, 302, 1175–1177. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging Threats and Persistent Conservation Challenges for Freshwater Biodiversity. Boil. Rev. 2018, 94, 849–873. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater Biodiversity: Importance, Threats, Status and Conservation Challenges. Boil. Rev. 2005, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Jelks, H.L.; Walsh, S.J.; Burkhead, N.M.; Contreras-Balderas, S.; Díaz-Pardo, E.; Hendrickson, D.A.; Lyons, J.; Mandrak, N.E.; McCormick, F.; Nelson, J.S.; et al. Conservation Status of Imperiled North American Freshwater and Diadromous Fishes. Fisheries 2008, 33, 372–407. [Google Scholar] [CrossRef]
- Burkhead, N.M. Extinction Rates in North American Freshwater Fishes, 1900–2010. BioScience 2012, 62, 798–808. [Google Scholar] [CrossRef]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No Saturation in the Accumulation of Alien Species Worldwide. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.S.; Zavaleta, E.S.; Eviner, V.T.; Naylor, R.L.; Vitousek, P.M.; Reynolds, H.L.; Hooper, D.U.; Lavorel, S.; Sala, O.E.; Hobbie, S.; et al. Consequences of Changing Biodiversity. Nature 2000, 405, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Sardain, A.; Sardain, E.; Leung, B. Global Forecasts of Shipping Traffic and Biological Invasions to 2050. Nat. Sustain. 2019, 2, 274–282. [Google Scholar] [CrossRef]
- Clout, M.N.; Veitch, C.R. Turning the tide of biological invasion: the potential for eradicating invasive species. In Turning the Tide: The Eradication of Invasive Species; IUCN SSC Invasive Species Specialist Group: Gland, Switzerland; Cambridge, UK, 2002; pp. 1–3. Available online: http://www.issg.org/pdf/publications/turning_the_tide.pdf (accessed on 1 June 2020).
- Simberloff, D. Eradication—Preventing Invasions at the Outset. Weed Sci. 2003, 51, 247–253. [Google Scholar] [CrossRef]
- Simberloff, D. We can eliminate invasions or live with them. Successful management projects. In Ecological Impacts of Non-Native Invertebrates and Fungi on Terrestrial Ecosystems; Langor, D., Sweeney, J., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 149–157. ISBN 978-1-4020-9680-8. [Google Scholar]
- Simberloff, D. Eradication: Pipe dream or real option? In Plant Invasions in Protected Areas; Foxcroft, L.C., Pyšek, P., Richardson, D.M., Genovesi, P., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 549–559. ISBN 978-94-007-7750-7. [Google Scholar]
- Leung, B.; Lodge, D.M.; Finnoff, D.; Shogren, J.F.; Lewis, M.A.; Lamberti, G. An Ounce of Prevention or a Pound of Cure: Bioeconomic Risk Analysis of Invasive Species. Proc. R. Soc. B Boil. Sci. 2002, 269, 2407–2413. [Google Scholar] [CrossRef]
- Finnoff, D.; Shogren, J.F.; Leung, B.; Lodge, D. Take a Risk: Preferring Prevention over Control of Biological Invaders. Ecol. Econ. 2007, 62, 216–222. [Google Scholar] [CrossRef]
- Zanden, M.J.V.; Olden, J.D. A Management Framework for Preventing the Secondary Spread of Aquatic Invasive Species. Can. J. Fish. Aquat. Sci. 2008, 65, 1512–1522. [Google Scholar] [CrossRef]
- Kocovsky, P.M.; Chapman, D.C.; Qian, S. “Asian Carp” Is Societally and Scientifically Problematic. Let’s Replace It. Fisheries 2018, 43, 311–316. [Google Scholar] [CrossRef]
- Kolar, C.S.; Chapman, D.C.; Courtenay, W.R.J.; Housel, C.M.; Williams, J.D.; Jennings, D.P. Asian Carps of the Genus Hypophthalmichthys (Pisces, Cyprinidae)—A Biological Synopsis and Environmental Risk Assessment; American Fisheries Society Special Publication: Bethesda, MD, USA, 2007; Volume 33, ISBN 978-1-888569-79-7. [Google Scholar]
- Whitledge, G.W.; Knights, B.; Vallazza, J.; Larson, J.; Weber, M.J.; Lamer, J.T.; Phelps, Q.E.; Norman, J.D. Identification of Bighead Carp and Silver Carp Early-Life Environments and Inferring Lock and Dam 19 Passage in the Upper Mississippi River: Insights from Otolith Chemistry. Boil. Invasions 2018, 21, 1007–1020. [Google Scholar] [CrossRef]
- Sass, G.G.; Hinz, C.; Erickson, A.C.; McClelland, N.N.; McClelland, M.A.; Epifanio, J.M. Invasive Bighead and Silver Carp Effects on Zooplankton Communities in the Illinois River, Illinois, USA. J. Great Lakes Res. 2014, 40, 911–921. [Google Scholar] [CrossRef]
- Kuznetsov, Y.A. Consumption of Bacteria by the Silver Carp (Hypophthalmichthys molitrix). J. Ichthyol. 1977, 17, 398–403. [Google Scholar]
- Fukushima, M.; Takamura, N.; Sun, L.; Nakagawa, M.; Matsushige, K.; Xie, P. Changes in the Plankton Community Following Introduction of Filter-Feeding Planktivorous Fish. Freshw. Boil. 1999, 42, 719–735. [Google Scholar] [CrossRef]
- Laws, E.A.; Weisburd, R. Use of Silver Carp to Control Algal Biomass in Aquaculture Ponds. Progress. Fish-Culturist 1990, 52, 1–8. [Google Scholar] [CrossRef]
- Lieberman, D.M. Use of Silver Carp (Hypophthalmichthys molotrix) and Bighead Carp (Aristichthys nobilis) for Algae Control in a Small Pond: Changes in Water Quality. J. Freshw. Ecol. 1996, 11, 391–397. [Google Scholar] [CrossRef]
- Irons, K.S.; Sass, G.G.; McClelland, M.A.; Stafford, J.D. Reduced Condition Factor of Two Native Fish Species Coincident with Invasion of Non-Native Asian Carps in the Illinois River, USA Is This Evidence for Competition and Reduced Fitness? J. Fish Boil. 2007, 71, 258–273. [Google Scholar] [CrossRef]
- Pendleton, R.M.; Schwinghamer, C.; Solomon, L.E.; Casper, A.F. Competition among River Planktivores: Are Native Planktivores Still Fewer and Skinnier in Response to the Silver Carp Invasion? Environ. Boil. Fishes 2017, 100, 1213–1222. [Google Scholar] [CrossRef]
- Chick, J.H.; Gibson-Reinemer, D.K.; Soeken-Gittinger, L.; Casper, A.F. Invasive Silver Carp is Empirically Linked to Declines of Native Sport Fish in the Upper Mississippi River System. Boil. Invasions 2019, 22, 723–734. [Google Scholar] [CrossRef]
- Asian Carp Regional Coordinating Committee. Asian Carp Monitoring and Response Plan. 2018. Available online: https://www.asiancarp.us/Documents/MRP2018.pdf (accessed on 1 June 2020).
- Hill, L. The Chicago River: A Natural and Unnatural History; Lake Claremont Press: Chicago, IL, USA, 2000. [Google Scholar]
- Moy, P.B.; Polls, I.; Dettmers, J.M. The Chicago Sanitary and Ship Canal aquatic nuisance species dispersal barrier. In Invasive Asian Carps in North America, American Fisheries Society Symposium; Chapman, D.C., Hoff, M.H., Eds.; American Fisheries Society: Bethesda, MD, USA, 2010; Volume 74, pp. 121–137. [Google Scholar]
- Rasmussen, J.L.; Regier, H.A.; Sparks, R.E.; Taylor, W.W. Dividing the Waters: The Case for Hydrologic Separation of the North American Great Lakes and Mississippi River Basins. J. Great Lakes Res. 2011, 37, 588–592. [Google Scholar] [CrossRef]
- Asian Carp Regional Coordinating Committee. Asian Carp Action Plan. 2020. Available online: https://www.asiancarp.us/Documents/2020-Action-Plan.pdf (accessed on 1 June 2020).
- Dettmers, J.M.; Boisvert, B.A.; Barkley, T.; Sparks, R.E. Potential Impact of Steel-Hulled Barges on Movement of Fish across an Electric Barrier to Prevent the Entry of Invasive Carp into Lake Michigan; Aquatic Ecology Technical Report 2005/19; Illinois Natural History Survey Center for Aquatic Ecology: Zion, IL, USA, 2005; Available online: https://www.ideals.illinois.edu/bitstream/handle/2142/10091/inhscaev02005i00019_opt.pdf?sequence=2&isAllowed=y (accessed on 1 June 2020).
- Sparks, R.E.; Barkley, T.L.; Creque, S.M.; Dettmers, J.M.; Stainbrook, K.M. Evaluation of an electric fish dispersal barrier in the Chicago Sanitary and Ship Canal. In Invasive Asian Carps in North America, American Fisheries Society Symposium; Chapman, D.C., Hoff, M.H., Eds.; American Fisheries Society: Bethesda, MD, USA, 2010; Volume 74, pp. 139–161. [Google Scholar]
- Evans, N.T.; Brouder, M.J. Asian Carp Entrainment, Retainment and Upstream Transport by Commercial Barge tows on the Illinois Waterway—2018 Trials; US Fish & Wildlife Service Report; US Fish and Wildlife Service Carterville Fish and Wildlife Conservation Office: Willmington, IL, USA, 2020. Available online: https://www.fws.gov/midwest/fisheries/carterville/documents/2018-Barge-Entrainment-Study-Report.pdf (accessed on 1 June 2020).
- Parker, A.D.; Rogers, P.B.; Finney, S.T.; Simmonds, R.L.J. Preliminary Results of Fixed DIDSON Evaluations at the Electric Dispersal Barrier in the Chicago Sanitary and Ship Canal; US Fish & Wildlife Service Report; US Fish and Wildlife Service, Carterville Fish and Wildlife Conservation Office: Willmington, IL, USA, 2013. Available online: https://www.fws.gov/midwest/fisheries/carterville/documents/DIDSON.pdf (accessed on 1 June 2020).
- Reynolds, J.B. Electrofishing. In Fisheries Techniques, 2nd ed.; Murphy, B.R., Willis, D.W., Eds.; American Fisheries Society: Bethesda, MD, USA, 1996; pp. 221–253. ISBN 9781888569001. [Google Scholar]
- Noatch, M.R.; Suski, C.D. Non-Physical Barriers to Deter Fish Movements. Environ. Rev. 2012, 20, 71–82. [Google Scholar] [CrossRef]
- USACE. The Great Lakes and Mississippi River Interbasin Study—Brandon Road Final Integrated Feasibility Study and Environmental Impact Statement—Will County, Illinois; US Army Corps of Engineers, Rock Island and Chicago Districts: Rock Island, TN, USA; Chicago, IL, USA, 2018; Available online: https://usace.contentdm.oclc.org/utils/getfile/collection/p16021coll7/id/11394 (accessed on 1 June 2020).
- Cooke, S.; Hill, W.R. Can Filter-Feeding Asian Carp Invade the Laurentian Great Lakes? A Bioenergetic Modelling Exercise. Freshw. Boil. 2010, 55, 2138–2152. [Google Scholar] [CrossRef]
- Cuddington, K.; Currie, W.J.S.; Koops, M.A. Could an Asian Carp Population Establish in the Great Lakes from a Small Introduction? Boil. Invasions 2013, 16, 903–917. [Google Scholar] [CrossRef]
- Wittmann, M.E.; Cooke, R.M.; Rothlisberger, J.D.; Rutherford, E.S.; Zhang, H.; Mason, D.M.; Lodge, D.M. Use of Structured Expert Judgment to Forecast Invasions by Bighead and Silver Carp in Lake Erie. Conserv. Boil. 2014, 29, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Lauber, T.B.; Stedman, R.C.; Connelly, N.A.; Rudstam, L.G.; Ready, R.C.; Poe, G.L.; Bunnell, D.B.; Höök, T.O.; Koops, M.A.; Ludsin, S.A.; et al. Using Scenarios to Assess Possible Future Impacts of Invasive Species in the Laurentian Great Lakes. N. Am. J. Fish. Manag. 2016, 36, 1292–1307. [Google Scholar] [CrossRef]
- Zhang, H.; Rutherford, E.S.; Mason, D.M.; Breck, J.T.; Wittmann, M.E.; Cooke, R.M.; Lodge, D.M.; Rothlisberger, J.D.; Zhu, X.; Johnson, T.B. Forecasting the Impacts of Silver and Bighead Carp on the Lake Erie Food Web. Trans. Am. Fish. Soc. 2015, 145, 136–162. [Google Scholar] [CrossRef]
- Cummins, E.P.; Strowitzki, M.J.; Taylor, C.T. Mechanisms and Consequences of Oxygen and Carbon Dioxide Sensing in Mammals. Physiol. Rev. 2020, 100, 463–488. [Google Scholar] [CrossRef]
- Cummins, E.P.; Selfridge, A.C.; Sporn, P.H.S.; Sznajder, J.I.; Taylor, C.T. Carbon Dioxide-Sensing in Organisms and Its Implications for Human Disease. Cell. Mol. Life Sci. 2013, 71, 831–845. [Google Scholar] [CrossRef]
- Thom, C.; Guerenstein, P.G.; Mechaber, W.L.; Hildebrand, J.G. Floral CO2 Reveals Flower Profitability to Moths. J. Chem. Ecol. 2004, 30, 1285–1288. [Google Scholar] [CrossRef]
- Seeley, T.D. Atmospheric Carbon Dioxide Regulation in Honey-Bee (Apis mellifera) Colonies. J. Insect Physiol. 1974, 20, 2301–2305. [Google Scholar] [CrossRef]
- Gillies, M.T. The Role of Carbon Dioxide in Host-Finding by Mosquitoes (Diptera: Culicidae): A Review. Bull. Entomol. Res. 1980, 70, 525–532. [Google Scholar] [CrossRef]
- Takken, W.; Knols, B.G.J. Odor-Mediated Behavior of Afrotropical Malaria Mosquitoes. Annu. Rev. Entomol. 1999, 44, 131–157. [Google Scholar] [CrossRef] [PubMed]
- Faucher, C. Behavioral Responses of Drosophila to Biogenic Levels of Carbon Dioxide Depend on Life-Stage, Sex and Olfactory Context. J. Exp. Boil. 2006, 209, 2739–2748. [Google Scholar] [CrossRef]
- Lahiri, S.; Forster II, R.E. CO2/H+ Sensing: Peripheral and Central Chemoreception. Int. J. Biochem. Cell Biol. 2003, 35, 1413–1435. [Google Scholar] [CrossRef]
- Shusterman, D. Individual Factors in Nasal Chemesthesis. Chem. Senses 2002, 27, 551–564. [Google Scholar] [CrossRef]
- Tresguerres, M.; Milsom, W.K.; Perry, S.F. CO2 and acid-base sensing. In Carbon Dioxide; Farrell, A.P., Brauner, C.J., Eds.; Elsevier: San Diego, CA, USA, 2019; Volume 37, pp. 33–68. ISBN 9780128176108. [Google Scholar]
- Eddy, F.B.; Lomholt, J.P.; Weber, R.E.; Johansen, K. Blood Respiratory Properties of Rainbow Trout (Salmo gairdneri) Kept in Water of High CO2 Tension. J. Exp. Boil. 1977, 67, 37–47. [Google Scholar]
- Brauner, C.J.; Baker, D.W. Patterns of acid–base regulation during exposure to hypercarbia in fishes. In Cardio-Respiratory Control in Vertebrates; Glass, M.L., Wood, S.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 43–63. ISBN 978-3-540-93984-9. [Google Scholar]
- Bernier, N.J.; Randall, D.J. Carbon Dioxide Anaesthesia in Rainbow Trout: Effects of Hypercapnic Level and Stress on Induction and Recovery from Anaesthetic Treatment. J. Fish Biol. 1998, 52, 621–637. [Google Scholar]
- Dennis, C.E.; Kates, D.F.; Noatch, M.R.; Suski, C.D. Molecular Responses of Fishes to Elevated Carbon Dioxide. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 187, 224–231. [Google Scholar] [CrossRef]
- Kates, D.; Dennis, C.; Noatch, M.R.; Suski, C.D. Responses of Native and Invasive Fishes to Carbon Dioxide: Potential for a Nonphysical Barrier to Fish Dispersal. Can. J. Fish. Aquat. Sci. 2012, 69, 1748–1759. [Google Scholar] [CrossRef]
- Iwama, G.K.; McGeer, J.C.; Pawluk, M.P. The Effects of Five Fish Anaesthetics on Acid-Base Balance, Hematocrit, Blood Gases, Cortisol, and Adrenaline in Rainbow Trout. Can. J. Zool. 1989, 67, 2065–2073. [Google Scholar] [CrossRef]
- Brauner, C.J.; Seidelin, M.; Madsen, S.S.; Jensen, F.B. Effects of Freshwater Hyperoxia and Hypercapnia and Their Influences on Subsequent Seawater Transfer in Atlantic Salmon (Salmo salar) Smolts. Can. J. Fish. Aquat. Sci. 2000, 57, 2054–2064. [Google Scholar] [CrossRef]
- Fish, F.F. The Anaesthesia of Fish by High Carbon-Dioxide Concentrations. Trans. Am. Fish. Soc. 1943, 72, 25–29. [Google Scholar] [CrossRef]
- Post, G. Carbonic Acid Anesthesia for Aquatic Organisms. Progress. Fish-Culturist 1979, 41, 142–144. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Yokoyama, Y.; Ueno, S.; Mitsuda, H. Changes of Blood Gas in Carp, Cyprinus Carpio, Anesthetized with Carbon Dioxide. Comp. Biochem. Physiol. Part A Physiol. 1991, 98, 431–436. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Kawai, F.; Kanamori, M. The Relationship between the EEG and Brain pH in Carp, Cyprinus carpio, Subjected to Environmental Hypercapnia at an Anesthetic Level. Comp. Biochem. Physiol. A Physiol. 1994, 107, 307–312. [Google Scholar]
- Beitinger, T.L. Behavioral Reactions for the Assessment of Stress in Fishes. J. Great Lakes Res. 1990, 16, 495–528. [Google Scholar] [CrossRef]
- Tierney, K.B. Chemical Avoidance Responses of Fishes. Aquat. Toxicol. 2016, 174, 228–241. [Google Scholar] [CrossRef]
- Shelford, V.E.; Allee, W.C. The Reactions of Fishes to Gradients of Dissolved Atmospheric Gases. J. Exp. Zool. 1913, 14, 207–266. [Google Scholar] [CrossRef]
- Powers, E.B.; Clark, R.T. Further Evidence on Chemical Factors Affecting the Migratory Movements of Fishes, Especially the Salmon. Ecology 1943, 24, 109–113. [Google Scholar] [CrossRef]
- Collins, B.G. Factors Influencing the Orientation of Migrating Anadromous Fishes. Fish. Bull. 1952, 52, 375–396. [Google Scholar]
- Bishai, H.M. Reactions of Larval and Young Salmonids to Different Hydrogen Ion Concentrations. ICES J. Mar. Sci. 1962, 27, 181–191. [Google Scholar] [CrossRef]
- Jones, K.A.; Hara, T.J.; Scherer, E. Locomotor Response by Arctic Char (Salvelinus alpinus) to Gradients of H+ and CO2. Physiol. Zool. 1985, 58, 413–420. [Google Scholar] [CrossRef]
- Ross, R.M.; Krise, W.F.; Redell, L.A.; Bennett, R.M. Effects of Dissolved Carbon Dioxide on the Physiology and Behavior of Fish in Artificial Streams. Environ. Toxicol. 2001, 16, 84–95. [Google Scholar] [CrossRef]
- Clingerman, J.; Bebak, J.; Mazik, P.M.; Summerfelt, S.T. Use of Avoidance Response by Rainbow Trout to Carbon Dioxide for Fish Self-Transfer between Tanks. Aquac. Eng. 2007, 37, 234–251. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Ishida, Y.; Ueno, S.; Mitsuda, H. Anesthetic Effect of CO2 on Fish. I. Changes in Depth of Anesthesia of the Carp Anesthetized with a Constant Level of CO2. Nippon. Suisan Gakkaishi 1988, 54, 457–462. [Google Scholar] [CrossRef]
- Pirhonen, J.; Schreck, C.B. Effects of Anaesthesia with MS-222, Clove Oil and CO2 on Feed Intake and Plasma Cortisol in Steelhead Trout (Oncorhynchus mykiss). Aquaculture 2003, 220, 507–514. [Google Scholar] [CrossRef]
- Dennis, C.E.; Adhikari, S.; Suski, C.D. Molecular and Behavioral Responses of Early-Life Stage Fishes to Elevated Carbon Dioxide. Boil. Invasions 2015, 17, 3133–3151. [Google Scholar] [CrossRef]
- Summerfelt, R.C.; Lewis, W.M. Repulsion of Green Sunfish by Certain Chemicals. J. Water Pollut. Control. Fed. 1967, 39, 2030–2038. [Google Scholar]
- Donaldson, M.R.; Amberg, J.; Adhikari, S.; Cupp, A.R.; Jensen, N.; Romine, J.; Wright, A.; Gaikowski, M.P.; Suski, C.D. Carbon Dioxide as a Tool to Deter the Movement of Invasive Bigheaded Carps. Trans. Am. Fish. Soc. 2016, 145, 657–670. [Google Scholar] [CrossRef]
- Cupp, A.R.; Erickson, R.; Fredricks, K.T.; Swyers, N.M.; Hatton, T.W.; Amberg, J.J. Responses of Invasive Silver and Bighead Carp to a Carbon Dioxide Barrier in Outdoor Ponds. Can. J. Fish. Aquat. Sci. 2017, 74, 297–305. [Google Scholar] [CrossRef]
- Cupp, A.R.; Smerud, J.; Tix, J.; Schleis, S.; Fredricks, K.; Erickson, R.A.; Amberg, J.; Morrow, W.; Koebel, C.; Murphy, E.; et al. Field Evaluation of Carbon Dioxide as a Fish Deterrent at a Water Management Structure along the Illinois River. Manag. Boil. Invasions 2018, 9, 299–308. [Google Scholar] [CrossRef]
- Hasler, C.T.; Woodley, C.M.; Schneider, E.V.; Hixson, B.K.; Fowler, C.J.; Midway, S.R.; Suski, C.D.; Smith, D.L. Avoidance of Carbon Dioxide in Flowing Water by Bighead Carp. Can. J. Fish. Aquat. Sci. 2019, 76, 961–969. [Google Scholar] [CrossRef]
- Sampson, S.J.; Chick, J.H.; Pegg, M.A. Diet Overlap among Two Asian Carp and Three Native Fishes in Backwater Lakes on the Illinois and Mississippi Rivers. Boil. Invasions 2008, 11, 483–496. [Google Scholar] [CrossRef]
- Deters, J.E.; Chapman, D.C.; McElroy, B. Location and Timing of Asian Carp spawning in the Lower Missouri River. Environ. Boil. Fishes 2012, 96, 617–629. [Google Scholar] [CrossRef]
- Réale, D.; Garant, D.; Humphries, M.M.; Bergeron, P.; Careau, V.; Montiglio, P.-O. Personality and the Emergence of the Pace-Of-Life Syndrome Concept at the Population Level. Philos. Trans. R. Soc. B Boil. Sci. 2010, 365, 4051–4063. [Google Scholar] [CrossRef]
- Myles-Gonzalez, E.; Burness, G.; Yavno, S.; Rooke, A.C.; Fox, M.G. To Boldly Go Where No Goby Has Gone Before: Boldness, Dispersal Tendency, and Metabolism at the Invasion Front. Behav. Ecol. 2015, 26, 1083–1090. [Google Scholar] [CrossRef]
- Cockrem, J.F. Stress, Corticosterone Responses and Avian Personalities. J. Ornithol. 2007, 148, 169–178. [Google Scholar] [CrossRef]
- Koolhaas, J.; Korte, S.M.; De Boer, S.; Van Der Vegt, B.; Van Reenen, C.; Hopster, H.; De Jong, I.; Ruis, M.; Blokhuis, H. Coping Styles in Animals: Current Status in Behavior and Stress-Physiology. Neurosci. Biobehav. Rev. 1999, 23, 925–935. [Google Scholar] [CrossRef]
- Réale, D.; Reader, S.M.; Sol, D.; McDougall, P.T.; Dingemanse, N.J. Integrating Animal Temperament within Ecology and Evolution. Boil. Rev. 2007, 82, 291–318. [Google Scholar] [CrossRef]
- Tucker, E.K.; Suski, C.D.; Philipp, M.A.; Jeffrey, J.D.; Hasler, C.T. Glucocorticoid and Behavioral Variation in Relation to Carbon Dioxide Avoidance across Two Experiments in Freshwater Teleost Fishes. Boil. Invasions 2018, 21, 505–517. [Google Scholar] [CrossRef]
- Tucker, E.K.; Suski, C.D. Presence of Conspecifics Reduces Between-Individual Variation and Increases Avoidance of Multiple Stressors in Bluegill. Anim. Behav. 2019, 158, 15–24. [Google Scholar] [CrossRef]
- Killen, S.S.; Marras, S.; Metcalfe, N.B.; McKenzie, D.J.; Domenici, P. Environmental Stressors Alter Relationships between Physiology and Behaviour. Trends Ecol. Evol. 2013, 28, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, N.B.; Van Leeuwen, T.E.; Killen, S.S. Does Individual Variation in Metabolic Phenotype Predict Fish Behaviour and Performance? J. Fish Boil. 2015, 88, 298–321. [Google Scholar] [CrossRef] [PubMed]
- Suski, C.D.; Philipp, M.A.; Hasler, C.T. Influence of Nutritional Status on Carbon Dioxide Tolerance and Avoidance Behavior in a Freshwater Teleost. Trans. Am. Fish. Soc. 2019, 148, 914–925. [Google Scholar] [CrossRef]
- Nadler, L.E.; Killen, S.S.; McClure, E.C.; Munday, P.L.; McCormick, M.I. Shoaling Reduces Metabolic Rate in a Gregarious Coral Reef Fish Species. J. Exp. Boil. 2016, 219, 2802–2805. [Google Scholar] [CrossRef] [PubMed]
- Dennis, C.E.; Adhikari, S.; Wright, A.W.; Suski, C.D.; Dennis, C.E. Molecular, Behavioral, and Performance Responses of Juvenile Largemouth Bass Acclimated to an Elevated Carbon Dioxide Environment. J. Comp. Physiol. B 2016, 186, 297–311. [Google Scholar] [CrossRef]
- Cupp, A.R.; Tix, J.; Smerud, J.; Erickson, R.A.; Fredricks, K.; Amberg, J.; Suski, C.D.; Wakeman, R. Using Dissolved Carbon Dioxide to Alter the Behavior of Invasive round Goby. Manag. Boil. Invasions 2017, 8, 567–574. [Google Scholar] [CrossRef]
- Tix, J.A.; Cupp, A.R.; Smerud, J.R.; Erickson, R.; Fredricks, K.T.; Amberg, J.J.; Suski, C.D. Temperature Dependent Effects of Carbon Dioxide on Avoidance Behaviors in Bigheaded Carps. Boil. Invasions 2018, 20, 3095–3105. [Google Scholar] [CrossRef]
- Beitinger, T.L.; Lutterschmidt, W.I. Measures of thermal tolerance. In Encyclopedia of Fish Physiology, 1st ed.; Farrell, A.P., Ed.; Elsevier: Waltham, MA, USA, 2011; pp. 1695–1702. ISBN 9780080923239. [Google Scholar]
- Somero, G. Temporal Patterning of Thermal Acclimation: From Behavior to Membrane Biophysics. J. Exp. Boil. 2015, 218, 167–169. [Google Scholar] [CrossRef]
- Hasler, C.T.; Bouyoucos, I.A.; Suski, C.D. Tolerance to Hypercarbia Is Repeatable and Related to a Component of the Metabolic Phenotype in a Freshwater Fish. Physiol. Biochem. Zool. 2017, 90, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Killen, S.S.; Adriaenssens, B.; Marras, S.; Claireaux, G.; Cooke, S.J. Context Dependency of Trait Repeatability and Its Relevance for Management and Conservation of Fish Populations. Conserv. Physiol. 2016, 4, cow007. [Google Scholar] [CrossRef] [PubMed]
- Gelwicks, K.R.; Zafft, D.J.; Bobbitt, J.P. Efficacy of Carbonic Acid as an Anesthetic for Rainbow Trout. N. Am. J. Fish. Manag. 1998, 18, 432–438. [Google Scholar] [CrossRef]
- Fivelstad, S.; Waagbø, R.; Stefansson, S.; Olsen, A.B. Impacts of Elevated Water Carbon Dioxide Partial Pressure at Two Temperatures on Atlantic Salmon (Salmo salar L.) Parr Growth and Haematology. Aquaculture 2007, 269, 241–249. [Google Scholar] [CrossRef]
- Neiffer, D.L.; Stamper, M.A. Fish Sedation, Anesthesia, Analgesia, and Euthanasia: Considerations, Methods, and Types of Drugs. ILAR J. 2009, 50, 343–360. [Google Scholar] [CrossRef]
- Fredricks, K.T.; Hubert, T.D.; Amberg, J.J.; Cupp, A.R.; Dawson, V.K. Chemical Controls for an Integrated Pest Management Program. N. Am. J. Fish. Manag. 2019. Available online: https://afspubs.onlinelibrary.wiley.com/doi/abs/10.1002/nafm.10339 (accessed on 2 June 2020). [CrossRef]
- Schneider, E.V.; Hasler, C.T.; Suski, C.D. Swimming Performance of a Freshwater Fish during Exposure to High Carbon Dioxide. Environ. Sci. Pollut. Res. 2018, 26, 3447–3454. [Google Scholar] [CrossRef]
- Ruebush, B.; Sass, G.; Chick, J.; Stafford, J. In-Situ Tests of Sound-Bubble-Strobe Light Barrier Technologies to Prevent Range Expansions of Asian Carp. Aquat. Invasions 2012, 7, 37–48. [Google Scholar] [CrossRef]
- Hasler, C.T.; Midway, S.R.; Jeffrey, J.D.; Tix, J.A.; Sullivan, C.; Suski, C.D. Exposure to Elevated pCO2 Alters Post-Treatment Diel Movement Patterns of Largemouth Bass over Short Time Scales. Freshw. Boil. 2016, 61, 1590–1600. [Google Scholar] [CrossRef]
- Hasler, C.T.; Jeffrey, J.D.; Butman, D.; Suski, C. Freshwater Biota and Rising pCO2? Ecol. Lett. 2016, 19, 98–108. [Google Scholar] [CrossRef]
- Jeffrey, J.D.; Hannan, K.D.; Hasler, C.T.; Suski, C.D. Hot and Bothered: Effects of Elevated pCO2 and Temperature on Juvenile Freshwater Mussels. Am. J. Physiol. Integr. Comp. Physiol. 2018, 315, R115–R127. [Google Scholar] [CrossRef] [PubMed]
- Hannan, K.D.; Jeffrey, J.D.; Hasler, C.T.; Suski, C.D. Physiological Responses of Three Species of Unionid Mussels to Intermittent Exposure to Elevated Carbon Dioxide. Conserv. Physiol. 2016, 4, cow066. [Google Scholar] [CrossRef] [PubMed]
- Hasler, C.T.; Jeffrey, J.D.; Schneider, E.V.; Hannan, K.D.; Tix, J.A.; Suski, C.D. Biological Consequences of Weak Acidification Caused by Elevated Carbon Dioxide in Freshwater Ecosystems. Hydrobiologia 2017, 806, 1–12. [Google Scholar] [CrossRef]
- Midway, S.R.; Hasler, C.T.; Wagner, T.; Suski, C.D. Predation of Freshwater Fish in Elevated Carbon Dioxide Environments. Mar. Freshwater Res. 2017, 68, 1585–1592. [Google Scholar] [CrossRef]
- Tix, J.A.; Hasler, C.T.; Sullivan, C.; Jeffrey, J.D.; Suski, C.D. Elevated Carbon Dioxide Has the Potential to Impact Alarm Cue Responses in Some Freshwater Fishes. Aquat. Ecol. 2016, 51, 59–72. [Google Scholar] [CrossRef]
- Robertson, M.; Hernandez, M.F.; Midway, S.R.; Hasler, C.T.; Suski, C.D. Shelter-Seeking Behavior of Crayfish, Procambarus Clarkii, in Elevated Carbon Dioxide. Aquat. Ecol. 2018, 52, 225–233. [Google Scholar] [CrossRef]



| Species | Avoidance Threshold | Test Environment | Temperature | pH | Mean Fish Size (mm) | Citation |
|---|---|---|---|---|---|---|
| Silver carp | 135 mg/L | Shuttle box | 18 °C | 8.0 | 460 | [66] |
| 125 mg/L | Shuttle box | 16 °C | 7.46 | 67 | [65] | |
| 59 mg/L (29,193 μatm) | Outdoor static pond | 16 °C | 8.25 | 254 | [86] | |
| 75 mg/L (29,532–41,393 μatm) | Outdoor flowing raceway | 8–13 °C | 7.5 | 278 | [87] | |
| Bighead carp | 180 mg/L | Shuttle box | 16 °C | 7.46 | 71 | [65] |
| 59 mg/L (29,193 μatm) | Outdoor static pond | 16 °C | 8.25 | 205 | [86] | |
| 75 mg/L (29,532–41,393 μatm) | Outdoor flowing raceway | 8–13 °C | 7.5 | 212 | [87] | |
| 160,000–186,000 μatm | Indoor flowing raceway | 21 °C | 8.3 | 145 | [89] |
| Factor | Outcome | Species | Citation | |
|---|---|---|---|---|
| Factors resulting in more CO2 needed for avoidance | Temperature | Higher concentrations of CO2 needed to induce avoidance at high temperatures relative to low temperatures. | Bighead carp, silver carp, round goby | [104,105] |
| Stress | Fish with artificially-elevated cortisol levels required more CO2 to induce shuttling than non-stressed controls. | Largemouth bass | [97] | |
| Factors requiring less CO2 for avoidance | Shoals | Shoals of fish avoided CO2 at lower thresholds than did individual fish. | Bluegill | [97] |
| Factors not influencing CO2 avoidance | Social personality | Preference for associating with conspecifics did not influence thresholds for CO2 avoidance. | Bluegill | [97] |
| Personality | Activity and boldness did not influence CO2 avoidance thresholds. | Bluegill | [97] | |
| Feeding | Fish that had been deprived food for 9 days avoided CO2 at the same threshold as fed conspecifics | Largemouth bass | [101] |
| Factor | Outcome | Species | Citation | |
|---|---|---|---|---|
| Factors resulting in a higher threshold for equilibrium loss in CO2 | Size | large fish lost equilibrium sooner (were more sensitive) at a given CO2 concentration than small fish. | Rainbow trout | [81] |
| Time × concentration interaction | Equilibrium loss occurs at extended exposure to low CO2 concentration, or brief exposure to high CO2 concentration. | Several species of salmonid (steelhead, chinook) | [69,70] | |
| Temperature | When CO2 was added to a tank at a constant rate, a higher CO2 concentration was required to induce equilibrium loss at high temperatures relative to low temperatures. | Silver carp, bighead carp, round goby. | [104,105] | |
| Food deprivation | Fish that had been deprived food for 14 days required more CO2 to induce equilibrium loss than fed conspecifics. | Largemouth bass | [101] | |
| Factors resulting in a lower threshold for equilibrium loss in CO2 | Temperature | Equilibrium loss occurs faster at higher temperature when CO2 concentration is held constant. | Several species of salmonid (steelhead, chinook) | [69,110] |
| Anaerobic swimming potential | Fish that required longer to become exhausted during burst swimming required less time to lose equilibrium at high CO2. | Largemouth bass | [108] | |
| Factors not influencing equilibrium loss in high CO2 environments | Standard metabolic rate | Variation in standard metabolic rate did not predict time to equilibrium loss in high CO2. | Largemouth bass | [108] |
| Aerobic scope | Variation in aerobic scope did not predict time to equilibrium loss in high CO2. | Largemouth bass | [108] | |
| Personality (activity, boldness) | Variation in activity and boldness did not influence time to equilibrium loss in high CO2. | Bluegill | [97] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suski, C.D. Development of Carbon Dioxide Barriers to Deter Invasive Fishes: Insights and Lessons Learned from Bigheaded Carp. Fishes 2020, 5, 25. https://doi.org/10.3390/fishes5030025
Suski CD. Development of Carbon Dioxide Barriers to Deter Invasive Fishes: Insights and Lessons Learned from Bigheaded Carp. Fishes. 2020; 5(3):25. https://doi.org/10.3390/fishes5030025
Chicago/Turabian StyleSuski, Cory D. 2020. "Development of Carbon Dioxide Barriers to Deter Invasive Fishes: Insights and Lessons Learned from Bigheaded Carp" Fishes 5, no. 3: 25. https://doi.org/10.3390/fishes5030025
APA StyleSuski, C. D. (2020). Development of Carbon Dioxide Barriers to Deter Invasive Fishes: Insights and Lessons Learned from Bigheaded Carp. Fishes, 5(3), 25. https://doi.org/10.3390/fishes5030025





