Study of Fishmeal Substitution on Growth Performance and Shelf-Life of Giltheadsea Bream (Sparusaurata)
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Trial
2.2. Diets and Experimental Design
2.3. Fish and Feeding
2.4. Shelf-Life Study
2.4.1. Sample Preparation
2.4.2. Chromogenic Array Preparation
- Preparation of probe 1, dye: thymol blue (40 mg), solvent: ethanol (8 mL), support: aluminum oxide.
- Preparation of probe 2, dye: bromothymol blue sodium salt (40 mg), solvent: ethanol (7 mL), support: silica gel.
- Preparation of probe 3, dye: bromocresol green (40 mg), solvent: ethanol (7 mL), support: silica gel.
- Preparation of probe 4, dye: dinuclear rhodium complex (40 mg), solvent: dichloromethane (6 mL), support: silica gel.
- Preparation of probe 5, dye: dinuclear rhodium complex (80 mg), solvent: dichloromethane (6 mL), support: silica gel.
- Preparation of probe 6, dye: dinuclear rhodium complex (140 mg), solvent: dichloromethane (6 mL), support: silica gel.
2.4.3. Analytical Determinations
2.5. Digital Imaging and Data Collection
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of Fishmeal on Fish Growth
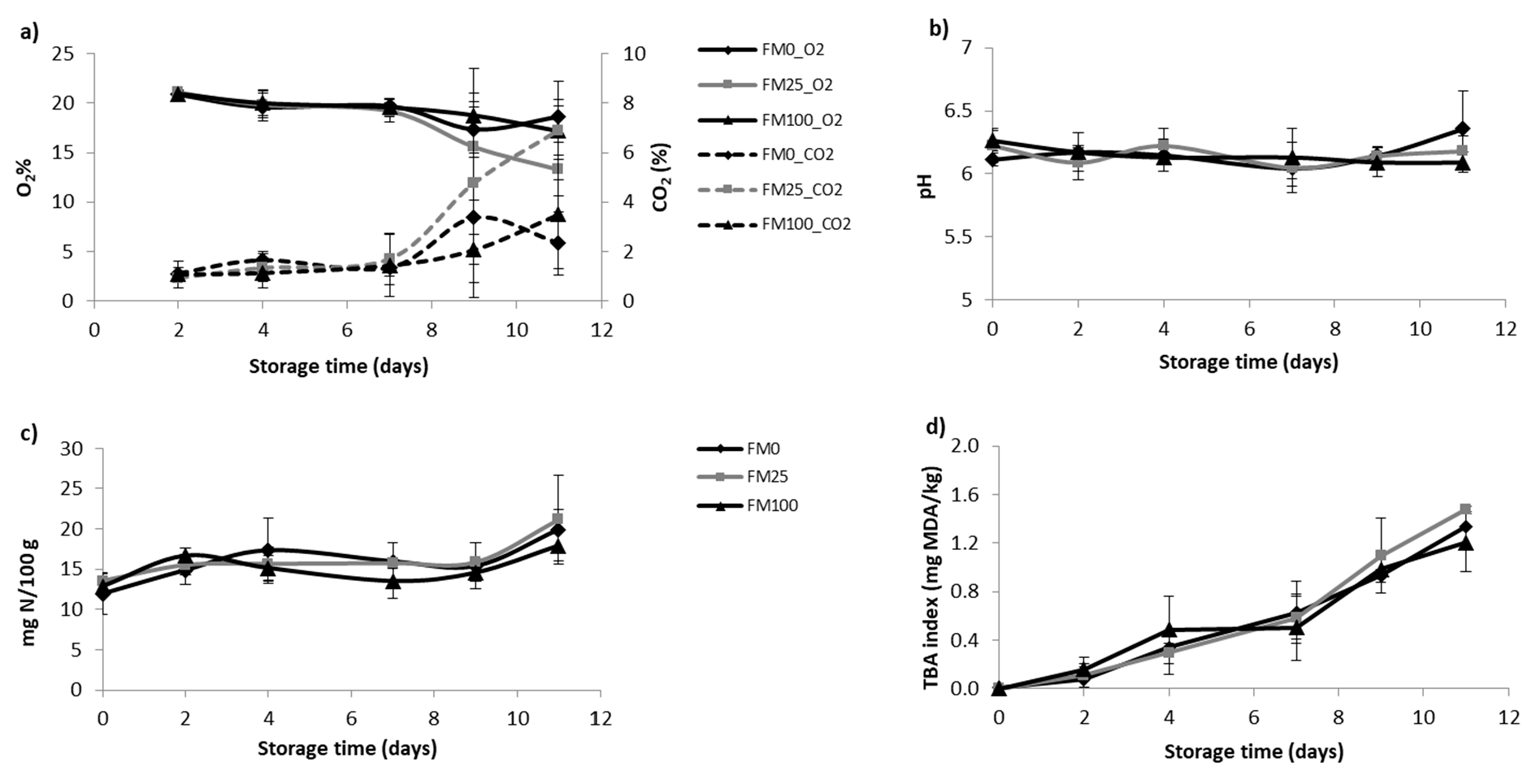
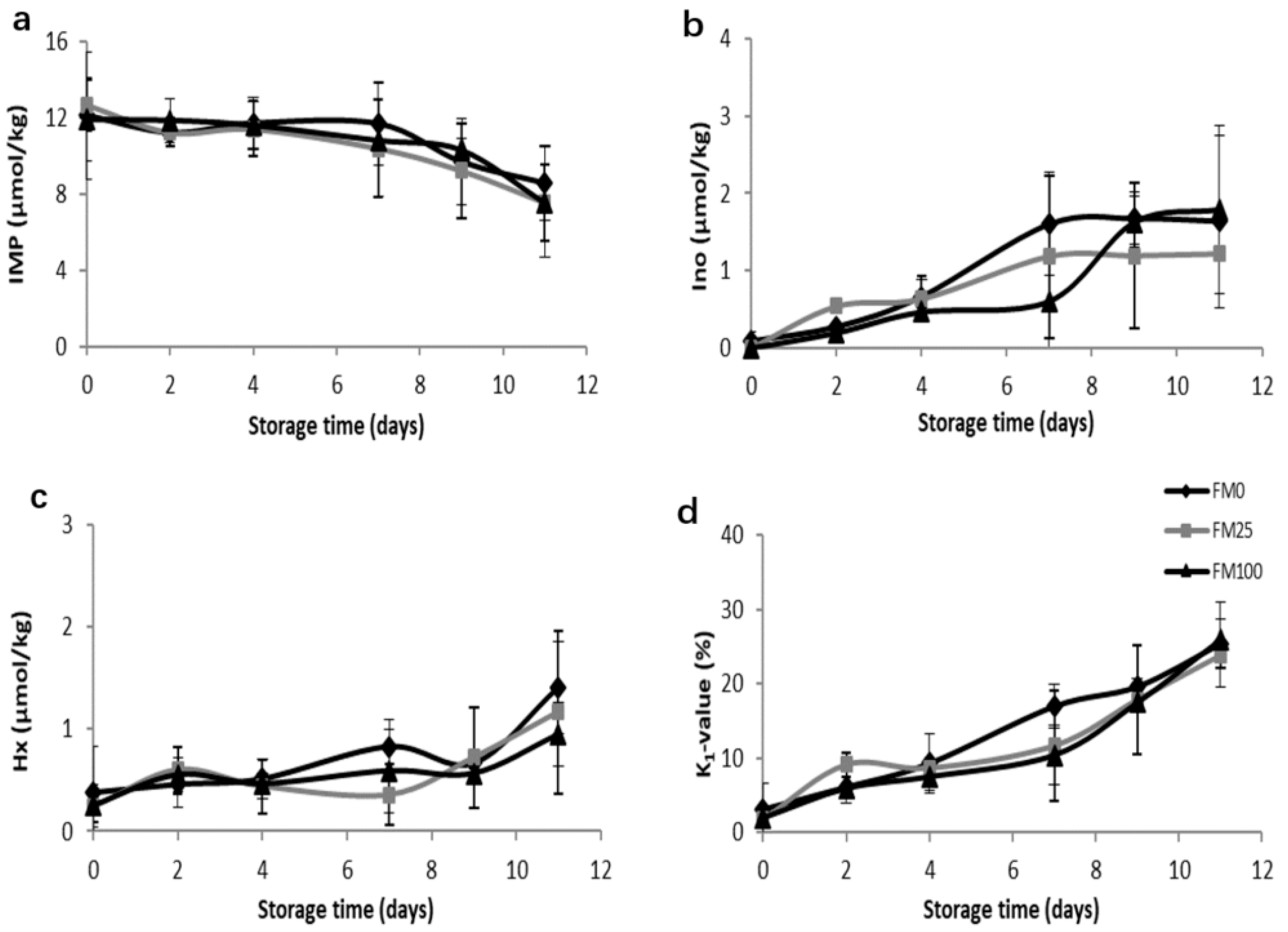
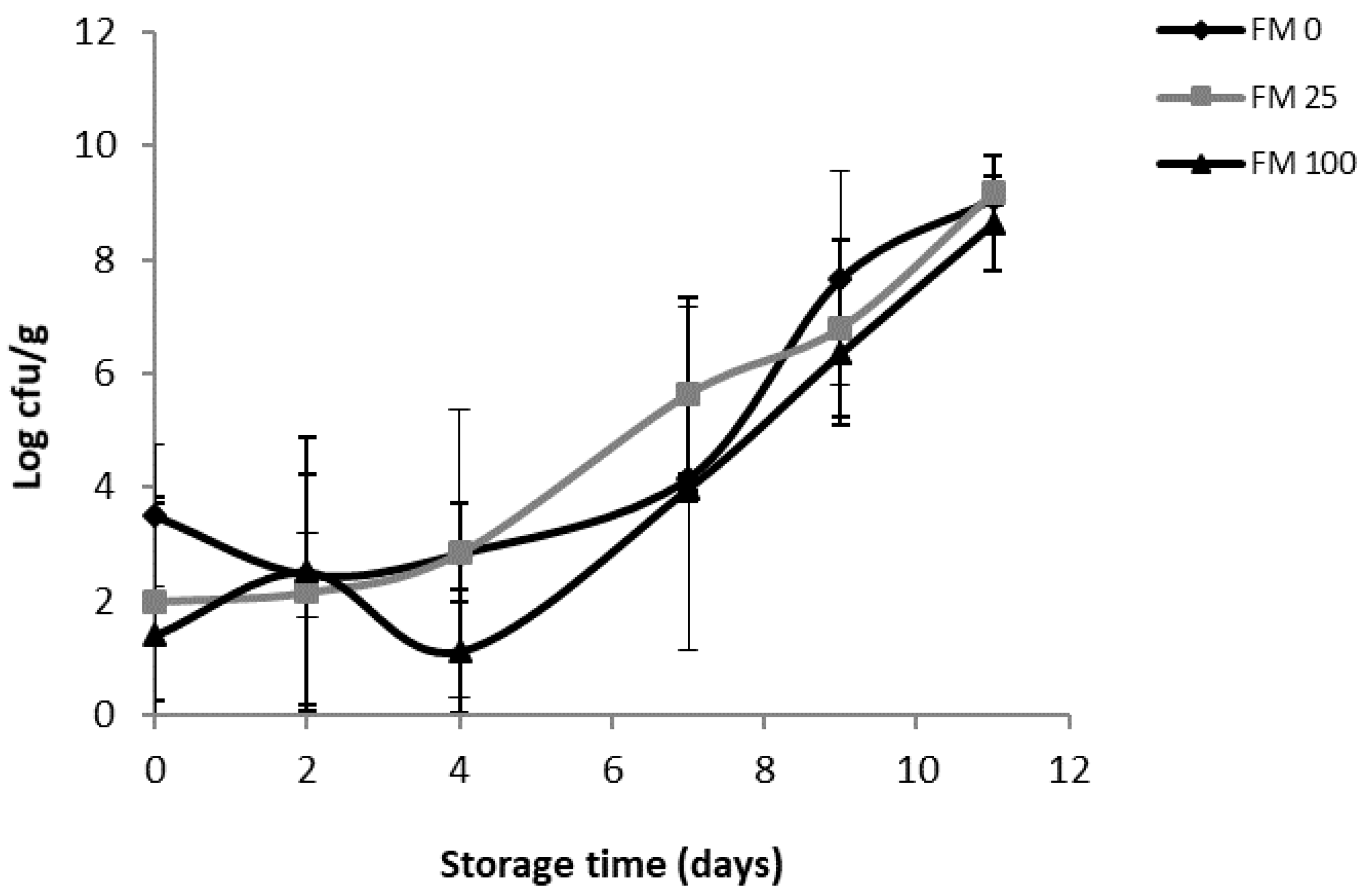
3.2. Shelf-Life Studies
3.2.1. Physicochemical Study
3.2.2. Microbial Analyses
3.2.3. Study of the Effect of Diets, Storage Time and Their Interaction on the Physicochemical and Microbial Parameters
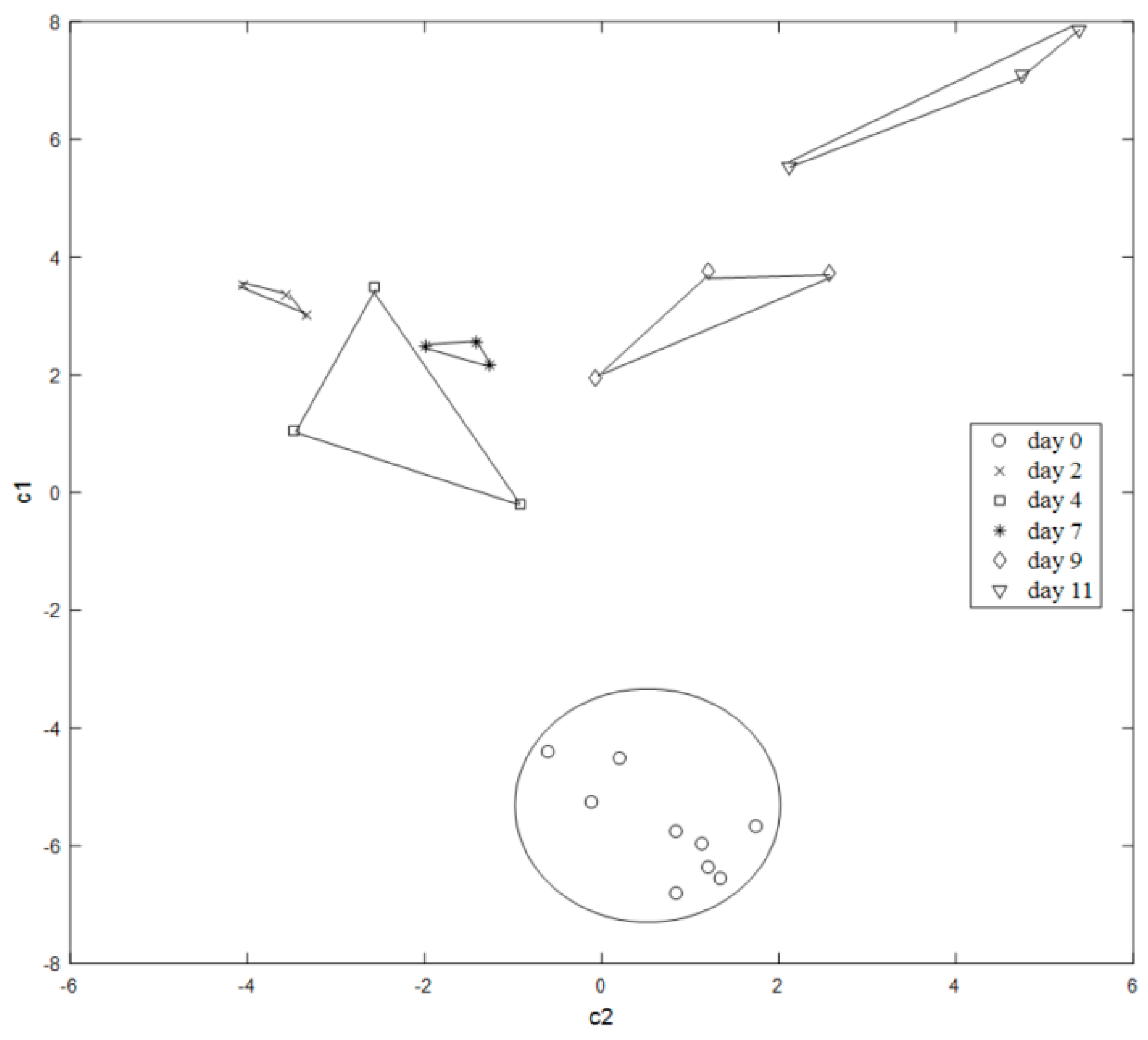
3.2.4. Changes in the Colorimetric Array
4. Conclusions
Author Contributions
Compliance with Ethical Standards
Funding
Acknowledgments
Conflicts of Interest
References
- Grigorakis, K. Compositional and organoleptic quality of farmed and wild gilthead sea bream (Sparusaurata) and sea bass (Dicentrarchuslabrax) and factors affecting it: A review. Aquaculture 2007, 272, 55–75. [Google Scholar] [CrossRef]
- Fezzardi, D.; Massa, F.; Àvila-Zaragoza, P.; Rad, F.; Yücel-Gier, G.; Deniz, H.; Salem, M.H.A.; Hamza, H.A.; Salem, S. Ben Indicators for sustainable aquaculture in Mediterranean and Black Sea countries: Guide for the use of indicators to monitor sustainable development of aquaculture. Gen. Fish. Comm. Mediterr. Stud. Rev. 2013, 93, 1–60. [Google Scholar]
- Martínez-Llorens, S.; Moñino, A.V.; Vidal, A.T.; Salvador, V.J.M.; Pla Torres, M.; JoverCerdá, M. Soybean meal as a protein source in gilthead sea bream (Sparusaurata L.) diets: Effects on growth and nutrient utilization. Aquac. Res. 2007, 38, 82–90. [Google Scholar] [CrossRef]
- Sánchez Lozano, N.B.; Tomás Vidal, A.; Martínez-Llorens, S.; Nogales Mérida, S.; Blanco, J.E.; Moñino López, A.; Pla Torres, M.; Cerdá, M.J. Growth and economic profit of gilthead sea bream (Sparusaurata, L.) fed sunflower meal. Aquaculture 2007, 272, 528–534. [Google Scholar] [CrossRef]
- Martínez-Llorens, S.; Baeza-Ariño, R.; Nogales-Mérida, S.; Jover-Cerdá, M.; Tomás-Vidal, A. Carob seed germ meal as a partial substitute in gilthead sea bream (Sparusaurata) diets: Amino acid retention, digestibility, gut and liver histology. Aquaculture 2012, 338, 124–133. [Google Scholar] [CrossRef]
- Sánchez-Lozano, N.B.; Martínez-Llorens, S.; Tomás-Vidal, A.; Cerdá, M.J. Effect of high-level fish meal replacement by pea and rice concentrate protein on growth, nutrient utilization and fillet quality in gilthead seabream (Sparusaurata, L.). Aquaculture 2009, 298, 83–89. [Google Scholar] [CrossRef]
- Estruch, G.; Tomás-Vidal, A.; El Nokrashy, A.M.; Monge-Ortiz, R.; Godoy-Olmos, S.; JoverCerdá, M.; Martínez-Llorens, S. Inclusion of alternative marine by-products in aquafeeds with different levels of plant-based sources for on-growing gilthead sea bream (Sparusaurata, L.): Effects on digestibility, amino acid retention, ammonia excretion and enzyme activity. Arch. Anim. Nutr. 2018, 72, 1–19. [Google Scholar] [CrossRef]
- Monge-Ortiz, R.; Llorens, S.M.; Márquez, L.; Moyano, F.J.; Jover-Cerdá, M.; Vidal, A.T. Potential use of high levels of vegetal proteins in diets for market-sized gilthead sea bream (Sparusaurata). Arch. Anim. Nutr. 2016, 70, 155–172. [Google Scholar] [CrossRef]
- Baeza-Ariño, R.; Martínez-Llorens, S.; Nogales-Mérida, S.; Jover-Cerda, M.; Tomás-Vidal, A. Study of liver and gut alterations in sea bream, Sparusaurata L., fed a mixture of vegetable protein concentrates. Aquac. Res. 2016, 47, 460–471. [Google Scholar] [CrossRef]
- Sitjá-Bobadilla, A.; Peña-Llopis, S.; Gómez-Requeni, P.; Médale, F.; Kaushik, S.; Pérez-Sánchez, J. Effect of fish meal replacement by plant protein sources on non-specific defence mechanisms and oxidative stress in gilthead sea bream (Sparusaurata). Aquaculture 2005, 249, 387–400. [Google Scholar] [CrossRef]
- Estruch, G.; Collado, M.C.; Monge-Ortiz, R.; Tomás-Vidal, A.; Jover-Cerdá, M.; Peñaranda, D.S.; Pérez Martínez, G.; Martínez-Llorens, S. Long-term feeding with high plant protein based diets in gilthead seabream (Sparusaurata, L.) leads to changes in the inflammatory and immune related gene expression at intestinal level. BMC Vet. Res. 2018, 14, 302. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, M.; Parisi, G.; Pérez-Sanchez, J.; Gomez-Réqueni, P.; Médale, F.; Kaushik, S.J.; Mecatti, M.; Poli, B.M. Effect of high-level fish meal replacement by plant proteins in gilthead sea bream (Sparusaurata) on growth and body/fillet quality traits. Aquac. Nutr. 2007, 13, 361–372. [Google Scholar] [CrossRef]
- Hernández, M.D.; López, M.B.; Álvarez, A.; Ferrandini, E.; García, B.G.; Garrido, M.D. Sensory, physical, chemical and microbiological changes in aquacultured meagre (Argyrosomusregius) fillets during ice storage. Food Chem. 2009, 114, 237–245. [Google Scholar] [CrossRef]
- Matos, E.; Silva, T.S.; Colen, R.; Dinis, M.T.; Dias, J. Plant protein and vegetable oil-based diets modulate gilthead seabream (Sparusaurata) muscle biochemical status and proteolytic enzymes at an early postmortem stage. Aquac. Nutr. 2014, 20, 153–162. [Google Scholar] [CrossRef]
- Álvarez Trujillo, A.M. Vida útil de la Dorada Almacenada en Refrigeración con Hielo: Influencia de Distintos Factores del Cultivo. Ph.D. Thesis, Universidad de Murcia, Murcia, Spain, 2012. [Google Scholar]
- Goulas, A.E.; Kontominas, M.G. Effect of salting and smoking-method on the keeping quality of chub mackerel (Scomberjaponicus): Biochemical and sensory attributes. Food Chem. 2005, 93, 511–520. [Google Scholar] [CrossRef]
- Olafsdottir, G.; Nesvadba, P.; Di Natale, C.; Careche, M.; Oehlenschläger, J.; Tryggvadottir, S.V.; Schubring, R.; Kroeger, M.; Heia, K.; Esaiassen, M. Multisensor for fish quality determination. Trends Food Sci. Technol. 2004, 15, 86–93. [Google Scholar] [CrossRef]
- Zaragozá, P.; Fuentes, A.; Fernández-Segovia, I.; Vivancos, J.-L.; Rizo, A.; Ros-Lis, J.V.; Barat, J.M.; Martínez-Máñez, R. Evaluation of sea bream (Sparusaurata) shelf life using an optoelectronic nose. Food Chem. 2013, 138, 1374–1380. [Google Scholar] [CrossRef]
- Gribbestad, I.S.; Aursand, M.; Martinez, I. High-resolution 1H magnetic resonance spectroscopy of whole fish, fillets and extracts of farmed Atlantic salmon (Salmo salar) for quality assessment and compositional analyses. Aquaculture 2005, 250, 445–457. [Google Scholar] [CrossRef]
- Folkestad, A.; Wold, J.P.; Rørvik, K.-A.; Tschudi, J.; Haugholt, K.H.; Kolstad, K.; Mørkøre, T. Rapid and non-invasive measurements of fat and pigment concentrations in live and slaughtered Atlantic salmon (Salmo salar L.). Aquaculture 2008, 280, 129–135. [Google Scholar] [CrossRef]
- Olsen, S.H.; Sørensen, N.K.; Larsen, R.; Elvevoll, E.O.; Nilsen, H. Impact of pre-slaughter stress on residual blood in fillet portions of farmed Atlantic cod (Gadusmorhua)—Measured chemically and by visible and near-infrared spectroscopy. Aquaculture 2008, 284, 90–97. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, C.; Zhao, J.; Ouyang, Q. Recent advances in emerging imaging techniques for non-destructive detection of food quality and safety. TrAC Trends Anal. Chem. 2013, 52, 261–274. [Google Scholar] [CrossRef]
- Salinas, Y.; Ros-Lis, J.V.; Vivancos, J.-L.; Martínez-Máñez, R.; Marcos, M.D.; Aucejo, S.; Herranz, N.; Lorente, I.; Garcia, E. A novel colorimetric sensor array for monitoring fresh pork sausages spoilage. Food Control 2014, 35, 166–176. [Google Scholar] [CrossRef]
- Salinas, Y.; Ros-Lis, J.V.; Vivancos, J.-L.; Martínez-Máñez, R.; Marcos, M.D.; Aucejo, S.; Herranz, N.; Lorente, I. Monitoring of chicken meat freshness by means of a colorimetric sensor array. Analyst 2012, 137, 3635–3643. [Google Scholar] [CrossRef]
- Peres, H.; Oliva-Teles, A. The optimum dietary essential amino acid profile for 560 gilthead seabream (Sparusaurata) juveniles. Aquaculture 2009, 296, 81–86. [Google Scholar] [CrossRef]
- Martínez-Llorens, S.; Vidal, A.T.; Moñino, A.V.; Torres, M.P.; JoverCerdá, M. Effects of dietary soybean oil concentration on growth, nutrient utilization and muscle fatty acid composition of gilthead sea bream (Sparusaurata L.). Aquac. Res. 2007, 38, 76–81. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Analytical Chemists International; Association of Official Analytical Chemists: Arlington, VA, USA, 2005. [Google Scholar]
- Moragues, M.E.; Esteban, J.; Ros-Lis, J.V.; Martínez-Máñez, R.; Marcos, M.D.; Martínez, M.; Soto, J.; Sancenón, F. Sensitive and selective chromogenic sensing of carbon monoxide via reversible axial CO coordination in binuclear rhodium complexes. J. Am. Chem. Soc. 2011, 133, 15762–15772. [Google Scholar] [CrossRef]
- Zaragozá, P.; Fernández-Segovia, I.; Fuentes, A.; Vivancos, J.-L.; Ros-Lis, J.V.; Barat, J.M.; Martínez-Máñez, R. Monitorization of Atlantic salmon (Salmo salar) spoilage using an optoelectronic nose. Sens. Actuators B Chem. 2014, 195, 478–485. [Google Scholar] [CrossRef]
- Vyncke, W. Direct determination of the thiobarbituric acid value in trichloracetic acid extracts of fish as a measure of oxidative rancidity. Fette Seifen Anstrichm. 1970, 72, 1084–1087. [Google Scholar] [CrossRef]
- Barat, J.M.; Gil, L.; García-Breijo, E.; Aristoy, M.-C.; Toldrá, F.; Martínez-Máñez, R.; Soto, J. Freshness monitoring of sea bream (Sparusaurata) with a potentiometric sensor. Food Chem. 2008, 108, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Segovia, I.; Fuentes, A.; Aliño, M.; Masot, R.; Alcañiz, M.; Barat, J.M. Detection of frozen-thawed salmon (Salmo salar) by a rapid low-cost method. J. Food Eng. 2012, 113, 210–216. [Google Scholar] [CrossRef]
- ISO. ISO 4833:2003—Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Microorganisms—Colony-Count Technique at 30 Degrees C. Available online: https://www.iso.org/standard/34524.html (accessed on 26 March 2020).
- Kaushik, S.J.; Coves, D.; Dutto, G.; Blanc, D. Almost total replacement of fish meal by plant protein sources in the diet of a marine teleost, the European seabass, Dicentrarchuslabrax. Aquaculture 2004, 230, 391–404. [Google Scholar] [CrossRef]
- Grigorakis, K.; Taylor, K.D.A.; Alexis, M.N. Seasonal patterns of spoilage of ice-stored cultured gilthead sea bream (Sparusaurata). Food Chem. 2003, 81, 263–268. [Google Scholar] [CrossRef]
- Bagni, M.; Civitareale, C.; Priori, A.; Ballerini, A.; Finoia, M.; Brambilla, G.; Marino, G. Pre-slaughter crowding stress and killing procedures affecting quality and welfare in sea bass (Dicentrarchuslabrax) and sea bream (Sparusaurata). Aquaculture 2007, 263, 52–60. [Google Scholar] [CrossRef]
- Fuentes, A.; Masot, R.; Fernández-Segovia, I.; Ruiz-Rico, M.; Alcañiz, M.; Barat, J.M. Differentiation between fresh and frozen-thawed sea bream (Sparusaurata) using impedance spectroscopy techniques. Innov. Food Sci. Emerg. Technol. 2013, 19, 210–217. [Google Scholar] [CrossRef]
- Dalgaard, P. Freshness, Quality and Safety in Seafoods F-FE 380A/00 [mAY 2000]; Danish Institute for Fisheries Research: Silkeborg, Denmark, 2000; ISBN 1841701106. [Google Scholar]
- Connell, J.J. Method of assessing and selecting for quality. In Controlfish Quality, 4th ed.; Fishing News Books Ltd: Surrey, UK, 1975; pp. 157, 159–160. [Google Scholar]
- Kamal-Eldin, A. Effect of fatty acids and tocopherols on the oxidative stability of vegetable oils. Eur. J. Lipid Sci. Technol. 2006, 108, 1051–1061. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef] [PubMed]
- Tyug, T.S.; Prasad, K.N.; Ismail, A. Antioxidant capacity, phenolics and isoflavones in soybean by-products. Food Chem. 2010, 123, 583–589. [Google Scholar] [CrossRef]
- López-Bote, C.J.; Diez, A.; Corraze, G.; Arzel, J.; Alvarez, M.; Dias, J.; Kaushik, S.J.; Bautista, J.M. Dietary protein source affects the susceptibility to lipid peroxidation of rainbow trout (Oncorhynchus mykiss) and sea bass (Dicentrarchuslabrax) muscle. Anim. Sci. 2001, 73, 443–449. [Google Scholar] [CrossRef]
- Hosseini, S.V.; Abedian-Kenari, A.; Rezaei, M.; Nazari, R.M.; Feás, X.; Rabbani, M. Influence of the in vivo addition of alpha-tocopheryl acetate with three lipid sources on the lipid oxidation and fatty acid composition of Beluga sturgeon, Husohuso, during frozen storage. Food Chem. 2010, 118, 341–348. [Google Scholar] [CrossRef]
- Ng, W.-K.; Wang, Y.; Ketchimenin, P.; Yuen, K.-H. Replacement of dietary fish oil with palm fatty acid distillate elevates tocopherol and tocotrienol concentrations and increases oxidative stability in the muscle of African catfish, Clariasgariepinus. Aquaculture 2004, 233, 423–437. [Google Scholar] [CrossRef]
- Regost, C.; Jakobsen, J.V.; Rørå, A.M.B. Flesh quality of raw and smoked fillets of Atlantic salmon as influenced by dietary oil sources and frozen storage. Food Res. Int. 2004, 37, 259–271. [Google Scholar] [CrossRef]
- Wang, Y.; Yuen, K.-H.; Ng, W.-K. Deposition of tocotrienols and tocopherols in the tissues of red hybrid tilapia, Oreochromis sp., fed a tocotrienol-rich fraction extracted from crude palm oil and its effect on lipid peroxidation. Aquaculture 2006, 253, 583–591. [Google Scholar] [CrossRef]
- Alasalvar, C.; Taylor, K.D.A.; Öksüz, A.; Garthwaite, T.; Alexis, M.N.; Grigorakis, K. Freshness assessment of cultured sea bream (Sparusaurata) by chemical, physical and sensory methods. Food Chem. 2001, 72, 33–40. [Google Scholar] [CrossRef]
- Surette, M.E.; Gill, T.A.; LeBlanc, P.J. Biochemical basis of postmortem nucleotide catabolism in cod (Gadusmorhua) and its relationship to spoilage. J. Agric. Food Chem. 1988, 36, 19–22. [Google Scholar] [CrossRef]
- Lougovois, V.P.; Kyranas, E.R.; Kyrana, V.R. Comparison of selected methods of assessing freshness quality and remaining storage life of iced gilthead sea bream (Sparusaurata). Food Res. Int. 2003, 36, 551–560. [Google Scholar] [CrossRef]
- Anderson, M.D.R.P.; Vicente, C.Y.P. Microbiología Alimentaria: Metodología Analítica para Alimentos y Bebidas; Diaz de Santos: Madrid, Spain, 1999; ISBN 9788479784249. [Google Scholar]
- Özyurt, G.; Kuley, E.; Özkütük, S.; Özogul, F. Sensory, microbiological and chemical assessment of the freshness of red mullet (Mullus barbatus) and goldband goatfish (Upeneusmoluccensis) during storage in ice. Food Chem. 2009, 114, 505–510. [Google Scholar] [CrossRef]
| Diets | |||
|---|---|---|---|
| Ingredients (g kg −1) | |||
| FM100 | FM25 | FM0 | |
| Fish meal herring | 589 | 150 | |
| Wheat | 260 | 60 | |
| Wheat gluten | 105 | 202 | |
| Fababean meal | 25 | 40 | |
| Soybeanmeal | 132 | 160 | |
| Krill meal | 50 | 50 | |
| Pea meal | 25 | 25 | |
| Sunflowermeal | 132 | 160 | |
| Fish oil | 65 | 78 | 90 |
| Soybean oil | 66 | 66 | 65 |
| Squid meal | 100 | 100 | |
| Mono calcium phosphate | 27 | 38 | |
| Soybean lecithin | 10 | 10 | 10 |
| Taurine | 20 | 20 | |
| Methionine a | 5 | 5 | |
| Lysine a | 5 | 10 | |
| Multivitamin and minerals mix b | 10 | 10 | 10 |
| Proximate composition (g kg−1 dry weight matter) | |||
| Dry matter DM | 881 | 902 | 928 |
| Crude protein CP | 442 | 445 | 446 |
| Crude lipid CL | 185 | 201 | 200 |
| Ash | 101 | 101 | 88 |
| Carbohydrates c | 271 | 252 | 265 |
| FM100 | FM25 | FM0 | SEM | |
|---|---|---|---|---|
| Initial weight (g) | 129.5 | 130 | 125.5 | 3.54 |
| Final weight (g) | 393.7 | 423.7 | 383 | 10.86 |
| Survival (%) | 88.33 | 88.33 | 86.66 | 5.53 |
| SGR (% day−1) a | 0.73 | 0.77 | 0.71 | 0.02 |
| Diets | Storage Time | DietsxStorage Time | ||||
|---|---|---|---|---|---|---|
| F-Ratio | F-Ratio | F-Ratio | ||||
| O2 | 1.39 | ns | 12.97 | *** | 1.14 | ns |
| CO2 | 1.97 | ns | 12.10 | *** | 1.00 | ns |
| pH | 0.04 | ns | 1.81 | ns | 1.30 | ns |
| TVB-N | 1.09 | ns | 7.85 | *** | 0.60 | ns |
| TBA index | 0.30 | ns | 77.40 | *** | 0.73 | ns |
| K1-value | 0.87 | ns | 33.26 | *** | 0.45 | ns |
| Mesophilic | 1.63 | ns | 26.12 | *** | 0.44 | ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaragozá, P.; Martínez-Llorens, S.; Fernández-Segovia, I.; Vivancos, J.-L.; Tomas-Vidal, A.; Fuentes, A.; Ros-Lis, J.V.; Martínez-Máñez, R.; Barat, J.M. Study of Fishmeal Substitution on Growth Performance and Shelf-Life of Giltheadsea Bream (Sparusaurata). Fishes 2020, 5, 15. https://doi.org/10.3390/fishes5020015
Zaragozá P, Martínez-Llorens S, Fernández-Segovia I, Vivancos J-L, Tomas-Vidal A, Fuentes A, Ros-Lis JV, Martínez-Máñez R, Barat JM. Study of Fishmeal Substitution on Growth Performance and Shelf-Life of Giltheadsea Bream (Sparusaurata). Fishes. 2020; 5(2):15. https://doi.org/10.3390/fishes5020015
Chicago/Turabian StyleZaragozá, Patricia, Silvia Martínez-Llorens, Isabel Fernández-Segovia, José-Luis Vivancos, Ana Tomas-Vidal, Ana Fuentes, José Vicente Ros-Lis, Ramón Martínez-Máñez, and José Manuel Barat. 2020. "Study of Fishmeal Substitution on Growth Performance and Shelf-Life of Giltheadsea Bream (Sparusaurata)" Fishes 5, no. 2: 15. https://doi.org/10.3390/fishes5020015
APA StyleZaragozá, P., Martínez-Llorens, S., Fernández-Segovia, I., Vivancos, J.-L., Tomas-Vidal, A., Fuentes, A., Ros-Lis, J. V., Martínez-Máñez, R., & Barat, J. M. (2020). Study of Fishmeal Substitution on Growth Performance and Shelf-Life of Giltheadsea Bream (Sparusaurata). Fishes, 5(2), 15. https://doi.org/10.3390/fishes5020015







