Zooplankton Feeding Induces Macroscopical Gonad Malformations in Whitefish (Coregonus ssp.) from Lake Thun, Switzerland
Abstract
1. Introduction
2. Results
3. Discussion
4. Material and Methods
4.1. Experimental Setup
4.2. Fish Stripping, Egg Fertilization and Incubation
4.3. Fish Rearing
4.4. Fish Feeding and Zooplankton Sampling
4.5. Fish Sampling and Assessment of Gonad Malformations
4.6. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Porter, T.R.; Corey, S. Hermaphroditic lake whitefish, Coregonus clupeaformis, from Lake Huron. J. Fish. Res. Board Can. 1974, 31, 1944–1945. [Google Scholar] [CrossRef]
- Scott, D.B.C. Hermaphrodite specimen of Coregonus lavaretus. from Loch Lomond, Scotland. J. Fish Biol. 1975, 7, 70. [Google Scholar] [CrossRef]
- Hunter, J.R.; Macewicz, B.J. Rates of atresia in the ovary of captive and wild Northern anchovy. Engraulis mordax. Fish. Bull. 1985, 83, 119–136. [Google Scholar]
- Jobling, S.; Nolan, M.; Tyler, C.R.; Brighty, G.; Sumpter, J.P. Widespread sexual disruption in wild fish. Environ. Sci. Technol. 1998, 32, 2498–2506. [Google Scholar] [CrossRef]
- Kinnison, M.T.; Unwin, M.J.; Jira, F. Macroscopic intersexuality in salmonid fishes. NZ J. Mar. Freshw. Res. 2000, 34, 125–134. [Google Scholar] [CrossRef]
- Blazer, V.S. Histopathological assessment of gonadal tissue in wild fishes. Fish Physiol. Biochem. 2002, 26, 85–101. [Google Scholar] [CrossRef]
- Devlin, R.H.; Nagahama, Y. Sexc determination and sex difference in fish: An overview of genetic, physiological, and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- Körner, O.; Vermeirssen, E.L.M. Burkhardt-Holm, P. Intersex in feral brown trout from Swiss midland rivers. J. Fish Biol. 2005, 67, 1734–1740. [Google Scholar] [CrossRef]
- Mikodina, E.V.; Sedova, M.A.; Smirnov, A.A. On abnormal gonads of the Gizhiga-Kamchatka population of the herring Clupea pallasi. J. Ichthyol. 2005, 45, 191–199. [Google Scholar]
- Papoulias, D.M.; Chapman, D.; Tillitt, D.E. Reproductive condition and occurrence of intersex in bighead carp and silver carp in the Missouri river. Hydrobiologia 2006, 571, 355–360. [Google Scholar] [CrossRef]
- Schwindt, A.R.; Kent, M.L.; Simonich, S.M.L.; Landers, D.H.; Blett, T.; Schreck, C.B. Reproductive abnormalities in trout from Western U.S. National Parks. Trans. Am. Fish. Soc. 2009, 138, 522–531. [Google Scholar] [CrossRef]
- Feist, S.W.; Stentiford, G.D.; Kent, M.L.; Ribeiro Santos, A.; Lorance, P. Histopathological assessment of liver and gonad pathology in continental slope fish from the northeast Atlantic Ocean. Mar. Environ. Res. 2015, 106, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Ojaveer, H.; Tomkiewicz, J.; Arula, T.; Klais, R. Female ovarian abnormalities and reproductive failure of autumn-spawning herring (Clupea harengus membras) in the Baltic Sea. ICES J. Mar. Sci. 2015, 72, 2332–2340. [Google Scholar] [CrossRef]
- Rajasilta, M.; Elfving, M.; Hänninen, J.; Laine, P.; Vuorinen, U.; Paranko, J. Morphological abnormalities in gonads of the Baltic herring (Clupea harengus membras): Description of types and prevalence in the Northern Baltic Sea. Ambio 2016, 45, 205–214. [Google Scholar] [CrossRef]
- Feist, G.W.; Webb, M.A.H.; Gundersen, G.T.; Foster, E.P.; Schreck, C.B.; Maule, A.G.; Fitzpatrick, M.S. Evidence of detrimental effects of environmental contaminants on growth and reproductive physiology of white sturgeon in impounded areas of the Columbia river. Environ. Health Persp. 2005, 113, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Abdel-moneim, A.; Coulter, D.P.; Mahapathra, C.T.; Sepulveda, M.S. Intersex in fishes and amphibians: Population implications, prevalence, mechanisms and molecular biomarker. J. Appl. Toxicol. 2015, 35, 1228–1240. [Google Scholar] [CrossRef]
- Baroiller, J.F.; D’Cotta, H.; Saillant, E. Environmental effects on fish sex determination and differentiation. Sex. Dev. 2009, 3, 118–135. [Google Scholar] [CrossRef]
- Hamaguchi, S. Bilaterally asymmetric testes in fishes of the genus Oryzias. Zool. Sci. 1996, 13, 757–763. [Google Scholar] [CrossRef]
- Park, I.S.; Nam, Y.K.; Kim, D.S. Growth performance, morphometric traits and gonad development of induced reciprocal diploid and triploid hybrids between the mud loach (Misgurnus mizolepis Günther) and cyprinid loach (Misgurnus anguillicaudatus Cntor). Aquacult. Res. 2006, 37, 1246–1253. [Google Scholar] [CrossRef]
- Secombes, C.J.; Needham, E.A.; Laird, L.M.; Lewis, A.E.; Priede, I.G. The long-term effects of auto-imunologically induce granulomas on the testes of rainbow trout, Salmo gairdneri Richardson. J. Fish Biol. 1985, 26, 483–489. [Google Scholar] [CrossRef]
- Luksiene, D.; Sandström, O. Reproductive disturbance in a roach (Rutilus rutilus) population affected by cooling water discharge. J. Fish Biol. 1994, 45, 613–625. [Google Scholar] [CrossRef]
- Yadrenkina, E.N. Appearance of hermaphrodite individuals in the crucian carp population (Carassius auratus, Cyprinida) during the regression phase of the water level in Chany lake (Western Sibiria). Limnology 2020, 21, 287–295. [Google Scholar] [CrossRef]
- Wicklund, T.; Lounasheimo, L.; Lom, J.; Bylund, G. Gonadal impairment in roach Rutilus rutilus from Finnish coastal areas of the northern Baltic Sea. Dis. Aquat. Org. 1996, 26, 163–171. [Google Scholar] [CrossRef]
- Harrod, C.; Griffiths, D. Parasitism, space constraints, and gonad asymmetry in the pollan (Coregonus autumnalis). Can. J. Fish. Aquat. Sci. 2005, 62, 2796–2801. [Google Scholar] [CrossRef]
- Noaksson, E.; Tjärnlund, U.; Bosveld, A.T.C.; Balk, L. Evidence for endocrine disruption in perch (Perca fluviatilis) and roach (Rutilus rutilus) in a remote Swedish lake in the vicinity of a public refuse dump. Toxicol. Appl. Pharmacol. 2001, 174, 160–176. [Google Scholar] [CrossRef]
- Noaksson, E.; Gustavson, B.; Linderoth, M.; Zebühr, Y.; Broman, D.; Balk, L. Gonad development and plasma steroid profiles by HRGC/HRMS during one reproductive cycle in reference and leachate-exposed female perch (Perca fluviatilis). Toxicol. Appl. Pharmacol. 2004, 195, 247–261. [Google Scholar] [CrossRef]
- Hecker, M.; Murphy, M.B.; Coady, K.K.; Villeneuve, D.L.; Jones, P.D.; Carr, J.A.; Solomon, K.R.; Smith, E.E.; Van Der Kraak, G.; Gross, T.; et al. Terminology of gonadal anomalies in fish and amphibians resulting from chemical exposures. Rev. Environ. Contam. Toxicol. 2006, 187, 103–131. [Google Scholar]
- Garcia, M.S.; Constantino, D.H.J.; Silva, A.P.G.; Perobelli, J.E. Fish pollutants MeHg and Aroclor cause permanent structural damage in male gonads and kidneys after prepubertal exposure. Int. J. Exp. Pathol. 2016, 97, 360–368. [Google Scholar] [CrossRef]
- Jordanova, M.; Rebok, K.; Dragun, Z.; Ramani, S.; Ivanova, L.; Kostov, V.; Valic, D.; Krasnici, N.; Marijic, V.F.; Kapetanovic, D. Histopathology investigation on the Vadar chub (Squalius vadarenis) populations captured from the rivers impacted by mining activities. Ecotox. Environ. Saf. 2016, 129, 35–42. [Google Scholar] [CrossRef]
- Chukwuka, A.; Ogbeide, O.; Uhunamure, G. Gonad pathology and intersex severity in pelagic (Tilpia zilli) and benthic (Neochanna diversus and Clarias gariepinus) species from a pesticide-impacted agrarian catchment, south-south Nigeria. Chemosphere 2019, 225, 535–547. [Google Scholar] [CrossRef]
- Bernet, D.; Wahli, T.; Kueng, C.; Segner, H. Frequent and unexplained gonadal abnormalities in whitefish (central alpine Coregonus sp.) from an alpine oligotrophic lake in Switzerland. Dis. Aquat. Org. 2004, 61, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Bittner, D.; Bernet, D.; Wahli, T.; Segner, H.; Küng, C.; Largiadèr, C.R. How normal is abnormal? Discriminating between deformations and natural variation in gonad morphology of European whitefish Coregonus lavaretus (L). J. Fish Biol. 2009, 74, 1594–1614. [Google Scholar] [CrossRef] [PubMed]
- Edge, T.A.; McAllister, D.E.; Quadri, S.U. Meristic and morphometric variation between the endangered Acadian whitefish, Coregonus huntsman, and the Lake whitefish, Coregonus clupeafomis, in the Canadian maritime provinces and the State of Maine, USA. Can. J. Fish. Aquat. Sci. 1991, 48, 2140–2151. [Google Scholar] [CrossRef]
- Lu, G.; Bernantchez, L. Correlated trophic specialisation and genetic divergence in sympatric lake whitefish ecotpyes (Coregonus clupeaformis). Support for the ecological speciation hypothesis. Evolution 1999, 53, 1491–1505. [Google Scholar]
- Bittner, D.; Excoffier, L.; Largiadèr, C.R. Patterns of morphological changes and hybridization between sympatric whitefish morphs (Coregonus spp.) in a Swiss lake: A role for eutrophication? Molec. Ecol. 2010, 19, 2152–2167. [Google Scholar] [CrossRef]
- Kahilainin, K.K.; Siwertson, A.; Gjelland, K.O.; Knudsen, R.; Bohn, T.; Amundsen, P.A. The role of gill raker number variability in adaptive radiation of coregonids. Evol. Ecol. 2011, 25, 573–588. [Google Scholar] [CrossRef]
- Volanthen, P.; Bittner, D.; Hudson, A.G.; Young, K.A.; Müller, R.; Lundsgaard-Hansen, B.; Roy, D.; Di Piazza, S.; Largiader, C.R.; Seehausen, O. Eutrophication causes speciation reversal in whitefish adaptive radiations. Nature 2012, 482, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Mikaelian, I.; de Lafontaine de, Y.; Harhsbarger, J.C.; Lee, L.L.J.; Martineau, D. Health of Lake whitefish (Coregonus clupeaformis) with elevated tissue levels of environmental contaminants. Environ. Toxicol. Chem. 2002, 21, 532–541. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A. Why most published research findings are false. PLoS Med. 2005, 2, e124. [Google Scholar] [CrossRef]
- Kirchhofer, A. Growth characteristics of coregonid populations in three lakes with different trophic states and decreasing nutrient concentrations. Arch. Hydrobiol. Spec. Issues Advanc. Limnol. 1995, 46, 61–70. [Google Scholar]
- Urbach, D.; Britschgi, A.; Jacob, A.; Bittner, D.; Bernet, D.; Wahli, T.; Yoccoz, N.G.; Wedekind, C. Gonadal alterations in male whitefish Coregonus fatioi: No evidence for genetic damage reducing viability in early life stages. Dis. Aquat. Org. 2008, 81, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Bittner, D.; Cossins, A.R.; Segner, H.; Excoffier, L.; Largiader, C.R. Identification of candidate genese and physiologcial pathways involved in gonad deformation in whitefish (Coregonus spp.) from Lake Thun, Switzerland. Int. J. Environ. Res. Public Health 2011, 8, 2706–2733. [Google Scholar] [CrossRef] [PubMed]
- Börjeson, H.; Norrgren, L. M74 syndrome: A review of potential etiological factorsChemically Induced Alterations in Functional Development and Reproduction of Fishes. In Proceedings of the 1995 Wingspread Conference, Wingspread, USA, 1976; Rolland, R.M., Gilbertson, M., Peterson, R.E., Eds.; SETAC Press: Pensacola, FL, USA, 1997; pp. 153–166. [Google Scholar]
- Fitzsimons, J.D.; Brown, S.B.; Honeyfield, D.C.; Hanth, J.G. A review of early mortality syndrome (EMS) in great lakes salmonids: Relationship with thiamine deficiency. Ambio 1999, 28, 9–15. [Google Scholar]
- Riley, S.C.; Evans, A.N. Phylogenetic and ecological characteristics associated with thiaminase activity in Laurentian Great Lakes fishes. Trans. Amer. Fish. Soc. 2008, 137, 147–157. [Google Scholar] [CrossRef]
- Eckmann, R.; Rösch, R.; Ortlepp, J.; Kleifeld, G. Survival and growth of coregonid larvae from Lake Constance fed on zooplankton of different origin. Arch. Hydrobiol. 1986, 22, 203–214. [Google Scholar]
- Eckmann, R. Histopathological alterations in the intestine of whitefish (Coregonus sp.) larvae reared on zooplankton from Lake Constance. Dis. Aquat. Org. 1985, 1, 11–17. [Google Scholar] [CrossRef]
- Eckmann, R. Pathological changes in the midgut epithelium of grayling, Thymallus thymallus L., larvae reared on different kinds of food, and their relation to mortality and growth. J. Fish Diseas. 1987, 10, 91–99. [Google Scholar] [CrossRef]
- Guthruf, K.; Maurer, V.; Pokorni, B.; Zeh, M. Entwicklung des Phyto- und Crustaceenplanktons in Brienzer-, Thuner, Murten-, Neuenburger-und Bielersee. Amt für Wasser und Abfall, Gewässer- und Bodenschutzlabor GBL, Bern, Service de l’Environnement, Fribourg, Service de la Protection de l’Environment Neuchâtel; Kanton Bern, Cantone Fribourg: Bern, Fribourg, Switzerland, 2009; p. 112. [Google Scholar]
- Guthruf, K.; Maurer, V.; Zeh, M. Entwicklung des Phyto- und Crustaceenplanktons in Brienzer-, Thuner, Bieler-, Neuenburger- und Murtensee. Amt für Wasser und Abfall, Gewässer- und Bodenschutzlabor GBL, Bern; Service de l’Environnement, Fribourg; Service de la Protection de l’Environnement: Neuchâtel, Switzerland, 2019; p. 87. [Google Scholar]
- Rufli, H. Ernährung und Wachstum der Felchenpopulationen (Coregonus spp.) des Thuner-und Bielersees. Schweiz. Zr Hydrol. 1979, 41, 64–93. [Google Scholar] [CrossRef]
- Rodger, H.D.; Turnbull, T.; Edwards, C.; Coda, G.A. Cyanobacterial (blue-green algal) bloom associated pathology in brown trout, Salmo trutta, in Lake Lever, Scotland. J. Fish Dis. 1994, 17, 177–181. [Google Scholar] [CrossRef]
- Ernst, B.; Hoeger, S.J.; O’Brien, E.; Dietrich, D.R. Physiological stress and pathology in European whitefish (Coregonus lavaretus) induced by subchronic exposure to environmentally relevant densities of Planktothrix rubescens. Aquat. Toxicol. 2007, 82, 15–26. [Google Scholar] [CrossRef]
- Fischer, W.J.; Dietrich, D.R. Pathological and biochemical characterization of micocystin-indcued hepatopancreas and kidney damage in carp (Cyprinus carpio). Toxicol. Appl. Pharmacol 2000, 164, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Andersson, T.; Förlin, L.; Härdig, J.; Larsson, A. Physiological disturbances in fish living in coastal water polluted with bleached kraft pulp mill effluents. Can. J. Fish. Aquat. Sci. 1988, 45, 1525–1536. [Google Scholar] [CrossRef]
- Lye, C.M.; Frid, C.L.J.; Gill, M.E.; McCormick, D. Abnormalities in the reproductive health of flounder Paralichthys flesus exposed to effluent from a sewage treatment works. Mar. Poll. Bull. 1997, 34, 34–41. [Google Scholar] [CrossRef]
- Munkittrick, K.R.; McMaster, M.E.M.; McCarthy, L.H.; Servos, M.R.; van der Kraak, G.L. An overview of recent studies on the potential of pulp mill effluents to alter reproductive parameters in fish. J. Toxicol. Environ. Health B 1998, 1, 347–371. [Google Scholar] [CrossRef]
- Liedtke, A.; Schönenberger, R.; Eggen, R.I.L.; Suter, M.J.-F. Internal exposure of whitefish (Coregonus lavaretus) to estrogens. Aquat. Toxicol. 2009, 93, 158–165. [Google Scholar] [CrossRef]
- Bernet, D.; Liedtke, A.; Bittner, D.; Eggen, R.I.L.; Kipfer, S.; Küng, C.; Largiader, C.; Suter, M.J.-F.; Wahli, T.; Segner, H. Gonadal Deformations in Whitefish from Lake Thun: Defining the Case and Evaluating the Role of EDCs. Chimia 2008, 62, 383–388. [Google Scholar] [CrossRef]
- Kipfer, S.; Segner, H.; Wenger, M.; Wahli, T.; Bernet, D. Long term laboratory estrogen (E2) exposure of whitefish (central alpine Coregonus lavaretus) induces intersex but not the Lake Thun-typical gonad deformations. Dis. Aquat. Org. 2009, 84, 43–56. [Google Scholar] [CrossRef]
- Bogdal, C.; Naef, M.; Schmid, P.; Kohler, M.; Zennegg, M.; Bernet, D.; Scheringer, M.; Hungerbühler, K. Unexplained gonad alterations in whitefish (Coregonus spp.) from Lake Thun, Switzerland: Levels of Persistent Organic Pollutants in Different Morphs. Chemosphere 2009, 74, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Berset, J.D.; Ochsenbein, U.; Zeh, M. Are Lake Thun and Lake Brienz contaminated with explosive residues? Chimia 2007, 61, 532. [Google Scholar]
- Ochsenbein, U.; Zeh, M.; Berset, J.D. Comparing solid phase extraction and direct injection for the analysis of ultra-trace levels of relevant explosives in lake water and tributaries using liquid chromatography–electrospray tandem mass spectrometry. Chemosphere 2008, 72, 974–980. [Google Scholar] [CrossRef]
- Brem, D.; Gälli, R. Beurteilung des Einsatzes von Bauchemikalien beim Bau der NEAT am Lötschberg–Stoffflussanalyse und grobe Abschätzung der ökologischen Risiken Short report for the Authority of Water Protection (Amt für Gewässerschutz und Abfallwirtschaft des Kantons Bern, Gewässer- & Bodenschutzlabor. 2005. Available online: http://www.bve.be.ch/site/bve_gsa_gwq_seen_allgInfo_NEAT_Bauchemikalien_Kurzbericht.pdf (accessed on 24 October 2019).
- Bernet, D.; Wahli, T.; Gerecke, A.; Segner, H. Exposure of whitefish (Coregonus lavaretus) eggs to native or chemically spiked sediments from Lake Thun does not lead to abnormal gonad development. Environ.Biotechnol. 2011, 7, 17–29. [Google Scholar]
- Bernet, D.; Wahli, T.; Kipfer, S.; Segner, H. Macroscopic gonadal deviations and intersex in developing whitefish. Coregonus Lavaretus. Aquat. Biol. 2009, 6, 1–13. [Google Scholar] [CrossRef]
- Steinmann, P. Monographie der Schweizer Koregonen. Schweiz Zr Hydrol 1950, 12, 340–491. [Google Scholar]
- Belk, M.C.; Benson, L.J.; Rasmussen, J.; Peck, S.L. Hatchery-induced morphological variation in an endangered fish: A challenge for hatchery-based recovery efforts. Can. J. Fish. Aquat. Sci. 2008, 65, 401–408. [Google Scholar] [CrossRef]
- Thrushfield, M.; Ortega, C.; de Blas, I.; Noordhuizen, J.P.; Frankena, K. WIN EPISCOPE 2.0: Improved epidemiological software for veterinary medicine. Vet. Rec. 2001, 148, 567–572. [Google Scholar] [CrossRef]
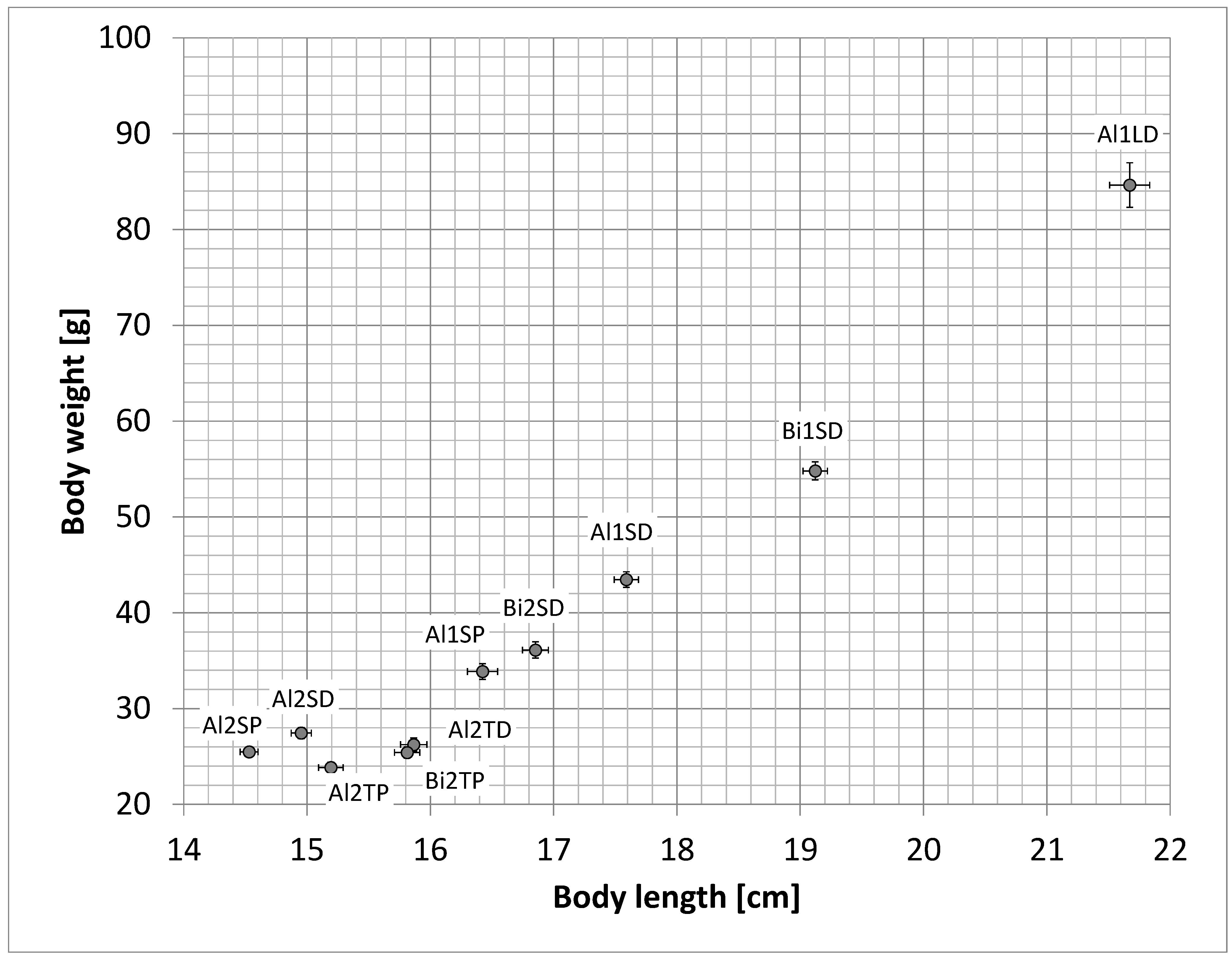
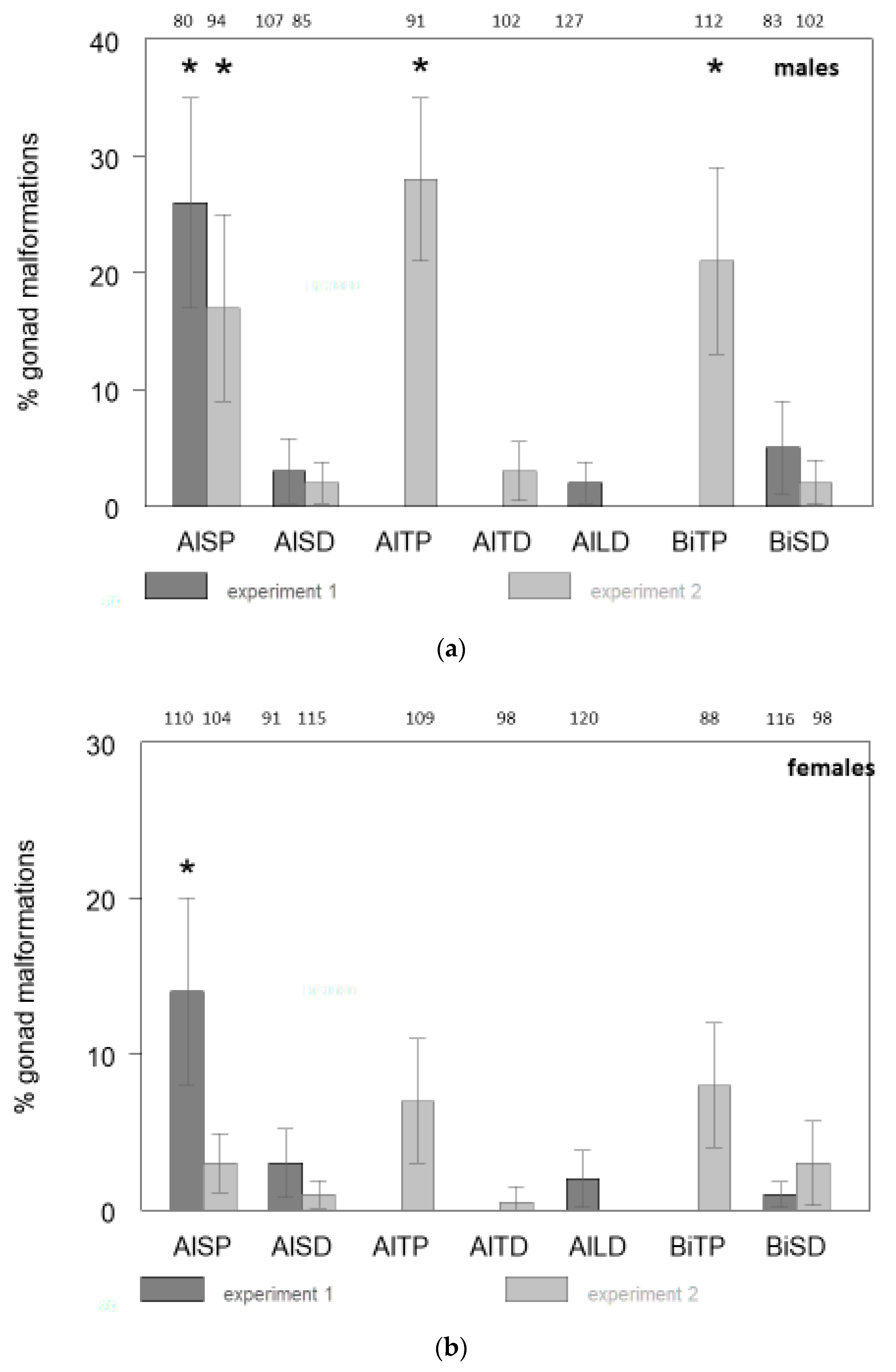
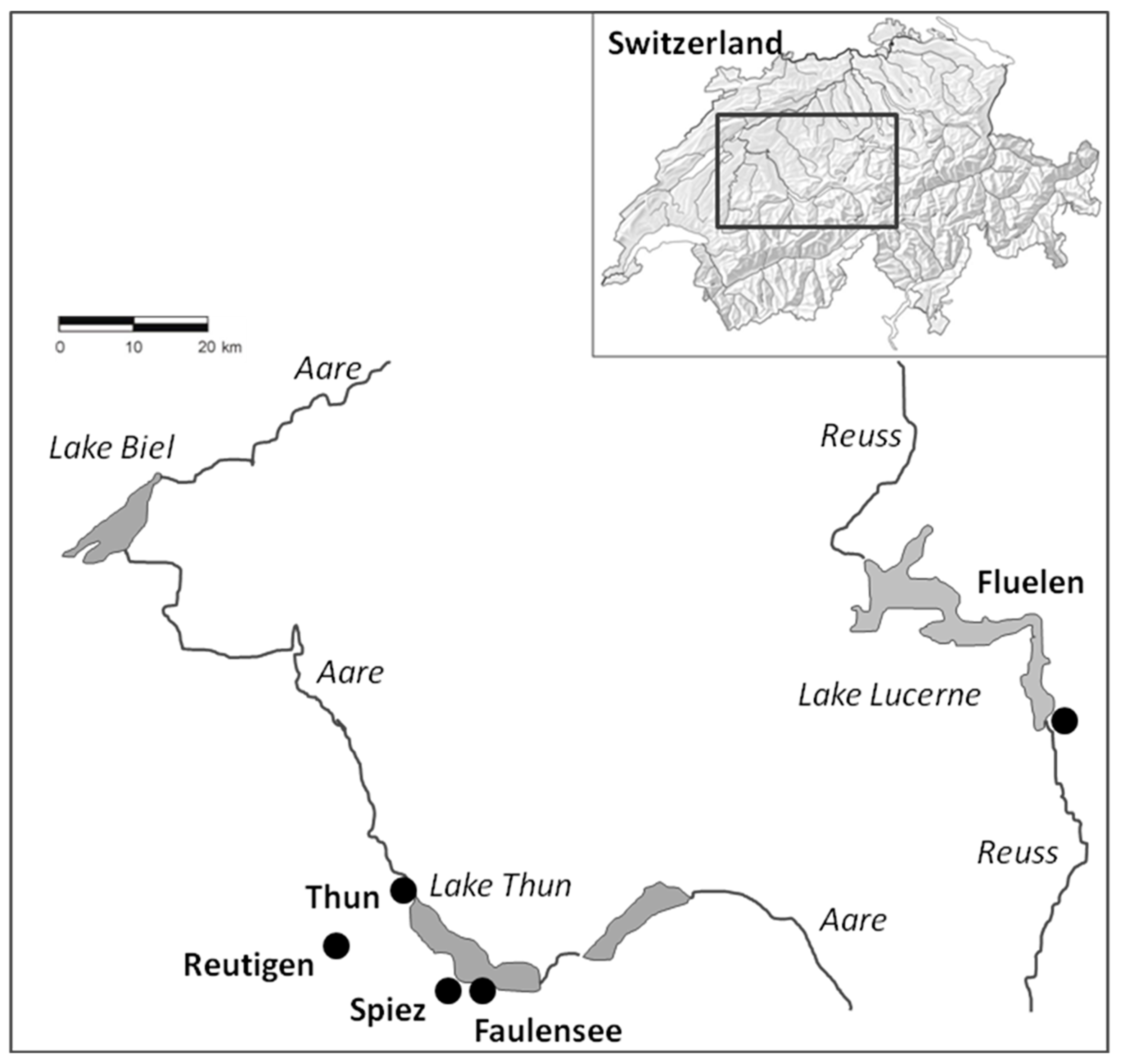
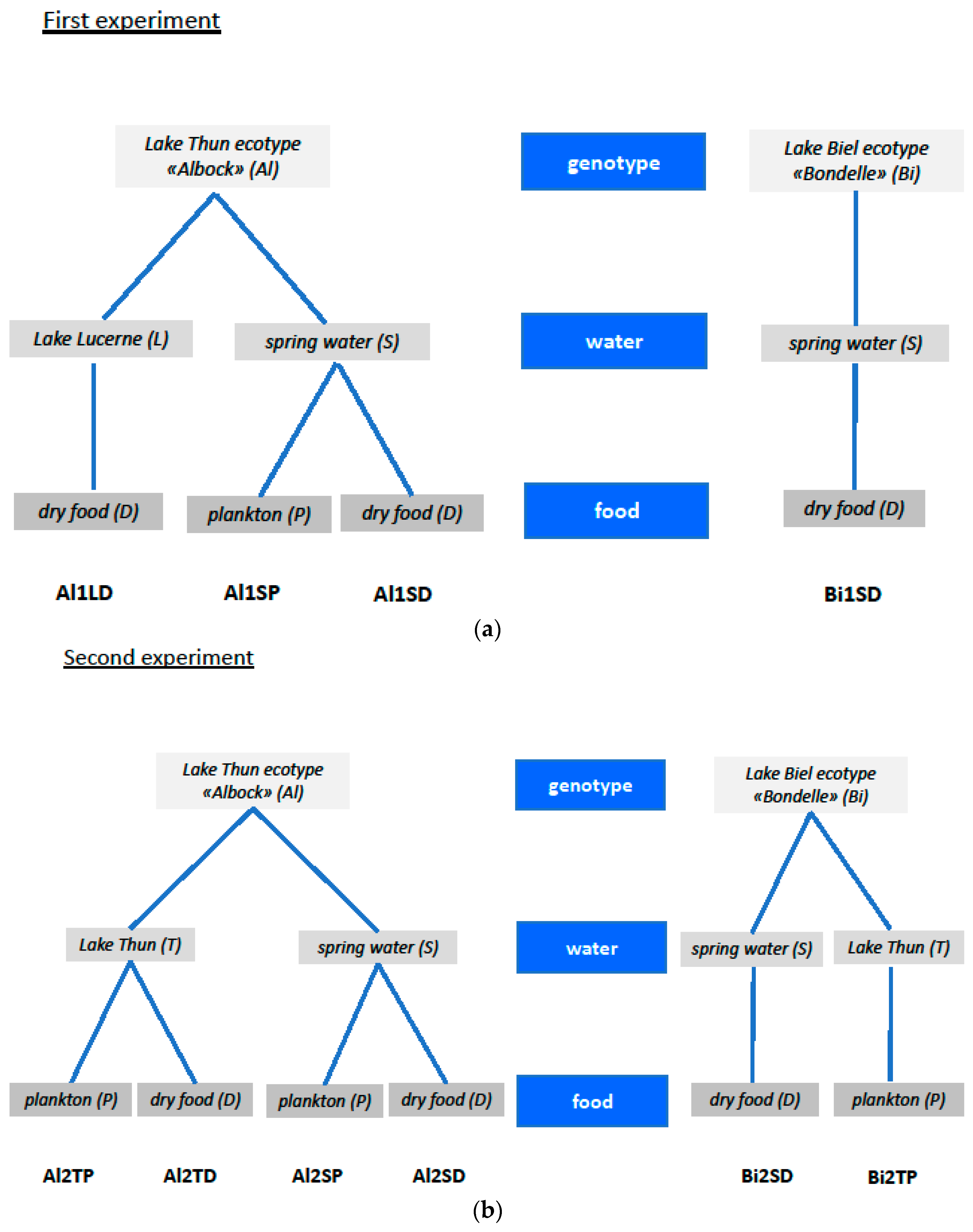
| Abbreviations Used for Treatment Groups | Description of the Treatment Group |
|---|---|
| 1st experiment | |
| Al1LD | Whitefish of the ecotype “Albock” from Lake Thun, fertilized and reared in Lake Lucerne water, and fed with artificial dry feed (Al = Albock, L = Lake Lucerne water, D = dry food). |
| Al1SP | Whitefish of the ecotype “Albock” from Lake Thun, fertilized and reared in spring water, and fed with zooplankton from Lake Thun (Al = Albock, S = spring water, P = zooplankton). |
| Al1SD | Whitefish of the ecotype “Albock” from Lake Thun, fertilized and reared in spring water, and fed with artificial dry feed (Al = Albock, S = spring water, D = dry food). |
| Bi1SD | Whitefish of the ecotype “Bondelle” from Lake Biel, fertilized and reared in spring water, and fed with artificial dry feed (Bi = Lake Biel whitefish, S = spring water, D = dry food). |
| 2nd experiment | |
| Al2TP | Whitefish of the ecotype “Albock” from Lake Thun, fertilized and reared in Lake Thun water, and fed with zooplankton from Lake Thun (Al = Albock, T = Lake Thun water, P = zooplankton). |
| Al2TD | Whitefish of the ecotype “Albock” from Lake Thun, fertilized and reared in Lake Thun water, and fed with artificial dry feed (Al = Albock, T = Lake Thun water, D = dry food). |
| Al2SP | Whitefish of the ecotype “Albock” from Lake Thun, fertilized and reared in spring water, and fed with zooplankton from Lake Thun (Al = Albock, S = spring water, P = zooplankton). This is a repetition of Al1SP. |
| Al2SD | Whitefish of the ecotype “Albock” from Lake Thun, fertilized and reared in spring water, and fed with artificial dry feed (Al = Albock, S = spring water, D = dry food). This is a repetition of Al1SD. |
| Bi2TP | Whitefish ecotype “Bondelle” from Lake Biel, fertilized and reared in Lake Thun water, and fed with zooplankton from Lake Thun (Bi = Lake Biel whitefish, T = Lake Thun water, P = zooplankton). |
| Bi2SD | Whitefish of the ecotype “Bondelle” from Lake Biel, fertilized and reared in spring water, and fed with artificial dry feed (Bi = Lake Biel whitefish, S = spring water, D = dry food). This is a repetition of Bi1SD. |
| Group | Origin of Parental Fish | N of Parental Fish | f of Gonad Deformations in Parental Fish | Hatchery | Water for Fertilization | Fish Farm | Water Source | Feed | N Fish | Age of Fish [dph] | Age of Fish [d°C] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al1LD | Lake Thun | 126 | 6 | Fluelen | Lake Lucerne | Fluelen | Lake Lucerne | Dry feed | 247 | 982 | 10,747 |
| Al1SP | Lake Thun | 126 | 6 | Spiez | Spring water | Reutigen | Spring water | Plankton | 200 | 974 | 8296 |
| Al1SD | Lake Thun | 126 | 6 | Spiez | Spring water | Reutigen | Spring water | Dry feed | 200 | 974 | 8296 |
| Bi1SD | Lake Biel | 89 | 1 | Spiez | Spring water | Reutigen | Spring water | Dry feed | 200 | 987 | 8407 |
| Al2TP | Lake Thun | 52 | 15 | Faulensee | Lake Thun | Thun | Lake Thun | Plankton | 200 | 964 | 10,324 |
| Al2TD | Lake Thun | 52 | 15 | Faulensee | Lake Thun | Thun | Lake Thun | Dry feed | 200 | 964 | 10,324 |
| Al2SP | Lake Thun | 52 | 15 | Reutigen | Spring water | Reutigen | Spring water | Plankton | 200 | 1002 | 8526 |
| Al2SD | Lake Thun | 52 | 15 | Reutigen | Spring water | Reutigen | Spring water | Dry feed | 200 | 1002 | 8526 |
| Bi2SD | Lake Biel | 70 | 0 | Reutigen | Spring water | Reutigen | Spring water | Dry feed | 200 | 1020 | 8687 |
| Bi2TP | Lake Biel | 70 | 0 | Faulensee | Lake Thun | Thun | Lake Thun | Plankton | 200 | 973 | 10,389 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernet, D.; Wahli, T.; Küng, C.; Segner, H. Zooplankton Feeding Induces Macroscopical Gonad Malformations in Whitefish (Coregonus ssp.) from Lake Thun, Switzerland. Fishes 2020, 5, 26. https://doi.org/10.3390/fishes5030026
Bernet D, Wahli T, Küng C, Segner H. Zooplankton Feeding Induces Macroscopical Gonad Malformations in Whitefish (Coregonus ssp.) from Lake Thun, Switzerland. Fishes. 2020; 5(3):26. https://doi.org/10.3390/fishes5030026
Chicago/Turabian StyleBernet, Daniel, Thomas Wahli, Christoph Küng, and Helmut Segner. 2020. "Zooplankton Feeding Induces Macroscopical Gonad Malformations in Whitefish (Coregonus ssp.) from Lake Thun, Switzerland" Fishes 5, no. 3: 26. https://doi.org/10.3390/fishes5030026
APA StyleBernet, D., Wahli, T., Küng, C., & Segner, H. (2020). Zooplankton Feeding Induces Macroscopical Gonad Malformations in Whitefish (Coregonus ssp.) from Lake Thun, Switzerland. Fishes, 5(3), 26. https://doi.org/10.3390/fishes5030026






